Clostridium butyricum Improves Rumen Fermentation and Growth Performance of Heat-Stressed Goats In Vitro and In Vivo
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diet and Treatment
2.2. Modeling of Heat-Stressed Goats
2.3. Measurement of Physiological Indices of Goats
2.4. Gene Expression Analysis Using Real-Time Quantitative PCR
2.5. Cortisol Concentration Measurement
2.6. Rumen Fermentation Experiments In Vitro
2.7. Rumen Fermentation Experiments In Vivo
2.8. Sample Analysis
2.9. Statistical Analysis
3. Results
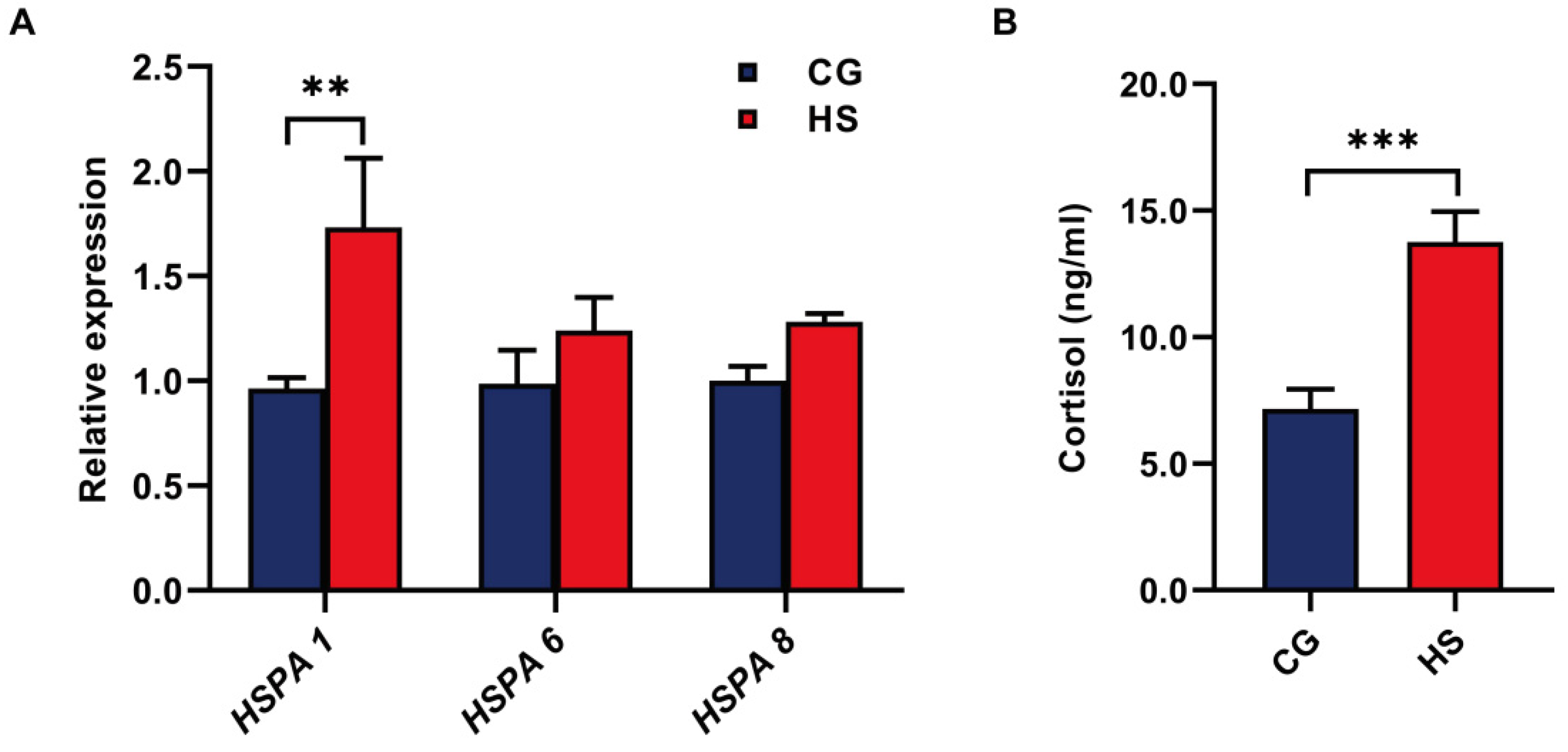
3.1. Evaluation of the Model of Heat-Stressed Goats
3.2. Rumen Fermentation In Vitro with Clostridium butyricum Supplement
3.3. Rumen Fermentation In Vivo with Clostridium butyricum Supplement
3.4. Growth Performance of Heat-Stressed Goats with Clostridium butyricum Supplement
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cai, L.Y.; Yu, J.K.; Zhang, J.; Qi, D.S. The effects of slatted floors and manure scraper systems on the concentrations and emission rates of ammonia, methane and carbon dioxide in goat buildings. Small Rumin. Res. 2015, 132, 103–110. [Google Scholar] [CrossRef]
- Cai, L.Y.; Yu, J.K.; Hartanto, R.; Zhang, J.; Yang, A.; Qi, D.S. Effects of heat challenge on growth performance, ruminal, blood and physiological parameters of Chinese crossbred goats. Small Rumin. Res. 2019, 174, 125–130. [Google Scholar] [CrossRef]
- Tajima, K.I.; Nonaka, K.; Higuchi, N.; Takusari, M.; Kurihara, A.; Takenak, M.; Mitsumori, H.; Kajikawa, R.I.; Aminov, R.I. Influence of high temperature and humidity on rumen bacterial diversity in Holstein heifers. Anaerobe 2007, 2, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Uyeno, Y.; Sekiguchi, Y.; Tajima, K.; Takenaka, A.; Kurihar, M.; Kamagata, Y. An rRNA-based analysis for evaluating the effect of heat stress on the rumen microbial composition of Holstein heifers. Anaerobe 2010, 1, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, S.; Salama, A.A.K.; Caja, G.; Albanel, E.; Such, X. Supplementation with soybean oil increase milk fat and improves milk fatty acid profile in heat stressed dairy goats. J. Dairy Sci. 2013, 96, 124–127. [Google Scholar]
- He, Y.Q.; Li, Y.Q.; Yang, Y.K.; Liu, X.Y.; Liu, G.B.; Sun, B.L.; Liu, D.W. Research Advances on Heat Stress in Goats. Chin. Anim. Husb. Vet. Med. 2018, 45, 1120–1126. [Google Scholar]
- Shehata, H.; Newmaster, S.G. Combined targeted and non-targeted pcr based methods reveal high levels of compliance in probiotic products sold as dietary supplements in united states and canada. Front. Microbiol. 2020, 11, 1095. [Google Scholar] [CrossRef] [PubMed]
