Phenology, Seasonal Abundance, and Host-Plant Association of Spittlebugs (Hemiptera: Aphrophoridae) in Vineyards of Northwestern Italy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
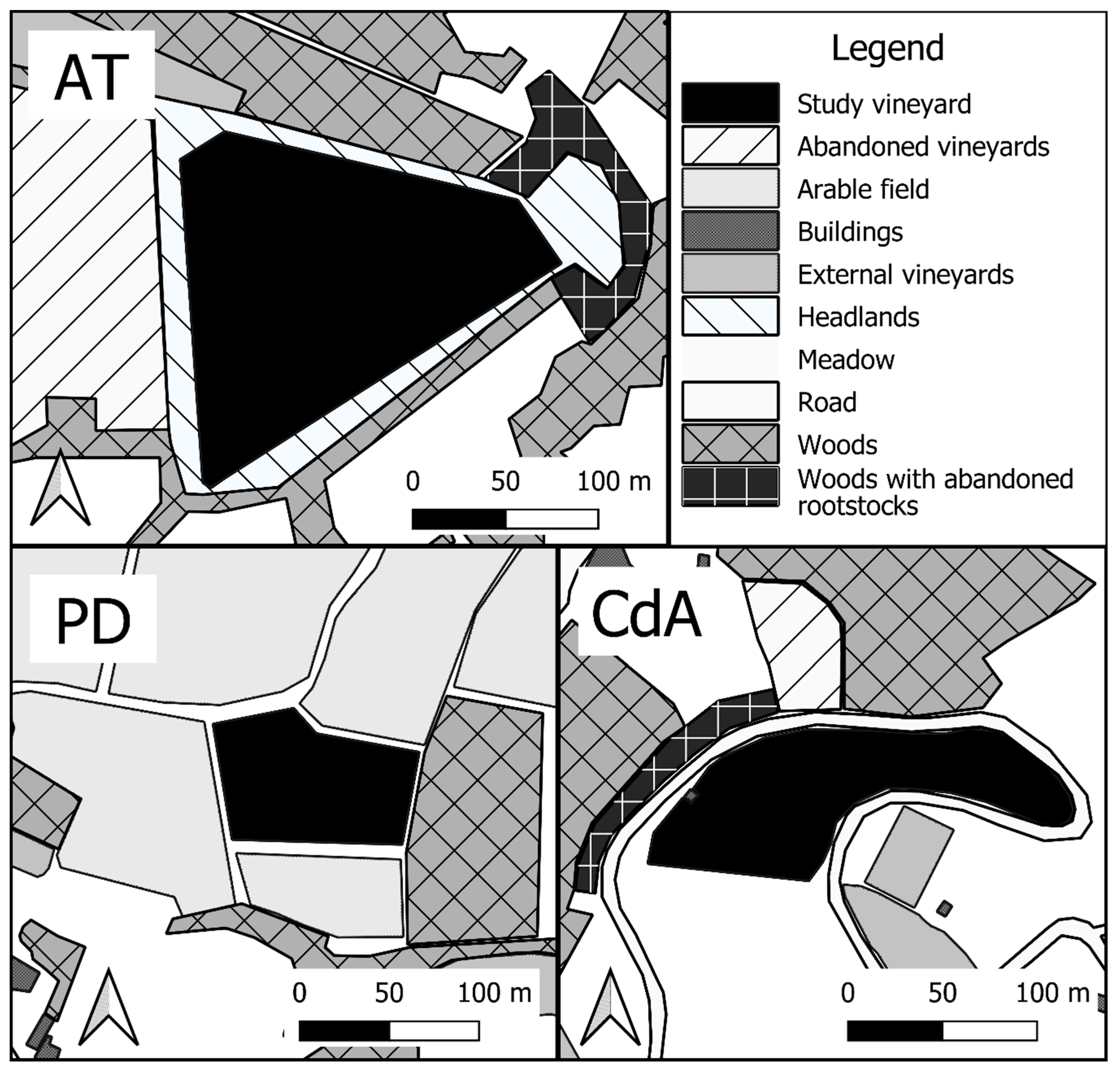
2.1. Survey Sites
2.2. Sampling of Nymphal Stages
2.3. Sampling of Adult Stage
2.4. Degree-Days
2.5. Statistical Analysis
3. Results
3.1. Species Composition
3.2. Nymphal Stages of Spittlebugs
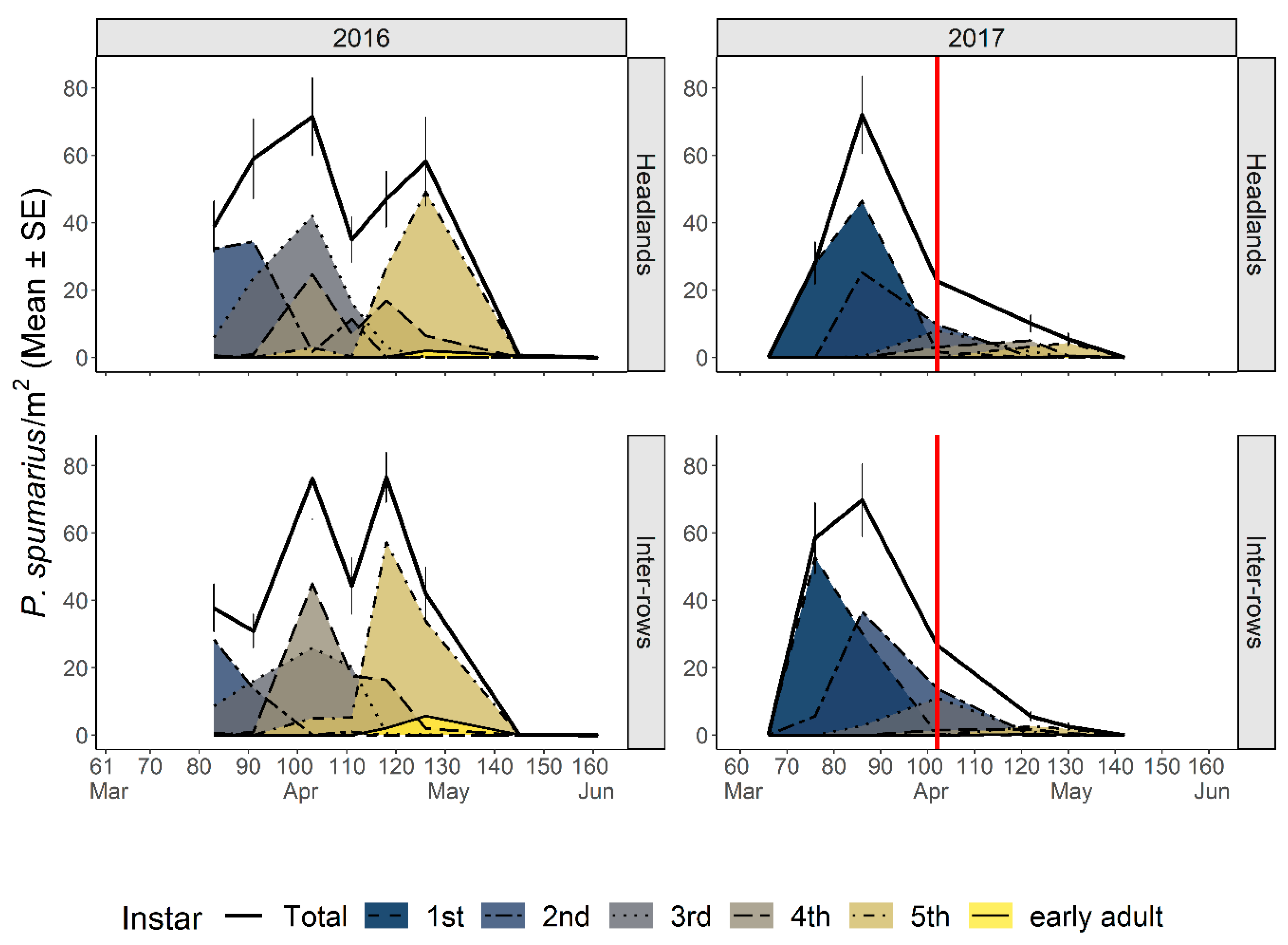
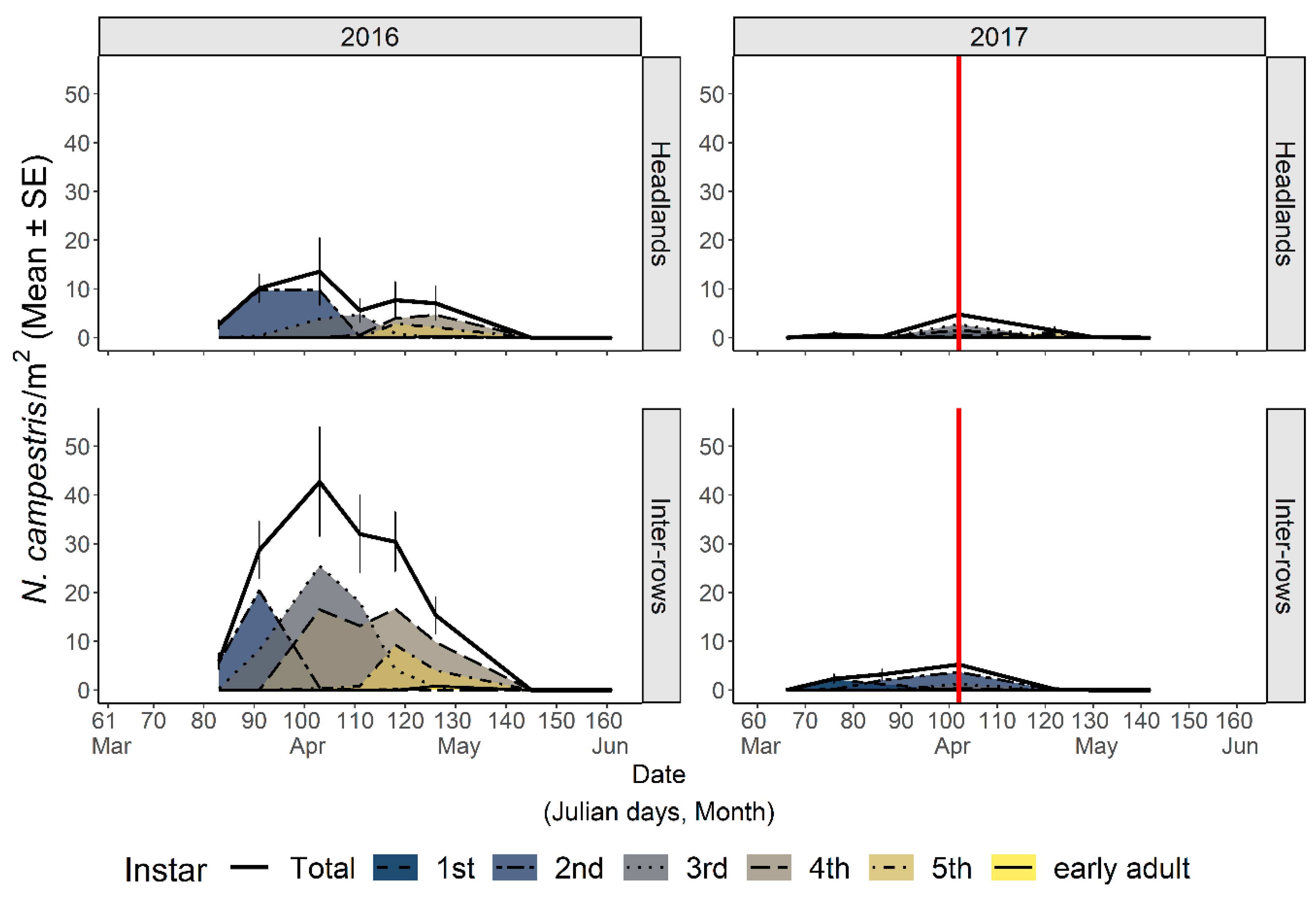
3.2.1. Abundance and Phenology
3.2.2. Chronological and Physiological Timing of P. spumarius Nymphs
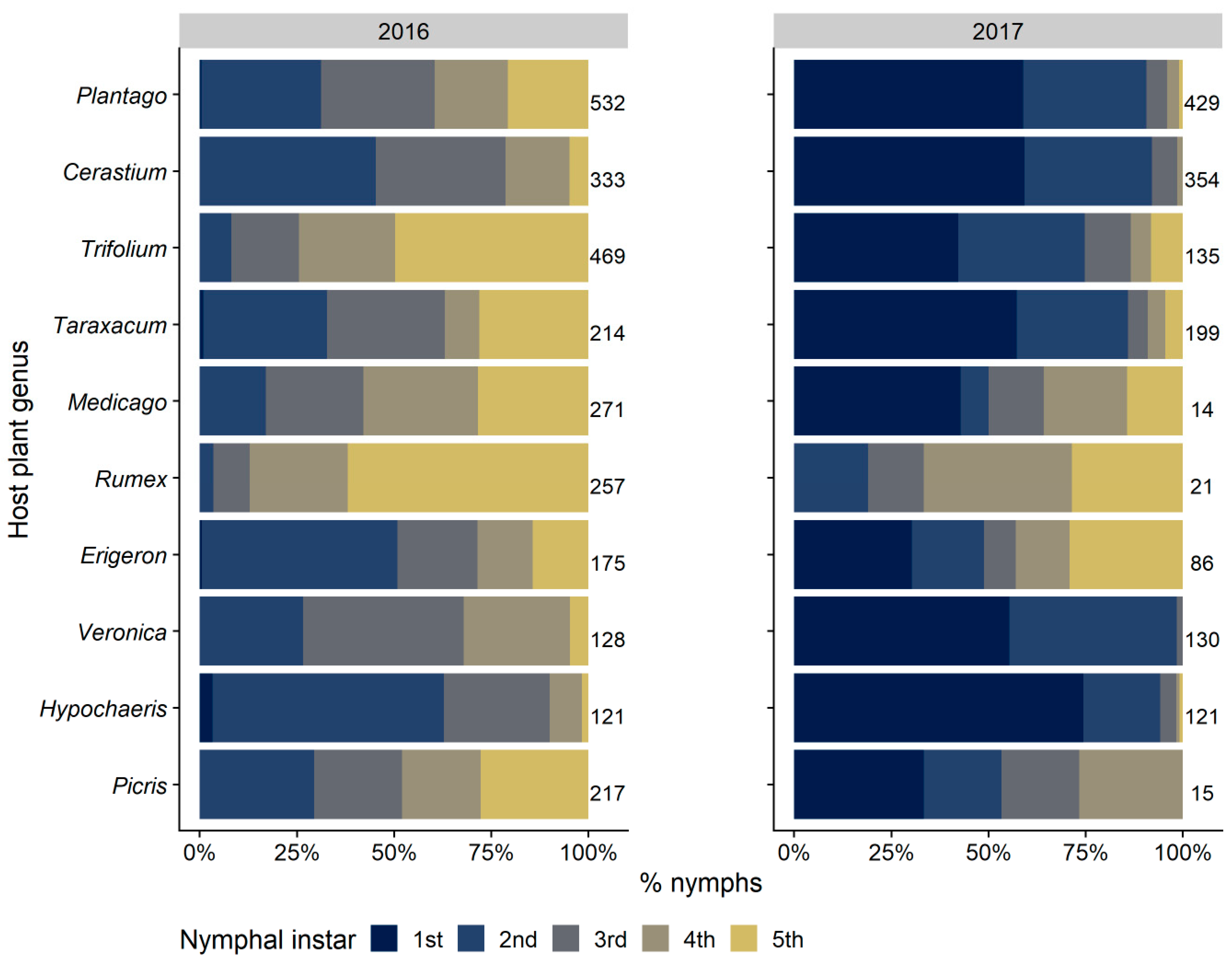
3.2.3. Selection and Exploitation of Host Plants
3.3. Adults
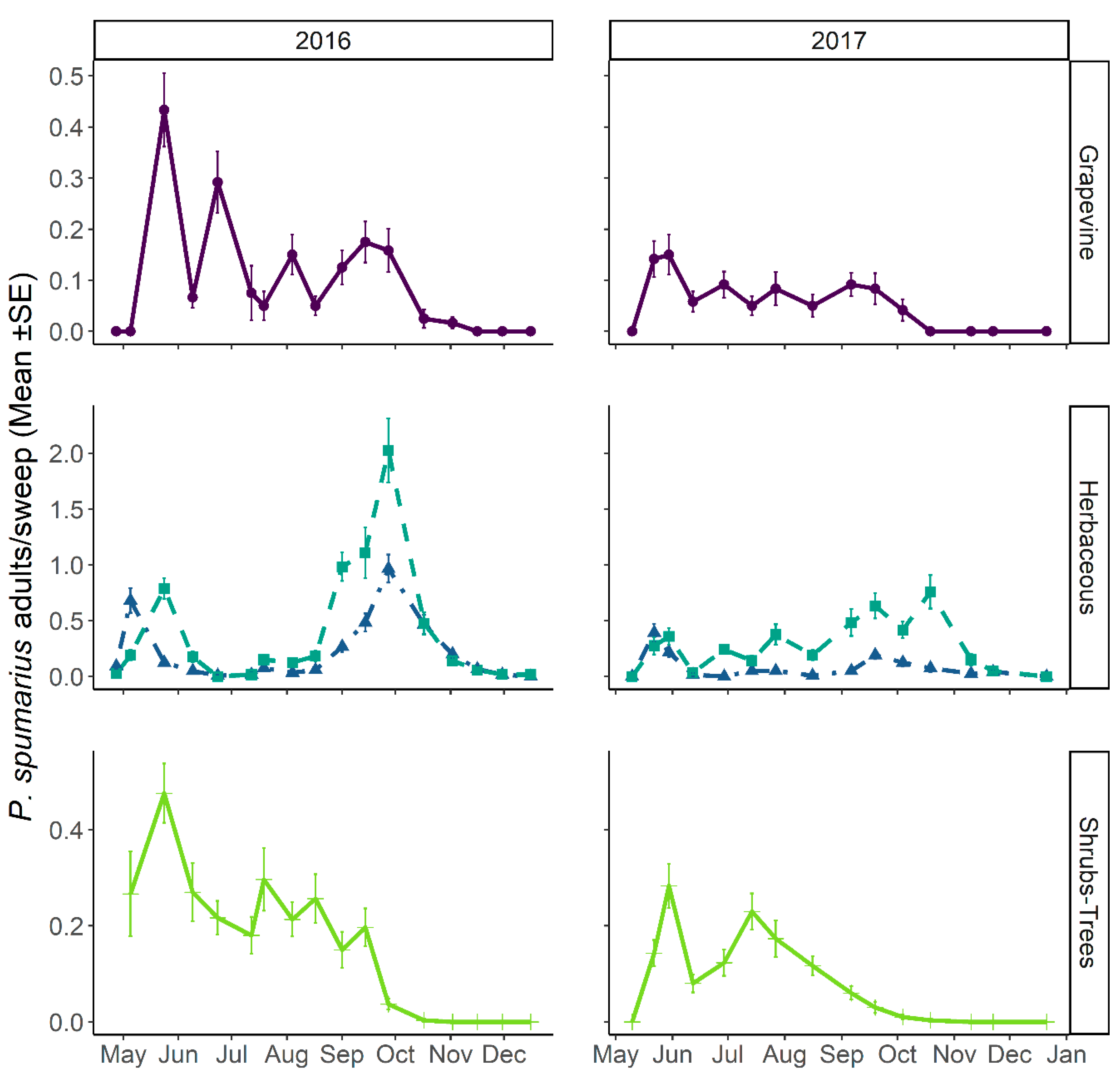
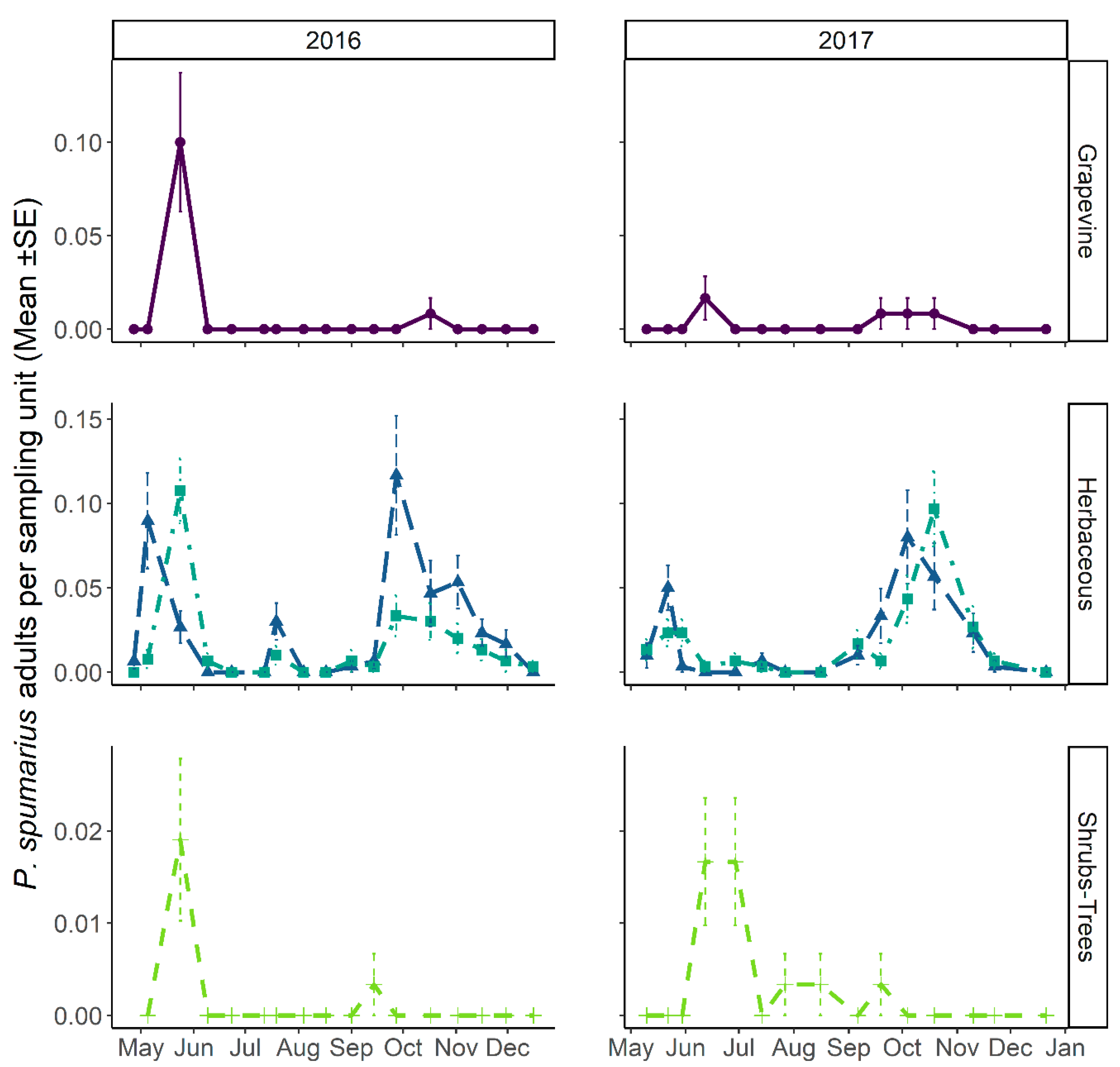
3.3.1. Abundance and Temporal Dynamics
3.3.2. Distribution on Woody Plants
3.3.3. Sex Ratio
3.3.4. Color Morphs of Philaenus spumarius Adults
3.4. The 2018 Survey
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liebhold, A.M.; Brockerhoff, E.G.; Kalisz, S.; Nuñez, M.A.; Wardle, D.; Wingfield, M.J. Biological invasions in forest ecosystems. Biol. Invasions 2017, 19, 3437–3458. [Google Scholar] [CrossRef]
