Calcium-Based Biomineralization: A Smart Approach for the Design of Novel Multifunctional Hybrid Materials
Abstract
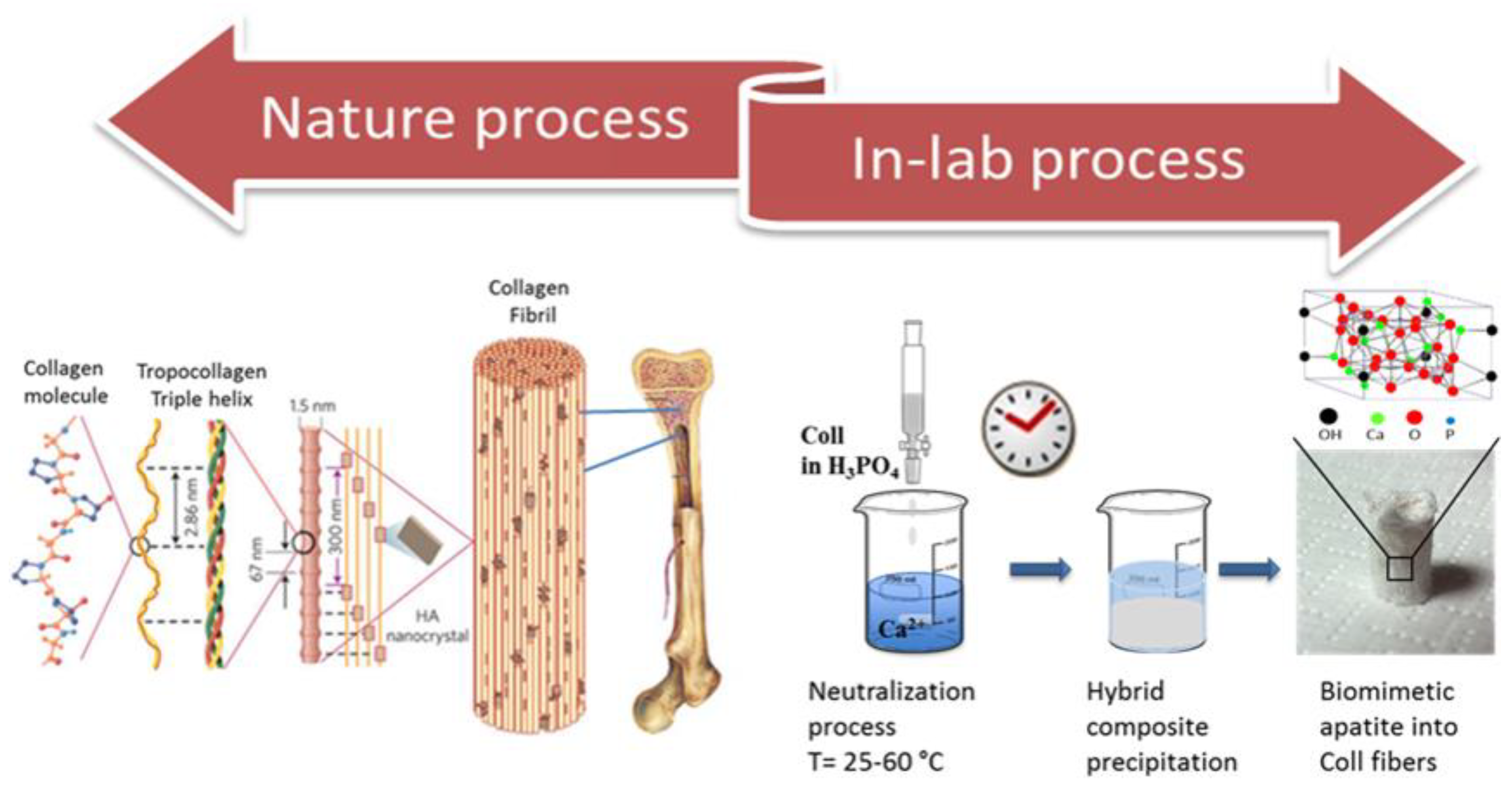
:1. Introduction
Mimicking Biomineralization in the Lab
2. Features of Biomimetic and Hybrid Biomaterials
| Physic-Chemical Parameter | Scaffold | Nanosystems |
|---|---|---|
| Biocompatibility | Absence of cytotoxicity [44]; Support and stimulation of cellular activity [43] | Absence of cytotoxicity Support and stimulation of cellular activity [1] |
| Biodegradability | Controlled biodegradability [46] | High bioabsorption and biodegradability Absence of bioaccumulation of ions [1] |
| Architecture | Stability under physiological condition Highly porous and interconnected [47] Hierarchical design structure [9,57] | Stability under physiological conditions [1] |
| Porosity and pore size | Mixture of macro- and micro-porosity [48,49,50,51,52] | / |
| Mechanical properties | Mechanical integrity [53] | Stability under physiological conditions [1] |
| Surface properties | Support and stimulation of cellular activity Tissue-specific functionalization [54,55,56] | Tissue-specific functionalization Target-specific functionalization [1] |
| Bioactivity | Osteoinductive Osteoconductive [6,9,57] | Controlled drug release and distribution [1,58,59] |
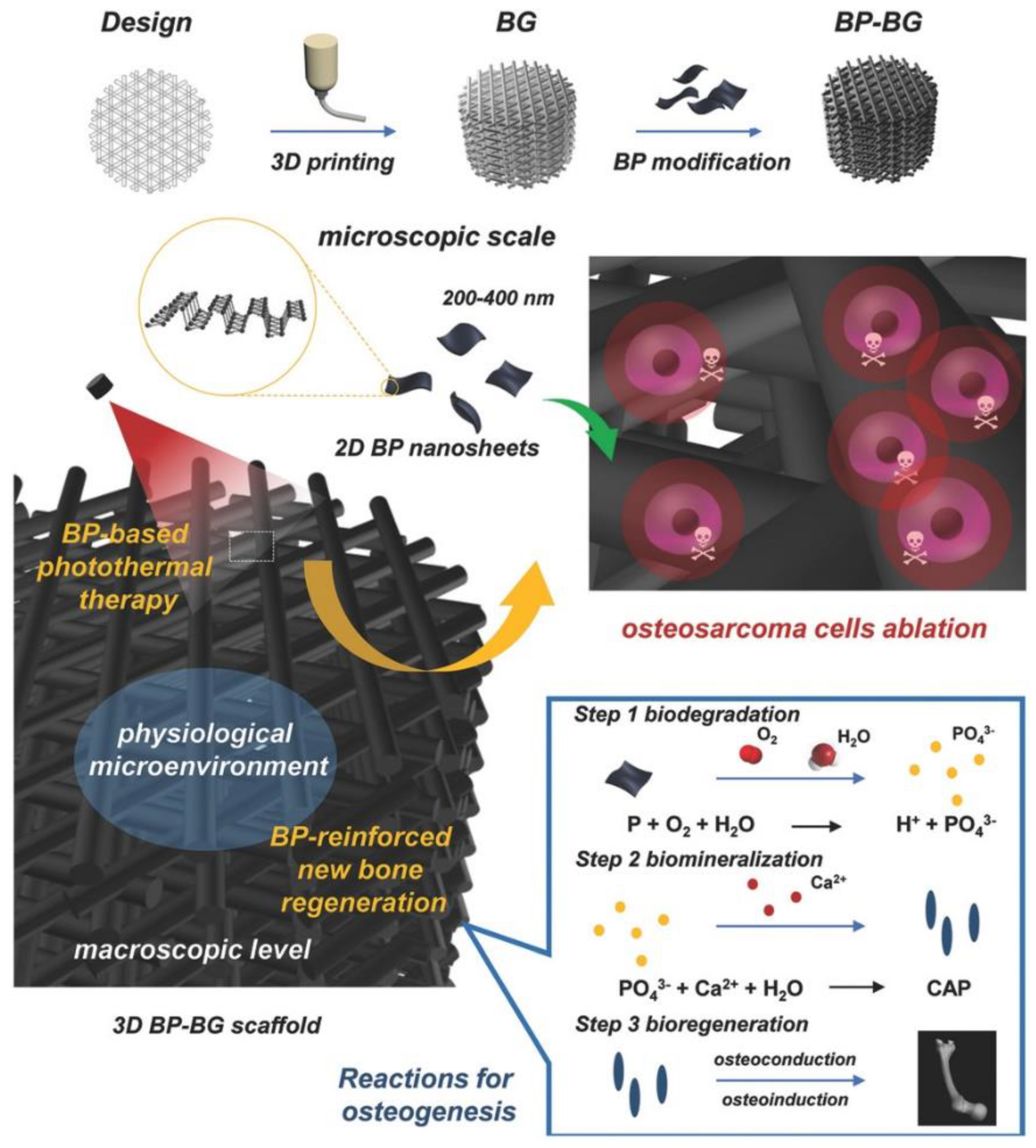
3. Applications in Biomedical Field: Tissue Regeneration and Many More
3.1. Biomineralization, 3D Printing and 3D Bio-Printing
3.2. 3D Predictive Models: From Cancer Study to Drug Testing
3.3. Physical Filters against Solar Radiations
| Physical Filter | Advantages | Drawbacks |
|---|---|---|
| TiO2-Oxiol [112] |
|
|
| HA-Ascorbic Acid [115] |
|
|
| HA-Chitosan [117] |
|
|
| ions-doped HA (Cr3+, Fe3+, Zn2+, Mn2+, Ti4+) [120,121,125] |
|
| Chemical Filter | Physical Filter | HA-Based Physical Filter |
|---|---|---|
3.4. Nano and Micro Drug Delivery Systems
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Wang, W.; Liu, X.; Zheng, X.; Jin, H.J.; Li, X. Biomineralization: An Opportunity and Challenge of Nanoparticle Drug Delivery Systems for Cancer Therapy. Adv. Heal. Mater. 2020, 9, e2001117. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Y.; Deveaux, J.G.; Masoud, M.A.; Chandra, F.S.; Chen, H.; Zhang, D.; Feng, L. Biomineralization Forming Process and Bio-inspired Nanomaterials for Biomedical Application: A Review. Minerals 2019, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Tampieri, A.; Ruffini, A.; Ballardini, A.; Montesi, M.; Panseri, S.; Salamanna, F.; Fini, M.; Sprio, S. Heterogeneous chemistry in the 3-D state: An original approach to generate bioactive, mechanically-competent bone scaffolds. Biomater. Sci. 2018, 7, 307–321. [Google Scholar] [CrossRef]
- Campodoni, E.; Patricio, T.; Montesi, M.; Tampieri, A.; Sandri, M.; Sprio, S. Biomineralization Process Generating Hybrid Nano- and Micro-Carriers; Woodhead Publishing: Sawston, UK, 2018; pp. 19–42. [Google Scholar] [CrossRef]
- Tampieri, A.; D’Alessandro, T.; Sandri, M.; Sprio, S.; Landi, E.; Bertinetti, L.; Panseri, S.; Pepponi, G.; Goettlicher, J.; Bañobre-López, M.; et al. Intrinsic magnetism and hyperthermia in bioactive Fe-doped hydroxyapatite. Acta Biomater. 2012, 8, 843–851. [Google Scholar] [CrossRef]
- Luz, G.; Mano, J. Nanoscale Design in Biomineralization for Developing New Biomaterials for Bone Tissue Engineering (BTE); Woodhead Publishing: Sawston, UK, 2014; pp. 153–195. [Google Scholar] [CrossRef]
- Luz, G.; Mano, J.F. Mineralized structures in nature: Examples and inspirations for the design of new composite materials and biomaterials. Compos. Sci. Technol. 2010, 70, 1777–1788. [Google Scholar] [CrossRef] [Green Version]
- Preti, L.; Lambiase, B.; Campodoni, E.; Sandri, M.; Ruffini, A.; Pugno, N.; Tampieri, A.; Sprio, S. Nature-Inspired Processes and Structures: New Paradigms to Develop Highly Bioactive Devices for Hard Tissue Regeneration; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Elisabetta, C.; Maria, D.S.; Manuela, M.; Margherita, M.; Monica, M.; Silvia, P.; Simone, S.; Anna, T.; Monica, S. Biomimetic Approaches for the Design and Development of Multifunctional Bioresorbable Layered Scaffolds for Dental Regeneration; CRC Press: Boca Raton, FL, USA, 2020; pp. 104–119. [Google Scholar] [CrossRef]
- Tampieri, A.; Sandri, M.; Landi, E.; Pressato, D.; Francioli, S.; Quarto, R.; Martin, I. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 2008, 29, 3539–3546. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Morales, J.; Iafisco, M.; Delgado-López, J.M.; Sarda, S.; Drouet, C. Progress on the preparation of nanocrystalline apatites and surface characterization: Overview of fundamental and applied aspects. Prog. Cryst. Growth Charact. Mater. 2013, 59, 1–46. [Google Scholar] [CrossRef] [Green Version]
- Pereira, D.D.M.; Habibovic, P. Biomineralization-Inspired Material Design for Bone Regeneration. Adv. Health Mater. 2018, 7, e1800700. [Google Scholar] [CrossRef] [PubMed]
