Density and Abundance Estimation of Amazonian River Dolphins: Understanding Population Size Variability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection
2.3. Analysis
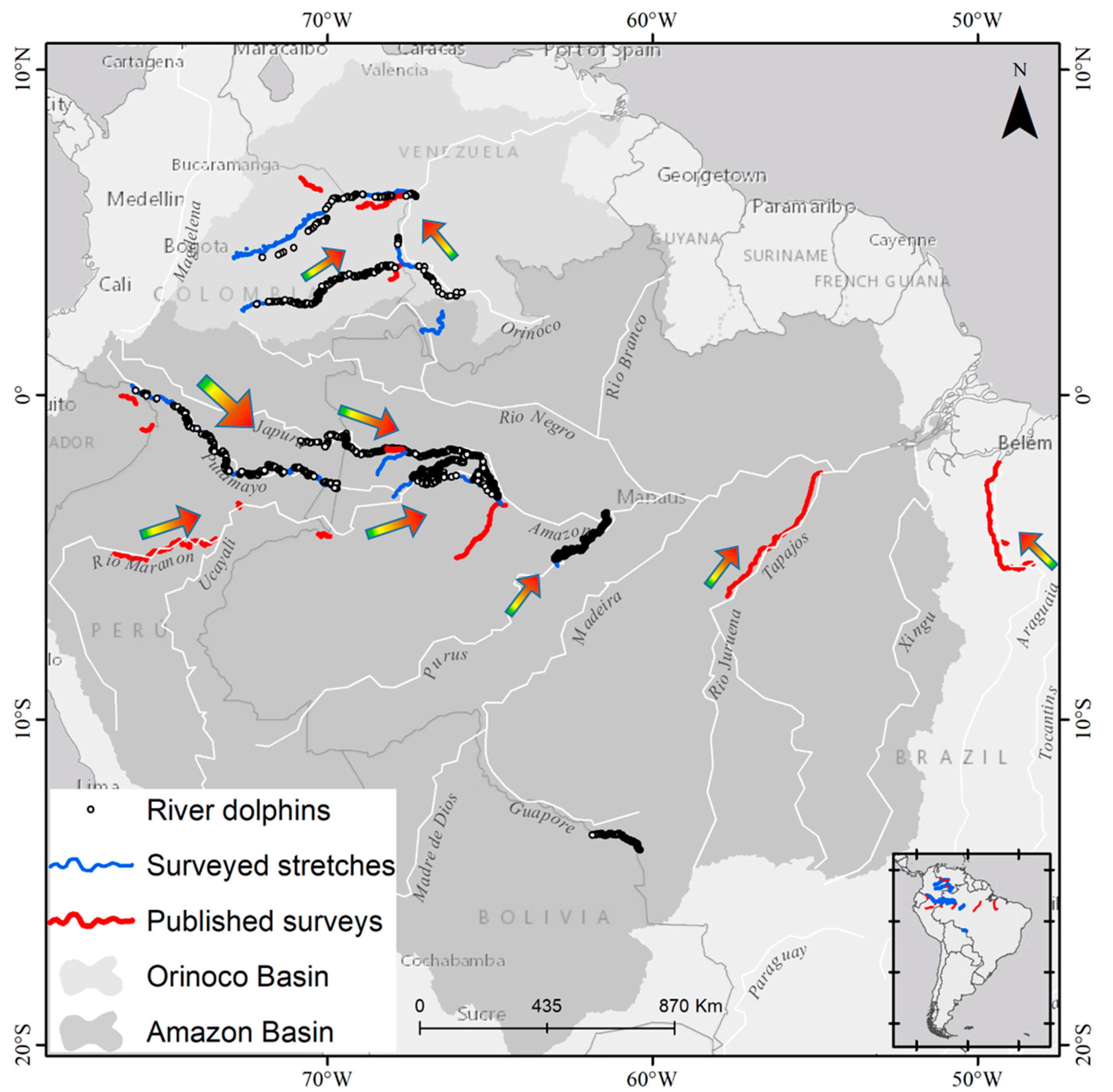
3. Results
4. Discussion
4.1. Density and Population Size
4.1.1. River Basin and Water Type
4.1.2. Drainage Position in the River Basin
4.2. Level and Range of Human Activities
4.3. Survey-Specific g(0) and Variances in Estimates of Density
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Best, R.C.; da Silva, V.M.F. Amazon river dolphin, boto Inia geoffrensis (de Blainville, 1817). In Handbook of Marine Mammals 1989; Ridgway, S.H., Harrison, R., Eds.; River Dolphins and the Larger Toothed Whales; Academic Press: London, UK, 1989; Volume 4, pp. 1–23. [Google Scholar]
- Best, R.C.; da Silva, V.M.F. Biology, status and conservation of Inia geoffrensis in the Amazon and Orinoco river basins. Occas. Pap. IUCN Species Surviv. Comm. SSC 1989, 3, 23–33. [Google Scholar]
- Pilleri, G.; Gihr, M. Observations on the Bolivian (Inia geoffrensis boliviensis d’Orbigny, 1834) and the Amazonian bufeo (Inia geoffrensis geoffrensis de Blainville, 1817) with description of a new subespecies (Inia geoffrensis humboltiana). Investig. Cetacea 1977, 8, 11–76. [Google Scholar]
- Rice, D.W. Marine Mammals of the World: Systematics and Distribution; Special Publication Number 4. 1998. The Society for Marine Mammalogy. Available online: http://www.marinemammalscience.org (accessed on 10 November 2019).