- Almeida, R.P.P. Emerging plant disease epidemics: Biological research is key but not enough. PLoS Biol. 2018, 16, e2007020. [Google Scholar] [CrossRef] [PubMed]
- Battisti, A.; Roques, A.; Colombari, F.; Frigimelica, G.; Guido, M. Efficient Transmission of an Introduced Pathogen Via an Ancient Insect-Fungus Association. Naturwissenschaften 1999, 86, 479–483. [Google Scholar] [CrossRef]
- Wingfield, M.J.; Garnas, J.; Hajek, A.; Hurley, B.; de Beer, Z.W.; Taerum, S.J. Novel and co-evolved associations between insects and microorganisms as drivers of forest pestilence. Biol. Invasions 2016, 18, 1045–1056. [Google Scholar] [CrossRef]
- Cruaud, A.; Gonzalez, A.-A.; Godefroid, M.; Nidelet, S.; Streito, J.-C.; Thuillier, J.-M.; Rossi, J.-P.; Santoni, S.; Rasplus, J.-Y. Using insects to detect, monitor and predict the distribution of Xylella fastidiosa: A case study in Corsica. Sci. Rep. 2018, 8, 15628. [Google Scholar] [CrossRef] [Green Version]
- Morente, M.; Cornara, D.; Plaza, M.; Durán, J.M.; Capiscol, C.; Trillo, R.; Ruiz, M.; Ruz, C.; Sanjuan, S.; Pereira, J.A.; et al. Distribution and Relative Abundance of Insect Vectors of Xylella fastidiosa in Olive Groves of the Iberian Peninsula. Insects 2018, 9, 175. [Google Scholar] [CrossRef] [Green Version]
