Habitat Modelling on the Potential Impacts of Shipping Noise on Fin Whales (Balaenoptera physalus) in Offshore Irish Waters off the Porcupine Ridge
Abstract
1. Introduction
2. Materials and Methods
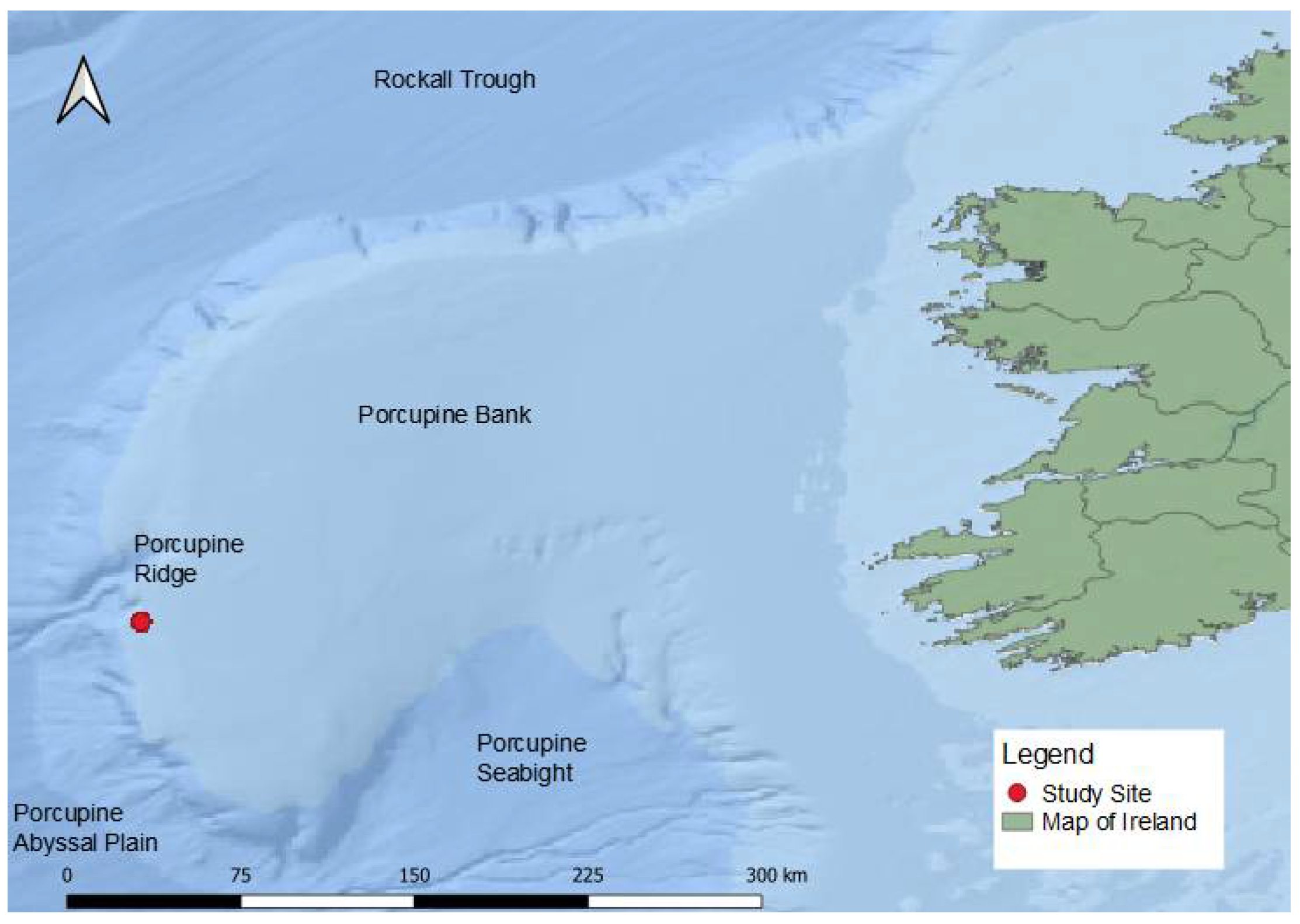
2.1. Data Collection
2.2. Acoustic Detections
2.2.1. Fin Whale Call Detections
2.2.2. Vessel Detections
2.3. Environmental Data
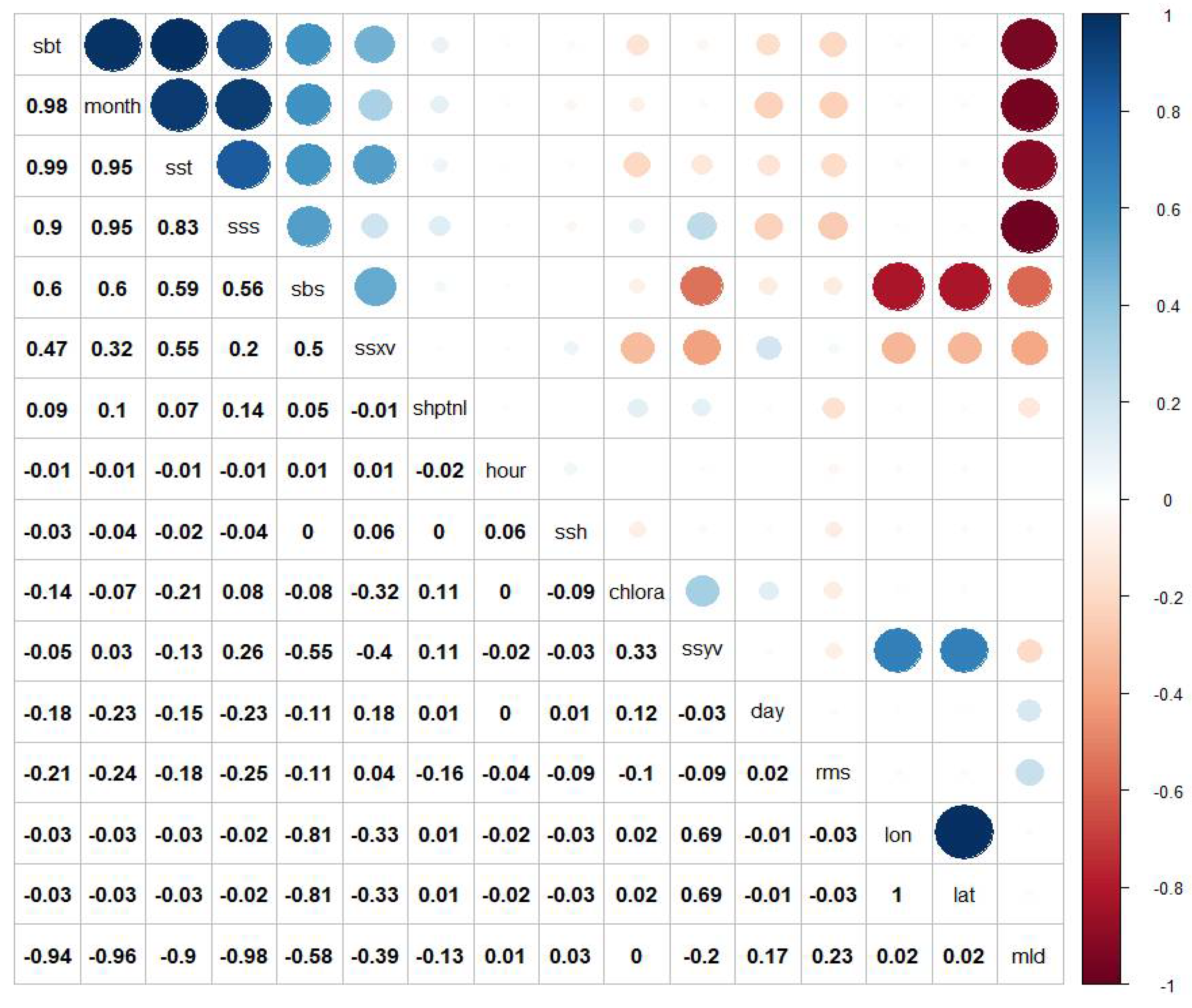
2.4. Statistical Analysis
2.5. Habitat Modelling
3. Results
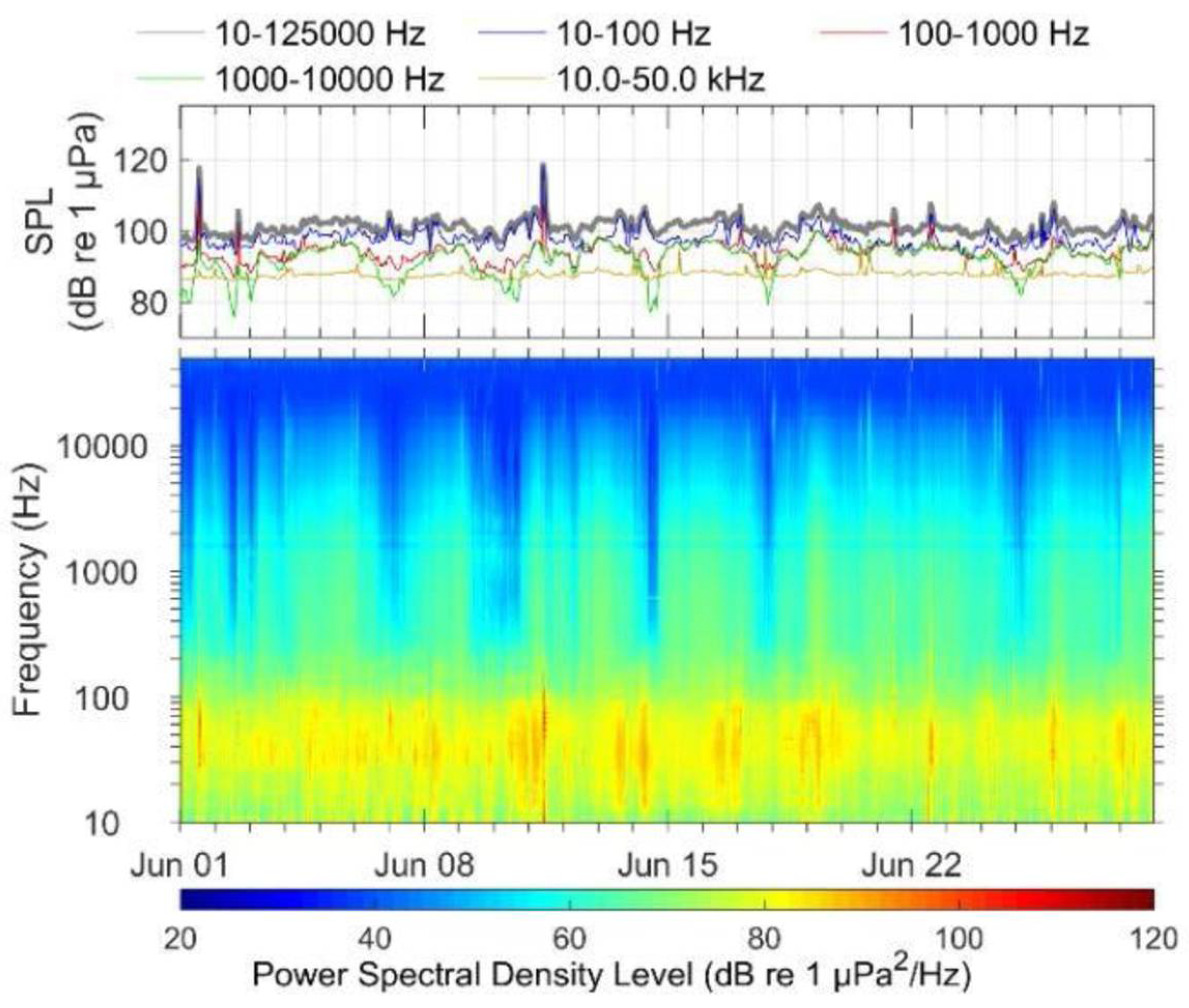
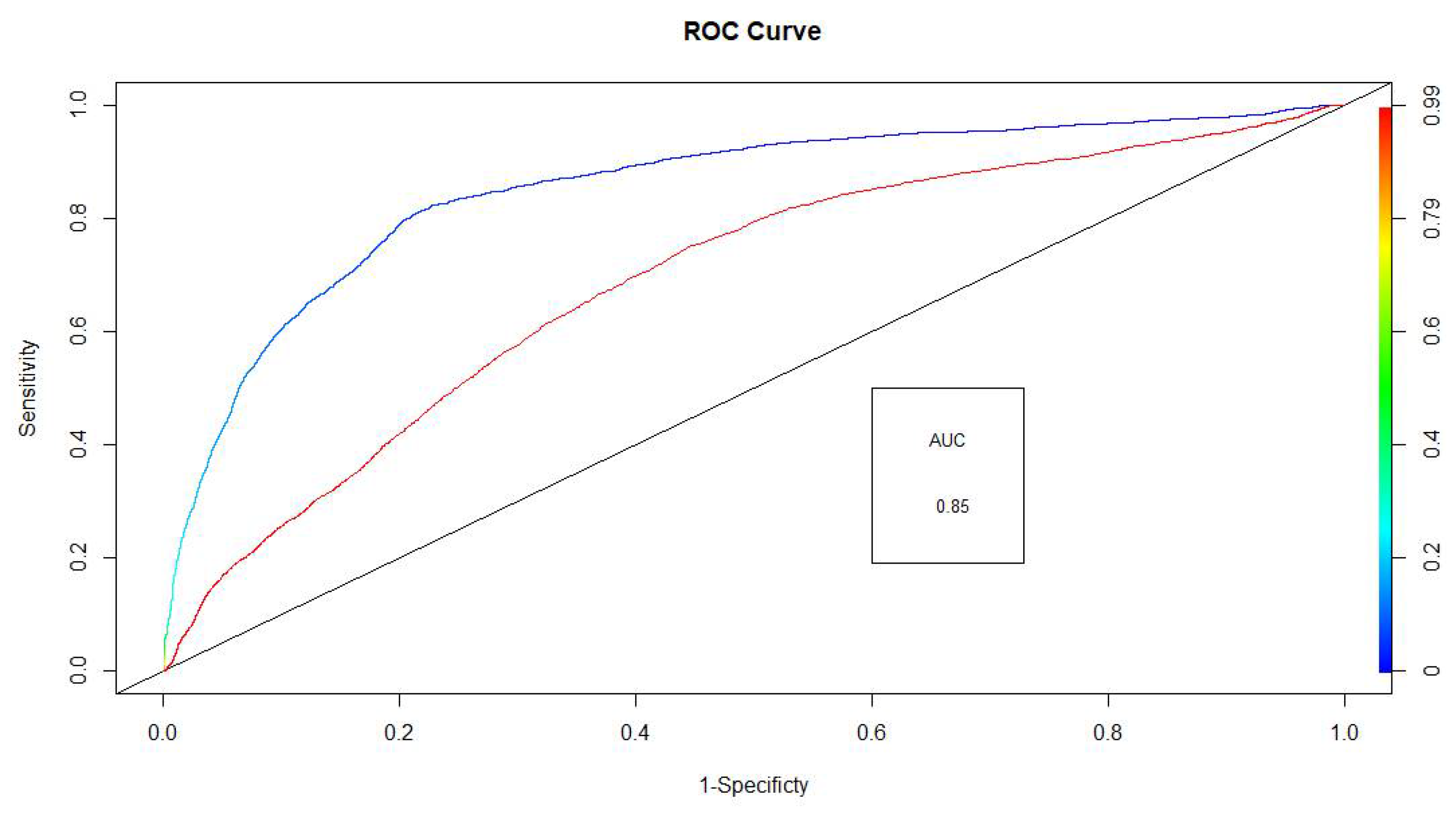
3.1. Detector Performance
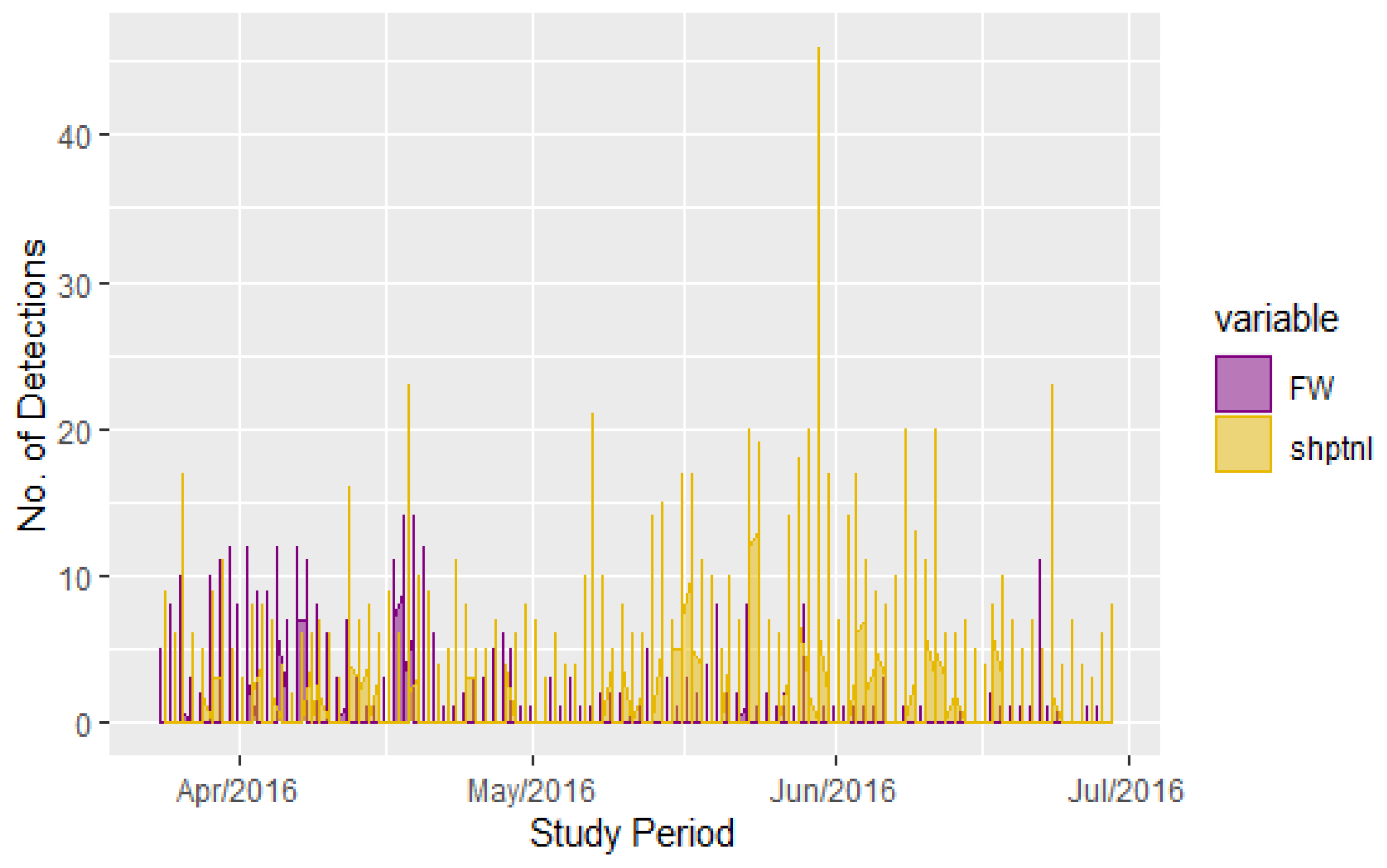
3.2. Observed Trends
3.3. Habitat Modelling Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatch, L.T.; Clark, C.W.; Van Parijs, S.M.; Frankel, A.S.; Ponirakis, D.W. Quantifying Loss of Acoustic Communication Space for Right Whales in and around a U.S. National Marine Sanctuary. Conserv. Biol. 2012, 26, 983–994. [Google Scholar] [CrossRef]
- André, M.; Van der Schaar, M.; Zaugg, S.; Houégnigan, L.; Sánchez, A.M.; Castell, J.V. Listening to the Deep: Live monitoring of ocean noise and cetacean acoustic signals. Mar. Pollut. Bull. 2011, 63, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.; O’Brien, J.; Berrow, S.; O’Connor, I.; Wall, D. Assessment of Impulsive and Continuous Low-Frequency Noise in Irish Waters. In The Effects of Noise on Aquatic Life II; Popper, A.N., Hawkins, A., Eds.; Springer: New York, NY, USA, 2016; pp. 73–81. Available online: http://link.springer.com/10.1007/978-1-4939-2981-8_9 (accessed on 24 May 2020).
- Clark, C.W.; Ellison, W.T.; Southall, B.L.; Hatch, L.; Van Parijs, S.M.; Frankel, A.; Ponirakis, D. Acoustic masking in marine ecosystems: Intuitions, analysis, and implication. Mar. Ecol. Progress Ser. 2009, 395, 201–222. [Google Scholar] [CrossRef]
- Southall, B.; Bowles, A.; Ellison, W.; Finneran, J.; Gentry, R.; Greene, C., Jr.; Kastak, D.; Ketten, D.; Miller, J.; Nachtigall, P.; et al. Marine Mammal Noise Exposure Criteria: Initial Scientific Recommendations. Bioacoustics 2008, 17, 273–275. [Google Scholar] [CrossRef]
- Studds, G.E.; Wright, A.J. A Brief Review of Anthropogenic Sound in the Oceans. Int. J. Comp. Psychol. 2007, 20, 14. [Google Scholar]
- Wright, A.J.; Deak, T.; Parsons, E.C.M. Concerns related to chronic stress in marine mammals. Int. Whal. Comm. 2009, 2, 1–7. [Google Scholar]