- Trujillo, F.; Portocarrero, M.; Gómez-Salazar, C.; Diazgranados, M.C.; Castellanos-Mora, L.; Ruíz-García, M.; Caballero, S. Status and conservation of river dolphins Inia geoffrensis and Sotalia fluviatilis in the Amazon and Orinoco basins in Colombia, 2010. In The Action Plan for South American River Dolphins 2020; SOLAMAC: Bogota, DC, Colombia, 2010; pp. 29–57. [Google Scholar]
- Hrbek, T.; da Silva, V.M.F.; Dutra, N.; Gravena, W.; Martin, A.R.; Farias, I.P. A new species of river dolphin from Brazil or: How little do we know our biodiversity. PLoS ONE 2014, 9, e83623. [Google Scholar] [CrossRef] [Green Version]
- Latrubesse, E.M.; Stevaux, J.C.; Sinha, R. Tropical Rivers. Geomorphology 2005, 70, 187–206. [Google Scholar] [CrossRef]
- Godoy, J.R.; Petts, G.; Salo, J. Riparian flooded forests of the Orinoco and Amazon basins: A comparative review. Biodivers. Conserv. 1999, 8, 551–586. [Google Scholar] [CrossRef]
- Winemiller, K.O.; Jepsen, D.B. Effects of seasonality and fish movement on tropical river food webs. J. Fish Biology. 1998, 53, 267–296. [Google Scholar] [CrossRef]
- Goulding, M.; Barthem, R.B.; Ferreira, E.J.G. The Smithsonian Atlas of the Amazon; Smithsonian Institution Press: Washington, DC, USA, 2003. [Google Scholar]
- Goulding, M. Amazon: The Flooded Forest; BBC Books: London, UK, 1989. [Google Scholar]
- Martin, A.R.; da Silva, V.M.F.; Salmon, D. Riverine habitat preferences of botos (Inia geoffrensis) and tucuxis (Sotalia fluviatilis) in the central Amazon. Mar. Mammal Science. 2004, 20, 189–200. [Google Scholar] [CrossRef]
- Gómez-Salazar, C.; Coll, M.; Whitehead, H. River dolphins as indicators of ecosystem degradation in large tropical rivers. Ecological. Indicators. 2012, 23, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Mosquera-Guerra, F.; Trujillo, F.; Oliveira-da-Costa, M.; Marmontel, M.; Van Damme, P.A.; Franco, N.; Córdova, L.; Campbell, E.; Alfaro-Shigueto, J.; Mena, J.L.; et al. Home range and movements of Amazon river dolphins (Inia geoffrensis) in the Amazon and Orinoco river basins. Endanger. Species 2021, 45, 269–282. [Google Scholar] [CrossRef]
- Ward, J.V.; Tockner, K. Biodiversity: Towards a unifying theme for river ecology. Freshw. Biol. 2001, 46, 807–820. [Google Scholar] [CrossRef]
- Naiman, R.J.; Bunn, S.E.; Nilsson, C.; Petts, G.E.; Pinay, G.; Thompson, L.C. Legitimizing fluvial ecosystems as users of water: An overview. Environ. Manag. 2002, 30, 455–467. [Google Scholar] [CrossRef]
- Dale, V.H.; Beyeler, S.C. Challenges in the development and use of ecological indicators. Ecol. Indic. 2001, 1, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Elmqvist, T.; Folke, C.; Nyström, M.; Peterson, G.; Bengtsson, J.; Walker, B.; Norberg, J. Response diversity, ecosystem change, and resilience. Front. Ecol. Environ. 2003, 1, 488–494. [Google Scholar] [CrossRef]
- Anganuzzi, A.A.; Buckland, S.T. Post-Stratification as a Bias Reduction Technique. J. Wildlife. Management. 1993, 57. [Google Scholar] [CrossRef]
- Martin, A.R.; da Silva, V.M.F. River dolphins and flooded forest: Seasonal habitat use and sexual segregation of botos (Inia geoffrensis) in an extreme cetacean environment. J. Zool. 2004, 263, 295–305. [Google Scholar] [CrossRef]
