Effectiveness of Ethyl Formate as a Fumigant of Blattella germanica and Periplaneta americana (Blattodea: Ectobiidae, Blattidae) in Cross-Border Trade Transportation
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Fumigant
2.3. Fumigation Experiments
2.4. Gas Concentration and Sorption Measurement
2.5. Statistical Analysis
3. Results
3.1. Inhibitory Effects of EF on Hatching
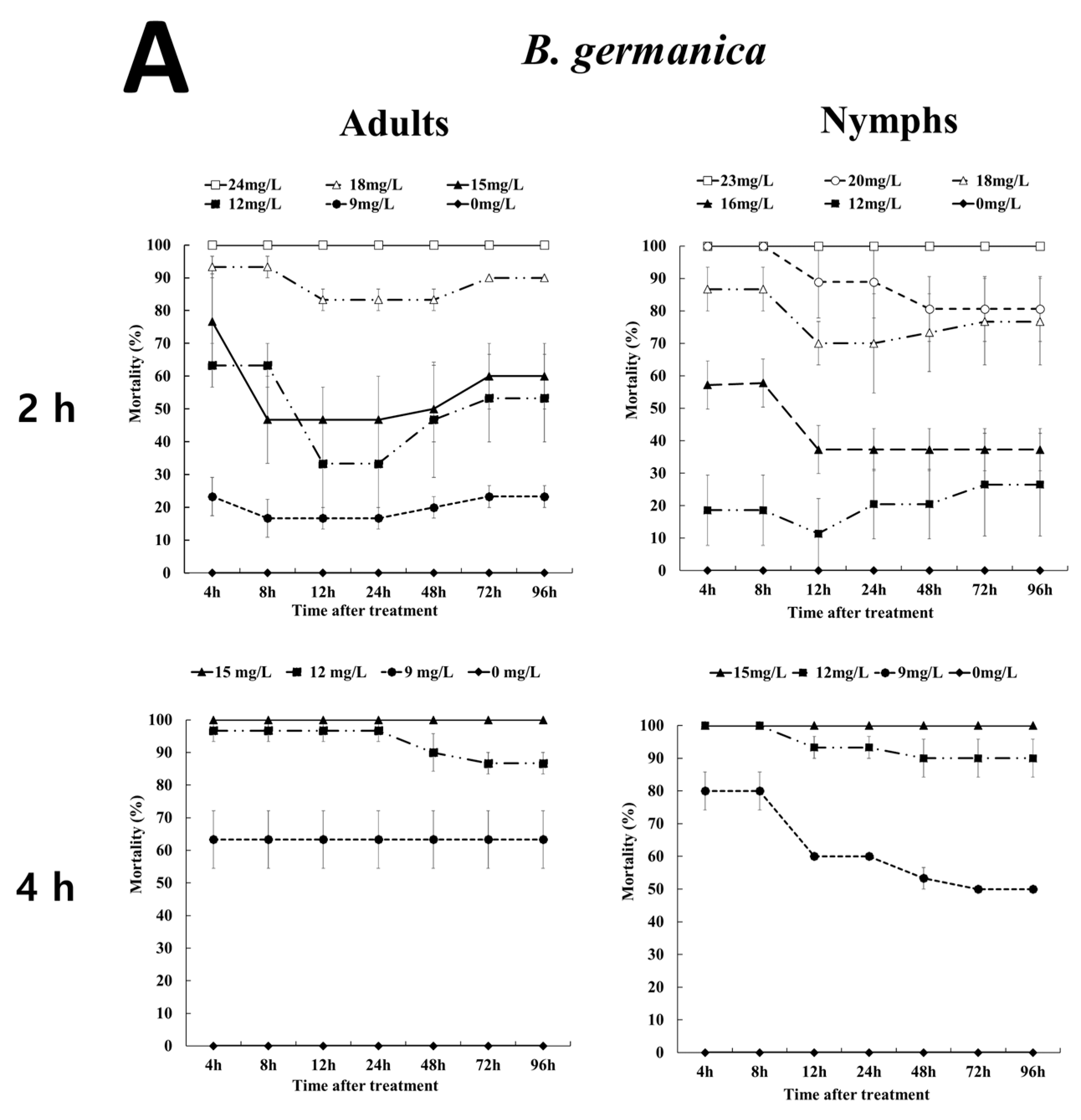
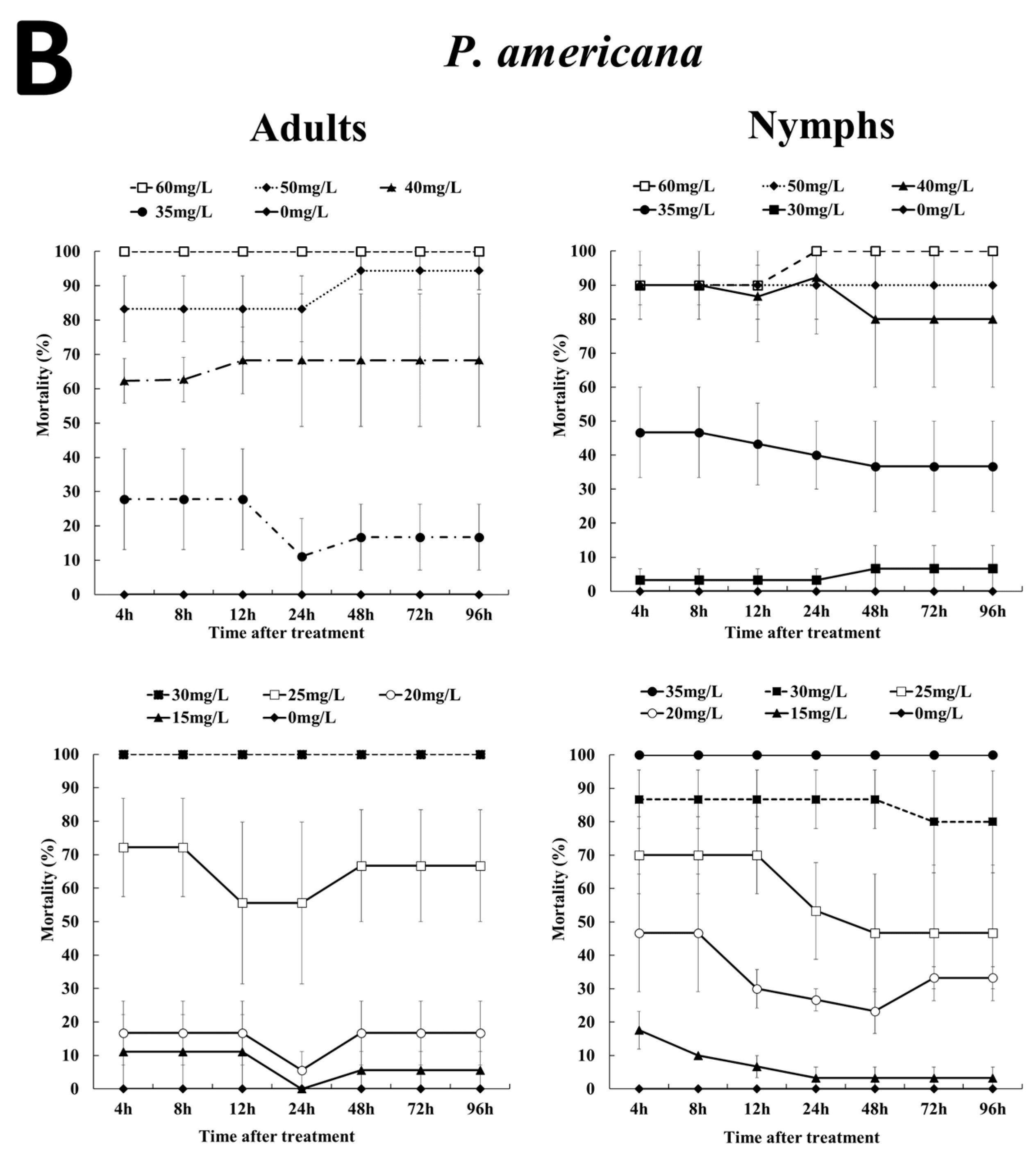
3.2. Effects of EF Fumigation on Adults and Nymphs
