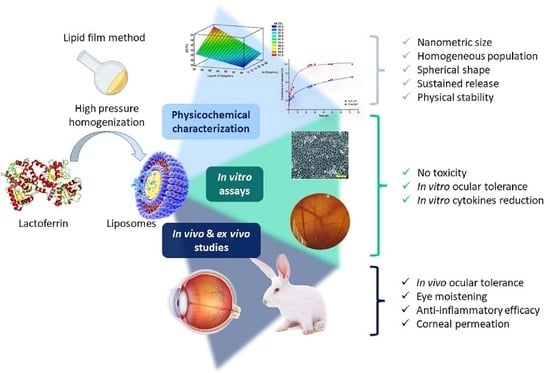
Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lactoferrin Loaded Liposomes Production
2.3. Optimization of Lactoferrin Loaded Liposomes
2.4. Physicochemical Characterization
2.5. Morphological Characterization and Interaction Studies of Optimized Liposomes
2.6. Stability Studies
2.7. Biopharmaceutical Behaviour
2.8. Cytotoxicity
2.9. Ocular Tolerance
2.10. Induction and Treatment of Dry Eye
2.11. Anti-Inflammatory Efficacy Assays
2.12. Statistical Analysis
3. Results and Discussion
3.1. Optimization Study
3.2. Morphological Characterization
3.3. Interaction Studies
3.4. Stability of Lactoferrin Loaded Liposomes
3.5. Biopharmaceutical Behaviour of bLF-LIP
3.6. Cytotoxicity
3.7. Ocular Tolerance
3.8. Therapeutic Efficacy against Dry Eye Disease
3.9. Anti-Inflammatory Efficacy
3.9.1. In Vitro Assays: IL-8 and TNF-α Determination
3.9.2. In Vivo Assays
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Structure | Injury | Evaluation | Score |
|---|---|---|---|
| CORNEA | (A) Degree of cloudiness or opacity | Corneal score: A × B × 5 Maximum score: 80 | |
| -Absence of ulceration | 0 | ||
| -Diffuse areas | 1 | ||
| -Translucent areas | 2 | ||
| -Opalescent areas | 3 | ||
| -Full opacity | 4 | ||
| (B) Affected areas | |||
| -None | 0 | ||
| -A quarter or less | 1 | ||
| -More than a quarter but without means | 2 | ||
| -More than three quarters up a whole plane | 3 | ||
| -More than half but less than three quarters | 4 | ||
| IRIS | (A) Iris injury score | Iris score: A·× 5 Maximum score: 10 | |
| -Normal | 0 | ||
| -Deep folds, congestion, swelling, moderate circumcorneal injection | 1 | ||
| -No reaction to light, haemorrhage, great destruction | 2 | ||
| CONJUNCTIVA | (A) Redness | Conjunctival score: (A + B + C) × 2 Maximum score: 20 | |
| -Normal glasses | 0 | ||
| -Some clearly injected vessels | 1 | ||
| -Diffuse redness | 2 | ||
| -Big diffuse redness | 3 | ||
| (B) Chemosis or Inflammation | |||
| -None | 0 | ||
| -Some | 1 | ||
| -Marked with partial disorder of the eyelids | 2 | ||
| -Eyelid more or less closed | 3 | ||
| -Semi eyelids | 4 | ||
| (C) Sweat | |||
| -None | 0 | ||
| -Any amount anomalous | 1 | ||
| -Wetting and eyelid hairs | 2 | ||
| -Periocular wetting | 3 | ||
| Calculation of the Ocular Irritation Index | OII | Classification | |
| OII = Cornea (A·× B·× 5) + Iris (A × 5) + Conjunctiva ((A + B + C) × 2) | 0 | Non-irritant | |
| 0–15 | Weakly irritant | ||
| >15–30 | Moderately irritant | ||
| >30–50 | Irritant | ||
| >50 | Extremely irritant | ||
References
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Roda, M.; Corazza, I.; Reggiani, M.L.B.; Pellegrini, M.; Taroni, L.; Giannaccare, G.; Versura, P. Dry Eye Disease and Tear Cytokine Levels—A Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 3111. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, P.; Craig, J.; Rupenthal, I. Formulation Considerations for the Management of Dry Eye Disease. Pharmaceutics 2021, 13, 207. [Google Scholar] [CrossRef]
- Joossen, C.; Baán, A.; Moreno-Cinos, C.; Joossens, J.; Cools, N.; Lanckacker, E.; Moons, L.; Lemmens, K.; Lambeir, A.-M.; Fransen, E.; et al. A novel serine protease inhibitor as potential treatment for dry eye syndrome and ocular inflammation. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Seen, S.; Tong, L. Dry eye disease and oxidative stress. Acta Ophthalmol. 2018, 96, e412–e420. [Google Scholar] [CrossRef] [Green Version]