- Gómez-Salazar, C.; Trujillo, F.; Portocarrero-Aya, M.; Whitehead, H. Population, density estimates, and conservation of river dolphins (Inia and Sotalia) in the Amazon and Orinoco river basins. Mar. Mammal Sci. 2012, 28, 124–153. [Google Scholar] [CrossRef] [Green Version]
- Sioli, H. The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and Its Basin; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 56. [Google Scholar]
- Junk, W.J.; Wittmann, F.; Schöngart, J.; Piedade, M.T. A classification of the major habitats of Amazonian black-water river floodplains and a comparison with their white-water counterparts. Wetl. Ecol. Manag. 2015, 23, 677–693. [Google Scholar] [CrossRef]
- Gregory, K.J. The human role in changing river channels. Geomorphology 2006, 79, 172–191. [Google Scholar] [CrossRef]
- Mosquera-Guerra, F.; Trujillo, F.; Parks, D.; Oliveira-da-Costa, M.; Marmontel, M.; Armenteras-Pascual, D.; Usma, S.; Willems, D.; Carvajal-Castro, J.D.; Mantilla-Meluk, H.; et al. Analysis of distribution of river dolphins (Inia and Sotalia) in protected and transformed areas in the Amazon and Orinoco basins. In Scientific Committee/Meetings/SC67B|Slovenia 2018/SM; International Whaling Commission: Cambridge, UK, 2018. [Google Scholar]
- Mosquera-Guerra, F.; Trujillo, F.; Parks, D.; Oliveira-da-Costa, M.; Van Damme, P.A.; Echeverria, A.; Franco, N.; Carvajal-Castro, J.D.; Mantilla-Meluk, H.; Marmontel, M.; et al. Mercury in Populations of River Dolphins of the Amazon and Orinoco basins. EcoHealth 2019, 4, 743–759. [Google Scholar] [CrossRef]
- Mosquera-Guerra, F.; Trujillo, F.; Aya-Cuero, C.; Franco-León, N.; Valencia, K.; Vásquez, A.; Duran-Prieto, C.; Morales, M.D.J.; Pachón-Bejarano, G.A.; Mantilla-Meluk, H.; et al. Population estimate and identification of major conservation threats for the river dolphin (Inia geoffrensis humboldtiana) at the Colombian Orinoquia. Therya 2019, 11, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Paschoalini, M.; Almeida, R.M.; Trujillo, F.; Melo-Santos, G.; Marmontel, M.; Pavanato, H.J.; Mosquera-Guerra, F.; Ristau, N.; Zerbini, A.N. On the brink of isolation: Population estimates of the Araguaian river dolphin in a human-impacted region in Brazil. PLoS ONE 2020, 15, e0231224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braulik, G.T.; Smith, B.D. Platanista gangetica (amended version of 2017 assessment). IUCN Red List. Threat. Species 2019, E.T41758A151913336. [Google Scholar] [CrossRef]
- da Silva, V.; Trujillo, F.; Martin, A.; Zerbini, A.N.; Crespo, E.; Aliaga-Rossel, E.; Reeves, R. Inia geoffrensis. IUCN Red List. Threat. Species 2018, E.T10831A50358152. [Google Scholar] [CrossRef]
- da Silva, V.; Martin, A.; Fettuccia, D.; Bivaqua, L.; Trujillo, F. Sotalia fluviatilis. IUCN Red List. Threat. Species 2020, E.T190871A50386457. [Google Scholar] [CrossRef]
- IWC. South Asian River Dolphin Task Team Workshop Report; University of Nottingham: Kuala Lumpur, Malaysia, 19–21 July 2019. [Google Scholar]
- Khan, U.; Willems, D. Report of the Trinational Workshop on the Irrawaddy Dolphin; WWF: Zeist, Netherlands; Sindh, Pakistan, 2021; virtual. [Google Scholar]
- Wang, D.; Turvey, S.T.; Zhao, X.; Mei, Z. Neophocaena asiaeorientalis spp. asiaeorientalis. IUCN Red List. Threat. Species 2013, E.T43205774A45893487. [Google Scholar] [CrossRef]
- Smith, B.D.; Beasley, I. Orcaella brevirostris (Mekong River subpopulation). IUCN Red List. Threat. Species 2004, E.T44555A10919444. [Google Scholar] [CrossRef]