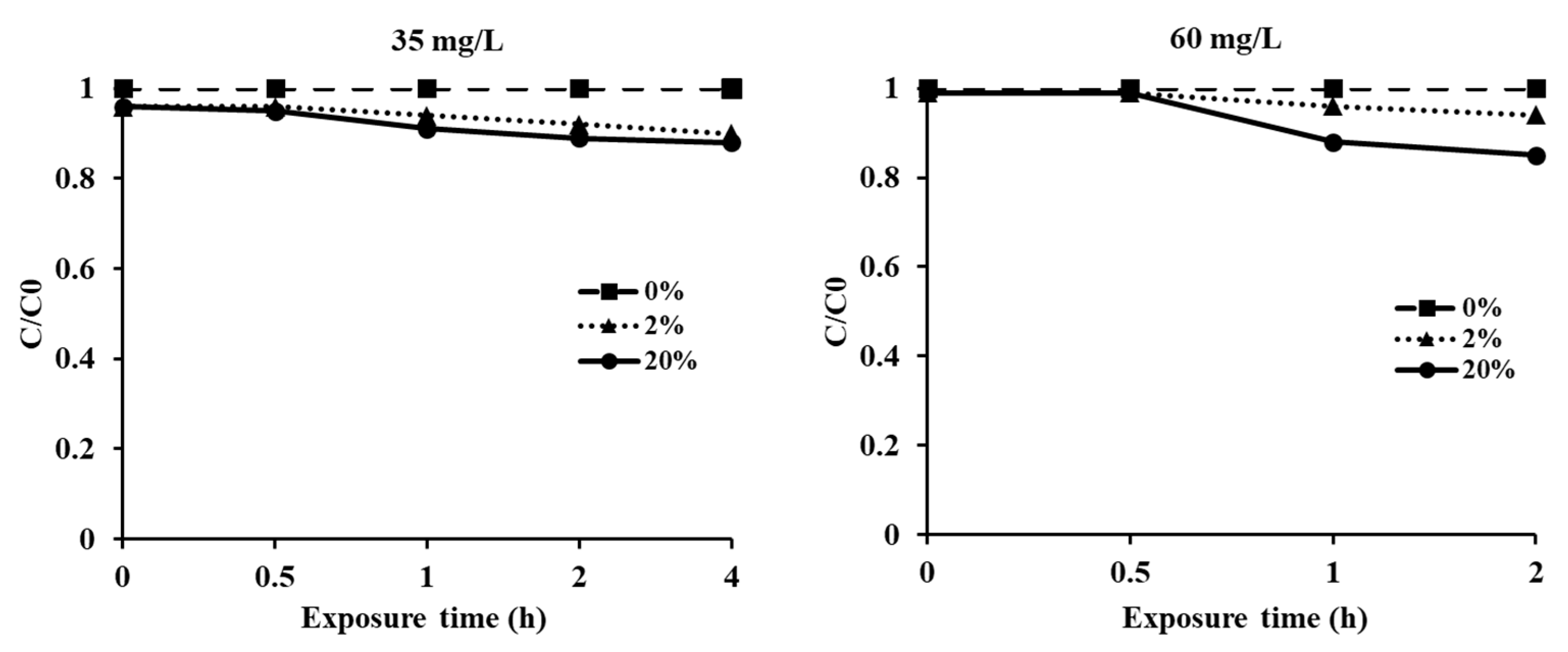
3.3. Timber Sorption Rate of EF
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Beccaloni, G.W. Cockroach Species File Online. Version 5.0/5.0. 2014. Available online: http://Cockroach.SpeciesFile.org (accessed on 22 September 2021).
