The Involvement of Macrophage Colony Stimulating Factor on Protein Hydrolysate Injection Mediated Hematopoietic Function Improvement
Abstract
1. Introduction
2. Materials and Methods
2.1. PH Preparation
2.2. Molecular Mass and Amino Acid Analyses
2.3. Cell Experiments
2.3.1. Cell Culture
2.3.2. Transfection of M-CSF siRNA in K562 Cells
2.4. Animal Experiments and Drug Treatment Protocols
2.5. Assessment of Peripheral Blood Physiological Indexes
2.6. Flow Cytometry
2.7. Histopathological Analysis
2.8. Cytokine Assays
2.9. Western Blotting
2.10. Statistical Analysis
3. Results
3.1. Molecular Mass Distribution and Amino Acid Content of PH
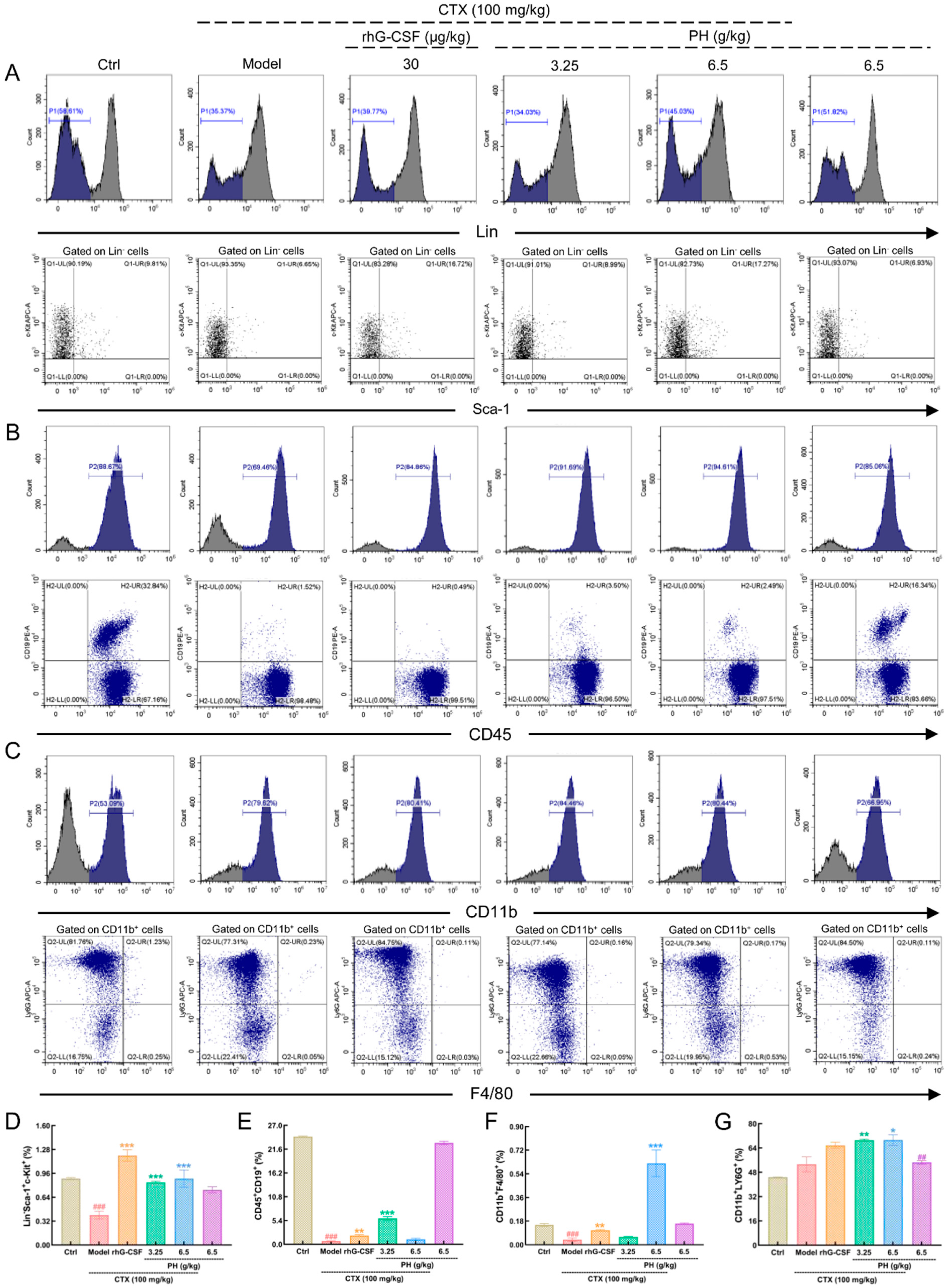
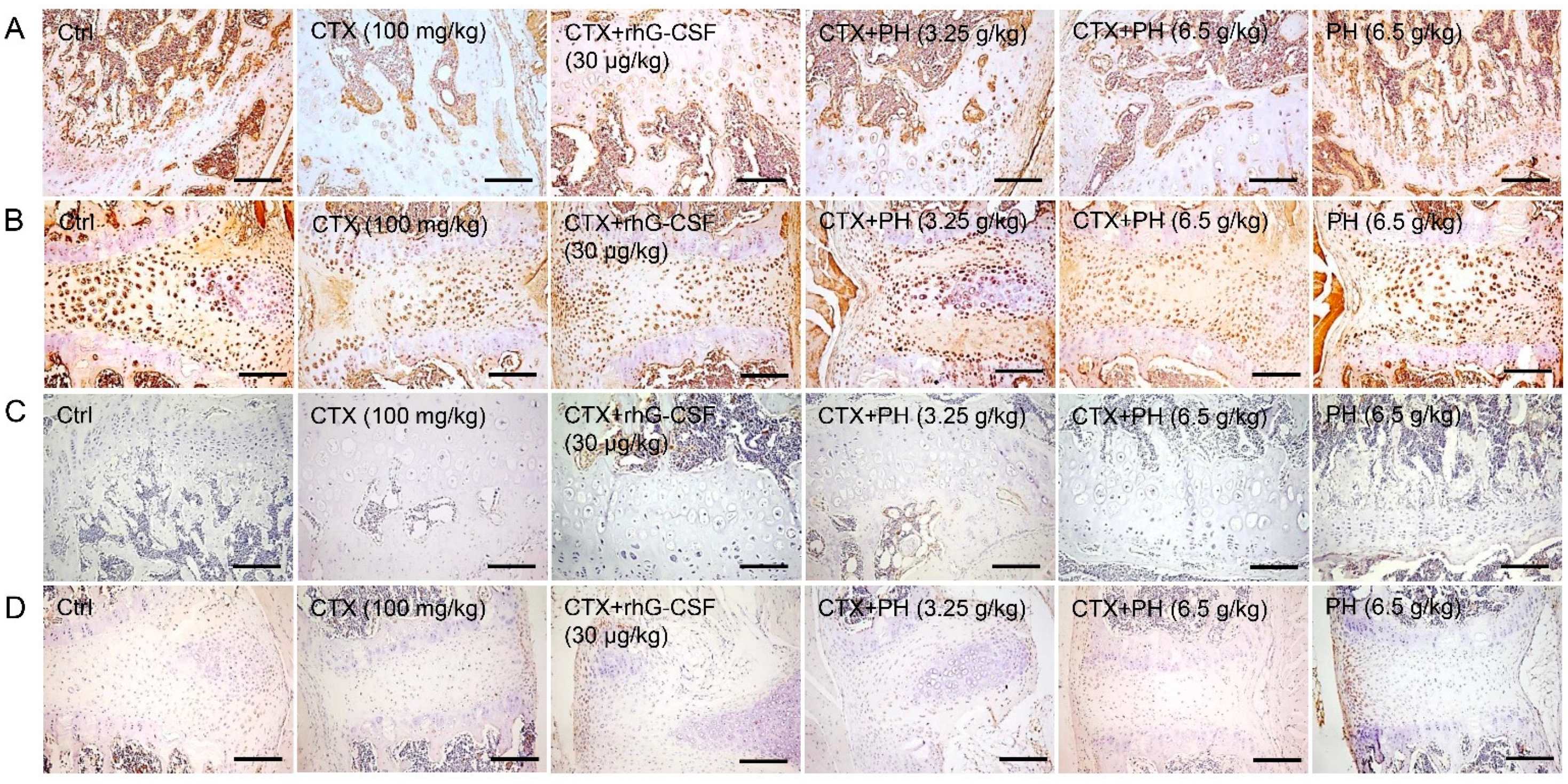
3.2. PH promoted the Hematopoietic Function of Mice
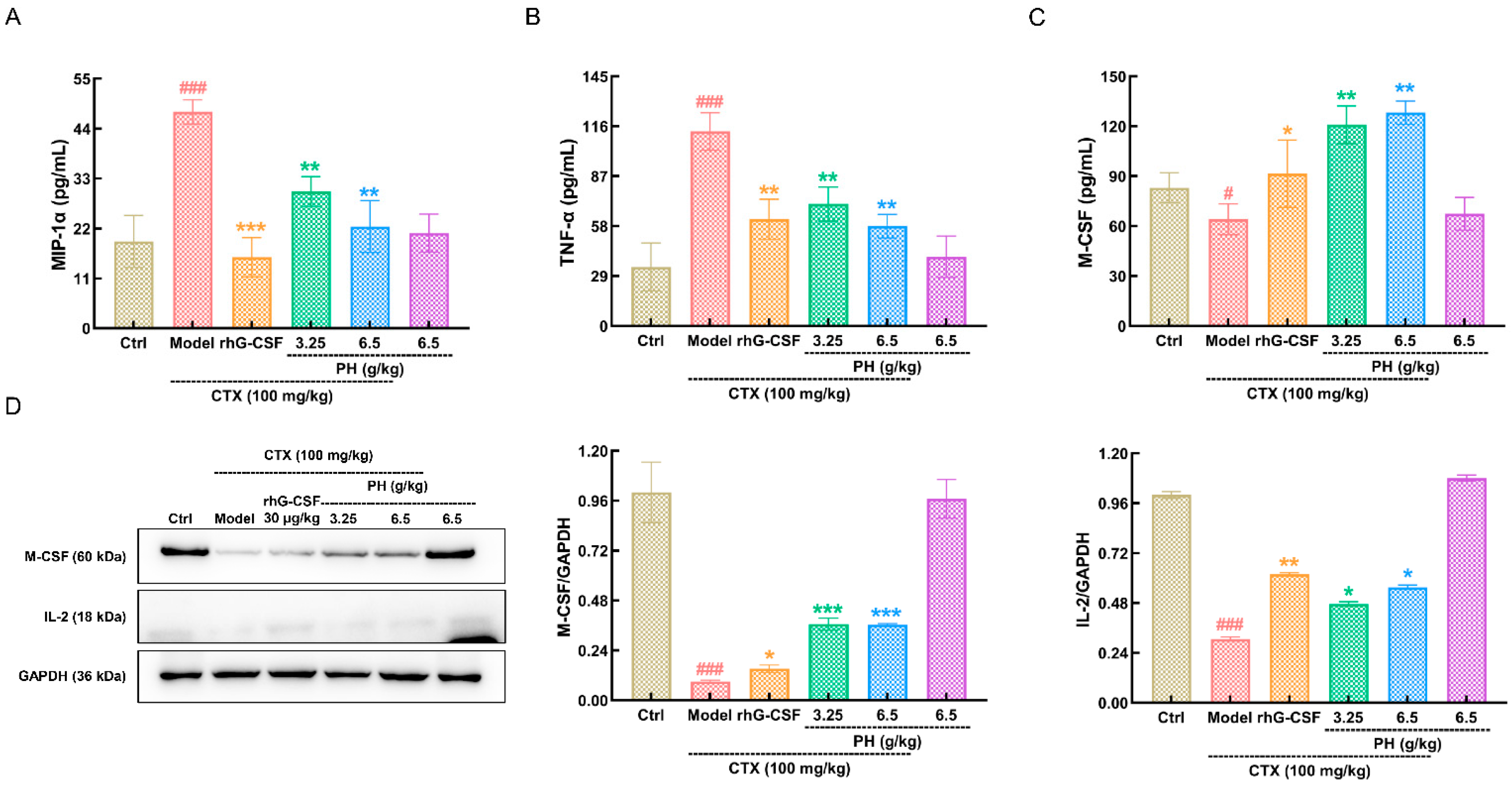
3.3. PH Regulates Hematopoietic Cytokine Expression
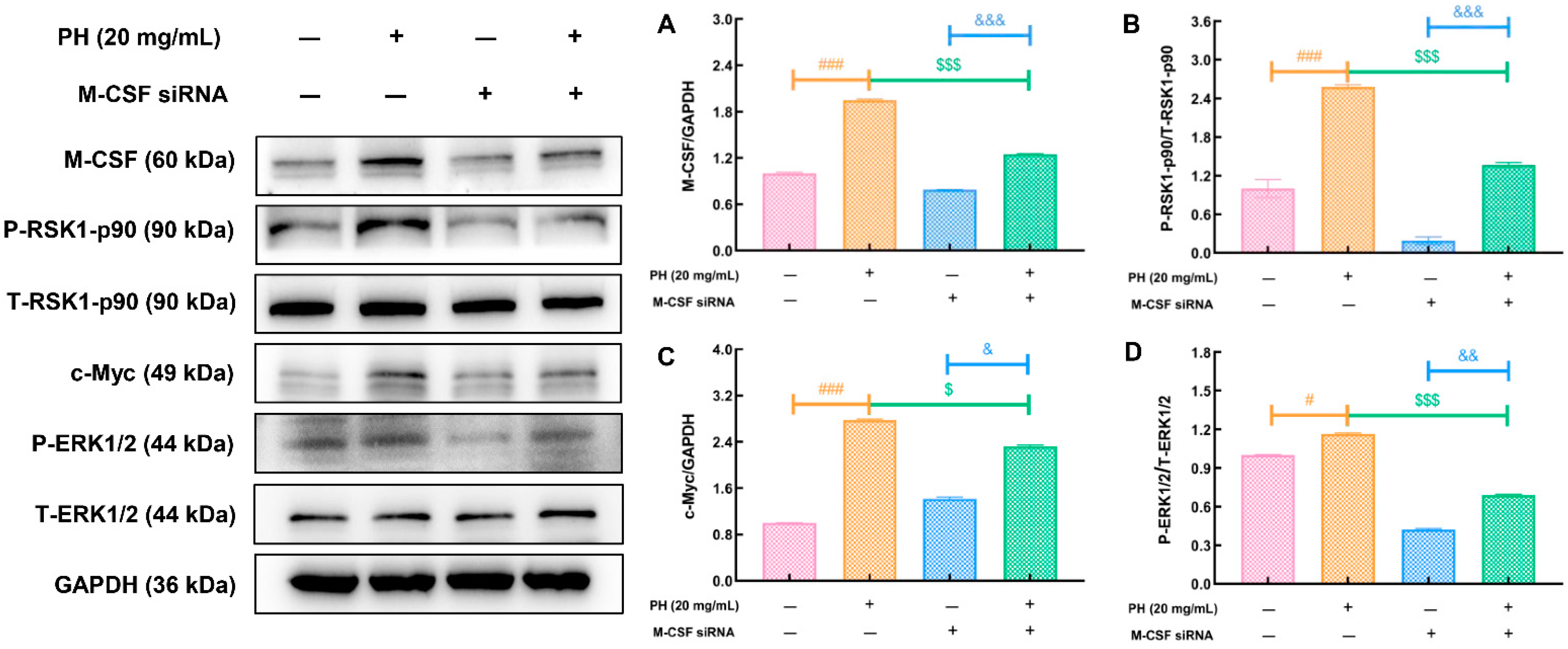
3.4. PH Regulates M-CSF to Improve Hematopoietic Function
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Carey, P.J. Drug-Induced Myelosuppression. Drug Saf. 2003, 26, 691–706. [Google Scholar] [CrossRef]
- Brigle, K.; Pierre, A.; Finley-Oliver, E.; Faiman, B.; Tariman, J.; Miceli, T.; Board, A. Myelosuppression, Bone Disease, and Acute Renal Failure: Evidence-Based Recommendations for Oncologic Emergencies. Clin. J. Oncol. Nurs. 2017, 21, 60–76. [Google Scholar] [CrossRef]
- He, M.; Wang, N.; Zheng, W.; Cai, X.; Qi, D.; Zhang, Y.; Han, C. Ameliorative effects of ginsenosides on myelosuppression induced by chemotherapy or radiotherapy. J. Ethnopharmacol. 2020, 268, 113581. [Google Scholar] [CrossRef]
- Chen, X.-Y.; Zhao, A.-B.; Yu, B.; Wu, X.-L.; Cao, K.-J.; Li, E.-Q.; Li, Q.-M. Protective effects on myelosuppression mice treated by three different classic chinese medicine formulae. Pharmacogn. Mag. 2011, 7, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Li, L.; Wang, Y.; Liu, T. Hematopoietic stem cells: Multiparameter regulation. Hum. Cell 2016, 29, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Du, H.; Yao, M.; Cui, X.; Shi, Q.; Wang, Y.; Yang, Y. Chinese Herbal Medicine for Myelosuppression Induced by Chemotherapy or Radiotherapy: A Systematic Review of Randomized Controlled Trials. Evid. -Based Complement. Altern. Med. 2015, 2015, 1–12. [Google Scholar] [CrossRef]