- Cavalieri, V.; Altamura, G.; Fumarola, G.; Di Carolo, M.; Saponari, M.; Cornara, D.; Bosco, D.; Dongiovanni, C. Transmission of Xylella fastidiosa Subspecies Pauca Sequence Type 53 by Different Insect Species. Insects 2019, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Plazio, E.; Saladini, M.A.; Volani, S.; Simonetto, A.; Fumarola, G.; Di Carolo, M.; Porcelli, F.; et al. Phenology, seasonal abundance and stage-structure of spittlebug (Hemiptera: Aphrophoridae) populations in olive groves in Italy. Sci. Rep. 2019, 9, 17725. [Google Scholar] [CrossRef]
- Sicard, A.; Zeilinger, A.R.; Vanhove, M.; Schartel, T.E.; Beal, D.J.; Daugherty, M.P.; Almeida, R.P. Xylella fastidiosa: Insights into an Emerging Plant Pathogen. Annu. Rev. Phytopathol. 2018, 56, 181–202. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Plant Health (PLH). Vitis sp. response to Xylella fastidiosa strain CoDiRO. EFSA J. 2015, 13, 4314. [Google Scholar] [CrossRef] [Green Version]
- Purcell, A.H. Xylella fastidiosa, a Regional Problem or a Global Threat? J. Plant Pathol. 1997, 79, 99–105. [Google Scholar]
- Tumber, K.P.; Alston, J.M.; Fuller, K.B. Pierce’s disease costs California $104 million per year. Calif. Agric. 2014, 68, 20–29. [Google Scholar] [CrossRef]