- Thula, T.T.; Rodriguez, D.E.; Lee, M.H.; Pendi, L.; Podschun, J.; Gower, L.B. In vitro mineralization of dense collagen substrates: A biomimetic approach toward the development of bone-graft materials. Acta Biomater. 2011, 7, 3158–3169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, S.; George, A.; Tooth, B.; Genetics, D. Engineering Mineralized and Load Bearing Tissues; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; Volume 881, pp. 129–142. [Google Scholar] [CrossRef] [Green Version]
- Tampieri, A.; Sprio, S.; Sandri, M.; Valentini, F. Mimicking natural bio-mineralization processes: A new tool for osteochondral scaffold development. Trends Biotechnol. 2011, 29, 526–535. [Google Scholar] [CrossRef]
- Nudelman, F.; Sonmezler, E.; Bomans, P.H.H.; de With, G.; Sommerdijk, N.A.J.M. Stabilization of amorphous calcium carbonate by controlling its particle size. Nanoscale 2010, 2, 2436–2439. [Google Scholar] [CrossRef]
- Walker, J.M.; Marzec, B.; Nudelman, F. Solid-State Transformation of Amorphous Calcium Carbonate to Aragonite Captured by CryoTEM. Angew. Chem. 2017, 129, 11902–11905. [Google Scholar] [CrossRef] [Green Version]
- Dellaquila, A.; Campodoni, E.; Tampieri, A.; Sandri, M. Overcoming the Design Challenge in 3D Biomimetic Hybrid Scaffolds for Bone and Osteochondral Regeneration by Factorial Design. Front. Bioeng. Biotechnol. 2020, 8, 743. [Google Scholar] [CrossRef]
- Bharadwaz, A.; Jayasuriya, A.C. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater. Sci. Eng. C 2020, 110, 110698. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Y.; Zhang, J.; Bao, S.; Xian, L.; Dong, X.; Zheng, W.; Li, Y.; Gao, H.; Zhou, W. Bioactive and Biocompatible Macroporous Scaffolds with Tunable Performances Prepared Based on 3D Printing of the Pre-Crosslinked Sodium Alginate/Hydroxyapatite Hydrogel Ink. Macromol. Mater. Eng. 2019, 304, 1800698. [Google Scholar] [CrossRef]
- Turnbull, G.; Clarke, J.; Picard, F.; Riches, P.; Jia, L.; Han, F.; Li, B.; Shu, W. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 2017, 3, 278–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.; He, W.; Shuai, Y.; Min, S.; Zhu, L. Nucleation of hydroxyapatite crystals by self-assembled Bombyx mori silk fibroin. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 742–748. [Google Scholar] [CrossRef]
- Patrício, T.M.F.; Panseri, S.; Sandri, M.; Tampieri, A.; Sprio, S. New bioactive bone-like microspheres with intrinsic magnetic properties obtained by bio-inspired mineralisation process. Mater. Sci. Eng. C 2017, 77, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Chatzipanagis, K.; Baumann, C.G.; Sandri, M.; Sprio, S.; Tampieri, A.; Kröger, R. In situ mechanical and molecular investigations of collagen/apatite biomimetic composites combining Raman spectroscopy and stress-strain analysis. Acta Biomater. 2016, 46, 278–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Shi, X.; Miao, M.; He, T.; Dong, Z.H.; Zhan, K.; Yang, J.H.; Zhao, B.; Xia, B.Y. Bio-inspired design of hierarchical FeP nanostructure arrays for the hydrogen evolution reaction. Nano Res. 2017, 11, 3537–3547. [Google Scholar] [CrossRef]
- Hou, Y.-K.; Pan, G.-L.; Sun, Y.-Y.; Gao, X.-P. LiMn0.8Fe0.2PO4/Carbon Nanospheres@Graphene Nanoribbons Prepared by the Biomineralization Process as the Cathode for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 16500–16510. [Google Scholar] [CrossRef]
- Wu, M.; Chen, K.; Yang, S.; Wang, Z.; Huang, P.-H.; Mai, J.; Li, Z.-Y.; Huang, T.J. High-throughput cell focusing and separation via acoustofluidic tweezers. Lab Chip 2018, 18, 3003–3010. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, S.; Sun, J.; Meng, X.; Luo, J.; Zhou, D.; Crittenden, J.C. Impact of Chloride Ions on UV/H2O2 and UV/Persulfate Advanced Oxidation Processes. Environ. Sci. Technol. 2018, 52, 7380–7389. [Google Scholar] [CrossRef]
- Chen, W.; Wang, G.; Tang, R. Nanomodification of living organisms by biomimetic mineralization. Nano Res. 2014, 7, 1404–1428. [Google Scholar] [CrossRef]
- Walsh, P.J.; Fee, K.; Clarke, S.A.; Julius, M.L.; Buchanan, F.J. Blueprints for the Next Generation of Bioinspired and Biomimetic Mineralised Composites for Bone Regeneration. Mar. Drugs 2018, 16, 288. [Google Scholar] [CrossRef] [Green Version]
- Osorio, R.; Alfonso-Rodríguez, C.A.; Osorio, E.; Medina-Castillo, A.L.; Alaminos, M.; Toledano-Osorio, M.; Toledano, M. Novel potential scaffold for periodontal tissue engineering. Clin. Oral Investig. 2017, 21, 2695–2707. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Filardo, G.; Kon, E.; Panseri, S.; Montesi, M.; Iafisco, M.; Savini, E.; Sprio, S.; Cunha, C.; Giavaresi, G.; et al. Fabrication and Pilot In Vivo Study of a Collagen-BDDGE-Elastin Core-Shell Scaffold for Tendon Regeneration. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulghani, S.; Mitchell, G.R. Biomaterials for In Situ Tissue Regeneration: A Review. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczmarek, B.; Sionkowska, A.; Kozlowska, J.; Osyczka, A. New composite materials prepared by calcium phosphate precipitation in chitosan/collagen/hyaluronic acid sponge cross-linked by EDC/NHS. Int. J. Biol. Macromol. 2018, 107, 247–253. [Google Scholar] [CrossRef]