- Memona, H.; Manzoor, F.; Anjum, A. Cockroaches (Blattodea: Blattidae): A reservoir of pathogenic microbes in human-dwelling localities in Lahore. J. Med. Entomol. 2017, 54, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Sriwichai, P.; Nacapunchai, D.; Pasuralertsakul, S.; Rongsriyam, Y.; Thavara, U. Survey of indoor cockroaches in some dwellings in Bangkok. Southeast Asian J. Trop. Med. Public Health 2002, 33, 36–40. [Google Scholar] [PubMed]
- Chompoosri, J.; Thavara, U.; Tawatsin, A.; Sathantriphop, S.; Yi, T. Cockroach surveys in the Northern region of Thailand and Guangxi Province of China. Southeast Asian J. Trop. Med. Public Health 2004, 35, 46–49. [Google Scholar]
- Memona, H.; Manzoor, F.; Riaz, S. Species diversity and distributional pattern of cockroaches in Lahore, Pakistan. J. Arthropod-Borne Dis. 2017, 11, 249–259. [Google Scholar] [PubMed]
- Gore, J.C.; Schal, C. Cockroach allergen biology and mitigation in the indoor environment. Annu. Rev. Entomol. 2007, 52, 439–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, T.S.; Hogan, M.B.; Welch, J.E.; Corder, W.T.; Wilson, N.W. Modern prevalence of insect sensitization in rural asthma and allergic rhinitis patients. Allergy Asthma Proc. 2005, 26, 356–360. [Google Scholar]
- Sohn, M.H.; Kim, K.E. The cockroach and allergic diseases. Allergy Asthma Immunol. Res. 2012, 4, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Hamu, H.; Debalke, S.; Zemene, E.; Birlie, B.; Mekonnen, Z.; Yewhalaw, D. Isolation of intestinal parasites of public health importance from cockroaches (Blattella germanica) in Jimma Town, Southwestern Ethiopia. J. Parasitol. Res. 2014, 2014, 186240. [Google Scholar] [CrossRef] [Green Version]
- Wada-Katsumata, A.; Zurek, L.; Nalyanya, G.; Roelofs, W.L.; Zhang, A.; Schal, C. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl. Acad. Sci. USA 2015, 112, 15678–15683. [Google Scholar] [CrossRef] [Green Version]
- Pomés, A.; Mueller, G.A.; Randall, T.A.; Chapman, M.D.; Arruda, L.K. New insights into cockroach allergens. Curr. Allergy Asthma Rep. 2017, 17, 25. [Google Scholar] [CrossRef] [Green Version]
- Hughes, J.H. Aircraft and Public Health Service. Foreign Quarantine Entomology. Public Health Rep. 1949, 64 (Suppl. 210), 38. [Google Scholar]
- Eads, R.B.; Campos, E.G.; Trevino, H.A. Quarantine problems associated with the importation of bananas from Mexico. J. Econ. Entomol. 1966, 59, 896–899. [Google Scholar] [CrossRef]
- Norman, K.N.T. The persistence of methyl bromide residues in rice, dried fruit, seeds and nuts following laboratory fumigation. Pest Manag. Sci. 2000, 56, 154–158. [Google Scholar] [CrossRef]
- Oldenburg, M.; Baur, X. Cockroach infestation on seagoing ships. Arch. Environ. Occup. Health 2008, 63, 41–46. [Google Scholar] [CrossRef]
- Oldenburg, M.; Latza, U.; Baur, X. Occupational health risks due to shipboard cockroaches. Int. Arch. Occup. Environ. Health 2008, 81, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.; Lee, C. Sustainable cockroach management using insecticidal baits: Formulations, behavioral responses and issues. In Urban Insect Pests-Sustainable Management Strategies; Dhang, P., Ed.; CAB International: Oxfordshire, UK, 2014; pp. 65–85. [Google Scholar]
- Zhu, F.; Lavine, L.; O’Neal, S.; Lavine, M.; Foss, C.; Walsh, D. Insecticide resistance and management strategies in urban ecosystems. Insects 2016, 7, 2. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Methods and Operating Procedures for Aircraft Disinsection: Report of a WHO Consultation; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Bond, E.J.; Dumas, T.; Hobbs, S. Corrosion of metals by the fumigant phosphine. J. Stored Prod. Res. 1984, 20, 57–63. [Google Scholar] [CrossRef]