- Redak, R.A.; Purcell, A.H.; Lopes, J.R.; Blua, M.J.; Iii, R.F.M.; Andersen, P.C. The Biology Of Xylem Fluid–Feeding Insect Vectors of Xylella fastidiosa And Their Relation to Disease Epidemiology. Annu. Rev. Entomol. 2004, 49, 243–270. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A. Paradigms: Examples from the Bacterium Xylella fastidiosa. Annu. Rev. Phytopathol. 2013, 51, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Cornara, D.; Morente, M.; Markheiser, A.; Bodino, N.; Tsai, C.-W.; Fereres, A.; Redak, R.A.; Perring, T.M.; Lopes, J.R.S. An overview on the worldwide vectors of Xylella fastidiosa. Entomol. Gen. 2019, 39, 157–181. [Google Scholar] [CrossRef]
- Severin, H.H.P. Spittle-Insect Vectors of Pierce’s Disease Virus. II. Life History and Virus Transmission. Hilgardia 1950, 19, 357–376. [Google Scholar] [CrossRef] [Green Version]
- Purcell, A.H. Almond Leaf Scorch: Leafhopper and Spittlebug Vectors12. J. Econ. Entomol. 1980, 73, 834–838. [Google Scholar] [CrossRef]
- Daane, K.M.; Wistrom, C.M.; Shapland, E.B.; Sisterson, M.S. Seasonal abundance of Draeculacephala minerva and other Xylella fastidiosa vectors in California almond orchards and vineyards. J. Econ. Entomol. 2011, 104, 367–374. [Google Scholar] [CrossRef]