- Jagannathan-Bogdan, M.; Zon, L.I. Hematopoiesis. Development 2013, 140, 2463–2467. [Google Scholar] [CrossRef]
- Salem, M.L.; Al-Khami, A.A.; El-Nagaar, S.A.; Zidan, A.-A.; Al-Sharkawi, I.M.; Díaz-Montero, C.M.; Cole, D.J. Kinetics of rebounding of lymphoid and myeloid cells in mouse peripheral blood, spleen and bone marrow after treatment with cyclophosphamide. Cell. Immunol. 2012, 276, 67–74. [Google Scholar] [CrossRef][Green Version]
- Han, J.; Wang, Y.; Cai, E.; Zhang, L.; Zhao, Y.; Sun, N.; Zheng, X.; Wang, S. Study of the Effects and Mechanisms of Ginsenoside Compound K on Myelosuppression. J. Agric. Food Chem. 2019, 67, 1402–1408. [Google Scholar] [CrossRef]
- Peláez, B.; Campillo, J.A.; López-Asenjo, J.A.; Subiza, J.L. Cyclophosphamide Induces the Development of Early Myeloid Cells Suppressing Tumor Cell Growth by a Nitric Oxide-Dependent Mechanism. J. Immunol. 2001, 166, 6608–6615. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Teng, S.; Zhang, L.; Teng, L.; Wang, D. Calf thymus polypeptide improved hematopoiesis via regulating colony-stimulating factors in BALB/c mice with hematopoietic dysfunction. Int. J. Biol. Macromol. 2020, 156, 204–216. [Google Scholar] [CrossRef]
- Trueblood, E.S.; Brown, W.C.; Palmer, G.H.; Davis, W.C.; Stone, D.M.; McElwain, T.F. B-Lymphocyte Proliferation during Bovine Leukemia VirusInduced Persistent Lymphocytosis Is Enhanced by T-Lymphocyte-Derived Interleukin-2. J. Virol. 1998, 72, 3169–3177. [Google Scholar] [CrossRef]
- Mishra, S.; Patel, D.D.; Bansal, D.D.; Kumar, R. Semiquinone glucoside derivative provides protection against γ-radiation by modulation of immune response in murine model. Environ. Toxicol. 2014, 31, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Misago, M.; Tsukada, J.; Ogawa, R.; Kikuchi, M.; Hanamura, T.; Chiba, S.; Oda, S.; Morimoto, I.; Eto, S. Enhancing effects of IL-2 on M-CSF production by human peripheral blood monocytes. Int. J. Hematol. 1993, 58, 43–51. [Google Scholar]
- Sturgill, M.G.; Huhn, R.D.; Drachtman, R.A.; Ettinger, A.G.; Ettinger, L.J. Pharmacokinetics of intravenous recombinant hu-man granulocyte colony-stimulating factor (rhG-CSF) in children receiving myelosuppressive cancer chemotherapy: Clearance increases in relation to absolute neutrophil count with repeated dosing. Am. J. Hematol. 1997, 54, 124–130. [Google Scholar] [CrossRef]