- Croll, D.A.; Clark, C.W.; Calambokidis, J.; Ellison, W.T.; Tershy, B.R. Effect of anthropogenic low-frequency noise on the foraging ecology of Balaenoptera whales. Anim. Conserv. 2001, 4, 13–27. [Google Scholar] [CrossRef]
- Tennessen, J.; Parks, S. Acoustic propagation modeling indicates vocal compensation in noise improves communication range for North Atlantic right whales. Endang. Species Res. 2016, 30, 225–237. [Google Scholar] [CrossRef]
- Nowacek, D.P.; Thorne, L.H.; Johnston, D.W.; Tyack, P.L. Responses of cetaceans to anthropogenic noise. Mammal Rev. 2007, 37, 81–115. [Google Scholar] [CrossRef]
- Tyack, P.L. Implications for marine mammals of large-scale changes in the marine acoustic environment. J. Mammal. 2008, 89, 549–558. [Google Scholar] [CrossRef]
- Baines, M.; Reichelt, M.; Griffin, D. An autumn aggregation of fin (Balaenoptera physalus) and blue whales (B. musculus) in the Porcupine Seabight, southwest of Ireland. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 168–177. [Google Scholar] [CrossRef]
- Širović, A.; Williams, L.N.; Kerosky, S.M.; Wiggins, S.M.; Hildebrand, J.A. Temporal separation of two fin whale call types across the eastern North Pacific. Mar. Biol. 2013, 160, 47–57. [Google Scholar] [CrossRef]
- Víkingsson, G.A.; Pike, D.G.; Desportes, G.; Øien, N.; Gunnlaugsson, T.; Bloch, D. Distribution and abundance of fin whales (Balaenoptera physalus) in the Northeast and Central Atlantic as inferred from the North Atlantic Sightings Surveys 1987–2001. NAMMCO Sci. Publ. 2009, 7, 49–72. [Google Scholar] [CrossRef]
- Hammond, P.S.; Macleod, K.; Berggren, P.; Borchers, D.L.; Burt, L.; Cañadas, A.; Desportes, G.; Donovan, G.P.; Gilles, A.; Gillespie, D.; et al. Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biol. Conserv. 2013, 164, 107–122. [Google Scholar] [CrossRef]
- McCauley, R.D. Offshore Irish Noise Logger Program (March to September 2014): Analysis of Cetacean Presence, and Ambient and Anthropogenic Noise Sources. Centre for Marine Science and Technology (CMST), Curtin University For RPS MetOcean/Woodside Energy (Ireland) Pty Ltd. 2015. Available online: https://cmst.curtin.edu.au/wp-content/uploads/sites/4/2015/06/CMST-2015-01-1296-Ireland-DRIMS_10574111.pdf (accessed on 14 August 2020).