- Williams, R.; Moore, J.E.; Gomez-Salazar, C.; Trujillo, F.; Burt, L. Searching for trends in river dolphin abundance: Designing surveys for looming threats, and evidence for opposing trends of two species in the Colombian Amazon. Biol. Conservation. 2016, 195, 136–145. [Google Scholar] [CrossRef]
- Brum, S.; Rosas-Ribeiro, P.; Amaral, R.D.S.; de Souza, D.A.; Castello, L.; da Silva, V.M.F. Conservation of Amazonian aquatic mammals. Aquatic. Conserv. Marine. Freshw. Ecosystem. 2021, 31, 1068–1086. [Google Scholar] [CrossRef]
- Hastie, G.D.; Barton, T.R.; Grellier, K.; Hammond, P.S.; SwIFT, R.J.; Thompson, P.M.; Wilson, B. Distribution of small cetaceans within a candidate Special Area of Conservation; implications for management. J. Cetacean Res. Manag. 2003, 5, 261–266. [Google Scholar]
- Lewis, W.M.; Hamilton, S.K.; Lasi, M.A.; Rodríguez, M.; Saunders, J.F. Ecological determinism on the Orinoco floodplain. AIBS Bull. 2000, 50, 681–692. [Google Scholar] [CrossRef] [Green Version]
- Barthem, R.B.; Charvet-Almeida, P.; Montag, L.F.A.; Lanna, A.E. Amazon Basin. In GIWA Regional Assessment 40b; University of Kalmar: Kalmar, Sweden, 2004. [Google Scholar]
- Meade, R. Suspended sediments of the modern Amazon and Orinoco Rivers. Quat. Int. 1994, 21, 29–39. [Google Scholar] [CrossRef]
- Junk, W.J.; Furch, K. A general review of tropical South American floodplains. Wetl. Ecol. Manag. 1993, 2, 231–238. [Google Scholar] [CrossRef]
- Martinelli, L.; Reynaldo, V.; Devol, A.; Richey, J.; Folsberg, B. Suspended sediment load in the Amazon basin: An overview. GeoJournal 1989, 19, 381–389. [Google Scholar] [CrossRef]
- Meade, R.; Weibezahn, F.; Lewis, W., Jr.; Perez, H.D. Suspended sediment budget for the Orinoco River. In The Orinoco River as an Ecosystem; Weibezahn, F., Alvarez, H., Lewis, W., Jr., Eds.; Universidad Simón Bolívar: Caracas, Venezuela, 1990; pp. 55–79. [Google Scholar]
- Hamilton, S.; Lewis, W. Physical characteristics of the fringing floodplain of the Orinoco River, Venezuela. Interciencia 1990, 15, 491–500. [Google Scholar]
- Sippel, S.; Hamilton, S.; Melack, J.; Choudhury, B. Determination of inundation area in the Amazon River floodplain using SMMR 37GHz polarization difference. Remote Sens. Environ. 1994, 48, 70–77. [Google Scholar] [CrossRef]
- Thomas, L.; Buckland, S.T.; Rexstad, E.A.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Applied. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Miller, D.L. Distance: Distance Sampling Detection Function and Abundance Estimation. R Package Version 0.9.7. 2017. Available online: https://CRAN.R-project.org/package=Distance (accessed on 12 February 2019).
- Laake, J.; Borchers, D.; Thomas, L.; Miller, D.; Bishop, J. mrds: Mark-Recapture Distance Sampling. R Package Version 2.1.18. 2017. Available online: https://CRAN.R-project.org/package=mrds (accessed on 12 February 2020).
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Frias, M.P. Estimação dos parâmetros populacionais de densidade e abundância para os golfinhos de rio da América do Sul boto (Inia spp.) e tucuxi (Sotalia fluviatilis): Aperfeiçoamento do método e abordagens ecológicas [Estimating Density and Population Size for South American River Dolphins Boto and Tucuxi: Improving Methods and Ecological Approaches]. Ph.D. Thesis, Universidade Federal de Juiz de Fora, Juiz de Fora, Brazil, 2019; 144p.