- Puhalla, S.; Bhattacharya, S.; Davidson, N.E. Hematopoietic growth factors: Personalization of risks and benefits. Mol. Oncol. 2012, 6, 237–241. [Google Scholar] [CrossRef]
- Lu, W.; Jia, D.; An, S.; Mu, M.; Qiao, X.; Liu, Y.; Li, X.; Wang, D. Calf Spleen Extractive Injection protects mice against cyclophosphamide-induced hematopoietic injury through G-CSF-mediated JAK2/STAT3 signaling. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Bioactive properties of milk proteins in humans: A review. Peptides 2015, 73, 20–34. [Google Scholar] [CrossRef]
- Daliri, E.B.M.; Oh, D.H.; Lee, B.H. Bioactive Peptides. Foods 2017, 6, 32. [Google Scholar] [CrossRef]
- Liu, H.; Tong, X. Research Progress of Antioxidant Peptides in Hydrolyzed Soybean Protein. China Condiment 2021, 46, 191–195. [Google Scholar]
- Zeng, S.; Pang, Y.; He, L.; Liu, Y. Effects of Soybean Peptides from Soybean Protein Isolate Hydrolyzed by Recombinant Aspergillus sojae Alkaline Protease on Immune Function and Antioxidation in Mice. Food Sci. Biotechnol. 2021, 40, 97–102. [Google Scholar]
- Lian, Y.; Zhong, P. Determination and evaluation of amino acids in protein hydrolysate injection. Chin. J. Pharm. Anal. 2012, 32, 61–66. [Google Scholar]
- Loken, M.R.; Shah, V.O.; Dattilio, K.L.; Civin, C.I. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood 1987, 70, 1316–1324. [Google Scholar] [CrossRef]
- Ostrand-Rosenberg, S. Myeloid-derived suppressor cells: More mechanisms for inhibiting antitumor immunity. Cancer Immunol. Immunother. 2010, 59, 1593–1600. [Google Scholar] [CrossRef]
- Wang, X.; Chu, Q.; Jiang, X.; Yu, Y.; Wang, L.; Cui, Y.; Lu, J.; Teng, L.; Wang, D. Sarcodon imbricatus polysaccharides improve mouse hematopoietic function after cyclophosphamide-induced damage via G-CSF mediated JAK2/STAT3 pathway. Cell Death Dis. 2018, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; He, D.; Zeng, H.-L.; Liu, J.; Yang, H.; Xu, L.-B.; Liang, H.; Wan, D.; Tang, C.-Y.; Cai, P.; et al. Siwu Paste protects bone marrow hematopoietic function in rats with blood deficiency syndrome by regulating TLR4/NF-κB/NLRP3 signaling pathway. J. Ethnopharmacol. 2020, 262, 113160. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xie, X.; Du, Y.; Ma, G.; Xu, X.; Sun, G.; Sun, X. Protective Effects of Biscoclaurine Alkaloids on Leukopenia Induced by 60Co-γ Radiation. Evid. -Based Complement. Altern. Med. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Weischendorff, S.; Kielsen, K.; Nederby, M.; Schmidt, L.; Burrin, D.; Heilmann, C.; Ifversen, M.; Sengeløv, H.; Mølgaard, C.; Müller, K. Reduced Plasma Amino Acid Levels During Allogeneic Hematopoietic Stem Cell Transplantation Are Associated with Systemic Inflammation and Treatment-Related Complications. Biol. Blood Marrow Transplant. 2019, 25, 1432–1440. [Google Scholar] [CrossRef]
- Jaako, P.; Debnath, S.; Olsson, K.; Bryder, D.; Flygare, J.; Karlsson, S. Dietary L-leucine improves the anemia in a mouse model for Diamond-Blackfan anemia. Blood 2012, 120, 2225–2228. [Google Scholar] [CrossRef]
- Eales, K.L.; Hollinshead, K.E.R.; A Tennant, D. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef]
- Jarosz, L.; Kwiecień, M.; Marek, A.; Grądzki, Z.; Winiarska-Mieczan, A.; Kalinowski, M.; Laskowska, E. Effects of feed supplementation with glycine chelate and iron sulfate on selected parameters of cell-mediated immune response in broiler chickens. Res. Vet. Sci. 2016, 107, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Miwa, Y.; Abe-Suzuki, S.; Abe, S.; Kirimura, S.; Onishi, I.; Kitagawa, M.; Kurata, M. Extramedullary hematopoiesis: Elucidating the function of the hematopoietic stem cell niche (Review). Mol. Med. Rep. 2015, 13, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Konoplev, S.; Hsieh, P.-P.; Chang, C.-C.; Medeiros, L.J.; Lin, P. Janus kinase 2 V617F mutation is detectable in spleen of patients with chronic myeloproliferative diseases suggesting a malignant nature of splenic extramedullary hematopoiesis. Hum. Pathol. 2007, 38, 1760–1763. [Google Scholar] [CrossRef]
- Short, C.; Lim, H.K.; Tan, J.; O’Neill, H.C. Targeting the Spleen as an Alternative Site for Hematopoiesis. BioEssays 2019, 41, e1800234. [Google Scholar] [CrossRef]
- Sultana, D.A.; Zhang, S.L.; Todd, S.P.; Bhandoola, A. Expression of Functional P-Selectin Glycoprotein Ligand 1 on Hematopoietic Progenitors Is Developmentally Regulated. J. Immunol. 2012, 188, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, B.A.; Bhandoola, A. Trafficking from the bone marrow to the thymus: A prerequisite for thymopoiesis. Immunol. Rev. 2006, 209, 47–57. [Google Scholar] [CrossRef]