- O’Brien, J.; Berrow, S.; McGrath, D.; Evans, P. Cetaceans In Irish Waters: A Review of Recent Research. Biol. Environ. Proc. R. Ir. Acad. 2009, 109B, 63–88. [Google Scholar] [CrossRef]
- Wall, D.; O’Brien, J.; Meade, J.; Allen, B.M. Summer Distribution and Relative Abundance of Cetaceans off the West Coast of Ireland. Biol. Environ. Proc. R. Ir. Acad. 2006, 106, 135–142. [Google Scholar] [CrossRef][Green Version]
- Clapham, P.J.; Young, S.B.; Brownell, R.L. Baleen whales: Conservation issues and the status of the most endangered populations. Mammal Rev. 1999, 29, 37–62. [Google Scholar] [CrossRef]
- Payne, R.; Webb, D. Orientation by means of long range acoustic signalling in baleen whales. Ann. N. Y. Acad. Sci. 1971, 188, 110–141. [Google Scholar] [CrossRef]
- Castellote, M.; Clark, C.W.; Lammers, M.O. Acoustic and behavioural changes by fin whales (Balaenoptera physalus) in response to shipping and airgun noise. Biol. Conserv. 2012, 147, 115–122. [Google Scholar] [CrossRef]
- Romagosa, M.; Pérez-Jorge, S.; Cascão, I.; Mouriño, H.; Lehodey, P.; Pereira, A.; Marques, T.A.; Matias, L.; Silva, M.A. Food talk: 40-Hz fin whale calls are associated with prey biomass. Proc. R. Soc. B 2021, 288, 20211156. [Google Scholar] [CrossRef]
- Cholewiak, D.; Clark, C.W.; Ponirakis, D.; Frankel, A.; Hatch, L.T.; Risch, D.; Stanistreet, J.E.; Thompson, M.; Vu, E.; Van Parijs, S.M. Communicating amidst the noise: Modeling the aggregate influence of ambient and vessel noise on baleen whale communication space in a national marine sanctuary. Endang. Species Res. 2018, 36, 59–75. [Google Scholar] [CrossRef]
- Praca, E.; Gannier, A.; Das, K.; Laran, S. Modelling the habitat suitability of cetaceans: Example of the sperm whale in the northwestern Mediterranean Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2009, 56, 648–657. [Google Scholar] [CrossRef]
- Redfern, J.V.; Ferguson, M.C.; Becker, E.A.; Hyrenbach, K.D.; Good, C.; Barlow, J.; Kaschner, K.; Baumgartner, M.F.; Forney, K.A.; Ballance, L.T.; et al. Techniques for cetacean–habitat modeling. Mar. Ecol. Progress Ser. 2006, 310, 271–295. [Google Scholar] [CrossRef]
- Berrow, S.; O’Brien, J.; Meade, R.; Delarue, J.; Kowarski, K.; Martin, B.; Moloney, J.; Wall, D.; Gillespie, D.; Leaper, R.; et al. Acoustic Surveys of Cetaceans in the Irish Atlantic Margin in 2015–2016: Occurrence, Distribution and Abundance; Department of Communication, Climate Action and Environment: Dublin, Ireland, 2018; 354p. [Google Scholar]
- Mellinger, D.; Barlow, J. Future Directions for Acoustic Marine Mammal Surveys: Stock Assessment and Habitat Use: Report of a workshop Held in La Jolla, California, 20-22 November 2002; NOAA Pacific Marine Environmental Laboratory: Seattle, WA, USA, 2003; p. 45, Report No.: Technical contribution No. 2557. [Google Scholar]
- O’Brien, J.; Beck, S.; Wall, D.; Pierini, A. Marine Mammals and Megafauna in Irish Waters—Behaviour, Distribution and Habitat Use-WP 2: Developing Acoustic Monitoring Techniques; Marine Institute: Galway, Ireland, 2013. [Google Scholar]
- Van der Graaf, A.; Ainslie, M.; André, M.; Brensing, K.; Dalen, J.; Dekeling, R.P.; Robinson, S.; Tasker, M.L.; Thomsen, F.; Werner, S. 2012, p. 27. Available online: https://ec.europa.eu/environment/marine/pdf/MSFD_reportTSG_Noise.pdf (accessed on 15 July 2021).
- Moloney, J.; Hillis, C.; Mouy, X.; Urazghildiiev, I.; Dakin, T. Autonomous Multichannel Acoustic Recorders on the VENUS Ocean Observatory. In 2014 Oceans—St John’s; IEEE: St. John’s, NL, Canada, 2014; pp. 1–6. Available online: http://ieeexplore.ieee.org/document/7003201/ (accessed on 30 October 2019).
- Kowarski, K.; Delarue, J.; Martin, B.; O’Brien, J.; Meade, R.; Ó Cadhla, O.; Berrow, S. Signals from the deep: Spatial and temporal acoustic occurrence of beaked whales off western Ireland. PLoS ONE 2018, 13, e0199431. [Google Scholar] [CrossRef] [PubMed]
- Barile, C.; Berrow, S.; O’Brien, J. Oceanographic Drivers of Cuvier’s (Ziphius cavirostris) and Sowerby’s (Mesoplodon bidens) Beaked Whales Acoustic Occurrence along the Irish Shelf Edge. J. Mar. Sci. Eng. 2021, 9, 1081. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing (Computer Software). Vienna, Austria. 2020. Available online: https://www.R-project.org (accessed on 15 July 2021).
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning; Springer: New York, NY, USA, 2013; Springer Texts in Statistics; Volume 103, Available online: http://link.springer.com/10.1007/978-1-4614-7138-7 (accessed on 12 May 2020).
- Brotons, L.; Thuiller, W.; Araújo, M.B.; Hirzel, A.H. Presence-absence versus presence-only modelling methods for predicting bird habitat suitability. Ecography 2004, 27, 437–448. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Smith, G.M. Analysing Ecological Data; Statistics for Biology and Health; Springer: New York, NY, USA; London, UK, 2007; 672p. [Google Scholar]
- Manning, C. Logistic regression (with R). Changes 2007, 4, 1–15. [Google Scholar]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecol. 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Hjort, J.; Luoto, M. Can abundance of geomorphological features be predicted using presence–absence data? Earth Surf. Process. Landforms 2008, 33, 741–750. [Google Scholar] [CrossRef]
- Louviere, J.J.; Hensher, D.A.; Swait, J.D.; Adamowicz, W. Stated Choice Methods: Analysis and Applications, 1st ed.; Cambridge University Press: Cambridge, UK, 2000; Available online: https://www.cambridge.org/core/product/identifier/9780511753831/type/book (accessed on 18 May 2020).