- Weiner, S.; Dove, P.M. An Overview of Biomineralization Processes and the Problem of the Vital Effect. Biomineralization 2003, 54, 1–30. [Google Scholar] [CrossRef]
- Zhong, L.; Qu, Y.; Shi, K.; Chu, B.; Lei, M.; Huang, K.; Gu, Y.; Qian, Z. Biomineralized polymer matrix composites for bone tissue repair: A review. Sci. China Ser. B Chem. 2018, 61, 1553–1567. [Google Scholar] [CrossRef]
- Campodoni, E.; Dozio, S.M.; Panseri, S.; Montesi, M.; Tampieri, A.; Sandri, M. Mimicking Natural Microenvironments: Design of 3D-Aligned Hybrid Scaffold for Dentin Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 836. [Google Scholar] [CrossRef]
- Salama, A. Cellulose/calcium phosphate hybrids: New materials for biomedical and environmental applications. Int. J. Biol. Macromol. 2019, 127, 606–617. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Y.; Ke, Q.; Yin, W.; Zhang, C.; Guo, Y.; Guan, J. Magnetic lanthanum-doped hydroxyapatite/chitosan scaffolds with endogenous stem cell-recruiting and immunomodulatory properties for bone regeneration. J. Mater. Chem. B 2020, 8, 5280–5292. [Google Scholar] [CrossRef]
- Tang, Y.-Q.; Wang, Q.-Y.; Ke, Q.-F.; Zhang, C.-Q.; Guan, J.-J.; Guo, Y.-P. Mineralization of ytterbium-doped hydroxyapatite nanorod arrays in magnetic chitosan scaffolds improves osteogenic and angiogenic abilities for bone defect healing. Chem. Eng. J. 2020, 387, 124166. [Google Scholar] [CrossRef]
- Furlani, F.; Marfoglia, A.; Marsich, E.; Donati, I.; Sacco, P. Strain Hardening in Highly Acetylated Chitosan Gels. Biomacromolecules 2021, 22, 2902–2909. [Google Scholar] [CrossRef]
- Ucar, S.; Bjørnøy, S.H.; Bassett, D.C.; Strand, B.L.; Sikorski, P.; Andreassen, J.-P. Nucleation and Growth of Brushite in the Presence of Alginate. Cryst. Growth Des. 2015, 15, 5397–5405. [Google Scholar] [CrossRef]
- Lloyd, A.W. Interfacial bioengineering to enhance surface biocompatibility. Med. Device Technol. 2002, 13, 18–21. [Google Scholar] [PubMed]
- Finkemeier, C.G. Bone-grafting and bone-graft substitutes. J. Bone Jt. Surg. 2002, 84, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317. [Google Scholar]
- Mountziaris, P.M.; Mikos, A.G. Modulation of the Inflammatory Response for Enhanced Bone Tissue Regeneration. Tissue Eng. Part B Rev. 2008, 14, 179–186. [Google Scholar] [CrossRef]
- Cukierman, E.; Pankov, R.; Stevens, D.R.; Yamada, K.M. Taking Cell-Matrix Adhesions to the Third Dimension. Science 2001, 294, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.; Harley, B.; Yannas, I.; Gibson, L. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, E.; Takita, H.; Itoh, H.; Wakisaka, Y.; Kuboki, Y. Pore Size of Porous Hydroxyapatite as the Cell-Substratum Controls BMP-Induced Osteogenesis. J. Biochem. 1997, 121, 317–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, H.; Haniu, H.; Takeuchi, A.; Ueda, K.; Sano, M.; Tanaka, M.; Takizawa, T.; Sobajima, A.; Kamanaka, T.; Saito, N. In Vitro and In Vivo Evaluation of Starfish Bone-Derived β-Tricalcium Phosphate as a Bone Substitute Material. Materials 2019, 12, 1881. [Google Scholar] [CrossRef] [Green Version]
- Habibovic, P.; Yuan, H.; van der Valk, C.M.; Meijer, G.; van Blitterswijk, C.; de Groot, K. 3D microenvironment as essential element for osteoinduction by biomaterials. Biomaterials 2005, 26, 3565–3575. [Google Scholar] [CrossRef]
- Whang, K.; Healy, K.E.; Elenz, D.R.; Nam, E.K.; Tsai, D.C.; Thomas, C.H.; Nuber, G.W.; Glorieux, F.H.; Travers, R.; Sprague, S.M. Engineering Bone Regeneration with Bioabsorbable Scaffolds with Novel Microarchitecture. Tissue Eng. 1999, 5, 35–51. [Google Scholar] [CrossRef]
- Boccaccio, A.; Ballini, A.; Pappalettere, C.; Tullo, D.; Cantore, S.; Desiate, A. Finite Element Method (FEM), Mechanobiology and Biomimetic Scaffolds in Bone Tissue Engineering. Int. J. Biol. Sci. 2011, 7, 112–132. [Google Scholar] [CrossRef]
- Cipitria, A.; Wagermaier, W.; Zaslansky, P.; Schell, H.; Reichert, J.; Fratzl, P.; Hutmacher, D.; Duda, G. BMP delivery complements the guiding effect of scaffold architecture without altering bone microstructure in critical-sized long bone defects: A multiscale analysis. Acta Biomater. 2015, 23, 282–294. [Google Scholar] [CrossRef] [Green Version]
- Kaigler, D.; Wang, Z.; Horger, K.; Mooney, D.J.; Krebsbach, P.H. VEGF Scaffolds Enhance Angiogenesis and Bone Regeneration in Irradiated Osseous Defects. J. Bone Miner. Res. 2006, 21, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Tampieri, A.; Landi, E.; Valentini, F.; Sandri, M.; D’Alessandro, T.; Dediu, V.; Marcacci, M. A conceptually new type of bio-hybrid scaffold for bone regeneration. Nanotechnology 2010, 22, 015104. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2011, 8, 147–166. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Wang, X.; Nie, S.; Chen, Z.; Shin, D.M. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clin. Cancer Res. 2008, 14, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Papakostidis, C.; Kanakaris, N.K.; Pretel, J.; Faour, O.; Morell, D.J.; Giannoudis, P.V. Prevalence of complications of open tibial shaft fractures stratified as per the Gustilo-Anderson classification. Injury 2011, 42, 1408–1415. [Google Scholar] [CrossRef]