- Cornara, D.; Sicard, A.; Zeilinger, A.R.; Porcelli, F.; Purcell, A.H.; Almeida, R.P.P. Transmission of Xylella fastidiosa to Grapevine by the Meadow Spittlebug. Phytopathology 2016, 106, 1285–1290. [Google Scholar] [CrossRef] [Green Version]
- Beal, D.J.; Cooper, M.; Daugherty, M.P.; Purcell, A.H.; Almeida, R.P.P. Seasonal Abundance and Infectivity of Philaenus spumarius (Hemiptera: Aphrophoridae), a Vector of Xylella fastidiosa in California Vineyards. Environ. Entomol. 2021, 50, 467–476. [Google Scholar] [CrossRef]
- Beal, D.J.; Adams, A.G.; Cooper, M.L.; Varela, L.G.; Smith, R.J.; Kron, C.R.; Almeida, R.P.P.; Daugherty, M.P. Assessment of Nymphal Ecology and Adult Xylella fastidiosa Transmission Biology of Aphrophora Nr. Permutata (Hemiptera: Aphrophoridae) in California Vineyards. Environ. Entomol. 2021. [Google Scholar] [CrossRef]
- European Union Council Directive 2000/29/EC of 8 May 2000 on Protective Measures against the Introduction into the Community of Organisms Harmful to Plants or Plant Products and against Their Spread within the Community. Off. J. Eur. Communities Legis. L169 2000, 43, 1–112.