- Mazet, R.; Yaméogo, J.B.G.; Wouessidjewe, D.; Choisnard, L.; Gèze, A. Recent Advances in the Design of Topical Ophthalmic Delivery Systems in the Treatment of Ocular Surface Inflammation and Their Biopharmaceutical Evaluation. Pharmaceutics 2020, 12, 570. [Google Scholar] [CrossRef] [PubMed]
- Anfuso, C.D.; Olivieri, M.; Fidilio, A.; Lupo, G.; Rusciano, D.; Pezzino, S.; Gagliano, C.; Drago, F.; Bucolo, C. Gabapentin Attenuates Ocular Inflammation: In vitro and In vivo Studies. Front. Pharmacol. 2017, 8, 173. [Google Scholar] [CrossRef]
- Foster, C.S.; Kothari, S.; Anesi, S.D.; Vitale, A.T.; Chu, D.; Metzinger, J.L.; Cerón, O. The Ocular Immunology and Uveitis Foundation preferred practice patterns of uveitis management. Surv. Ophthalmol. 2016, 61, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.; Fett, N.; Rosenbach, M.; Werth, V.P.; Micheletti, R.G. Prevention and management of glucocorticoid-induced side effects: A comprehensive review. J. Am. Acad. Dermatol. 2017, 76, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Carnahan, M.C.; Goldstein, D.A. Ocular complications of topical, peri-ocular, and systemic corticosteroids. Curr. Opin. Ophthalmol. 2000, 11, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.; Blanch, E.; Adhikari, B. Characteristics of bovine lactoferrin powders produced through spray and freeze drying processes. Int. J. Biol. Macromol. 2017, 95, 985–994. [Google Scholar] [CrossRef] [PubMed]
- González-Chávez, S.A.; Arévalo-Gallegos, S.; Rascon-Cruz, Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents 2009, 33, 301.e1–301.e8. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.; Lee, S.; Ahmad, T.; Perikamana, S.K.M.; Kim, E.M.; Lee, S.W.; Shin, H. Bioactive Membrane Immobilized with Lactoferrin for Modulation of Bone Regeneration and Inflammation. Tissue Eng. Part A 2020, 26, 1243–1258. [Google Scholar] [CrossRef] [Green Version]
- Kanyshkova, T.G.; Buneva, V.N.; Nevinsky, G.A. Lactoferrin and Its biological functions. Biochemestry 2001, 66, 1–7. [Google Scholar] [CrossRef]
- Tamhane, M.; Cabrera-Ghayouri, S.; Abelian, G.; Viswanath, V. Review of Biomarkers in Ocular Matrices: Challenges and Opportunities. Pharm. Res. 2019, 36, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Hanstock, H.G.; Edwards, J.P.; Walsh, N.P. Tear Lactoferrin and Lysozyme as Clinically Relevant Biomarkers of Mucosal Immune Competence. Front. Immunol. 2019, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Rageh, A.A.; Ferrington, D.; Roehrich, H.; Yuan, C.; Terluk, M.R.; Nelson, E.F.; Montezuma, S.R. Lactoferrin Expression in Human and Murine Ocular Tissue. Curr. Eye Res. 2016, 41, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Lu, C.; Zhang, J. Lactoferrin and Its Detection Methods: A Review. Nutrients 2021, 13, 2492. [Google Scholar] [CrossRef]
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on bovine lactoferrin. EFSA J. 2012, 10, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Håversen, L.; Ohlsson, B.G.; Hahn-Zoric, M.; Hanson, L. Å; Mattsby-Baltzer, I. Lactoferrin down-regulates the LPS-induced cytokine production in monocytic cells via NF-κB. Cell. Immunol. 2002, 220, 83–95. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Wong, H.; Ashida, K.-Y.; Schryvers, A.B.; Lönnerdal, B. The N1 Domain of Human Lactoferrin Is Required for Internalization by Caco-2 Cells and Targeting to the Nucleus. Biochemistry 2008, 47, 10915–10920. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.; Wu, J. Bovine lactoferrin-derived ACE inhibitory tripeptide LRP also shows antioxidative and anti-inflammatory activities in endothelial cells. J. Funct. Foods 2016, 25, 375–384. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, J.; Kelly, M.; Holbein, B.E.; Lehmann, C. Iron chelation for the treatment of uveitis. Med. Hypotheses 2017, 103, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kanwar, J.R.; Roy, K.; Patel, Y.; Zhou, S.-F.; Singh, M.R.; Singh, D.; Nasir, M.; Sehgal, R.; Sehgal, A.; Singh, R.S.; et al. Multifunctional Iron Bound Lactoferrin and Nanomedicinal Approaches to Enhance Its Bioactive Functions. Molecules 2015, 20, 9703–9731. [Google Scholar] [CrossRef]
- López, E.S.; Egea, M.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.; Silva, A.; García, M. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen—In vitro, ex vivo and in vivo characterization. Colloids Surfaces B: Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumari, S.; Dandamudi, M.; Rani, S.; Behaeghel, E.; Behl, G.; Kent, D.; O’Reilly, N.; O’Donovan, O.; McLoughlin, P.; Fitzhenry, L. Dexamethasone-Loaded Nanostructured Lipid Carriers for the Treatment of Dry Eye Disease. Pharmaceutics 2021, 13, 905. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Sánchez-López, E.; Espina, M.; Egea, M.A.; García, M.L. Polymeric nanoparticles of (-)-epigallocatechin gallate: A new formulation for the treatment of ocular diseases. J. Control. Release 2017, 259, e7. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Lebrón, J.A.; López-López, M.; García-Calderón, C.B.; Rosado, I.V.; Balestra, F.R.; Huertas, P.; Rodik, R.V.; Kalchenko, V.I.; Bernal, E.; Moyá, M.L.; et al. Multivalent Calixarene-Based Liposomes as Platforms for Gene and Drug Delivery. Pharmaceutics 2021, 13, 1250. [Google Scholar] [CrossRef]
- Navarro-Partida, J.; Castro-Castaneda, C.; Cruz-Pavlovich, F.S.; Aceves-Franco, L.; Guy, T.; Santos, A. Lipid-Based Nanocarriers as Topical Drug Delivery Systems for Intraocular Diseases. Pharmaceutics 2021, 13, 678. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, N.; Rho, J.G.; Um, W.; Ek, P.K.; Nguyen, V.Q.; Oh, B.H.; Kim, W.; Park, J.H. Hyaluronic Acid Nanoparticles as Nanomedicine for Treatment of Inflammatory Diseases. Pharmaceutics 2020, 12, 931. [Google Scholar] [CrossRef]
- Xiang, B.; Cao, D.-Y. Preparation of Drug Liposomes by Thin-Film Hydration and Homogenization. In Liposome-Based Drug Delivery Systems; Lu, W.-L., Qi, X.-R., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 25–35. [Google Scholar]
- Nekkanti, V.; Marwah, A.; Pillai, R. Media milling process optimization for manufacture of drug nanoparticles using design of experiments (DOE). Drug Dev. Ind. Pharm. 2013, 41, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Anaraki, N.I.; Sadeghpour, A.; Iranshahi, K.; Toncelli, C.; Cendrowska, U.; Stellacci, F.; Dommann, A.; Wick, P.; Neels, A. New approach for time-resolved and dynamic investigations on nanoparticles agglomeration. Nano Res. 2020, 13, 2847–2856. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Silva-Abreu, M.; Calpena, A.C.; Egea, M.A.; Espina, M.; García, M.L. Development of fluorometholone-loaded PLGA nanoparticles for treatment of inflammatory disorders of anterior and posterior segments of the eye. Int. J. Pharm. 2018, 547, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, M.-I. HPLC of Peptides and Proteins. Methods and Protocols, 1st ed.; Humana Press: Totowa, NJ, USA, 2004. [Google Scholar]