- Fremah, A.S.K.; Ekwemalo, E.K.; Asiamah, H.; Ismail, S.; Ibrahim, H.; Worku, M. Effect of probiotic supplementation on growth and global gene expression in dairy cows. J. Appl. Anim. Res. 2017, 3, 257–263. [Google Scholar]
- Wang, R.Z.; Sun, Y.; Chen, J.W.; Huang, Y.Q.; Jiang, Z.H.; Yang, Q.F.; Meng, G.M.; Chen, X.P. Effects of Clostridium butyricum on performance of breed ducks. J. Henan Agri. Sci. 2009, 10, 143–145. [Google Scholar]
- Yang, C.M.; Cao, G.T.; Ferket, P.R.; Liu, T.T.; Zhou, L.; Zhang, L.; Xiao, Y.P.; Chen, A. Effects of probiotic, Clostridium butyricum, on growth performance, immune function, and cecal microflora in broiler chickens. Poul. Sci. 2012, 91, 2121–2129. [Google Scholar] [CrossRef]
- Liao, X.D.; Wu, R.J.; Ma, L.M.; Zhao, L.M.; Zheng, Z.J.; Zhang, R.J. Effects of Clostridium butyricum on antioxidant properties, meat quality and fatty acid composition of broiler birds. Lipids Health Dis. 2015, 14, 36. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, M.; Tu, Y.; Zhang, N.F.; Deng, K.D.; Ma, T.; Diao, Q.Y. Effect of oral administration of probiotics on growth performance, apparent nutrient digestibility and stress-related indicators in Holstein calves. J. Anim. Physiol. Anim. Nutr. 2016, 100, 33–38. [Google Scholar] [CrossRef]
- Li, W.Q. Effects of Clostridium butyricum on Growth Performance, Blood Index and Rumen Fermentation in Calves and Bred Cattle. Master’s Thesis, Northeast Agricultural University, Harbin, China, 2019. [Google Scholar]
- Zhang, J.; Sun, J.; Chen, X.; Nie, C.; Zhao, J.B.; Guan, W.Y.; Lei, L.H.; He, T.; Chen, Y.Q.; Johnston, L.J. Combination of Clostridium butyricum and corn bran optimized intestinal microbial fermentation using a weaned pig model. Front. Microbiol. 2018, 9, 3091. [Google Scholar] [CrossRef]
- Gan, F.; Ren, F.; Chen, X.X.; Lv, C.H.; Pan, C.L.; Ye, G.P.; Shi, J.; Shi, X.L.; Zhou, H.; Shituleni, A.; et al. Effects of selenium-enriched probiotics on heat shock protein mRNA levels in piglet under heat stress conditions. J. Agr. Food Chem. 2013, 61, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Zaneb, H.; Masood, S.; Khan, R.U.; Mobashar, M.; Khan, I.; Din, S.; Khan, M.S.; Rehman, H.U.; Tinelli, A. Single or combined applications of zinc and multi-strain probiotic on intestinal histomorphology of broilers under cyclic heat stress. Probiotics Antimicro. 2020, 12, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.J.; Li, L. Effects of active dry yeast in diet on ruminants. Feed Res. 2009, 3, 29. [Google Scholar]