- Marques, F.F.; Buckland, S.T. Incorporating covariates into standard line transect analyses. Biometrics 2003, 59, 924–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvitz, D.G.; Thompson, D.J. A generalization of sampling without replacement from a finite universe. J. Am. Stat. Assoc. 1952, 47, 663–685. [Google Scholar] [CrossRef]
- Innes, S.; Heide-Jørgensen, M.P.; Laake, J.L.; Laidre, K.L.; Cleator, H.J.; Richard, P.; Stewart, R.E.A. Surveys of belugas and narwhals in the Canadian High Arctic in 1996. NAMMCO Sci. Publ. 2002, 4, 169–190. [Google Scholar] [CrossRef] [Green Version]
- Vidal, O.; Barlow, J.; Hurtado, L.; Torre, J.; Cendon, P.; Ojeda, Z. Distribution and abundance of the Amazon river dolphin (Inia geoffrensis) and the tucuxi (Sotalia fluviatilis) in the upper Amazon River. Mar. Mammal Science. 1997, 13, 427–445. [Google Scholar] [CrossRef]
- Aliaga-Rossel, E. Distribution and abundance of the pink river dolphin, bufeo (Inia geoffrensis) in the Tijamuchi River, Beni-Bolivia. Aquat. Mammal. 2002, 28, 312–323. [Google Scholar]
- Aliaga-Rossel, E.; McGuire, T.L.; Hamilton, H. Distribution and encounter rates of the river dolphin (Inia geoffrensis boliviensis) in the central Bolivian Amazon. J. Cetacean Res. Manag. 2006, 8, 87. [Google Scholar]
- Pavanato, H.J.; Melo-Santos, G.; Lima, D.S.; Portocarrero-Aya, M.; Paschoalini, M.; Mosquera, F.; Trujillo, F.; Meneses, R.; Marmontel, M.; Maretti, C. Risks of dam construction for South American river dolphins: A case study of the Tapajós River. Endanger. Species Reearchs 2016, 31, 47–60. [Google Scholar] [CrossRef] [Green Version]
- Pavanato, H.J.; Salazar, C.G.; Lima, D.; Paschoalini, M.; Ristau, N.; Marmontel, M. Density, abundance and group size of river dolphins (Inia geoffrensis and Sotalia fluviatilis) in central Amazonia, Brazil. J. Cetacean Res. Manag. 2019, 20, 93–100. [Google Scholar] [CrossRef]
- Guyot, J.L.; Jouanneau, J.M.; Soare, L.; Boaventura, G.R.; Maillet, N.; Lagane, C. Clay mineral composition of river sediments in the Amazon Basin. Catena 2007, 71, 340–356. [Google Scholar] [CrossRef]
- Mosquera-Guerra, F.; Trujillo, F.; Meluk, H.M.; Valencia, A.V.; Valderrama, D.H.R.; Navarro, F.A.V.; Garzón, J.G.A.; Londoño, I. Tamaño poblacional, densidad y distribución de Inia geoffrensis y Sotalia fluviatilis en la cuenca media del río Caquetá. Momentos De Cienc. 2015, 12, 107–115, ISSN 1692–5491. [Google Scholar]
- Marmontel, M.; de Melo, J.F.; Frias, M.P.; Giovanni, A.; de Almeida-Coelho, H.I.C.; Pérez, H.P.J. Amazonian River Dolphin Density Estimates in the Mamirauá Sustainable Development Reserve 2017–2019. In Proceedings of the International Whaling Commission, Scientific Committee Meeting, Virtual, 12–24 May 2020. 68SC. [Google Scholar]
- Hamilton, S.K.; Lewis, W.M.; Sippel, S.J. Energy sources for aquatic animals in the Orinoco River floodplain: Evidence from stable isotopes. Oecologia 1992, 89, 324–330. [Google Scholar] [CrossRef]