- Cano, A.; Ettcheto, M.; Espina, M.; Auladell, C.; Calpena, A.C.; Folch, J.; Barenys, M.; López, E.S.; Camins, A.; García, M.L. Epigallocatechin-3-gallate loaded PEGylated-PLGA nanoparticles: A new anti-seizure strategy for temporal lobe epilepsy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1073–1085. [Google Scholar] [CrossRef]
- Cano, A.; Ettcheto, M.; Chang, J.-H.; Barroso, E.; Espina, M.; Kühne, B.A.; Barenys, M.; Auladell, C.; Folch, J.; Souto, E.B.; et al. Dual-drug loaded nanoparticles of Epigallocatechin-3-gallate (EGCG)/Ascorbic acid enhance therapeutic efficacy of EGCG in a APPswe/PS1dE9 Alzheimer’s disease mice model. J. Control. Release 2019, 301, 62–75. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Parrotta, G.; Vera, R.; Sánchez-López, E.; Galindo, R.; Kjeldsen, F.; Badia, J.; Baldoma, L.; Espina, M.; García, M.L. Ocular penetration of fluorometholone-loaded PEG-PLGA nanoparticles functionalized with cell-penetrating peptides. Nanomedicine 2019, 14, 3089–3104. [Google Scholar] [CrossRef]
- Derouiche, M.T.T.; Abdennour, S. HET-CAM test. Application to shampoos in developing countries. Toxicol. Vitr. 2017, 45, 393–396. [Google Scholar] [CrossRef]
- Shalom, Y.; Perelshtein, I.; Perkas, N.; Gedanken, A.; Banin, E. Catheters coated with Zn-doped CuO nanoparticles delay the onset of catheter-associated urinary tract infections. Nano Res. 2016, 10, 520–533. [Google Scholar] [CrossRef]
- Li, C.; Song, Y.; Luan, S.; Wan, P.; Li, N.; Tang, J.; Han, Y.; Xiong, C.; Wang, Z. Research on the Stability of a Rabbit Dry Eye Model Induced by Topical Application of the Preservative Benzalkonium Chloride. PLOS ONE 2012, 7, e33688. [Google Scholar] [CrossRef] [Green Version]
- Wadhwa, S.; Paliwal, R.; Paliwal, S.; Vyas, S. Nanocarriers in Ocular Drug Delivery: An Update Review. Curr. Pharm. Des. 2009, 15, 2724–2750. [Google Scholar] [CrossRef] [PubMed]
- Kandzija, N.; Khutoryanskiy, V.V. Delivery of Riboflavin-5′-Monophosphate Into the Cornea: Can Liposomes Provide Any Enhancement Effects? J. Pharm. Sci. 2017, 106, 3041–3049. [Google Scholar] [CrossRef]
- Fangueiro, J.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Silva, A.; Souto, E.B. Design of cationic lipid nanoparticles for ocular delivery: Development, characterization and cytotoxicity. Int. J. Pharm. 2014, 461, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Aboali, F.A.; Habib, D.A.; Elbedaiwy, H.M.; Farid, R.M. Curcumin-loaded proniosomal gel as a biofreindly alternative for treatment of ocular inflammation: In-vitro and in-vivo assessment. Int. J. Pharm. 2020, 589, 119835. [Google Scholar] [CrossRef]
- Yi, X.; Zheng, Q.; Pan, M.-H.; Chiou, Y.-S.; Li, Z.; Li, L.; Chen, Y.; Hu, J.; Duan, S.; Wei, S.; et al. Liposomal vesicles-protein interaction: Influences of iron liposomes on emulsifying properties of whey protein. Food Hydrocoll. 2019, 89, 602–612. [Google Scholar] [CrossRef]
- Mazyed, E.A.; Abdelaziz, A.E. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics 2020, 12, 465. [Google Scholar] [CrossRef]
- Ibrahim, S.S. The Role of Surface Active Agents in Ophthalmic Drug Delivery: A Comprehensive Review. J. Pharm. Sci. 2019, 108, 1923–1933. [Google Scholar] [CrossRef]