- Schingoethe, D.J.; Linke, K.N.; Kalscheur, K.F.; Hippen, A.R.; Rennich, D.R.; Yoon, I. Feed effeciency of mid-lactation dairy cows fed yeast culture during summer. J. Dairy Sci. 2004, 87, 4178–4181. [Google Scholar] [CrossRef]
- LPHSI. Livestock and Poultry Heat Stress Indices Agriculture Engineering Technology Guide; Clemson University: Clemson, SC, USA, 1990. [Google Scholar]
- Marai, I.F.M.; EI-Darawany, A.A.; Fadie, A.; Abdel-Hafez, M.A.M. Physiological traits as affected by heat stress in sheep—A review. Small Rumin. Res. 2007, 1, 1–12. [Google Scholar] [CrossRef]
- Peng, X.K.; Zhao, T.; Huang, X.Y.; Zhang, Y.; Xing, X.N.; Zahng, E.P. Effects of acute heat stress on blood biochemistry indices ans ecpression of HSP 70 family genes in bloos lymphocytes in goats. Acta Vet. Zootech. Sin. 2019, 50, 1219–1229. [Google Scholar]
- Wang, Z.X.; Wang, Z.S.; Wang, L.Z.; Liu, J.H.; Xu, L.X. Effects of temperature and humidity index in different seasons on production performance and physiological and biochemical indexes of dairy cows. J. Dairy Sci. 2009, 45, 60–63. [Google Scholar]
- Ramakersm, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef]
- McDougall, E.I. Studies on ruminant saliva. 1. The composition and output of sheep’s saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Maitisaiyidi, T.; Yibureyimu, A.; Yang, K. Determination of ammonia-nitrogen in ruminal fluid treated with methanol by alkaline hypochlorite-phenol speetrophotometry. Xinjiang Agr. Sci. 2012, 3, 565–570. [Google Scholar]
- Yang, W.Z.; Beauchemin, K.A.; Rode, L.M. Effects of grain processing, forage to concentrate ratio, and forage particle size on rumen pH and digestion by dairy cows. J. Dairy Sci. 2001, 2, 203–216. [Google Scholar] [CrossRef]
- Zhang, L.Y. Feed Analysis and Feed Quality Testing Technology; China Agricultural University Press: Beijing, China, 2007; pp. 270–274. [Google Scholar]
- Martin, S.A.; Nisbet, D.J. Effect of direct-fed microbials on rumen microbial fermentation. J. Dairy Sci. 1992, 75, 1736–1744. [Google Scholar] [CrossRef]
- Ghorbani, G.R.; Morgavi, D.P.; Beauchemin, K.A.; Leedle, J.A.Z. Effects of bacterial direct-fed microbials on ruminal fermentation, blood variables, and the microbial populations of feedlot cattle. J. Anim. Sci. 2002, 80, 1977–1985. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Yang, W.Z.; Morgavi, D.P.; Ghorbani, G.R.; Kautz, W.; Leedle, J.A. Effects of bacterial directfed microbials and yeast on site and extent of digestion, blood chemistry, and subclinical ruminal acidosis in feedlot cattle. J. Anim. Sci. 2003, 81, 1628–1640. [Google Scholar] [CrossRef]