- Hamilton, H.; Caballero, S.; Collins, A.G.; Brownell Jr, R.L. Evolution of river dolphins. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2001, 268, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Medina, E.; Silva, J.F. Savannas of Northern South America: A Steady State Regulated by Water-Fire Interactions on a Background of Low Nutrient Availability. J. Biogeogr. 1990, 17, 403. [Google Scholar] [CrossRef]
- Savage, K.M.; Potter, P.E. Petrology of modern sands of the rios Guaviare and Inirida, southern Colombia: Tropical climate and sand composition. J. Geol. 1991, 99, 289–298. [Google Scholar] [CrossRef]
- Chitty, C.H.I. Phytoplankton in the upper and middle Orinoco river. Internationale Vereinigung für theoretische und angewandte Limnologie: Verhandlungen 1994, 25, 1856–1861. [Google Scholar]
- Lasso, C.A.; Machado-Allison, A.; Taphorn, D.C. Fishes and aquatic habitats of the Orinoco River Basin: Diversity and conservation. J. Fish Biol. 2016, 89, 174–191. [Google Scholar] [CrossRef]
- Sioli, H. The Amazon and its main affluents: Hydrography, morphology of the river courses, and river types. In The Amazon: Limnology and Landscape Ecology of a Mighty Tropical River and Its Basin; Sioli, H., Ed.; Dr. W. Junk Publishers: Dordrecht, The Netherlands, 1984; pp. 127–165. [Google Scholar]
- Gravena, W.; Da Silva, V.M.F.; Da Silva, M.N.; Farias, I.P.; Hrbek, T. Living between rapids: Genetic structure and hybridization in botos (Cetacea: Iniidae: Inia spp.) of the Madeira River, Brazil. Biolical J. Linnean. Soc. 2015, 114, 764–777. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, J.S. Developing flow–ecology relationships: Implications of nonlinear biological responses for water management. Freshw. Biol. 2017, 62, 1305–1324. [Google Scholar] [CrossRef] [Green Version]
- Gravena, W.; Farias, I.P.; da Silva, M.N.; da Silva, V.M.F.; Hrbek, T. Looking to the past and the future: Were the Madeira River rapids a geographical barrier to the boto (Cetacea: Iniidae)? Conservation. Genetics. 2014, 15, 619–629. [Google Scholar] [CrossRef]
- de Paiva, R.C.D.; Buarque, D.C.; Collischonn, W.; Bonnet, M.P.; Frappart, F.; Calmant, S.; Bulhoes, M.C.A. Large-scale hydrologic and hydrodynamic modeling of the Amazon River basin. Water Resour. Reearch 2013, 49, 1226–1243. [Google Scholar] [CrossRef] [Green Version]
- Mintzer, V.J.; Martin, A.R.; da Silva, V.M.F.; Barbour, A.B.; Lorenzen, K.; Frazer, T.K. Effect of illegal harvest on apparent survival of Amazon River dolphins (Inia geoffrensis). Biol. Conservation. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Mintzer, V.J.; Schmink, M.; Lorenzen, K.; Frazer, T.K.; Martin, A.R.; da Silva, V.M. Attitudes and behaviors toward Amazon River dolphins (Inia geoffrensis) in a sustainable use protected area. Biodivers. Conserv. 2015, 24, 247–269. [Google Scholar] [CrossRef]
- Iriarte, V.; Marmontel, M. Insights on the use of dolphins (boto, Inia geoffrensis and tucuxi, Sotalia fluviatilis) for bait in the piracatinga (Calophysus macropterus) fishery in the western Brazilian Amazon. J. Cetacean Res. Manag. 2013, 13, 163–173. [Google Scholar]
- Rivero, D.; Liu, Y. Venezuela’s Orinoco Mining Arc: A literature review of Environmental Impacts. Int. J. Sci. Res. Publ. 2020, 10, 202. [Google Scholar] [CrossRef]