- Ismail, A.; Nasr, M.; Sammour, O. Nanoemulsion as a feasible and biocompatible carrier for ocular delivery of travoprost: Improved pharmacokinetic/pharmacodynamic properties. Int. J. Pharm. 2020, 583, 119402. [Google Scholar] [CrossRef]
- Youshia, J.; Kamel, A.O.; El Shamy, A.; Mansour, S. Gamma sterilization and in vivo evaluation of cationic nanostructured lipid carriers as potential ocular delivery systems for antiglaucoma drugs. Eur. J. Pharm. Sci. 2021, 163, 105887. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Lee, C.D.; Bin Ahn, J.; Kim, D.-H.; Lee, J.K.; Lee, J.-Y.; Choi, J.-S.; Park, J.-S. Hyaluronic acid-coated solid lipid nanoparticles to overcome drug-resistance in tumor cells. J. Drug Deliv. Sci. Technol. 2019, 50, 365–371. [Google Scholar] [CrossRef]
- González-Fernández, F.; Bianchera, A.; Gasco, P.; Nicoli, S.; Pescina, S. Lipid-Based Nanocarriers for Ophthalmic Administration: Towards Experimental Design Implementation. Pharmaceutics 2021, 13, 447. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Ghadi, Z.; Ebrahimnejad, P.; Amiri, F.T.; Nokhodchi, A. Improved oral delivery of quercetin with hyaluronic acid containing niosomes as a promising formulation. J. Drug Target. 2021, 29, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, H.; Longman, M.R.; Alany, R.G.; Pierscionek, B. Phytosome-hyaluronic acid systems for ocular delivery of L-carnosine. Int. J. Nanomed. 2016, 11, 2815–2827. [Google Scholar] [CrossRef] [Green Version]
- Carvajal-Vidal, P.; Fábrega, M.-J.; Espina, M.; Calpena, A.C.; García, M.L. Development of Halobetasol-loaded nanostructured lipid carrier for dermal administration: Optimization, physicochemical and biopharmaceutical behavior, and therapeutic efficacy. Nanomed. Nanotechnol. Biol. Med. 2019, 20, 102026. [Google Scholar] [CrossRef]
- Marín, R.R.; Babick, F.; Hillemann, L. Zeta potential measurements for non-spherical colloidal particles – Practical issues of characterisation of interfacial properties of nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 532, 516–521. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef]
- Fangueiro, J.; Calpena, A.C.; Clares, B.; Andreani, T.; Egea, M.A.; Veiga, F.; Garcia, M.L.; Silva, A.; Souto, E.B. Biopharmaceutical evaluation of epigallocatechin gallate-loaded cationic lipid nanoparticles (EGCG-LNs): In vivo, in vitro and ex vivo studies. Int. J. Pharm. 2016, 502, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.Y.; Bala, S.; Škalko-Basnet, N.; di Cagno, M.P. Interpreting non-linear drug diffusion data: Utilizing Korsmeyer-Peppas model to study drug release from liposomes. Eur. J. Pharm. Sci. 2019, 138, 105026. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Romero, A.-M.; Maestrelli, F.; Mura, P.A.; Rabasco, A.M.; González-Rodríguez, M.L. Novel Findings about Double-Loaded Curcumin-in-HPβcyclodextrin-in Liposomes: Effects on the Lipid Bilayer and Drug Release. Pharmaceutics 2018, 10, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez-Segura, L.; Parra, A.; Calpena-Campmany, A.C.; Gimeno, Á.; De Aranda, I.G.; Boix-Montañes, A. Ex Vivo Permeation of Carprofen Vehiculated by PLGA Nanoparticles through Porcine Mucous Membranes and Ophthalmic Tissues. Nanomaterials 2020, 10, 355. [Google Scholar] [CrossRef] [Green Version]
- Soni, V.; Pandey, V.; Tiwari, R.; Asati, S.; Tekade, R.K. Design and Evaluation of Ophthalmic Delivery Formulations. In Basic Fundamentals of Drug Delivery; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 473–538. [Google Scholar]