- Chiquette, J.; Allison, J.; Rasmussen, M.A. Propellantbryantii 25A used as a probiotic in early lactation dairy cows: Effect on ruminal fermentation characteristics, milk production, and milk composition. J. Dairy Sci. 2008, 91, 3536–3543. [Google Scholar] [CrossRef] [PubMed]
- Chiquette, J.; Allison, M.J.; Rasmussen, M. Use of Prevotella bryantii 25A and a commercial probiotic during subacute acidosis challenge in midlactation dairy cows. J. Dairy Sci. 2012, 95, 5985–5995. [Google Scholar] [CrossRef]
- Wang, J.Y.; Su, X.D.; Pan, K.C. Biological characteristics of Clostridium butyricum and its application in animal production. In Proceedings of the 13th National Symposium on animal Microecology, Beijing, China, 3 July 2018. [Google Scholar]
- Li, Y.P.; Li, H.H.; Wang, L.Y.; Zhu, Q.; Chen, L.B.; Qiao, J.Y.; Wang, W.J. Effects of Clostridium butyricum on growth performance, intestinal barrier function and serum cytokine contents of weaned piglets. Chin. J. Anim Nutr. 2019, 29, 2961–2968. [Google Scholar]
- Qadis, A.Q.; Goya, S.; Ikuta, K.; Yatsu, M.; Kimura, A.; Nakanishi, S.; Sato, S. Effects of a bacteria-based probiotic on ruminal ph, volatile fatty acids and bacterial flora of holstein calves. J. Vet. Med. Sci. 2004, 76, 877–885. [Google Scholar] [CrossRef][Green Version]
- Nocek, J.E.; Kautz, W.P.; Leedle, J.A.Z.; Allman, J.G. Ruminal supplementation of direct-fed microbials on diurnal pH variation and in situ digestion in dairy cattle. J. Dairy Sci. 2002, 85, 429–433. [Google Scholar] [CrossRef]
- Galip, N. Effect of supplemental yeast culture and sodium bicarbonate on ruminal fermentation and blood variables in rams. J. Anim. Physiol. Anim. Nutr. 2006, 90, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Marden, J.P.; Julien, C.; Monteils, V.; Auclair, E.; Moncoulon, R.; Bayourthe, C. How does live yeast differ from sodium bicarbonate to stabilize ruminal pH in high-yielding dairy cows? J. Dairy Sci. 2008, 91, 3528–3535. [Google Scholar] [CrossRef] [PubMed]
- Křižova, L.; Richter, M.; Třinacty, J.; Řiha, J.; Kumprechtová, D. The effect of feeding live yeast cultures on ruminal pH and redox potential in dry cows as continuously measured by a new wireless device. Czech J. Anim. Sci. 2011, 56, 37–45. [Google Scholar] [CrossRef]