- Miller, D.M.; Meek, F. Cost and efficacy comparison of integrated pest management strategies with monthly spray insecticide applications for German cockroach (Dictyoptera: Blattellidae) control in public housing. J. Econ. Entomol. 2004, 97, 559–569. [Google Scholar] [CrossRef]
- Williams, G.M.; Linker, H.M.; Waldvogel, M.G.; Leidy, R.B.; Schal, C. Comparison of conventional and integrated pest management programs in public schools. J. Econ. Entomol. 2005, 98, 1275–1283. [Google Scholar] [CrossRef]
- DeVries, Z.C.; Santangelo, R.G.; Crissman, J.; Mick, R.; Schal, C. Exposure risks and ineffectiveness of total release foggers (TRFs) used for cockroach control in residential settings. BMC Public Health 2019, 19, 96. [Google Scholar] [CrossRef] [Green Version]
- Simpson, T.; Bikoba, V.; Tipping, C.; Mitcham, E.J. Ethyl formate as a postharvest fumigant for selected pests of table grapes. J. Econ. Entomol. 2007, 100, 1084–1090. [Google Scholar] [CrossRef]
- Van Epenhuijsen, C.; Hedderley, D.; Somerfield, K.; Brash, D. Efficacy of ethyl formate and ethyl acetate for the control of onion thrips (Thrips tabaci). N. Z. J. Crop Hortic. Sci. 2007, 35, 267–274. [Google Scholar] [CrossRef]
- Cho, S.W.; Kim, H.K.; Kim, B.S.; Yang, J.O.; Kim, G.H. Combinatory effect of ethyl formate and phosphine fumigation on Pseudococcus longispinus and P. orchidicola (Hemiptera: Pseudococcidae) mortality and phytotoxicity to 13 foliage nursery plants. J. Asia Pac. Entomol. 2020, 23, 152–158. [Google Scholar] [CrossRef]
- Desmarchelier, J.M.; Johnston, F.M.; Vu, L.T. Ethyl formate, formic acid and ethanol in air, wheat, barley and sultanas: Analysis of natural levels and fumigant residues. Pestic. Sci. 1999, 55, 815–824. [Google Scholar] [CrossRef]
- Emekci, M.; Navarro, S.; Donahaye, E.; Rindner, M.; Azrieli, A. Respiration of Rhyzopertha dominica (F.) at reduced oxygen concentrations. J. Stored Prod. Res. 2004, 40, 27–38. [Google Scholar] [CrossRef]
- Vu, T.L.; Ren, Y.L. Natural levels of ethyl formate in stored grains determined using an improved method of analysis. J. Stored Prod. Res. 2004, 40, 77–85. [Google Scholar] [CrossRef]
- Ren, Y.; Mahon, D. Fumigation trials on the application of ethyl formate to wheat, split faba beans and sorghum in small metal bins. J. Stored Prod. Res. 2006, 42, 277–289. [Google Scholar] [CrossRef]
- SAS Institute. SAS Institute SAS User’s Guide, Statistics Version 9, 1st ed.; SAS Institute: Cary, NC, USA, 2009. [Google Scholar]
- Annis, P.C.; Waterford, C.J. Alternatives-Chemicals. In The Methyl Bromide Issue; Bell, C.H., Price, N., Chakrabarti, B., Eds.; Wiley and Sons.: New York, NY, USA, 1996; pp. 275–321. [Google Scholar]
- Bell, C.H.; Wontner Smith, T.J.; Savvidou, N. Some properties of sulphuryl fluoride in relation to its use as a fumigant in the cereals industry. In Advances in Stored Product Protection, Proceedings of the 8th International Working Conference on Stored Product Protection, York, UK, 22–26 July 2002; Credland, P.F., Armitage, D.M., Bell, C.H., Cogan, P.M., Highley, E., Eds.; CAB International: Wallingford, UK, 2003; pp. 910–915. [Google Scholar]
- Athanassiou, C.G.; Phillips, T.W.; Aikins, M.J.; Hasan, M.M.; Throne, J.E. Effectiveness of sulfuryl fluoride for control of different life stages of stored-product psocids (Psocoptera). J. Econ. Entomol. 2012, 105, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Environmental Protection Agency (EPA). Compatibility of Material and Electronic Equipment with Methyl Bromide and Chlorine Dioxide Fumigation. 2012. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?dirEntryId=246831 (accessed on 22 September 2021).