- Aliaga-Rossel, E.; Mariana, E.-W. Translocation of trapped Bolivian river dolphins (Inia boliviensis). J. Cetacean Res. Manag. 2020, 21, 17–23. [Google Scholar] [CrossRef]
- Campbell, E.; Mangel, J.C.; Alfaro-Shigueto, J.; Mena, J.L.; Thurstan, R.H.; Godley, B.J. Coexisting in the Peruvian Amazon: Interactions between fisheries and river dolphins. J. Nat. Conserv. 2020, 56, 125859. [Google Scholar] [CrossRef]
- Taylor, B.L.; Wade, P.R. “Best” abundance estimates and best management: Why they are not the same. In Quantitative Methods for Conservation Biology; Springer: New York, NY, USA, 2000; pp. 96–108. [Google Scholar]
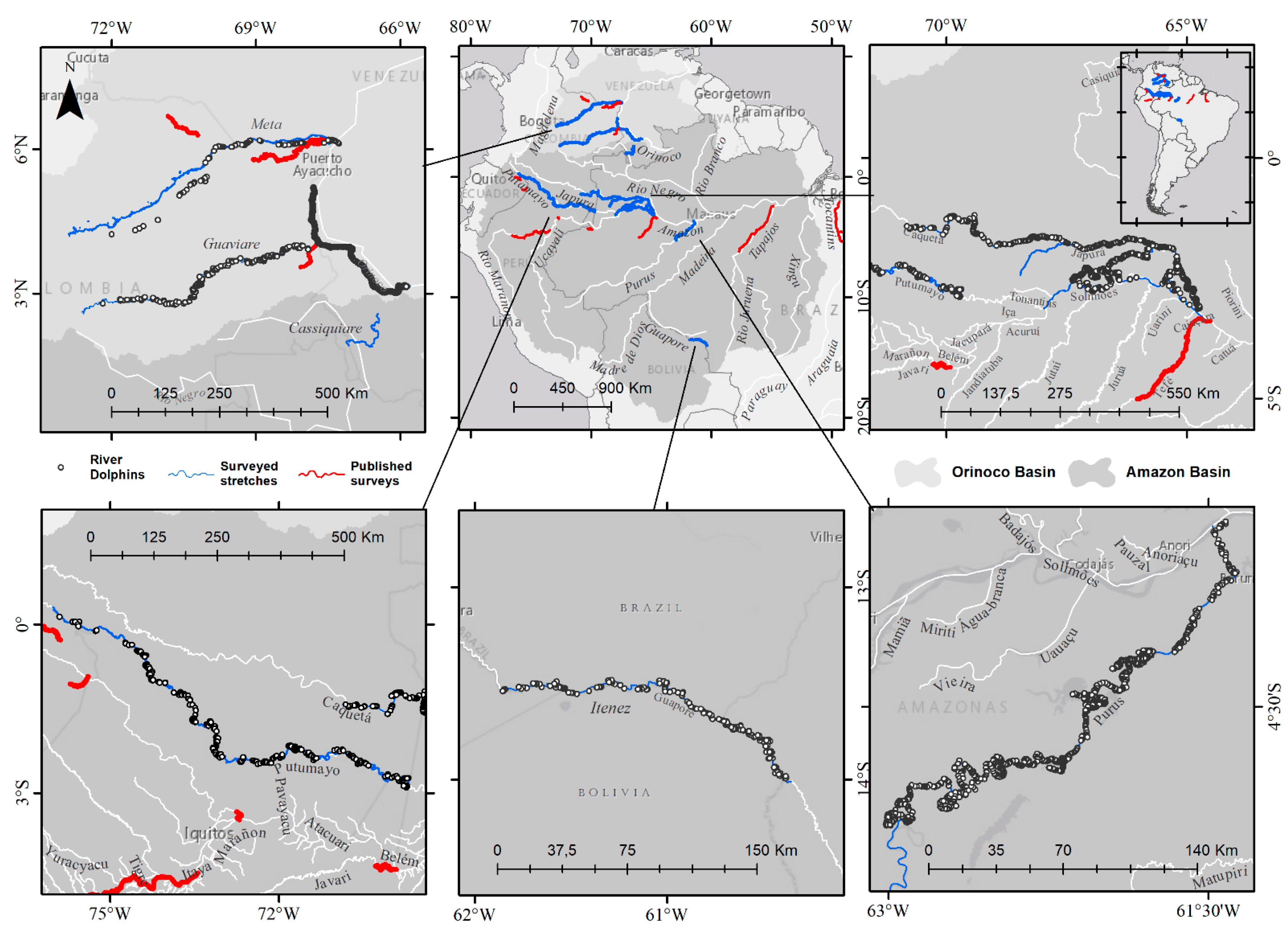
| River | Year | Season | Water Type | Basin | Coordinates Beginning | Coordinates End | * River Length (km) | Country | Sampling Effort (km) | Area (km²) |
|---|---|---|---|---|---|---|---|---|---|---|
| Purus | 2012 | Rising | White | Amazon | 3°40′55.75” S/ 61°28′25.42” W | 5°16′34.79” S/ 62°55′20.59” W | 3211 | Brazil | 512.05 | 538.72 |
| Meta | 2012 | Rising | White | Orinoco | 6°12′12.05” N/ 67°28′4.98” W | 4°21′30.86” N/ 72°5′2.86” W | 804 | Colombia/Venezuela | 584.84 | 969 |
| ** Orinoco/Cassiquiare | 2013 | Receding | White | Orinoco | 2°39′41.18” N/ 67°28′53.48” W | 4°3′43.63” N/ 67°42′10.87” W | 326 | Colombia/Venezuela | 454 | 482.06 |
| Japurá (Caquetá) + tributaries (Apaporis, Cahuinari, Miriti) | 2014 | Rising | White (Japurá River), black (tributaries) | Amazon | 3°9′46.89” S/ 64°47′37.13” W | 1°26′11.28” S/ 70°51′33.17” W | 2100 | Brazil/Colombia | 1027.53 | 1767.67 |
| Auati-Paranã channel | 2014 | Rising | Mixed | Amazon | 2°32′43.04” S/ 67°22′30.14” W | 1°51′4.89” S/ 65°42′37.48” W | 432.8 | Brazil | 432.8 | 55.74 |
| Solimões | 2014 | Rising | White | Amazon | 3°9′27.35” S/ 67°57′54.33” W | 3°21′47.71” S/ 64°39′49.92” W | 1700 | Brazil | 581.39 | 1745.39 |
| *** Juami and Japurá rivers | 2015 | Rising | Black (Juami River), White (Japurá River) | Amazon | 1°46′49.90” S/ 67°35′39.4” W | 2°27′54.28” S/ 68°27′57.7” W | Brazil | 386.95 | 254 | |
| Guaviare | 2016 | Receding | White | Orinoco | 2°36′58.61” N/ 72°38′8.65” W | 3°52′23.56” N/ 67°56′5.03” W | 1497 | Colombia | 968 | 593.75 |
| **** RDSM | 2016 | Receding | Predominantly White with mixed water in confluences | Amazon | 3°9′35” S/ 64°47′37” W | 2°32′50” S/ 67°22′8” W | 1422.81 | Brazil | 1422.81 | 2652 |
| Putumayo | 2017 | Receding | White | Amazon | 0°6′46.97” N/ 75°52′10.97” W | 2°53′39.76” S/ 69°44′2.22” W | 1610 | Cross-border: Ecuador, Colombia, Peru, Brazil | 1186 | 1108.66 |
| Iténez (Guaporé) | 2017 | Rising | Clear | Amazon | 13°32′21.44” S/ 61°51′13.28” W | 4°3′43.63” S/ 67°42′10.87” W | 1260 | Bolivia/Brazil | 481 | 40.18 |
| Meta | 2018 | Rising | White | Orinoco | 6°12′12.05” N/ 67°28′4.98” W | 4°21′30.86” N/ 72°5′2.86” W | 804 | Colombia/Venezuela | 630.44 | 969 |
| River | Inia geoffrensis | Sotalia fluviatilis | ||||
|---|---|---|---|---|---|---|
| D | N | CV | D | N | CV | |
| Purus | 14.5 | 7672 | 0.37 | 17.14 | 9238 | 0.49 |
| Meta | 1.04 | 972 | 0.56 | - | - | - |
| Orinoco—Cassiquiare channel | 0.9 | 435 | 1.36 | - | - | - |