- Askmyr, M.; Quach, J.; Purton, L.E. Effects of the bone marrow microenvironment on hematopoietic malignancy. Bone 2011, 48, 115–120. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Wang, L.-Y.; Wang, J.-X.; Wang, C.; Wang, C.-L.; Zhao, D.-P.; Wang, Z.-C.; Zhang, J.-J. Protective effects of paeoniflorin and albiflorin on chemotherapy-induced myelosuppression in mice. Chin. J. Nat. Med. 2016, 14, 599–606. [Google Scholar] [CrossRef]
- Salva, S.; Marranzino, G.; Villena, J.; Agüero, G.; Alvarez, S. Probiotic Lactobacillus strains protect against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 2014, 22, 209–221. [Google Scholar] [CrossRef]
- Kim, J.S.; Jang, W.S.; Lee, S.; Son, Y.; Park, S.; Lee, S.S. A study of the effect of sequential injection of 5-androstenediol on irradiation-induced myelosuppression in mice. Arch. Pharmacal. Res. 2014, 38, 1213–1222. [Google Scholar] [CrossRef]
- Han, X.; Gao, P.; Zhang, Y.; Wang, J.; Sun, F.; Liu, Q.; Zhang, S. Protective effect of the antioxidative peptide SS31 on ionizing radiation-induced hematopoietic system damage in mice. Blood Cells Mol. Dis. 2019, 77, 82–87. [Google Scholar] [CrossRef]
- Suman, S.; Datta, K.; Doiron, K.; Ren, C.; Kumar, R.; Taft, D.R.; Fornace, A.J., Jr.; Maniar, M. Radioprotective Effects of ON 01210.Na upon Oral Administration. J. Radiat. Res. 2012, 53, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.E.; Wagers, A.J.; Gulati, A.P.; Johnson, F.L.; Weissman, I.L. Physiological Migration of Hematopoietic Stem and Progenitor Cells. Science 2001, 294, 1933–1936. [Google Scholar] [CrossRef]
- Zhang, J.; Li, H.; Lu, L.; Yan, L.; Yang, X.; Shi, Z.; Li, D. The Yiqi and Yangyin Formula ameliorates injury to the hematopoietic system induced by total body irradiation. J. Radiat. Res. 2017, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zustakova, M.; Kratochvilova, L.; Slama, P. Apoptosis of Eosinophil Granulocytes. Biology 2020, 9, 457. [Google Scholar] [CrossRef]
- Zhang, Y.; Harada, A.; Bluethmann, H.; Wang, J.B.; Nakao, S.; Mukaida, N.; Matsushima, K. Tumor necrosis factor (TNF) is a physiologic regulator of hematopoietic progenitor cells: Increase of early hematopoietic progenitor cells in TNF receptor p55-deficient mice in vivo and potent inhibition of progenitor cell proliferation by TNF alpha in vitro. Blood 1995, 86, 2930–2937. [Google Scholar] [PubMed]