- Fadel Elseed, A.M.A.; Abusamra, M.A.R. Effect of supplemental yeast (Saccharomyces cerevisiae) culture on NDF digestibility and rumen fermentation of forage sorghum hay in Nubian goat’s. Res. J. Agr. Biol. Sci. 2007, 3, 133–137. [Google Scholar]
- Oeztuerk, H.; Sagmanligil, V. Role of live yeast in rumen ecosystem. DTW Dtsch. Tierarztl. Wochenschr. 2009, 116, 244–248. [Google Scholar]
- Kiran, R.R.; Kumar, D.S. Influence of yeast culture supplementation on rumen fermentation of bulls fed complete rations. Int. J. Agric. Sci. Vet. Med. 2013, 1, 8–15. [Google Scholar]
- Uyeno, Y.; Akiyama, K.; Hasunum, T.; Yamamoto, H.; Yokokawa, H.; Yamaguchi, T.; Kawashima, K.; Itoh, M.; Kushibiki, S.; Hirako, M. Effects of supplementing an active dry yeast product on rumen microbial community composition and on subsequent rumen fermentation of lactating cows in the mid-to-late lactation period. J. Anim. Sci. 2017, 88, 119–124. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority). Scientifific opinion on miya-golds (Clostridium butyricum) as a feed additive for weaned piglets, minor weaned porcine species and minor avian species. Eur. Food Saf. Auth. 2011, 9, 1951. [Google Scholar]
| Composition | Content |
|---|---|
| Alfalfa | 562 |
| Ground corn | 264 |
| Soybean meal | 84 |
| Wheat barn | 73 |
| Ca2HPO4 | 7 |
| Premix * | 10 |
| Nutrition level | |
| Dry matter | 951 |
| Organic matter | 854 |
| Crude protein | 173 |
| Neutral detergent fiber | 434 |
| Acid detergent fiber | 257 |
| Ca | 5.9 |
| P | 3.2 |
| Gene | Primer Sequence | Product Length | Annealing Temperature | GenBank Accession No. |
|---|---|---|---|---|
| β-actin | F: TCTGGCACCACACCTTCTAC | 102 | 60 | XM_018039831.1 |
| R:TCTTCTCACGGTTGGGCCTTG | ||||
| HSPA 1 | F: CGACCAGGGAAACCGGCAC | 151 | 60 | NM_005677146.3 |
| R: CGGGTCGCCGAACTTGC | ||||
| HSPA 6 | F: TCTGCCGCAACAGGATAAA | 239 | 60 | NM_001314233.1 |
| R: CGCCCACGCACGAGTAC | ||||
| HSPA 8 | F: ACCTCTATTACCCGTGCCC | 203 | 60 | XM_018039831.1 |
| R:CTCTTATTCAGTTCCTTCCCATT |
| Treatment | |||
|---|---|---|---|
| CG | HS | SEM | |
| Rectal temperature (°C) | 39.2 | 39.4 | 0.11 |
| Skin temperature (°C) | 34.1 a | 35.9 b | 0.21 |
| Pulse (beats/min) | 76.6 a | 82.1 b | 1.07 |
| Respiratory rate (breaths/min) | 27.5 a | 33.7 b | 2.43 |
| Treatment | ||||||
|---|---|---|---|---|---|---|
| Parameters | CG | CB1 | CB2 | CB3 | CB4 | SEM |
| pH | 6.43 a | 6.53 a | 6.60 b | 6.62 b | 6.52 a | 0.04 |
| ORP mV | −230.3 a | −236.4 a | −256.9 b | −255.7 b | −246.7 a | 5.24 |