| Japurá (Caquetá) + tributaries | 2.19 | 3871 | 0.94 | 1.79 | 3164 | 0.98 |
| Auati-Paranã channel | 5.5 | 307 | 0.51 | 5.8 | 324 | 0.55 |
| Solimões | 1.01 | 1763 | 1.34 | 1.34 | 2339 | 1.03 |
| Juami-Japurá rivers | 1.77 | 440 | 1.38 | 2.4 | 599 | 1.79 |
| Guaviare | 3.28 | 1138 | 0.32 | - | - | - |
| RDSM | 3.17 | 8407 | 0.74 | 3.35 | 8876 | 0.65 |
| Putumayo | 3.49 | 3897 | 0.61 | 0.49 | 546 | 0.95 |
| Iténez (Guaporé) | 5.07 | 204 | 0.88 | - | - | - |
| Meta | 1.49 | 1397 | 0.95 | - | - | - |
| River | Water Type | River Basin | g(0) | |
|---|---|---|---|---|
| Inia geoffrensis | Sotalia fluviatilis | |||
| Purus | White | Amazon | 0.86 (0.09) | 0.99 (0.008) |
| Meta—2012 | White | Orinoco | 0.95 (0.04) | |
| Orinoco—Cassiquiare channel | White | Orinoco | 0.81 (0.05) * | |
| Japurá (Caquetá) + tributaries (Apaporis, Cahuinari, Miriti) | White (Japurá River), Black (tributaries) | Amazon | 0.69 (0.04) | 0.83 (0.02) |
| Auati-Paranã channel | Mixed (Black and White) | Amazon | ||
| Solimões | White | Amazon | ||
| Juami and Japurá rivers | Black (Juami River), White (Japurá River) | Amazon | 0.81 (0.05) * | 0.98 (0.006) * |
| Guaviare | White | Orinoco | 0.71 (0.53) | |
| RDSM | Predominantly White with mixed water in confluences | Amazon | 0.79 (0.08) | 0.98 (0.01) |
| Putumayo | White | Amazon | 0.56 (0.08) | 0.59 (0.13) |
| Iténez (Guaporé) | Clear | Amazon | 0.81 (0.05) * | |
| Meta—2018 | White | Orinoco | 0.85 (0.11) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschoalini, M.; Trujillo, F.; Marmontel, M.; Mosquera-Guerra, F.; Paitach, R.L.; Julião, H.P.; dos Santos, G.M.A.; Van Damme, P.A.; Coelho, A.G.d.A.; Escobar Wilson White, M.; et al. Density and Abundance Estimation of Amazonian River Dolphins: Understanding Population Size Variability. J. Mar. Sci. Eng. 2021, 9, 1184. https://doi.org/10.3390/jmse9111184
Paschoalini M, Trujillo F, Marmontel M, Mosquera-Guerra F, Paitach RL, Julião HP, dos Santos GMA, Van Damme PA, Coelho AGdA, Escobar Wilson White M, et al. Density and Abundance Estimation of Amazonian River Dolphins: Understanding Population Size Variability. Journal of Marine Science and Engineering. 2021; 9(11):1184. https://doi.org/10.3390/jmse9111184
Chicago/Turabian StylePaschoalini, Mariana, Fernando Trujillo, Miriam Marmontel, Federico Mosquera-Guerra, Renan Lopes Paitach, Heloise Pavanato Julião, Gabriel Melo Alves dos Santos, Paul André Van Damme, André Giovanni de Almeida Coelho, Mariana Escobar Wilson White, and et al. 2021. "Density and Abundance Estimation of Amazonian River Dolphins: Understanding Population Size Variability" Journal of Marine Science and Engineering 9, no. 11: 1184. https://doi.org/10.3390/jmse9111184
APA StylePaschoalini, M., Trujillo, F., Marmontel, M., Mosquera-Guerra, F., Paitach, R. L., Julião, H. P., dos Santos, G. M. A., Van Damme, P. A., Coelho, A. G. d. A., Escobar Wilson White, M., & Zerbini, A. N. (2021). Density and Abundance Estimation of Amazonian River Dolphins: Understanding Population Size Variability. Journal of Marine Science and Engineering, 9(11), 1184. https://doi.org/10.3390/jmse9111184