- Newton, P. Direct effects of increasing carbon dioxide on pasture plants and communities. New Zealand J. Agric. Res. 1991, 34, 1–24. [Google Scholar] [CrossRef]
- Gannon, B.; Le Patourel, G.; Young, R. Effect of carbon dioxide on the Oriental cockroach, Blatta orientalis. Med. Vet. Entomol. 2001, 15, 68–72. [Google Scholar] [CrossRef]
- Reierson, D.A.; Rust, M.K.; Kennedy, J.M.; Daniel, V.; Maekawa, S. Enhancing the effectiveness of modified atmospheres to control insect pests in museums and similar sensitive areas. Proceeding of the Second International Conference on Insect Pests in the Urban Environment, Edinburgh, Scotland, 7–10 July 1996; Wildey, K.B., Ed.; Heriot-Watt University: Edinburgh, Scotland, 1996; pp. 319–327. [Google Scholar]
- Liu, B.; Zhang, F.; Wang, Y. Toxicity of phosphine to Carposina niponensis (Lepidoptera: Carposinadae) at low temperature. J. Econ. Entomol. 2010, 103, 1988–1993. [Google Scholar]
- Llácer, E.; Dembilio, O.; Jacas, J. Evaluation of the efficacy of an insecticidal paint based on chlorpyrifos and pyriproxyfen in a microencapsulated formulation against Rhynchophorus ferrugineus (Coleoptera: Curculionidae). J. Econ. Entomol. 2010, 103, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Lee, S.W.; Kim, J.I.; Yang, J.O.; Koo, H.N.; Kim, G.H. Synergistic effects of oxygen on phosphine and ethyl formate for the control of Phthorimaea operculella (Lepidoptera: Gelechiidae). J. Econ. Entomol. 2015, 108, 2572–2580. [Google Scholar] [CrossRef] [PubMed]
- Kyung, Y.; Kim, H.K.; Lee, J.S.; Kim, B.S.; Yang, J.O.; Lee, B.H.; Koo, H.N.; Kim, G.H. Efficacy and phytotoxicity of phosphine as fumigants for Frankliniella occidentalis (Thysanoptera: Thripidae) on asparagus. J. Econ. Entomol. 2018, 111, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Kim, H.K.; Kyung, Y.; Park, G.H.; Lee, B.H.; Yang, J.O.; Koo, H.N.; Kim, G.H. Fumigation activity of ethyl formate and phosphine against Tetranychus urticae (Acari: Tetranychidae) on imported sweet pumpkin. J. Econ. Entomol. 2018, 111, 1625–1632. [Google Scholar] [CrossRef]
- Williams, C.L. Effect of Fumigation on Cockroaches on Ships. Public Health Rep. 1931, 46, 1680–1694. [Google Scholar] [CrossRef]
- Schal, C.; Gautier, J.Y.; Bell, W.J. Behavioural ecology of cockroaches. Biol. Rev. 1984, 59, 209–254. [Google Scholar] [CrossRef]
| Species | Concentration (mg/L) | CT (mg·h/L) | na | Hatched Ootheca (%, Mean ± SE) b | No. of Hatched Nymphs | Hatching Inhibition Rate (%, Mean ± SE) |
|---|---|---|---|---|---|---|
| B. germanica | 70 | 219.6 | 15 | 6.7 ± 6.7 c | 13 | 97.4 ± 2.6 bc |
| 60 | 178.9 | 15 | 6.7 ± 6.7 c | 16 | 96.8 ± 3.2 c | |
| 50 | 137.5 | 15 | 46.7 ± 6.7 b | 154 | 68.5 ± 4.4 bc | |
| 40 | 115.2 | 15 | 53.3 ± 13.3 b | 174 | 64.2 ± 9.4 abc | |
| 30 | 77.4 | 15 | 73.3 ± 6.7 ab | 265 | 46.0 ± 8.8 ab | |
| 20 | 50.0 | 15 | 80.0 ± 11.5 ab | 355 | 27.9 ± 13.1 a | |
| 10 | 27.5 | 15 | 73.3 ± 6.7 ab | 313 | 36.2 ± 5.7 a | |
| Control | 15 | 100.0 ± 0.0 a | 492 | - | ||
| P. americana | 60 | 178.9 | 15 | 86.7 ± 6.7 a | 137 | 13.9 ± 11.7 a |
| 40 | 115.2 | 15 | 100.0 ± 0.0 a | 151 | 6.6 ± 1.2 a | |
| 30 | 77.4 | 15 | 80.0 ± 11.5 a | 134 | 17.8 ± 12.0 a | |
| 10 | 27.5 | 15 | 86.7 ± 6.7 a | 128 | 20.4 ± 7.6 a | |
| Control | 15 | 100.0 ± 0.0 a | 162 | - | ||