- Sprio, S.; Campodoni, E.; Sandri, M.; Preti, L.; Keppler, T.; Müller, F.A.; Pugno, N.M.; Tampieri, A. A Graded Multifunctional Hybrid Scaffold with Superparamagnetic Ability for Periodontal Regeneration. Int. J. Mol. Sci. 2018, 19, 3604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; O’Neill, H.; Rawn, C.J. Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 2006, 27, 4661–4670. [Google Scholar] [CrossRef]
- Yue, K.; de Santiago, G.T.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Alfano, M.; Nebuloni, M.; Allevi, R.; Zerbi, P.; Longhi, E.; Lucianò, R.; Locatelli, I.; Pecoraro, A.; Indrieri, M.; Speziali, C.; et al. Linearized texture of three-dimensional extracellular matrix is mandatory for bladder cancer cell invasion. Sci. Rep. 2016, 6, 36128. [Google Scholar] [CrossRef]
- Murphy, C.A.; Costa, J.; Correia, J.S.; Oliveira, J.M.; Reis, R.L.; Collins, M. Biopolymers and polymers in the search of alternative treatments for meniscal regeneration: State of the art and future trends. Appl. Mater. Today 2018, 12, 51–71. [Google Scholar] [CrossRef]
- Hao, Y.; Song, J.; Ravikrishnan, A.; Dicker, K.T.; Fowler, E.W.; Zerdoum, A.B.; Li, Y.; Zhang, H.; Rajasekaran, A.K.; Fox, J.M.; et al. Rapid Bioorthogonal Chemistry Enables in Situ Modulation of the Stem Cell Behavior in 3D without External Triggers. ACS Appl. Mater. Interfaces 2018, 10, 26016–26027. [Google Scholar] [CrossRef]
- Li, W.; Xu, R.; Huang, J.; Bao, X.; Zhao, B. Treatment of rabbit growth plate injuries with oriented ECM scaffold and autologous BMSCs. Sci. Rep. 2017, 7, 44140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Simões, M.D.J.; Cerri, P. Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Seeman, E. Bone Modeling and Remodeling. Crit. Rev. Eukaryot. Gene Expr. 2009, 19, 219–233. [Google Scholar] [CrossRef]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Menale, C.; Campodoni, E.; Palagano, E.; Mantero, S.; Erreni, M.; Inforzato, A.; Fontana, E.; Schena, F.; Hof, R.V.; Sandri, M.; et al. Mesenchymal Stromal Cell-Seeded Biomimetic Scaffolds as a Factory of Soluble RANKL in Rankl-Deficient Osteopetrosis. STEM CELLS Transl. Med. 2018, 8, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Dvorakova, J.; Dobra, J.K.; Babuska, V. Biological Evaluation of Polyvinyl Alcohol Hydrogels Enriched by Hyaluronic Acid and Hydroxyapatite. Int. J. Mol. Sci. 2020, 21, 5719. [Google Scholar] [CrossRef] [PubMed]
- Bassi, G.; Panseri, S.; Dozio, S.M.; Sandri, M.; Campodoni, E.; Dapporto, M.; Sprio, S.; Tampieri, A.; Montesi, M. Scaffold-based 3D cellular models mimicking the heterogeneity of osteosarcoma stem cell niche. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Ma, P.X. The engineering of patient-specific, anatomically shaped, digits. Biomaterials 2009, 30, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, I.; Kumar, A.; Joddar, B. A Bioactive Hydrogel and 3D Printed Polycaprolactone System for Bone Tissue Engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Wang, J.; Li, X.; Hu, X.; Zhou, W.; Dong, X.; Wang, C.; Yang, Z.; Binks, B.P. Facile preparation of bioactive nanoparticle/poly(ε-caprolactone) hierarchical porous scaffolds via 3D printing of high internal phase Pickering emulsions. J. Colloid Interface Sci. 2019, 545, 104–115. [Google Scholar] [CrossRef]
- Huang, T.; Fan, C.; Zhu, M.; Zhu, Y.; Zhang, W.; Li, L. 3D-printed scaffolds of biomineralized hydroxyapatite nanocomposite on silk fibroin for improving bone regeneration. Appl. Surf. Sci. 2019, 467–468, 345–353. [Google Scholar] [CrossRef]
- Romanazzo, S.; Molley, T.G.; Nemec, S.; Lin, K.; Sheikh, R.; Gooding, J.J.; Wan, B.; Li, Q.; Kilian, K.A.; Roohani, I. Synthetic Bone-Like Structures Through Omnidirectional Ceramic Bioprinting in Cell Suspensions. Adv. Funct. Mater. 2021, 31. [Google Scholar] [CrossRef]
- Liu, X.; Miller, A.L.; Park, S.; George, M.; Waletzki, B.E.; Xu, H.; Terzic, A.; Lu, L. Two-Dimensional Black Phosphorus and Graphene Oxide Nanosheets Synergistically Enhance Cell Proliferation and Osteogenesis on 3D Printed Scaffolds. ACS Appl. Mater. Interfaces 2019, 11, 23558–23572. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Yin, J.; Chen, Y.; Pan, S.; Yao, H.; Gao, Y.; Shi, J. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds: A Stepwise Countermeasure for Osteosarcoma. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Lin, S.; Zhong, Y.; Zhao, X.; Sawada, T.; Li, X.; Lei, W.; Wang, M.; Serizawa, T.; Zhu, H. Synthetic Multifunctional Graphene Composites with Reshaping and Self-Healing Features via a Facile Biomineralization-Inspired Process. Adv. Mater. 2018, 30, e1803004. [Google Scholar] [CrossRef]