- Prajapati, B.; Dunne, M.; Armstrong, R. Sample size estimation and statistical power analyses. Optom. Today 2010, 16, 10–18. [Google Scholar]
- Delarue, J.; Todd, S.K.; Van Parijs, S.M.; Di Iorio, L. Geographic variation in Northwest Atlantic fin whale (Balaenoptera physalus) song: Implications for stock structure assessment. J. Acoust. Soc. Am. 2009, 125, 1774–1782. [Google Scholar] [CrossRef] [PubMed]
- Morano, J.L.; Salisbury, D.P.; Rice, A.N.; Conklin, K.L.; Falk, K.L.; Clark, C.W. Seasonal and geographical patterns of fin whale song in the western North Atlantic Ocean. J. Acoust. Soc. Am. 2012, 132, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Whooley, P.; Berrow, S.; Barnes, C. Photo-identification of fin whales (Balaenoptera physalus L.) off the south coast of Ireland. Mar. Biodivers. Rec. 2011, 4, e8. [Google Scholar] [CrossRef]
- Berrow, S.; Whooley, P.; Irish Whale and Dolphin Group, Irish Scheme for Cetacean Observation and Public Education. Irish Cetacean Review (2000–2009): A Review of All Cetacean Sighting and Stranding Records Made during the Period 2000–2009 by the IWDG through ISCOPE, the Irish Scheme for Cetacean Observation and Public Education; IWDG: Kilrush, Ireland, 2010. [Google Scholar]
- Rogan, E.; Breen, P.; Mackey, M.; Cañadas, A.; Scheidat, M.; Geelhoed, S.C.V.; Jessopp, M. Aerial Surveys of Cetaceans and Seabirds in Irish waters: Occurrence, distribution and abundance in 2015–2017; Department of Communications, Climate Action & Environment: Dublin, Ireland, 2018. [Google Scholar]
- Chariff, R.A.; Clark, C.W. Acoustic Monitoring of Large Whales in Deep Waters North and West of the British Isles: 1996–2005. Cornell Laboratory of Ornithology. 2009; p. 40. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/196484/OES_UK_SOSUS_10year_report.pdf (accessed on 4 August 2020).
- Prieto, R.; Tobeña, M.; Silva, M.A. Habitat preferences of baleen whales in a mid-latitude habitat. Deep Sea Res. Part II Top. Stud. Oceanogr. 2017, 141, 155–167. [Google Scholar] [CrossRef]
- Watkins, W.A.; Tyack, P.; Moore, K.E.; Bird, J.E. The 20-Hz signals of finback whales (Balaenoptera physalus). J. Acoust. Soc. Am. 1987, 82, 1901–1912. [Google Scholar] [CrossRef]
- Panigada, S.; Zanardelli, M.; MacKenzie, M.; Donovan, C.; Mélin, F.; Hammond, P.S. Modelling habitat preferences for fin whales and striped dolphins in the Pelagos Sanctuary (Western Mediterranean Sea) with physiographic and remote sensing variables. Remote. Sens. Environ. 2008, 112, 3400–3412. [Google Scholar] [CrossRef]
- Scales, K.L.; Schorr, G.S.; Hazen, E.L.; Bograd, S.J.; Miller, P.I.; Andrews, R.D.; Zerbini, A.N.; Falcone, E.A. Should I stay or should I go? Modelling year-round habitat suitability and drivers of residency for fin whales in the California Current. Divers. Distrib. 2017, 23, 1204–1215. [Google Scholar] [CrossRef]
- Dransfeld, L.; Maxwell, H.W.; Moriarty, M.; Nolan, C.; Kelly, E.; Pedreschi, D.; Slattery, N.; Connolly, P. North Western Waters Atlas, 3rd ed.; Marine Institute: Galway, Ireland, 2015; Available online: https://oar.marine.ie/handle/10793/1075 (accessed on 4 June 2020).
- White, M.; Bowyer, P. The Shelf-Edge Current North-West of Ireland. In Annales Geophysicae; Springer: Berlin/Heidelberg, Germany, 1997; Volume 15, pp. 1076–1083. [Google Scholar]
- Belkin, I.M.; Cornillon, P.C. Fronts in the World Ocean’s Large Marine Ecosystems. ICES CM 2007, 500, 21–33. [Google Scholar]
- Baines, M.E.; Reichelt, M. Upwellings, canyons and whales: An important winter habitat for balaenopterid whales off Mauritania, northwest Africa. J. Cetacean Res. Manag. 2014, 14, 57–67. [Google Scholar]
- Shabangu, F.W.; Findlay, K.P.; Yemane, D.; Stafford, K.M.; Van den Berg, M.; Blows, B.; Andrew, R.K. Seasonal occurrence and diel calling behaviour of Antarctic blue whales and fin whales in relation to environmental conditions off the west coast of South Africa. J. Mar. Syst. 2019, 190, 25–39. [Google Scholar] [CrossRef]
- Shabangu, F.W.; Yemane, D.; Stafford, K.M.; Ensor, P.; Findlay, K.P. Modelling the effects of environmental conditions on the acoustic occurrence and behaviour of Antarctic blue whales. PLoS ONE 2017, 12, e0172705. [Google Scholar] [CrossRef] [PubMed]
- Víkingsson, G.A. Feeding of fin whales (Balaenoptera physalus) off Iceland—Diurnal and seasonal variation and possible rates. J. Northwest Atl. Fish. Sci. 1997, 77–89. [Google Scholar] [CrossRef]
- Keen, E.M.; Falcone, E.A.; Andrews, R.D.; Schorr, G.S. Diel Dive Behavior of Fin Whales (Balaenoptera physalus) in the Southern California Bight. Aquat. Mamm. 2019, 45, 233–243. [Google Scholar] [CrossRef]