| Concentration (mg/L) | Species | na | Location | Hatched Ootheca (%, Mean ± SE) b | No. of Hatched Nymphs | Hatching Inhibition Rate (%, Mean ± SE) | CT (mg·h/L) |
|---|---|---|---|---|---|---|---|
| 35 | B. germanica | 15 | Top | 80.0 ± 11.5 a | 287 | 34.7 ± 5.6 a | 78.5 |
| 15 | Middle | 66.7 ± 6.7 a | 272 | 37.6 ± 10.2 a | |||
| 15 | Bottom | 73.3 ± 6.7 a | 261 | 40.7 ± 6.2 a | |||
| 15 | Control | 93.3 ± 6.7 a | 445 | - | |||
| P. americana | 15 | Top | 80.0 ± 11.5 a | 131 | 11.1 ± 4.8 a | ||
| 15 | Middle | 73.3 ± 6.7 a | 113 | 22.2 ± 6.7 a | |||
| 15 | Bottom | 80.0 ± 0.0 a | 139 | 4.4 ± 8.8 a | |||
| 15 | Control | 86.7 ± 6.7 a | 148 | - |
| Exposure Time | Concentration (mg/L) | Species | Stage | n | Location | Mortality | CT (mg·h/L) |
|---|---|---|---|---|---|---|---|
| 4 h | 35 | B. germanica | Adult | 30 | Top | 100.0 ± 0.0 a | 78.5 |
| 30 | Middle | 100.0 ± 0.0 a | |||||
| 30 | Bottom | 100.0 ± 0.0 a | |||||
| 30 | Control | 6.7 ± 3.3 b | |||||
| Nymph | 30 | Top | 100.0 ± 0.0 a | ||||
| 30 | Middle | 100.0 ± 0.0 a | |||||
| 30 | Bottom | 100.0 ± 0.0 a | |||||
| 30 | Control | 6.7 ± 6.7 b | |||||
| P. americana | Adult | 30 | Top | 100.0 ± 0.0 a | |||
| 30 | Middle | 100.0 ± 0.0 a | |||||
| 30 | Bottom | 100.0 ± 0.0 a | |||||
| 30 | Control | 6.7 ± 3.3 b | |||||
| Nymph | 30 | Top | 100.0 ± 0.0 a | ||||
| 30 | Middle | 100.0 ± 0.0 a | |||||
| 30 | Bottom | 100.0 ± 0.0 a | |||||
| 30 | Control | 6.7 ± 3.3 b | |||||
| 2 h | 60 | B. germanica | Adult | 30 | Top | 100.0 ± 0.0 a | 70.8 |
| 30 | Middle | 100.0 ± 0.0 a | |||||
| 30 | Bottom | 100.0 ± 0.0 a | |||||
| 30 | Control | 6.7 ± 3.3 b | |||||
| Nymph | 30 | Top | 100.0 ± 0.0 a | ||||
| 30 | Middle | 100.0 ± 0.0 a | |||||
| 30 | Bottom | 100.0 ± 0.0 a | |||||
| 30 | Control | 6.7 ± 3.3 b | |||||
| P. americana | Adult | 30 | Top | 100.0 ± 0.0 a | |||
| 30 | Middle | 100.0 ± 0.0 a | |||||
| 30 | Bottom | 100.0 ± 0.0 a | |||||
| 30 | Control | 6.7 ± 3.3 b | |||||
| Nymph | 30 | Top | 100.0 ± 0.0 a | ||||
| 30 | Middle | 100.0 ± 0.0 a | |||||
| 30 | Bottom | 100.0 ± 0.0 a | |||||
| 30 | Control | 6.7 ± 3.3 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-K.; Park, H.; Seok, S.-J.; Kyung, Y.; Kim, G.-H. Effectiveness of Ethyl Formate as a Fumigant of Blattella germanica and Periplaneta americana (Blattodea: Ectobiidae, Blattidae) in Cross-Border Trade Transportation. Insects 2021, 12, 1010. https://doi.org/10.3390/insects12111010
Kim H-K, Park H, Seok S-J, Kyung Y, Kim G-H. Effectiveness of Ethyl Formate as a Fumigant of Blattella germanica and Periplaneta americana (Blattodea: Ectobiidae, Blattidae) in Cross-Border Trade Transportation. Insects. 2021; 12(11):1010. https://doi.org/10.3390/insects12111010
Chicago/Turabian StyleKim, Hyun-Kyung, Hanchan Park, Seung-Ju Seok, Yejin Kyung, and Gil-Hah Kim. 2021. "Effectiveness of Ethyl Formate as a Fumigant of Blattella germanica and Periplaneta americana (Blattodea: Ectobiidae, Blattidae) in Cross-Border Trade Transportation" Insects 12, no. 11: 1010. https://doi.org/10.3390/insects12111010
APA StyleKim, H.-K., Park, H., Seok, S.-J., Kyung, Y., & Kim, G.-H. (2021). Effectiveness of Ethyl Formate as a Fumigant of Blattella germanica and Periplaneta americana (Blattodea: Ectobiidae, Blattidae) in Cross-Border Trade Transportation. Insects, 12(11), 1010. https://doi.org/10.3390/insects12111010