- Unger, C.; Kramer, N.; Walzl, A.; Scherzer, M.; Hengstschläger, M.; Dolznig, H. Modeling human carcinomas: Physiologically relevant 3D models to improve anti-cancer drug development. Adv. Drug Deliv. Rev. 2014, 79–80, 50–67. [Google Scholar] [CrossRef]
- Antoni, D.; Burckel, H.; Josset, E.; Noel, G. Three-Dimensional Cell Culture: A Breakthrough In Vivo. Int. J. Mol. Sci. 2015, 16, 5517–5527. [Google Scholar] [CrossRef]
- Borella, G.; Da Ros, A.; Borile, G.; Porcù, E.; Tregnago, C.; Benetton, M.; Marchetti, A.; Bisio, V.; Montini, B.; Michielotto, B.; et al. Targeting mesenchymal stromal cells plasticity to reroute acute myeloid leukemia course. Blood 2021. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Du, D.; Lin, Y. Bioinspired nanoscale materials for biomedical and energy applications. J. R. Soc. Interface 2014, 11, 20131067. [Google Scholar] [CrossRef] [Green Version]
- Lopa, S.; Madry, H. Bioinspired Scaffolds for Osteochondral Regeneration. Tissue Eng. Part A 2014, 20, 2052–2076. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Luo, X.; Li, Z.; Zhuang, C.; Li, L.; Lu, L.; Ding, S.; Tian, J.; Zhou, C. Rapid biomimetic mineralization of collagen fibrils and combining with human umbilical cord mesenchymal stem cells for bone defects healing. Mater. Sci. Eng. C 2016, 68, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, M.A.; Alves, T.F.R.; De Lima, R.; Oliveira, J.M.; Vila, M.M.D.C.; Balcão, V.; Severino, P.; Chaud, M.V.; Oliveira, J.M., Jr. Scaffolds and tissue regeneration: An overview of the functional properties of selected organic tissues. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2015, 104, 1483–1494. [Google Scholar] [CrossRef]
- Yagüe, M.F.; Abbah, S.; McNamara, L.; Zeugolis, D.I.; Pandit, A.; Biggs, M.J. Biomimetic approaches in bone tissue engineering: Integrating biological and physicomechanical strategies. Adv. Drug Deliv. Rev. 2014, 84, 1–29. [Google Scholar] [CrossRef]
- Mravic, M.; Péault, B.; James, A.W. Current Trends in Bone Tissue Engineering. BioMed Res. Int. 2014, 2014, 865270. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int. 2016, 2016, 6180487. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.B.; Moncivais, K.; Caplan, A. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp. Mol. Med. 2013, 45, e54. [Google Scholar] [CrossRef] [Green Version]
- Lei, B.; Wang, L.; Chen, X.; Chae, S.-K. Biomimetic and molecular level-based silicate bioactive glass–gelatin hybrid implants for loading-bearing bone fixation and repair. J. Mater. Chem. B 2013, 1, 5153–5162. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Bourquin, M.; Hulliger, J. Mineralization of Calcium Phosphate Crystals in Starch Template Inducing a Brushite Kidney Stone Biomimetic Composite. Cryst. Growth Des. 2013, 13, 2166–2173. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, B.; Yang, X.; Xiao, Y.; Wang, X.; Shao, C.; Tang, R. A Drug-Free Tumor Therapy Strategy: Cancer-Cell-Targeting Calcification. Angew. Chem. Int. Ed. 2016, 55, 5225–5229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hauser, N.; Singer, G.; Trippel, M.; Kubik-Huch, R.A.; Schneider, C.; Stampanoni, M. Non-invasive classification of microcalcifications with phase-contrast X-ray mammography. Nat. Commun. 2014, 5, 3797. [Google Scholar] [CrossRef]
- Varsano, N.; Dadosh, T.; Kapishnikov, S.; Pereiro, E.; Shimoni, E.; Jin, X.; Kruth, H.S.; Leiserowitz, L.; Addadi, L. Development of Correlative Cryo-soft X-ray Tomography and Stochastic Reconstruction Microscopy. A Study of Cholesterol Crystal Early Formation in Cells. J. Am. Chem. Soc. 2016, 138, 14931–14940. [Google Scholar] [CrossRef]
- Vidavsky, N.; Kunitake, J.A.; Chiou, A.E.; Northrup, P.; Porri, T.J.; Ling, L.; Fischbach, C.; Estroff, L.A. Studying biomineralization pathways in a 3D culture model of breast cancer microcalcifications. Biomaterials 2018, 179, 71–82. [Google Scholar] [CrossRef]
- Chen, H.; Fu, S.; Fu, L.; Yang, H.; Chen, D. Simple Synthesis and Characterization of Hexagonal and Ordered Al–MCM–41 from Natural Perlite. Minerals 2019, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Miller, F.R.; Santner, S.J.; Tait, L.; Dawson, P.J. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J. Natl. Cancer Inst. 2000, 92, 1185–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santner, S.J.; Dawson, P.J.; Tait, L.; Soule, H.D.; Eliason, J.; Mohamed, A.N.; Wolman, S.R.; Heppner, G.H.; Miller, F.R. Malignant MCF10CA1 Cell Lines Derived from Premalignant Human Breast Epithelial MCF10AT Cells. Breast Cancer Res. Treat. 2001, 65, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Palm, M.D.; O’Donoghue, M.N. Update on photoprotection. Dermatol. Ther. 2007, 20, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Smaoui, S.; Ben Hlima, H.; Ben Chobba, I.; Kadri, A. Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arab. J. Chem. 2017, 10, S1216–S1222. [Google Scholar] [CrossRef] [Green Version]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorganica Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Antoniou, C.; Kosmadaki, M.; Stratigos, A.; Katsambas, A. Sunscreens—What’s important to know. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1110–1119. [Google Scholar] [CrossRef]