- Benson, E.; Goebl, M.G.; Yang, F.-C.; Kapur, R.; McClintick, J.; Sanghani, S.; Clapp, D.W.; Harrington, M.A. Loss of SIMPL compromises TNF-α-dependent survival of hematopoietic progenitors. Exp. Hematol. 2010, 38, 71–81. [Google Scholar] [CrossRef][Green Version]
- Suehiro, Y. Macrophage inflammatory protein 1α enhances in a different manner adhesion of hematopoietic progenitor cells from bone marrow, cord blood, and mobilized peripheral blood. Exp. Hematol. 1999, 27, 1637–1645. [Google Scholar] [CrossRef]
- Kondadasula, S.V.; Varker, K.A.; Lesinski, G.B.; Benson, D.M.; Lehman, A.; Olencki, T.; Monk, J.P.; Kendra, K.; Carson, W.E. Activation of extracellular signaling regulated kinase in natural killer cells and monocytes following IL-2 stimulation in vitro and in patients undergoing IL-2 immunotherapy: Analysis via dual parameter flow-cytometric assay. Cancer Immunol. Immunother. 2008, 57, 1137–1149. [Google Scholar] [CrossRef]
- Zhao, M.-F.; Qu, X.-J.; Qu, J.-L.; Jiang, Y.-H.; Zhang, Y.; Hou, K.-Z.; Deng, H.; Liu, Y.-P. The Role of E3 Ubiquitin Ligase Cbl Proteins in Interleukin-2-Induced Jurkat T-Cell Activation. BioMed Res. Int. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Lee, A.W.-M.; States, D.J. Both Src-Dependent and -Independent Mechanisms Mediate Phosphatidylinositol 3-Kinase Regulation of Colony-Stimulating Factor 1-Activated Mitogen-Activated Protein Kinases in Myeloid Progenitors. Mol. Cell. Biol. 2000, 20, 6779–6798. [Google Scholar] [CrossRef] [PubMed]
- Handra-Luca, A.; Bilal, H.; Bertrand, J.-C.; Fouret, P. Extra-Cellular Signal-Regulated ERK-1/ERK-2 Pathway Activation in Human Salivary Gland Mucoepidermoid Carcinoma. Am. J. Pathol. 2003, 163, 957–967. [Google Scholar] [CrossRef]
- Murphy, M.J.; Wilson, A.; Trumpp, A. More than just proliferation: Myc function in stem cells. Trends Cell Biol. 2005, 15, 128–137. [Google Scholar] [CrossRef]