- Simon, M.; Stafford, K.M.; Beedholm, K.; Lee, C.M.; Madsen, P.T. Singing behavior of fin whales in the Davis Strait with implications for mating, migration and foraging. J. Acoust. Soc. Am. 2010, 128, 3200–3210. [Google Scholar] [CrossRef]
- Christie, A.E.; Yu, A.; Pascual, M.G. Circadian signaling in the Northern krill Meganyctiphanes norvegica: In silico prediction of the protein components of a putative clock system using a publicly accessible transcriptome. Mar. Genomics 2018, 37, 97–113. [Google Scholar] [CrossRef]
- Baumgartner, M.F.; Fratantoni, D.M. Diel periodicity in both sei whale vocalization rates and the vertical migration of their copepod prey observed from ocean gliders. Limnol. Oceanogr. 2008, 53, 2197–2209. [Google Scholar] [CrossRef]
- Stafford, K.M.; Moore, S.E.; Fox, C.G. Diel variation in blue whale calls recorded in the eastern tropical Pacific. Anim. Behav. 2005, 69, 951–958. [Google Scholar] [CrossRef]
- Wiggins, S.; Oleson, E.; Mcdonald, M.; Hildebrand, J. Blue Whale (Balaenoptera musculus) Diel Call Patterns Offshore of Southern California. Aquat. Mamm. 2005, 31, 161–168. [Google Scholar] [CrossRef]
- Pastor, M.V.; Palter, J.B.; Pelegrí, J.L.; Dunne, J.P. Physical drivers of interannual chlorophyll variability in the eastern subtropical North Atlantic: Interannual Chlorophyll Variability. J. Geophys. Res. Oceans 2013, 118, 3871–3886. [Google Scholar] [CrossRef]
- Druon, J.N.; Panigada, S.; David, L.; Gannier, A.; Mayol, P.; Arcangeli, A.; Cañadas, A.; Laran, S.; Di Méglio, N.; Gauffier, P. Potential feeding habitat of fin whales in the western Mediterranean Sea: An environmental niche model. Mar. Ecol. Progress Ser. 2012, 464, 289–306. [Google Scholar] [CrossRef]
- Ryan, C.; Berrow, S.D.; McHugh, B.; O’Donnell, C.; Trueman, C.N.; O’Connor, I. Prey preferences of sympatric fin (Balaenoptera physalus) and humpback (Megaptera novaeangliae) whales revealed by stable isotope mixing models. Mar. Mamm. Sci. 2014, 30, 242–258. [Google Scholar] [CrossRef]
- Thomas, P.O.; Reeves, R.R.; Brownell, R.L. Status of the world’s baleen whales. Mar. Mamm. Sci. 2016, 32, 682–734. [Google Scholar] [CrossRef]
- Visser, F.; Hartman, K.; Pierce, G.; Valavanis, V.; Huisman, J. Timing of migratory baleen whales at the Azores in relation to the North Atlantic spring bloom. Mar. Ecol. Progress Ser. 2011, 440, 267–279. [Google Scholar] [CrossRef]
- NRC (Ed.) Marine Mammal Populations and Ocean Noise: Determining when Noise Causes Biologically Significant Effects; National Academies Press: Washington, DC, USA, 2005; 126p. [Google Scholar]
- Sciacca, V.; Caruso, F.; Beranzoli, L.; Chierici, F.; De Domenico, E.; Embriaco, D.; Favali, P.; Giovanetti, G.; Larosa, G.; Marinaro, G. Annual Acoustic Presence of Fin Whale (Balaenoptera physalus) Offshore Eastern Sicily, Central Mediterranean Sea. PLoS ONE 2015, 10, e0141838. [Google Scholar] [CrossRef] [PubMed]
- Romagosa, M.; Cascão, I.; Silva, M.A. Underwater noise levles and shipping off the Faial–Pico Channel, Azores, in relation to the acoustic presence of baleen whales. Acústica 2020, 23, 1–9. [Google Scholar]
- Romagosa, M.; Cascão, I.; Merchant, N.D.; Lammers, M.O.; Giacomello, E.; Marques, T.A.; Silva, M.A. Underwater Ambient Noise in a Baleen Whale Migratory Habitat Off the Azores. Front. Mar. Sci. 2017, 4, 109. [Google Scholar] [CrossRef]
- Aulanier, F.; Simard, Y.; Roy, N.; Bandet, M.; Gervaise, C. Groundtruthed probabilistic shipping noise modeling and mapping: Application to blue whale habitat in the Gulf of St. Lawrence. Proc. Mtgs. Acoust. 2016, 070006. Available online: http://asa.scitation.org/doi/abs/10.1121/2.0000258 (accessed on 28 May 2020).
- Gabriele, C.M.; Ponirakis, D.W.; Clark, C.W.; Womble, J.N.; Vanselow, P.B.S. Underwater Acoustic Ecology Metrics in an Alaska Marine Protected Area Reveal Marine Mammal Communication Masking and Management Alternatives. Front. Mar. Sci. 2018, 5, 270. [Google Scholar] [CrossRef]
- Dahlheim Marilyn, E.; Dean Fisher, H.; Schempp, J.D. The Gray Whale: Eschrichtius Robustus, 1st ed.; Academic Press: Cambridge, MA, USA, 1984; pp. 511–541. Available online: https://www.elsevier.com/books/the-gray-whale-eschrichtius-robustus/jones/978-0-08-092372-7 (accessed on 17 August 2020).
- Bryant, P.J.; Lafferty, C.M.; Lafferty, S.K. Reoccupation of Laguna Guerrero Negro, Baja California, Mexico, by Gray Whales. In The Gray Whale: Eschrichtius Robustus; Elsevier: Amsterdam, The Netherlands, 1984; pp. 375–387. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780080923727500212 (accessed on 17 August 2020).