- Zhong, X.; Downs, C.A.; Li, Y.; Zhang, Z.; Li, Y.; Liu, B.; Gao, H.; Li, Q. Comparison of toxicological effects of oxybenzone, avobenzone, octocrylene, and octinoxate sunscreen ingredients on cucumber plants (Cucumis sativus L.). Sci. Total Environ. 2020, 714, 136879. [Google Scholar] [CrossRef]
- Hiller, J.; Klotz, K.; Meyer, S.; Uter, W.; Hof, K.; Greiner, A.; Göen, T.; Drexler, H. Systemic availability of lipophilic organic UV filters through dermal sunscreen exposure. Environ. Int. 2019, 132, 105068. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Wiench, K.; Landsiedel, R.; Schulte, S.; Inman, A.O.; Riviere, J.E. Safety Evaluation of Sunscreen Formulations Containing Titanium Dioxide and Zinc Oxide Nanoparticles in UVB Sunburned Skin: An In Vitro and In Vivo Study. Toxicol. Sci. 2011, 123, 264–280. [Google Scholar] [CrossRef] [Green Version]
- Schulz, J.; Hohenberg, H.; Pflücker, F.; Gärtner, E.; Will, T.; Pfeiffer, S.; Wepf, R.; Wendel, V.; Gers-Barlag, H.; Wittern, K.-P. Distribution of sunscreens on skin. Adv. Drug Deliv. Rev. 2002, 54, S157–S163. [Google Scholar] [CrossRef]
- Shao, Y.; Schlossman, D. Effect of particle size on performance of physical sunscreen formulas. In Proceedings of the PCIA Conference, Shanghai, China, March 1999; pp. 1–9. [Google Scholar]
- Levine, A. Sunscreen use and awareness of chemical toxicity among beach goers in Hawaii prior to a ban on the sale of sunscreens containing ingredients found to be toxic to coral reef ecosystems. Mar. Policy 2020, 117, 103875. [Google Scholar] [CrossRef]
- Battistin, M.; Dissette, V.; Bonetto, A.; Durini, E.; Manfredini, S.; Marcomini, A.; Casagrande, E.; Brunetta, A.; Ziosi, P.; Molesini, S.; et al. A New Approach to UV Protection by Direct Surface Functionalization of TiO2 with the Antioxidant Polyphenol Dihydroxyphenyl Benzimidazole Carboxylic Acid. Nanomaterials 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bino, A.; Baldisserotto, A.; Scalambra, E.; Dissette, V.; Vedaldi, D.E.; Salvador, A.; Durini, E.; Manfredini, S.; Vertuani, S. Design, synthesis and biological evaluation of novel hydroxy-phenyl-1H-benzimidazoles as radical scavengers and UV-protective agents. J. Enzym. Inhib. Med. Chem. 2017, 32, 527–537. [Google Scholar] [CrossRef]
- Hossan, J.; Gafur, M.A.; Kadir, M.R.; Mainul, M. Preparation and Characterization of Gelatin- Hydroxyapatite Composite for Bone Tissue Engineering. Int. J. Eng. Technol. 2014, 57, 113–122. [Google Scholar]
- Amin, R.M.; Elfeky, S.; Verwanger, T.; Krammer, B. A new biocompatible nanocomposite as a promising constituent of sunscreens. Mater. Sci. Eng. C 2016, 63, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Selim, M.; Shea, C.R.; Grichnik, J.M.; Omar, M.M.; Monteiro-Riviere, N.; Pinnell, S.R. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J. Am. Acad. Dermatol. 2003, 48, 866–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morsy, R.; Ali, S.S.; El-Shetehy, M. Development of hydroxyapatite-chitosan gel sunscreen combating clinical multidrug-resistant bacteria. J. Mol. Struct. 2017, 1143, 251–258. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Mattioli-Belmonte, M.; Celotti, G.; Sandri, M.; Gigante, A.; Fava, P.; Biagini, G. Biomimetic Mg- and Mg,CO3-substituted hydroxyapatites: Synthesis characterization and in vitro behaviour. J. Eur. Ceram. Soc. 2006, 26, 2593–2601. [Google Scholar] [CrossRef]
- Landi, E.; Tampieri, A.; Celotti, G.; Sprio, S.; Sandri, M.; Logroscino, G. Sr-substituted hydroxyapatites for osteoporotic bone replacement. Acta Biomater. 2007, 3, 961–969. [Google Scholar] [CrossRef]
- de Araujo, T.; de Souza, S.; Miyakawa, W.; de Sousa, E. Phosphates nanoparticles doped with zinc and manganese for sunscreens. Mater. Chem. Phys. 2010, 124, 1071–1076. [Google Scholar] [CrossRef]
- De Araujo, T.S.; De Souza, S.O.; De Sousa, E.M.B. Effect of Zn2+, Fe3+ and Cr3+ addition to hydroxyapatite for its application as an active constituent of sunscreens. J. Physics: Conf. Ser. 2010, 249. [Google Scholar] [CrossRef]
- Piccirillo, C.; Rocha, C.; Tobaldi, D.M.; Pullar, R.C.; Labrincha, J.A.; Ferreira, M.O.; Castro, P.M.L.; Pintado, M.M.E. A hydroxyapatite–Fe2O3 based material of natural origin as an active sunscreen filter. J. Mater. Chem. B 2014, 2, 5999–6009. [Google Scholar] [CrossRef]
- Popov, A.; Priezzhev, A.V.; Lademann, J.; Myllylä, R. Alteration of skin light-scattering and absorption properties by application of sunscreen nanoparticles: A Monte Carlo study. J. Quant. Spectrosc. Radiat. Transf. 2011, 112, 1891–1897. [Google Scholar] [CrossRef]
- Morlando, A.; Cardillo, D.; Devers, T.; Konstantinov, K. Titanium doped tin dioxide as potential UV filter with low photocatalytic activity for sunscreen products. Mater. Lett. 2016, 171, 289–292. [Google Scholar] [CrossRef]