| NH3-N (mg 100 mL−1) | 16.78 a | 17.25 a | 17.32 b | 17.75 b | 16.67 a | 0.20 |
| TVFA (mmol L−1) | 42.77 a | 45.35 a | 55.01 b | 53.10 b | 43.43 a | 2.55 |
| Acetic acid (mmol L−1) | 17.38 a | 17.58 a | 22.33 b | 23.96 b | 18.08 a | 1.37 |
| Propionic acid (mmol L−1) | 14.04 a | 15.10 a | 16.01 b | 15.40 b | 14.08 a | 0.38 |
| Butyric acid (mmol L−1) | 11.35 | 12.67 | 14.67 | 13.74 | 11.49 | 0.64 |
| A/P ratio | 1.24 a | 1.16 a | 1.39 b | 1.55 b | 1.28 a | 0.07 |
| DM (%) | 44.60 a | 45.02 a | 56.78 b | 56.77 b | 45.05 a | 3.99 |
| NDF (%) | 30.1 a | 30.04 a | 36.45 b | 36.66 b | 30.77 a | 1.54 |
| ADF (%) | 25.78 a | 25.04 a | 28.31 b | 27.58 b | 26.07 a | 0.76 |
| Treatment | ||||
|---|---|---|---|---|
| Parameters | HS1 | HS2 | HS3 | SEM |
| pH | 6.53 a | 6.92 b | 6.71 a | 0.05 |
| ORP | −157.3 a | −203.0 b | −161.4 a | 6.33 |
| NH3-N (mg 100 mL−1) | 9.10 a | 13.47 b | 9.62 a | 0.88 |
| TVFA (mmol L−1) | 44.84 a | 64.73 b | 51.92 a | 3.46 |
| Acetic acid (mmol L−1) | 19.38 a | 30.78 b | 23.24 a | 2.79 |
| Propionic acid (mmol L−1) | 14.08 a | 20.27 b | 16.01 a | 1.64 |
| Butyric acid (mmol L−1) | 11.38 | 13.68 | 11.67 | 1.74 |
| A/P ratio | 1.31 a | 1.52 b | 1.45 a | 0.81 |
| Treatment | ||||
|---|---|---|---|---|
| Parameters | HS1 | HS2 | HS3 | SEM |
| DMI (kg) | 0.79 a | 0.87 b | 0.85 a | 0.02 |
| ADG (kg) | 0.08 a | 0.23 b | 0.11 a | 0.02 |
| DM (%) | 50.58 a | 66.46 b | 56.27 a | 3.43 |
| NDF (%) | 38.32 a | 54.13 b | 40.43 a | 3.09 |
| ADF (%) | 37.82 a | 50.06 b | 38.47 a | 3.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, L.; Hartanto, R.; Zhang, J.; Qi, D. Clostridium butyricum Improves Rumen Fermentation and Growth Performance of Heat-Stressed Goats In Vitro and In Vivo. Animals 2021, 11, 3261. https://doi.org/10.3390/ani11113261
Cai L, Hartanto R, Zhang J, Qi D. Clostridium butyricum Improves Rumen Fermentation and Growth Performance of Heat-Stressed Goats In Vitro and In Vivo. Animals. 2021; 11(11):3261. https://doi.org/10.3390/ani11113261
Chicago/Turabian StyleCai, Liyuan, Rudy Hartanto, Ji Zhang, and Desheng Qi. 2021. "Clostridium butyricum Improves Rumen Fermentation and Growth Performance of Heat-Stressed Goats In Vitro and In Vivo" Animals 11, no. 11: 3261. https://doi.org/10.3390/ani11113261
APA StyleCai, L., Hartanto, R., Zhang, J., & Qi, D. (2021). Clostridium butyricum Improves Rumen Fermentation and Growth Performance of Heat-Stressed Goats In Vitro and In Vivo. Animals, 11(11), 3261. https://doi.org/10.3390/ani11113261