- Soubeyrand, S.; De Jerphanion, P.; Martin, O.; Saussac, M.; Manceau, C.; Hendrikx, P.; Lannou, C. Inferring pathogen dynamics from temporal count data: The emergence of Xylella fastidiosan France is probably not recent. New Phytol. 2018, 219, 824–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moralejo, E.; Gomila, M.; Montesinos, M.; Borràs, D.; Pascual, A.; Nieto, A.; Adrover, F.; Gost, P.A.; Seguí, G.; Busquets, A.; et al. Phylogenetic inference enables reconstruction of a long-overlooked outbreak of almond leaf scorch disease (Xylella fastidiosa) in Europe. Commun. Biol. 2020, 3, 560. [Google Scholar] [CrossRef] [PubMed]
- Olmo, D.; Nieto, A.; Borràs, D.; Montesinos, M.; Adrover, F.; Pascual, A.; Gost, P.; Quetglas, B.; Urbano, A.; García, J.; et al. Landscape Epidemiology of Xylella fastidiosa in the Balearic Islands. Agronomy 2021, 11, 473. [Google Scholar] [CrossRef]
- Moralejo, E.; Borràs, D.; Gomila, M.; Montesinos, M.; Adrover, F.; Juan, A.; Nieto, A.; Olmo, D.; Seguí, G.; Landa, B.B. Insights into the epidemiology of Pierce’s disease in vineyards of Mallorca, Spain. Plant Pathol. 2019, 68, 1458–1471. [Google Scholar] [CrossRef]
- Lessio, F.; Tedeschi, R.; Alma, A. Influence of Foraging Strip Crops on the Presence of Leafhoppers and Planthoppers Associated to Grapevines’ Phytoplasmas. Bull. Insectology 2017, 70, 221–229. [Google Scholar]
- Trivellone, V.; Jermini, M.; Jakovljević, M.; Mitrović, M. Auchenorrhyncha Collected in Vineyard, Corn and Potato Fields in Switzerland and Their Potential Role as Vectors of Stolbur Phytoplasma. DGaaE Nachr. 2017, 31, 26–27. [Google Scholar]
- Quaglino, F.; Sanna, F.; Moussa, A.; Faccincani, M.; Passera, A.; Casati, P.; Bianco, P.A.; Mori, N. Identification and ecology of alternative insect vectors of ‘Candidatus Phytoplasma solani’ to grapevine. Sci. Rep. 2019, 9, 19522. [Google Scholar] [CrossRef]
- Di Serio, F.; Bodino, N.; Cavalieri, V.; Demichelis, S.; Di Carolo, M.; Dongiovanni, C.; Fumarola, G.; Gilioli, G.; Guerrieri, E.; Picciotti, U.; et al. Collection of data and information on biology and control of vectors of Xylella fastidiosa. EFSA Support. Publ. 2019, 16, 1628. [Google Scholar] [CrossRef]
- Avosani, S.; Tattoni, C.; Mazzoni, V.; Ciolli, M. Occupancy and detection of agricultural threats: The case of Philaenus spumarius, European vector of Xylella fastidiosa. Agric. Ecosyst. Environ. 2021, 324, 107707. [Google Scholar] [CrossRef]
- Pavan, F. Xylem-Feeding Auchenorrhyncha Potentially Involved in Pierce’s Disease of Grapevines in Europe. Boll. Zool. Agrar. Bachic. 2006, 38, 103–114. [Google Scholar]
- Braccini, P.; Pavan, F. Study of Auchennorhyncha fauna in Tuscany vineyards (Italy) [Vitis vinifera L.]. Petria (Italy) 2000, 10, 181–182. [Google Scholar]
- Mazzoni, V. Contribution to the Knowledge of the Auchenorrhyncha (Hemiptera Fulgoromorpha and Cicadomorpha) of Tuscany (Italy). Redia 2005, 88, 85–102. [Google Scholar]
- Whittaker, J.B. Density Regulation in a Population of Philaenus spumarius (L.) (Homoptera: Cercopidae). J. Anim. Ecol. 1973, 42, 163. [Google Scholar] [CrossRef]
- Dongiovanni, C.; Cavalieri, V.; Bodino, N.; Tauro, D.; Di Carolo, M.; Fumarola, G.; Altamura, G.; Lasorella, C.; Bosco, D. Plant Selection and Population Trend of Spittlebug Immatures (Hemiptera: Aphrophoridae) in Olive Groves of the Apulia Region of Italy. J. Econ. Entomol. 2018, 112, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Vilbaste, J. Preliminary Key for the Identification of the Nymphs of North European Homoptera Cicadinea. II. Cicadelloidea. Ann. Zool. Fenn. 1982, 19, 1–20. [Google Scholar]
- Pignatti, S. Flora d’Italia; Edagricole: Milano, Italy, 1982; ISBN 9788820623128 2302. [Google Scholar]
- Weaver, C.; King, D. Meadow Spittlebug Philaenus leucophthalmus (L.). Ohio Agricultural Experiment Station. Res. Bull. 1954, 741, 1–99. [Google Scholar]
- Lorenz, D.; Eichhorn, K.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Halkka, O.; Raatikainen, M.; Halkka, L. Conditions requisite for stability of polymorphic balance in Philaenus spumarius (L.) (Homoptera). Genetica 1990, 46, 67–76. [Google Scholar] [CrossRef]
- Stewart, A.; Lees, D.R. The colour/pattern polymorphism of Philaenus spumarius (L.) (Homoptera: Cercopidae) in England and Wales. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 69–89. [Google Scholar] [CrossRef]
- Drosopoulos, S. New data on the nature and origin of colour polymorphism in the spittlebug genus Philaenus (Hemiptera: Aphorophoridae). Ann. Société Entomol. Fr. (N.S.) 2003, 39, 31–42. [Google Scholar] [CrossRef]
- Chmiel, S.M.; Wilson, M.C. Estimation of the Lower and Upper Developmental Threshold Temperatures and Duration of the Nymphal Stages of the Meadow Spittlebug, Philaenus spumarius12. Environ. Entomol. 1979, 8, 682–685. [Google Scholar] [CrossRef]
- West, J.; Lees, D.R. Temperature and Egg Development in the Spittlebug Philaenus spumarius (L.) (Homoptera: Aphrophoridae). Entomologist 1988, 107, 46–51. [Google Scholar]
- Zajac, M.A.; Hall, F.R.; Wilson, M.C. Heat Unit Model for the Development of Meadow Spittlebug (Homoptera: Cercopidae) on Strawberry. Environ. Entomol. 1989, 18, 347–350. [Google Scholar] [CrossRef]
- Kullback, S.; Leibler, R.A. On Information and Sufficiency. Ann. Math. Stat. 1951, 22, 79–86. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020.