- Yasukawa, A.; Tamura, J. Preparation and structure of titanium-cerium-calcium hydroxyapatite particles and their ultraviolet protecting ability. Colloids Surf. A Physicochem. Eng. Asp. 2020, 609, 125705. [Google Scholar] [CrossRef]
- Tampieri, A.; Sandri, M.; Sprio, S. Physical Solar Filter Consisting of Substituted Hydroxyapatite in An Organic Matrix. US 10,813,856 B2, 27 October 2020. [Google Scholar]
- Burnett, M.E.; Wang, S.Q. Current sunscreen controversies: A critical review. Photodermatol. Photoimmunol. Photomed. 2011, 27, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Lamberti, G.; Galdi, I.; Vallet-Regí, M. Anti-Osteoporotic Drug Release from Ordered Mesoporous Bioceramics: Experiments and Modeling. AAPS PharmSciTech 2011, 12, 1193–1199. [Google Scholar] [CrossRef] [Green Version]
- Raja, N.; Park, H.; Choi, Y.-J.; Yun, H.-S. Multifunctional Calcium-Deficient Hydroxyl Apatite–Alginate Core–Shell-Structured Bone Substitutes as Cell and Drug Delivery Vehicles for Bone Tissue Regeneration. ACS Biomater. Sci. Eng. 2021, 7, 1123–1133. [Google Scholar] [CrossRef]
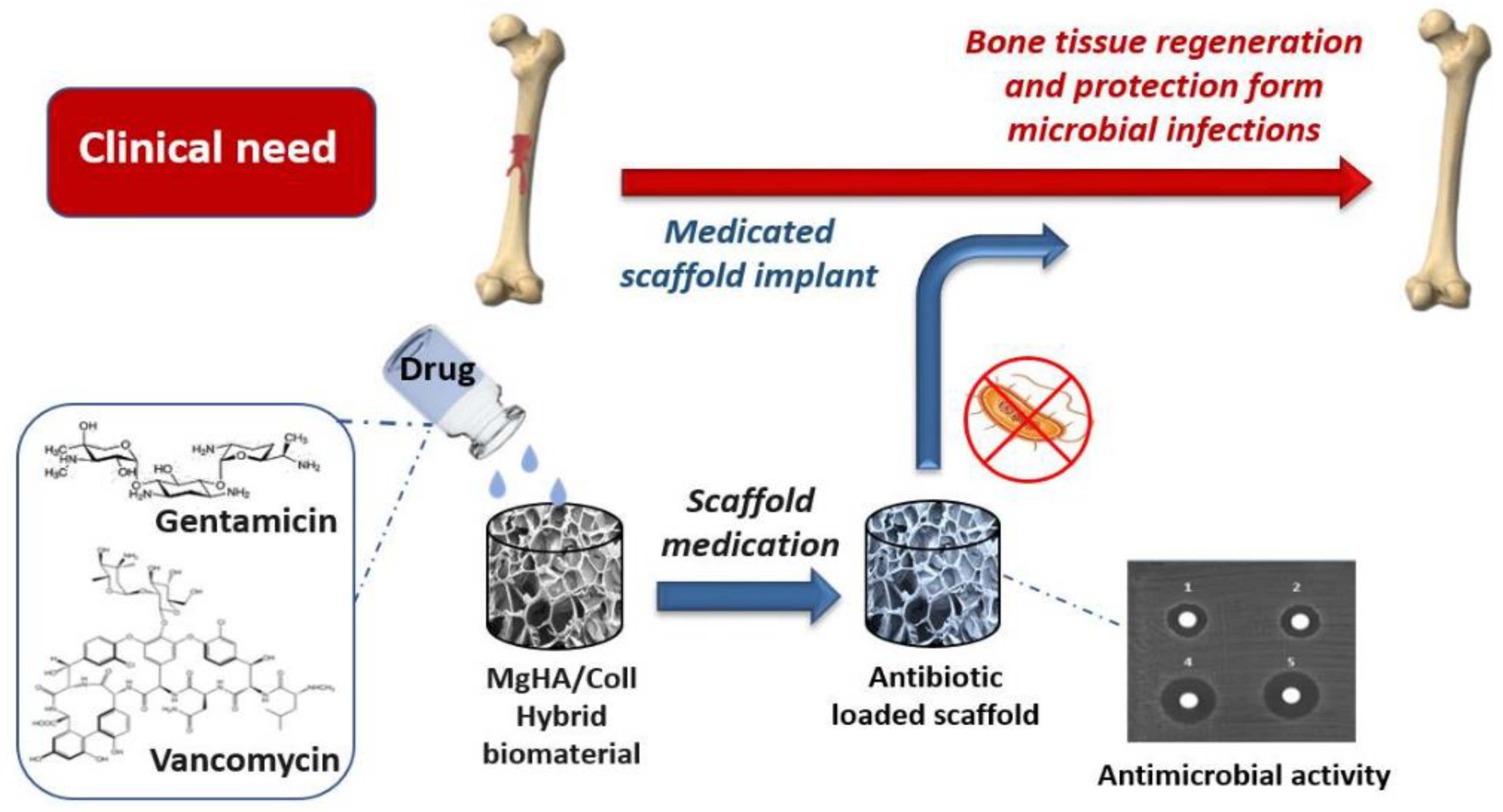
- Mulazzi, M.; Campodoni, E.; Bassi, G.; Montesi, M.; Panseri, S.; Bonvicini, F.; Gentilomi, G.A.; Tampieri, A.; Sandri, M. Medicated Hydroxyapatite/Collagen Hybrid Scaffolds for Bone Regeneration and Local Antimicrobial Therapy to Prevent Bone Infections. Pharmaceutics 2021, 13, 1090. [Google Scholar] [CrossRef] [PubMed]
- Barroug, A.; Glimcher, M.J. Hydroxyapatite crystals as a local delivery system for cisplatin: Adsorption and release of cisplatin in vitro. J. Orthop. Res. 2002, 20, 274–280. [Google Scholar] [CrossRef]
- Barroug, A.; Kuhn, L.T.; Gerstenfeld, L.C.; Glimcher, M.J. Interactions of cisplatin with calcium phosphate nanoparticles: In vitro controlled adsorption and release. J. Orthop. Res. 2004, 22, 703–708. [Google Scholar] [CrossRef]
- Chen, Q.; Liang, J.; Wang, S.; Wang, D.; Wang, R. An asymmetric approach toward chiral multicyclic spirooxindoles from isothiocyanato oxindoles and unsaturated pyrazolones by a chiral tertiary amine thiourea catalyst. Chem. Commun. 2013, 49, 1657–1659. [Google Scholar] [CrossRef]
- Srivastava, P.; Hira, S.K.; Srivastava, D.N.N.; Singh, V.K.; Gupta, U.; Singh, R.; Singh, R.A.; Manna, P.P. ATP-Decorated Mesoporous Silica for Biomineralization of Calcium Carbonate and P2 Purinergic Receptor-Mediated Antitumor Activity against Aggressive Lymphoma. ACS Appl. Mater. Interfaces 2018, 10, 6917–6929. [Google Scholar] [CrossRef]
- Descamps, M.; Duhoo, T.; Monchau, F.; Lu, J.; Hardouin, P.; Hornez, J.; Leriche, A. Manufacture of macroporous β-tricalcium phosphate bioceramics. J. Eur. Ceram. Soc. 2008, 28, 149–157. [Google Scholar] [CrossRef]
- Okazaki, Y.; Abe, Y.; Yasuda, K.; Hiasa, K.; Hirata, I. Osteoclast Response to Bioactive Surface Modification of Hydroxyapatite. Open J. Stomatol. 2014, 04, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Pokhrel, S. Hydroxyapatite: Preparation, Properties and Its Biomedical Applications. Adv. Chem. Eng. Sci. 2018, 08, 225–240. [Google Scholar] [CrossRef] [Green Version]
- Iafisco, M.; Sandri, M.; Panseri, S.; Delgado-López, J.M.; Gómez-Morales, J.; Tampieri, A. Magnetic Bioactive and Biodegradable Hollow Fe-Doped Hydroxyapatite Coated Poly(l-lactic) Acid Micro-nanospheres. Chem. Mater. 2013, 25, 2610–2617. [Google Scholar] [CrossRef]
- Iafisco, M.; Drouet, C.; Adamiano, A.; Pascaud, P.; Montesi, M.; Panseri, S.; Sarda, S.; Tampieri, A. Superparamagnetic iron-doped nanocrystalline apatite as a delivery system for doxorubicin. J. Mater. Chem. B 2015, 4, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ain, Q.-U.; Munir, H.; Jelani, F.; Anjum, F.; Bilal, M. Antibacterial potential of biomaterial derived nanoparticles for drug delivery application. Mater. Res. Express 2019, 6, 125426. [Google Scholar] [CrossRef]
- Caplin, J.D.; García, A.J. Implantable antimicrobial biomaterials for local drug delivery in bone infection models. Acta Biomater. 2019, 93, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Pal, U. 3D hydroxyapatite scaffold for bone regeneration and local drug delivery applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101131. [Google Scholar] [CrossRef]
- Dorati, R.; De Trizio, A.; Genta, I.; Merelli, A.; Modena, T.; Conti, B. Formulation and in vitro characterization of a composite biodegradable scaffold as antibiotic delivery system and regenerative device for bone. J. Drug Deliv. Sci. Technol. 2016, 35, 124–133. [Google Scholar] [CrossRef]
- El-Husseiny, M.; Patel, S.; Macfarlane, R.J.; Haddad, F.S. Biodegradable antibiotic delivery systems. J. Bone Jt. Surg. 2011, 93, 151–157. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Fan, W.; Gelinsky, M.; Xiao, Y.; Chang, J.; Friis, T.; Cuniberti, G. In situ preparation and protein delivery of silicate–alginate composite microspheres with core-shell structure. J. R. Soc. Interface 2011, 8, 1804–1814. [Google Scholar] [CrossRef] [Green Version]