- Grigorieva, I.; Grigoriev, V.G.; Rowney, M.K.; Hoover, R.G. Regulation of c-myc Transcription by Interleukin-2 (IL-2). J. Biol. Chem. 2000, 275, 7343–7350. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, X.; Li, S.; Xing, X.; Wang, H.; Lazarovici, P.; Zheng, W. Protective mechanism of artemisinin on rat bone marrow-derived mesenchymal stem cells against apoptosis induced by hydrogen peroxide via activation of c-Raf-Erk1/2-p90rsk-CREB pathway. Stem Cell Res. Ther. 2019, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Li, Y.; Deng, Z.; Zhang, Z.; Xie, Q.; Zhang, H.; Zhong, W.; Pan, B. IGHG1 Regulates Prostate Cancer Growth via the MEK/ERK/c-Myc Pathway. BioMed Res. Int. 2019, 2019, 7201562. [Google Scholar] [CrossRef]
- Kogkopoulou, O.; Tzakos, E.; Mavrothalassitis, G.; Baldari, C.T.; Paliogianni, F.; Young, H.A.; Thyphronitis, G. Conditional up-regulation of IL-2 production by p38 MAPK inactivation is mediated by increased Erk1/2 activity. J. Leukoc. Biol. 2006, 79, 1052–1060. [Google Scholar] [CrossRef]

| Description | Item Number | Molecular Weight (kDa) | Dilution Rate | Applications |
|---|---|---|---|---|
| Macrophage colony-stimulating factor (M-CSF) a | ab233387 | 60 | 1:1000/1:500 (WB/IHC) | WB, IHC |
| p90 ribosomal S6 kinases 1 (RSK1-p90) a | ab32526 | 90 | 1:1000 | WB |
| P-RSK1-p90 (phospho T359/S363) a | ab32413 | 90 | 1:1000 | WB |
| c-Myc b | A1309 | 49 | 1:1000 | WB |
| Extracellular regulated protein kinases (ERK)1/2 a | ab36991 | 44 | 1:2000 | WB |
| P-ERK1/2 (phospho T202/T185) b | AP0485 | 44 | 1:1000 | WB |
| Interleukin (IL)-2 b | A16317 | 18 | 1:1000/1:200 (WB/IHC) | WB, IHC |
| Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) c | E-AB-20032 | 36 | 1:1000 | WB |
| Goat Anti Mouse IgG (H+L) (peroxidase/HRP conjugated) c | E-AB-1001 | - | 1:1000 | WB |
| Goat Anti Rabbit IgG (H+L) c | E-AB-1025 | - | 1:1000 | WB |
| FITC anti-mouse Lineage Cocktail with Isotype Ctrl d | 78022 | - | 20 µL/106 cells in 100 µL | FC |
| PE anti-mouse Ly-6A/E (Sca-1) Clone: D7 d | 108108 | - | 2.5 mL/106 cells in 100 µL | FC |
| APC anti-mouse CD117 (c-Kit) Clone:2B8 d | 105812 | - | 5 μL/106 cells in 100 µL | FC |
| PE anti-mouse F4/80 Clone:BM8 d | 123110 | - | 5 μL/106 cells in 100 µL | FC |
| FITC anti-mouse/human CD11b Clone:M1/70 d | 101205 | - | 0.5 μL/106 cells in 100 µL | FC |
| APC anti-mouse Ly-6G Clone:1A8 d | 127613 | - | 0.3 μL/106 cells in 100 µL | FC |
| FITC-CD3e Monoclonal Antibody Clone:145-2C11 e | 11-0031-82 | - | 1 μL/106 cells in 100 µL | FC |