- Bittrich, V. Caryophyllaceae. In Flowering Plants Dicotyledons: Magnoliid, Hamamelid and Caryophyllid Families; Kubitzki, K., Rohwer, J.G., Bittrich, V., Eds.; The Families and Genera of Vascular Plants; Springer: Berlin/Heidelberg, Germany, 1993; pp. 206–236. ISBN 978-3-662-02899-5. [Google Scholar]
- Cornara, D.; Saponari, M.; Zeilinger, A.; De Stradis, A.; Boscia, D.; Loconsole, G.; Bosco, D.; Martelli, G.P.; Almeida, R.; Porcelli, F. Spittlebugs as vectors of Xylella fastidiosa in olive orchards in Italy. J. Pest Sci. 2017, 90, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Godefroid, M.; Cruaud, A.; Streito, J.-C.; Rasplus, J.-Y.; Rossi, J.-P. Xylella fastidiosa: Climate suitability of European continent. Sci. Rep. 2019, 9, 8844. [Google Scholar] [CrossRef] [Green Version]
- EFSA Panel on Plant Health (PLH); Bragard, C.; Dehnen-Schmutz, K.; Di Serio, F.; Gonthier, P.; Jacques, M.; Miret, J.A.J.; Justesen, A.F.; MacLeod, A.; Magnusson, C.S.; et al. Update of the Scientific Opinion on the risks to plant health posed by Xylella fastidiosa in the EU territory. EFSA J. 2019, 17, e05665. [Google Scholar] [CrossRef]
- Villa, M.; Rodrigues, I.; Baptista, P.; Fereres, A.; Pereira, J.A. Populations and Host/Non-Host Plants of Spittlebugs Nymphs in Olive Orchards from Northeastern Portugal. Insects 2020, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Witsack, W. Experimental and Ecological Investigations on Forms of Dormancy in Homoptera-Cicadina (Auchenorrhyncha). 2. On Ovarian Parapause and Obligatory Embryonic Diapause in Philaenus spumarius (L.) (Aphrophoridae). Zool. Jahrbücher Abt. Syst. Okologie Geogr. Tiere 1973, 100, 517–562. [Google Scholar]
- Morente, M.; Cornara, D.; Moreno, A.; Fereres, A. Parapause breakage as a key step for the continuous indoor rearing of Philaenus spumarius. J. Appl. Entomol. 2021. [Google Scholar] [CrossRef]
- Drosopoulos, S.; Maryańska-Nadachowska, A.; Kuznetsova, V.G. The Mediterranean: Area of origin of polymorphism and speciation in the spittlebug Philaenus (Hemiptera, Aphrophoridae). Zoosystematics Evol. 2010, 86, 125–128. [Google Scholar] [CrossRef]
- Drosopoulos, S.; Asche, M. Biosystematic studies on the spittlebug genus Philaenus with the description of a new species. Zool. J. Linn. Soc. 1991, 101, 169–177. [Google Scholar] [CrossRef]
- Wiegert, R.G. Population Energetics of Meadow Spittlebugs (Philaenus spumarius L.) as Affected by Migration and Habitat. Ecol. Monogr. 1964, 34, 217–241. [Google Scholar] [CrossRef]
- Bodino, N.; Cavalieri, V.; Dongiovanni, C.; Saladini, M.A.; Simonetto, A.; Volani, S.; Plazio, E.; Altamura, G.; Tauro, D.; Gilioli, G.; et al. Spittlebugs of Mediterranean Olive Groves: Host-Plant Exploitation throughout the Year. Insects 2020, 11, 130. [Google Scholar] [CrossRef] [Green Version]
- Cornara, D.; Panzarino, O.; Santoiemma, G.; Bodino, N.; Loverre, P.; Mastronardi, M.G.; Mattia, C.; de Lillo, E.; Addante, R. Natural Areas as Reservoir of Candidate Vectors of Xylella fastidiosa. Bull. Insectology 2021, 74, 173–180. [Google Scholar]
- Whittaker, J.B. The Distribution and Population Dynamics of Neophilaenus lineatus (L.) and N. exclamationis (Thun.) (Homoptera, Cercopidae) on Pennine Moorland. J. Anim. Ecol. 1965, 34, 277. [Google Scholar] [CrossRef]
- Thompson, V. Spittlebug indicators of nitrogen-fixing plants. Ecol. Entomol. 1994, 19, 391–398. [Google Scholar] [CrossRef]
- Halkka, O.; Raatikainen, M.; Vasarainen, A.; Heinonen, L. Others Ecology and Ecological Genetics of Philaenus spumarius (L.) (Homoptera). Ann. Zool. Fenn. 1967, 4, 1–18. [Google Scholar]
- Markheiser, A.; Cornara, D.; Fereres, A.; Maixner, M. Analysis of vector behavior as a tool to predict Xylella fastidiosa patterns of spread. Entomol. Gen. 2020, 40, 1–13. [Google Scholar] [CrossRef]
- Santoiemma, G.; Tamburini, G.; Sanna, F.; Mori, N.; Marini, L. Landscape composition predicts the distribution of Philaenus spumarius, vector of Xylella fastidiosa, in olive groves. J. Pest Sci. 2019, 92, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Sanna, F.; Mori, N.; Santoiemma, G.; D’Ascenzo, D.; Scotillo, M.A.; Marini, L. Ground Cover Management in Olive Groves Reduces Populations of Philaenus spumarius (Hemiptera: Aphrophoridae), Vector of Xylella fastidiosa. J. Econ. Entomol. 2021, 114, 1716–1721. [Google Scholar] [CrossRef] [PubMed]
- Purcell, A.H. Vector Preference and Inoculation Efficiency as Components of Resistance to Pierce’s Disease in European Grape Cultivars. Phytopathology 1981, 71, 429–435. [Google Scholar] [CrossRef]
- Feil, H.; Feil, W.S.; Purcell, A.H. Effects of Date of Inoculation on the Within-Plant Movement of Xylella fastidiosa and Persistence of Pierce’s Disease Within Field Grapevines. Phytopathology 2003, 93, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bodino, N.; Cavalieri, V.; Pegoraro, M.; Altamura, G.; Canuto, F.; Zicca, S.; Fumarola, G.; Almeida, R.P.P.; Saponari, M.; Dongiovanni, C.; et al. Temporal Dynamics of the Transmission of Xylella fastidiosa Subsp. Pauca by Philaenus spumarius to Olive Plants. Entomol. Gen. 2021, 41, 463–480. [Google Scholar] [CrossRef]