- Flanagan, J.; Willcox, M. Role of lactoferrin in the tear film. Biochimie 2009, 91, 35–43. [Google Scholar] [CrossRef]
- Lawrenson, J.G. Anterior Eye. In Contact Lens Practice; Elsevier: Amsterdam, The Netherlands, 2018; pp. 10–27.e2. [Google Scholar]
- Ponzini, E.; Scotti, L.; Grandori, R.; Tavazzi, S.; Zambon, A. Lactoferrin Concentration in Human Tears and Ocular Diseases: A Meta-Analysis. Investig. Opthalmology Vis. Sci. 2020, 61, 9. [Google Scholar] [CrossRef]
- Han, F.Y.; Thurecht, K.J.; Whittaker, A.K.; Smith, M.T. Bioerodable PLGA-Based Microparticles for Producing Sustained-Release Drug Formulations and Strategies for Improving Drug Loading. Front. Pharmacol. 2016, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ruan, S.; Wang, Z.; Feng, N.; Zhang, Y. Hyaluronic Acid Coating Reduces the Leakage of Melittin Encapsulated in Liposomes and Increases Targeted Delivery to Melanoma Cells. Pharmaceutics 2021, 13, 1235. [Google Scholar] [CrossRef]
- Kim, D.J.; Jung, M.-Y.; Pak, H.-J.; Park, J.-H.; Kim, M.; Chuck, R.S.; Park, C.Y. Development of a novel hyaluronic acid membrane for the treatment of ocular surface diseases. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Gonzalez-Pizarro, R.; Carvajal-Vidal, P.; Bellowa, L.H.; Calpena, A.C.; Espina, M.; García, M.L. In-situ forming gels containing fluorometholone-loaded polymeric nanoparticles for ocular inflammatory conditions. Colloids Surfaces B Biointerfaces 2019, 175, 365–374. [Google Scholar] [CrossRef]
- Eldesouky, L.; El-Moslemany, R.; Ramadan, A.; Morsi, M.; Khalafallah, N. Cyclosporine Lipid Nanocapsules as Thermoresponsive Gel for Dry Eye Management: Promising Corneal Mucoadhesion, Biodistribution and Preclinical Efficacy in Rabbits. Pharmaceutics 2021, 13, 360. [Google Scholar] [CrossRef]
- Yousry, C.; Elkheshen, S.A.; El Laithy, H.; Essam, T.; Fahmy, R.H. Studying the influence of formulation and process variables on Vancomycin-loaded polymeric nanoparticles as potential carrier for enhanced ophthalmic delivery. Eur. J. Pharm. Sci. 2017, 100, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Vagge, A.; Senni, C.; Bernabei, F.; Pellegrini, M.; Scorcia, V.; E Traverso, C.; Giannaccare, G. Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections. Int. J. Mol. Sci. 2020, 21, 6668. [Google Scholar] [CrossRef] [PubMed]
- Devendra, J. Effect of Oral Lactoferrin on Cataract Surgery Induced Dry Eye: A Randomised Controlled Trial. J. Clin. Diagn. Res. 2015, 9, NC06–NC09. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Aqrawi, L.A.; Utheim, T.P.; Tashbayev, B.; Utheim, Ø.A.; Reppe, S.; Hove, L.H.; Herlofson, B.B.; Singh, P.B.; Palm, Ø.; et al. Elevated cytokine levels in tears and saliva of patients with primary Sjögren’s syndrome correlate with clinical ocular and oral manifestations. Sci. Rep. 2019, 9, 7319. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Garrido, N.; Fabrega, M.J.; Vera, R.; Giménez, R.; Badia, J.; Baldomà, L. Membrane vesicles from the probiotic Nissle 1917 and gut resident Escherichia coli strains distinctly modulate human dendritic cells and subsequent T cell responses. J. Funct. Foods 2019, 61, 1–12. [Google Scholar] [CrossRef]
- Shih, K.C.; Fong, P.Y.; Lam, P.Y.; Chan, T.C.Y.; Jhanji, V.; Tong, L. Role of tear film biomarkers in the diagnosis and management of dry eye disease. Taiwan J. Ophthalmol. 2019, 9, 150–159. [Google Scholar] [CrossRef]