- Jones, M.L.; Swartz, S.L.; Leatherwood, S. The Gray Whale: Eschrichtius Robustus; Academic Press: Cambridge, MA, USA, 2012; 625p. [Google Scholar]
- Beale, C.M. The Behavioral Ecology of Disturbance Responses. Int. J. Comp. Psychol. 2007, 20, 11. [Google Scholar]
- Scales, K.L.; Hazen, E.L.; Jacox, M.G.; Edwards, C.A.; Boustany, A.M.; Oliver, M.J.; Bograd, S.J. Scale of inference: On the sensitivity of habitat models for wide-ranging marine predators to the resolution of environmental data. Ecography 2017, 40, 210–220. [Google Scholar] [CrossRef]
- Pirotta, E.; Matthiopoulos, J.; MacKenzie, M.; Scott-Hayward, L.; Rendell, L. Modelling sperm whale habitat preference: A novel approach combining transect and follow data. Mar. Ecol. Progress Ser. 2011, 436, 257–272. [Google Scholar] [CrossRef]
- Daly, E.; White, M. Bottom trawling noise: Are fishing vessels polluting to deeper acoustic habitats? Mar. Pollut. Bull. 2021, 162, 111877. [Google Scholar] [CrossRef] [PubMed]
- Katsanevakis, S.; Stelzenmüller, V.; South, A.; Sørensen, T.K.; Jones, P.J.; Kerr, S.; Badalamenti, F.; Anagnostou, C.; Breen, P.; Chust, G.; et al. Ecosystem-based marine spatial management: Review of concepts, policies, tools, and critical issues. Ocean Coast. Manag. 2011, 54, 807–820. [Google Scholar] [CrossRef]
- Merchant, N.D.; Pirotta, E.; Barton, T.R.; Thompson, P.M. Monitoring ship noise to assess the impact of coastal developments on marine mammals. Mar. Pollut. Bull. 2014, 78, 85–95. [Google Scholar] [CrossRef] [PubMed]
| Variable | Description | Unit | Sources | Variable Type |
|---|---|---|---|---|
| FWbi | Fin whale detection presence/absence | presence/absence per min | Recorded from AMAR | Independent |
| shptnl | Number of shipping tonal detections | Detections/min | Recorded from AMAR | Dependent |
| rms | Root mean square of sound pressure level | dB re 1microPa | Recorded from AMAR | Dependent |
| sss | Sea Surface Salinity | PSU | Marine Institute | Dependent |
| sbt | Sea Bottom Salinity | PSU | Marine Institute | Dependent |
| mld | Mixed Layer Depth | meters | Marine Institute | Dependent |
| ssxv | Sea surface current east-west | m/s | Marine Institute | Dependent |
| ssyv | Sea Surface current north-south | m/s | Marine Institute | Dependent |
| ssh | Sea Surface height | meters | European Union’s Copernicus Marine Environment Monitoring Service | Dependent |
| chlora | Chlorophyll-a concentration | mg m−3 | NASA Ocean Colour Data | Dependent |
| month | Calendar Month of Sampling | Dependent | ||
| day | Day of the month during sampling | Dependent | ||
| hour | Hour of the sampling | mins | Dependent | |
| partday | Day or Night | Factor | Dependent | |
| lat | Latitude of the mooring | Coordinates | Dependent | |
| lon | Longitude of the mooring | Coordinates | Dependent |
| Acoustic Detections | Average/Month | Average/Day | Average/hour | Average/min |
|---|---|---|---|---|
| Fin whales Shipping tonals | 1487.33 4,0945.05 | 46 1364.80 | 1.92 56.87 | 0.03 0.95 |
| Months | Average Shipping Noise (rms dB re 1 μPa/min) | Fin Whale Detections (Percentage) |
|---|---|---|
| 24 March–31 March | 102.41 | 1046 (23.44%) |
| 1 April–30 April | 100.24 | 2928 (65.62%) |
| 1 May–31 May | 99.21 | 360 (8.07%) |
| 1 June–29 June | 99.12 | 128 (2.87%) |
| Total | 99.75 | 4462 (100%) |
| Covariates | Odds Ratio | Std. Error |
|---|---|---|
| Shipping noise levels (rms) * | 1.73 | ±0.044 |
| Shipping tonal detections * | 0.95 | ±0.020 |
| Days * | 1.59 | ±0.070 |
| Hour * | 0.47 | ±0.067 |
| Daytime * | 0.00 | ±4.427 |
| Night-time * | 0.00 | ±0.990 |
| Months * | 96,248.35 | ±0.880 |
| Sea Surface height (ssh) * | 22.69 | ±0.415 |
| Chlorophyll-a Concentration (chl-a) * | 402.86 | ±0.451 |
| Variables with Interactions | Odds Ratio | Std. Error |
|---|---|---|
| ssh with months * | 0.75 | ±0.087 |
| ssh with days * | 0.94 | ±0.006 |
| ssh with hours * | 0.92 | ±0.006 |
| ssh with night * | 0.33 | ±0.093 |
| chl-a with months * | 0.31 | ±0.092 |
| chl-a with days * | 0.96 | ±0.006 |
| Shipping noise with months * | 0.88 | ±0.009 |
| Shipping noise with days * | 0.99 | ±0.001 |
| Shipping noise with hours * | 1.01 | ±0.001 |
| Shipping noise with night * | 1.06 | ±0.010 |
| Shipping tonals with days * | 1.01 | ±0.0009 |
| Shipping tonals with nights * | 1.06 | ±0.0168 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, K.; Berrow, S.; Meade, R.; O’Brien, J. Habitat Modelling on the Potential Impacts of Shipping Noise on Fin Whales (Balaenoptera physalus) in Offshore Irish Waters off the Porcupine Ridge. J. Mar. Sci. Eng. 2021, 9, 1207. https://doi.org/10.3390/jmse9111207
Ramesh K, Berrow S, Meade R, O’Brien J. Habitat Modelling on the Potential Impacts of Shipping Noise on Fin Whales (Balaenoptera physalus) in Offshore Irish Waters off the Porcupine Ridge. Journal of Marine Science and Engineering. 2021; 9(11):1207. https://doi.org/10.3390/jmse9111207
Chicago/Turabian StyleRamesh, Kavya, Simon Berrow, Rossa Meade, and Joanne O’Brien. 2021. "Habitat Modelling on the Potential Impacts of Shipping Noise on Fin Whales (Balaenoptera physalus) in Offshore Irish Waters off the Porcupine Ridge" Journal of Marine Science and Engineering 9, no. 11: 1207. https://doi.org/10.3390/jmse9111207
APA StyleRamesh, K., Berrow, S., Meade, R., & O’Brien, J. (2021). Habitat Modelling on the Potential Impacts of Shipping Noise on Fin Whales (Balaenoptera physalus) in Offshore Irish Waters off the Porcupine Ridge. Journal of Marine Science and Engineering, 9(11), 1207. https://doi.org/10.3390/jmse9111207