- Godefroid, M.; Morente, M.; Schartel, T.; Cornara, D.; Purcell, A.; Gallego, D.; Moreno, A.; Pereira, J.A.; Fereres, A. Climate Tolerances of Philaenus spumarius Should Be Considered in Risk Assessment of Disease Outbreaks Related to Xylella fastidiosa. J. Pest Sci. 2021. [Google Scholar] [CrossRef]



| Philaenus spumarius | Neophilaenus campestris | Aphrophora alni | ||||
|---|---|---|---|---|---|---|
| Host Plant Family | No. Nymphs | % | No. Nymphs | % | No. Nymphs | % |
| Asteraceae | 1745 | 27.45 | 27 | 1.81 | 12 | 25.53 |
| Plantaginaceae | 1230 | 19.35 | 9 | 0.60 | 3 | 6.38 |
| Fabaceae | 1066 | 16.77 | 21 | 1.41 | 1 | 2.13 |
| Caryophyllaceae | 753 | 11.85 | — | — | — | — |
| Poaceae | 386 | 6.07 | 1412 | 94.77 | 29 | 61.70 |
| Polygonaceae | 291 | 4.58 | 8 | 0.54 | — | — |
| Rosaceae | 59 | 0.93 | — | — | 1 | 2.13 |
| Rubiaceae | 37 | 0.58 | — | — | 1 | 2.13 |
| Others | 770 | 12.11 | 13 | 0.87 | — | — |
| Nymphs not on plants | 20 | 0.31 | — | — | — | — |
| Host Plants | Philaenus spumarius | Neophilaenus campestris | Aphrophora alni | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus | No. Samples | % | n | Mean/SSU | % | n | Mean/SSU | % | n | Mean/SSU | % |
| Quercus | 283 | 33.85 | 586 | 2.07 | 56.89 | 5 | 0.02 | 27.78 | 36 | 0.13 | 37.11 |
| Prunus | 58 | 6.94 | 63 | 1.09 | 6.12 | 1 | 0.02 | 5.56 | 13 | 0.22 | 13.40 |
| Cornus | 50 | 5.98 | 59 | 1.18 | 5.73 | 2 | 0.04 | 11.11 | 6 | 0.12 | 6.19 |
| Robinia | 63 | 7.54 | 55 | 0.87 | 5.34 | 4 | 0.06 | 22.22 | 3 | 0.05 | 3.09 |
| Ulmus | 55 | 6.58 | 46 | 0.84 | 4.47 | 1 | 0.02 | 5.56 | 19 | 0.35 | 19.59 |
| Crataegus | 46 | 5.50 | 41 | 0.89 | 3.98 | 2 | 0.04 | 11.11 | 7 | 0.15 | 7.22 |
| Pyrus | 37 | 4.43 | 37 | 1.00 | 3.59 | 1 | 0.03 | 5.56 | 1 | 0.03 | 1.03 |
| Morus | 18 | 2.15 | 17 | 0.94 | 1.65 | 0 | 0.00 | 0.00 | 8 | 0.44 | 8.25 |
| Carpinus | 10 | 1.20 | 5 | 0.50 | 0.49 | 0 | 0.00 | 0.00 | 2 | 0.20 | 2.06 |
| Arbusto | 2 | 0.24 | 2 | 1.00 | 0.19 | 0 | 0.00 | 0.00 | 1 | 0.50 | 1.03 |
| Alnus | 1 | 0.12 | 0 | 0.00 | 0.00 | 0 | 0.00 | 0.00 | 1 | 1.00 | 1.03 |
| Philaenus spumarius | Neophilaenus campestris | ||||||
|---|---|---|---|---|---|---|---|
| Site | No. SSUp | Mean | SEM | n | Mean | SEM | n |
| AT | 15 | 9.07 | 2.36 | 34 | 4.8 | 2.04 | 18 |
| CdA | 15 | 5.33 | 2.89 | 20 | 0 | 0 | 0 |
| PD | 15 | 138 | 26 | 516 | 46.1 | 23 | 173 |
| Philaenus spumarius | Neophilaenus campestris | Aphrophora alni | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Site | Month | Vegetation | n | Mean | SE | Mean | SE | Mean | SE |
| AT | |||||||||
| June | |||||||||
| Shrubs–Trees | 15 | 0.06 | 0.02 | ― | ― | ― | ― | ||
| Inter-rows | 16 | 0 | 0 | ― | ― | ― | ― | ||
| Grapevine | 15 | 0.08 | 0.04 | ― | ― | ― | ― | ||
| September | |||||||||
| Shrubs–Trees | 15 | 0.11 | 0.04 | 0.02 | 0.01 | 0.01 | 0.009 | ||
| Inter-rows | 15 | 0.32 | 0.09 | 0.07 | 0.03 | ― | ― | ||
| Grapevine | 15 | 0.08 | 0.03 | ― | ― | ― | ― | ||
| CdA | |||||||||
| June | |||||||||
| Shrubs–Trees | 15 | 0.01 | 0.01 | ― | ― | 0.007 | 0.007 | ||
| Inter-rows | 15 | 0.02 | 0.02 | ― | ― | ― | ― | ||
| Grapevine | 15 | 0.05 | 0.03 | ― | ― | ― | ― | ||
| September | |||||||||
| Shrubs–Trees | 15 | 0 | 0 | ― | ― | ― | ― | ||
| Inter-rows | 15 | 0.1 | 0.04 | ― | ― | ― | ― | ||
| Grapevine | 15 | 0.02 | 0.02 | ― | ― | ― | ― | ||
| PD | |||||||||
| June | |||||||||
| Shrubs–Trees | 15 | 0.09 | 0.03 | 0.007 | 0.007 | 0.007 | 0.007 | ||
| Inter-rows | 15 | 0.22 | 0.07 | 0.08 | 0.03 | ― | ― | ||
| Grapevine | 15 | 0.3 | 0.06 | 0.017 | 0.017 | ― | ― | ||
| September | |||||||||
| Shrubs–Trees | 15 | 0.15 | 0.05 | ― | ― | 0.02 | 0.01 | ||
| Inter-rows | 15 | 0.75 | 0.13 | 0.1 | 0.04 | ― | ― | ||
| Grapevine | 15 | 0.42 | 0.07 | ― | ― | ― | ― | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bodino, N.; Demichelis, S.; Simonetto, A.; Volani, S.; Saladini, M.A.; Gilioli, G.; Bosco, D. Phenology, Seasonal Abundance, and Host-Plant Association of Spittlebugs (Hemiptera: Aphrophoridae) in Vineyards of Northwestern Italy. Insects 2021, 12, 1012. https://doi.org/10.3390/insects12111012
Bodino N, Demichelis S, Simonetto A, Volani S, Saladini MA, Gilioli G, Bosco D. Phenology, Seasonal Abundance, and Host-Plant Association of Spittlebugs (Hemiptera: Aphrophoridae) in Vineyards of Northwestern Italy. Insects. 2021; 12(11):1012. https://doi.org/10.3390/insects12111012
Chicago/Turabian StyleBodino, Nicola, Stefano Demichelis, Anna Simonetto, Stefania Volani, Matteo Alessandro Saladini, Gianni Gilioli, and Domenico Bosco. 2021. "Phenology, Seasonal Abundance, and Host-Plant Association of Spittlebugs (Hemiptera: Aphrophoridae) in Vineyards of Northwestern Italy" Insects 12, no. 11: 1012. https://doi.org/10.3390/insects12111012
APA StyleBodino, N., Demichelis, S., Simonetto, A., Volani, S., Saladini, M. A., Gilioli, G., & Bosco, D. (2021). Phenology, Seasonal Abundance, and Host-Plant Association of Spittlebugs (Hemiptera: Aphrophoridae) in Vineyards of Northwestern Italy. Insects, 12(11), 1012. https://doi.org/10.3390/insects12111012