- Aslankoohi, N.; Mequanint, K. Intrinsically fluorescent bioactive glass-poly(ester amide) hybrid microparticles for dual drug delivery and bone repair. Mater. Sci. Eng. C 2021, 128, 112288. [Google Scholar] [CrossRef]
- Etter, J.N.; Karasinski, M.; Ware, J.; Oldinski, R.A. Dual-crosslinked homogeneous alginate microspheres for mesenchymal stem cell encapsulation. J. Mater. Sci. Mater. Med. 2018, 29, 143. [Google Scholar] [CrossRef]
- Radwan, N.H.; Nasr, M.; Ishak, R.A.; Awad, G.A. Moxifloxacin-loaded in situ synthesized Bioceramic/Poly(L-lactide-co-ε-caprolactone) composite scaffolds for treatment of osteomyelitis and orthopedic regeneration. Int. J. Pharm. 2021, 602, 120662. [Google Scholar] [CrossRef]
- Alshemary, A.Z.; Akram, M.; Goh, Y.-F.; Tariq, U.; Butt, F.K.; Abdolahi, A.; Hussain, R. Synthesis, characterization, in vitro bioactivity and antimicrobial activity of magnesium and nickel doped silicate hydroxyapatite. Ceram. Int. 2015, 41, 11886–11898. [Google Scholar] [CrossRef]
- Ballardini, A.; Montesi, M.; Panseri, S.; Vandini, A.; Balboni, P.G.; Tampieri, A.; Sprio, S. New hydroxyapatite nanophases with enhanced osteogenic and anti-bacterial activity. J. Biomed. Mater. Res. Part A 2017, 106, 521–530. [Google Scholar] [CrossRef]
- Huh, A.J.; Kwon, Y.J. “Nanoantibiotics”: A new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J. Control. Release 2011, 156, 128–145. [Google Scholar] [CrossRef]
- Sprio, S.; Sandri, M.; Iafisco, M.; Panseri, S.; Montesi, M.; Ruffini, A.; Adamiano, A.; Ballardini, A.; Tampieri, A.B.A.A. Nature-Inspired Nanotechnology and Smart Magnetic Activation: Two Groundbreaking Approaches toward a New Generation of Biomaterials for Hard Tissue Regeneration; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Campodoni, E.; Adamiano, A.; Dozio, S.M.; Panseri, S.; Montesi, M.; Sprio, S.; Tampieri, A.; Sandri, M. Development of innovative hybrid and intrinsically magnetic nanobeads as a drug delivery system. Nanomedicine 2016, 11, 2119–2130. [Google Scholar] [CrossRef] [PubMed]
- Patrício, T.M.F.; Panseri, S.; Montesi, M.; Iafisco, M.; Sandri, M.; Tampieri, A.; Sprio, S. Superparamagnetic hybrid microspheres affecting osteoblasts behaviour. Mater. Sci. Eng. C 2018, 96, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Patrício, T.M.F.; Mumcuoglu, D.; Montesi, M.; Panseri, S.; Witte-Bouma, J.; Garcia, S.F.; Sandri, M.; Tampieri, A.; Farrell, E.; Sprio, S. Bio-inspired polymeric iron-doped hydroxyapatite microspheres as a tunable carrier of rhBMP-2. Mater. Sci. Eng. C 2020, 119, 111410. [Google Scholar] [CrossRef] [PubMed]
- Tatiana, F.P.; Simone, S.; Monica, S.; Natalia, G.; Monica, M.; Silvia, P.; Bas, K.; Anna, T. Bio-inspired superparamagnetic microspheres for bone tissue engineering applications. Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Miragoli, M.; Ceriotti, P.; Iafisco, M.; Vacchiano, M.; Salvarani, N.; Alogna, A.; Carullo, P.; Ramirez-Rodríguez, G.B.; Patrício, T.; Degli Esposti, L.; et al. Inhalation of peptide-loaded nanoparticles improves heart failure. Sci. Transl. Med. 2018, 10, eaan6205. [Google Scholar] [CrossRef] [Green Version]
- Degli Esposti, L.; Carella, F.; Adamiano, A.; Tampieri, A.; Iafisco, M. Calcium phosphate-based nanosystems for advanced targeted nanomedicine. Drug Dev. Ind. Pharm. 2018, 44, 1223–1238. [Google Scholar] [CrossRef]
- Barbanente, A.; Nadar, R.A.; Degli Esposti, L.; Palazzo, B.; Iafisco, M.; Beucken, J.J.J.P.V.D.; Leeuwenburgh, S.C.G.; Margiotta, N. Platinum-loaded, selenium-doped hydroxyapatite nanoparticles selectively reduce proliferation of prostate and breast cancer cells co-cultured in the presence of stem cells. J. Mater. Chem. B 2020, 8, 2792–2804. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campodoni, E.; Montanari, M.; Artusi, C.; Bassi, G.; Furlani, F.; Montesi, M.; Panseri, S.; Sandri, M.; Tampieri, A. Calcium-Based Biomineralization: A Smart Approach for the Design of Novel Multifunctional Hybrid Materials. J. Compos. Sci. 2021, 5, 278. https://doi.org/10.3390/jcs5100278
Campodoni E, Montanari M, Artusi C, Bassi G, Furlani F, Montesi M, Panseri S, Sandri M, Tampieri A. Calcium-Based Biomineralization: A Smart Approach for the Design of Novel Multifunctional Hybrid Materials. Journal of Composites Science. 2021; 5(10):278. https://doi.org/10.3390/jcs5100278
Chicago/Turabian StyleCampodoni, Elisabetta, Margherita Montanari, Chiara Artusi, Giada Bassi, Franco Furlani, Monica Montesi, Silvia Panseri, Monica Sandri, and Anna Tampieri. 2021. "Calcium-Based Biomineralization: A Smart Approach for the Design of Novel Multifunctional Hybrid Materials" Journal of Composites Science 5, no. 10: 278. https://doi.org/10.3390/jcs5100278
APA StyleCampodoni, E., Montanari, M., Artusi, C., Bassi, G., Furlani, F., Montesi, M., Panseri, S., Sandri, M., & Tampieri, A. (2021). Calcium-Based Biomineralization: A Smart Approach for the Design of Novel Multifunctional Hybrid Materials. Journal of Composites Science, 5(10), 278. https://doi.org/10.3390/jcs5100278