| PE anti-mouse CD19 Clone:6D5 d | 115507 | - | 1.25 μL/106 cells in 100 µL | FC |
| APC anti-mouse CD45 Clone:30-F11 d | 103112 | - | 1.25 μL/106 cells in 100 µL | FC |
| FITC-conjugated anti-rat IgG2a d | 400505 | - | 0.3 μL/106 cells in 100 µL | FC |
| PerCP-conjugated anti-rat IgG2b d | 400629 | - | 1.25 μL/106 cells in 100 µL | FC |
| APC-conjugated anti-rat IgG2b d | 400611 | - | 5 μL/106 cells in 100 µL | FC |
| FITC-conjugated anti-rat IgG2b d | 400605 | - | 0.5 μL/106 cells in 100 µL | FC |
| Distribution of Molecular Mass (g/mol) | Percentage (%) |
|---|---|
| 185.0–264.0 | 38.6 |
| 264.0–475.0 | 58.9 |
| 475.0–622.0 | 2.5 |
| Amino Acid | Contents (g/100 g) |
|---|---|
| Aspartic Acid | 0.39 |
| Threonine | 0.18 |
| Serine | 0.19 |
| Glutamic acid | 0.64 |
| Glycine | 0.10 |
| Alanine | 0.27 |
| Cystine | 0.019 |
| Valine | 0.30 |
| Methionine | 0.074 |
| Isoleucine | 0.22 |
| Leucine | 0.42 |
| Tyrosine | 0.034 |
| Phenylalanine | 0.075 |
| Lysine | 0.45 |
| Histidine | 0.10 |
| Arginine | 0.068 |
| Proline | 0.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, Y.; Meng, W.; Dong, Y.; Zhang, S.; Teng, L.; Liu, Y.; Li, L.; Wang, D. The Involvement of Macrophage Colony Stimulating Factor on Protein Hydrolysate Injection Mediated Hematopoietic Function Improvement. Cells 2021, 10, 2776. https://doi.org/10.3390/cells10102776
Wang S, Zhang Y, Meng W, Dong Y, Zhang S, Teng L, Liu Y, Li L, Wang D. The Involvement of Macrophage Colony Stimulating Factor on Protein Hydrolysate Injection Mediated Hematopoietic Function Improvement. Cells. 2021; 10(10):2776. https://doi.org/10.3390/cells10102776
Chicago/Turabian StyleWang, Shimiao, Yuchong Zhang, Weiqi Meng, Yihao Dong, Sujie Zhang, Lesheng Teng, Yang Liu, Lanzhou Li, and Di Wang. 2021. "The Involvement of Macrophage Colony Stimulating Factor on Protein Hydrolysate Injection Mediated Hematopoietic Function Improvement" Cells 10, no. 10: 2776. https://doi.org/10.3390/cells10102776
APA StyleWang, S., Zhang, Y., Meng, W., Dong, Y., Zhang, S., Teng, L., Liu, Y., Li, L., & Wang, D. (2021). The Involvement of Macrophage Colony Stimulating Factor on Protein Hydrolysate Injection Mediated Hematopoietic Function Improvement. Cells, 10(10), 2776. https://doi.org/10.3390/cells10102776