- Ghasemi, H. Roles of IL-6 in Ocular Inflammation: A Review. Ocul. Immunol. Inflamm. 2018, 26, 37–50. [Google Scholar] [CrossRef]
- Kruzel, M.L.; Zimecki, M.; Actor, J.K. Lactoferrin in a Context of Inflammation-Induced Pathology. Front. Immunol. 2017, 8, 1438. [Google Scholar] [CrossRef]









| Independent Variables | Dependent Variables | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| cP80 | cbLF | cLipoid-S75 | Zav | PI | ZP | EE | ||||
| (mg·mL−1) | (mg·mL−1) | (mg·mL−1) | (nm) | (mV) | (%) | |||||
| 1 | −1 | 2.0 | −1 | 10.0 | −1 | 30.0 | 253.6 ± 2.2 | 0.121 ± 0.024 | 21.9 ± 0.6 | 49.2 ± 0.9 |
| 2 | 1 | 3.0 | −1 | 10.0 | −1 | 30.0 | 378.2 ± 1.4 | 0.317 ± 0.064 | 16.3 ± 0.3 | 40.6 ± 0.3 |
| 3 | −1 | 2.0 | 1 | 20.0 | −1 | 30.0 | 160.0 ± 3.9 | 0.179 ± 0.021 | 22.9 ± 0.2 | 55.4 ± 1.7 |
| 4 | 1 | 3.0 | 1 | 20.0 | −1 | 30.0 | 85.0 ± 2.4 | 0.165 ± 0.033 | 22.7 ± 0.3 | 50.0 ± 2.5 |
| 5 | −1 | 2.0 | −1 | 10.0 | 1 | 60.0 | 471.7 ± 2.3 | 0.383 ± 0.046 | 24.3 ± 1.9 | 39.6 ± 4.0 |
| 6 | 1 | 3.0 | −1 | 10.0 | 1 | 60.0 | 133.7 ± 1.4 | 0.292 ± 0.036 | 25.4 ± 0.6 | 33.1 ± 1.5 |
| 7 | −1 | 2.0 | 1 | 20.0 | 1 | 60.0 | 602.8 ± 6.2 | 0.282 ± 0.016 | 26.0 ± 1.2 | 35.3 ± 0.4 |
| 8 | 1 | 3.0 | 1 | 20.0 | 1 | 60.0 | 242.1 ± 2.3 | 0.484 ± 0.031 | 26.2 ± 0.3 | 37.4 ± 0.3 |
| Zav | PI | ZP | EE |
|---|---|---|---|
| (nm) | (mV) | (%) | |
| 90.5 ± 0.6 | 0.201 ± 0.070 | 20.5 ± 0.4 | 50.0 ± 3.0 |
| Models | bLF-NPs | Free bLF | ||
|---|---|---|---|---|
| AIC | R2 | AIC | R2 | |
| Zero Order | 94.76 | 0.84 | 115.87 | 0.64 |
| First Order | 94.33 | 0.84 | 93.67 | 0.94 |
| Higuchi | 77.93 | 0.96 | 104.86 | 0.86 |
| Hyperbola | 89.26 | 0.90 | 90.60 | 0.96 |
| Korsmeyer–Peppas | n = 0.014 | n = 0.022 | ||
| 67.83 | 0.98 | 93.91 | 0.94 | |
| Parameters | Free bLF | bLF-LIP |
|---|---|---|
| J (µg·h−1·cm−2) | 171.79 ± 9.83 | 317.50 ± 67.84 * |
| Kp · 103 (cm·h−1) | 8.59 ± 0.49 | 15.88 ± 3.39 * |
| Q24 (µg) | 2635.83 ± 151.49 | 4874.52 ± 1042.71 * |
| QR (µg·g−1·cm−2) | 1.12 ± 0.01 | 0.55 ± 0.02 ** |
| Formulation | Medium Score | Classification | |
|---|---|---|---|
| HET-CAM | Draize | ||
| bLF-LIP | 0.07 ± 0.00 | 0.00 ± 0.00 | Non-irritant |
| Free bLF (20 mg·mL−1) | 0.07 ± 0.00 | 0.00 ± 0.00 | Non-irritant |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Machado, A.; Díaz-Garrido, N.; Cano, A.; Espina, M.; Badia, J.; Baldomà, L.; Calpena, A.C.; Souto, E.B.; García, M.L.; Sánchez-López, E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics 2021, 13, 1698. https://doi.org/10.3390/pharmaceutics13101698
López-Machado A, Díaz-Garrido N, Cano A, Espina M, Badia J, Baldomà L, Calpena AC, Souto EB, García ML, Sánchez-López E. Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics. 2021; 13(10):1698. https://doi.org/10.3390/pharmaceutics13101698
Chicago/Turabian StyleLópez-Machado, Ana, Natalia Díaz-Garrido, Amanda Cano, Marta Espina, Josefa Badia, Laura Baldomà, Ana Cristina Calpena, Eliana B. Souto, María Luisa García, and Elena Sánchez-López. 2021. "Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation" Pharmaceutics 13, no. 10: 1698. https://doi.org/10.3390/pharmaceutics13101698
APA StyleLópez-Machado, A., Díaz-Garrido, N., Cano, A., Espina, M., Badia, J., Baldomà, L., Calpena, A. C., Souto, E. B., García, M. L., & Sánchez-López, E. (2021). Development of Lactoferrin-Loaded Liposomes for the Management of Dry Eye Disease and Ocular Inflammation. Pharmaceutics, 13(10), 1698. https://doi.org/10.3390/pharmaceutics13101698