3D-Printed Mucoadhesive Collagen Scaffolds as a Local Tetrahydrocurcumin Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixture Preparation
2.3. Rheological Evaluation
2.4. 3D Printing
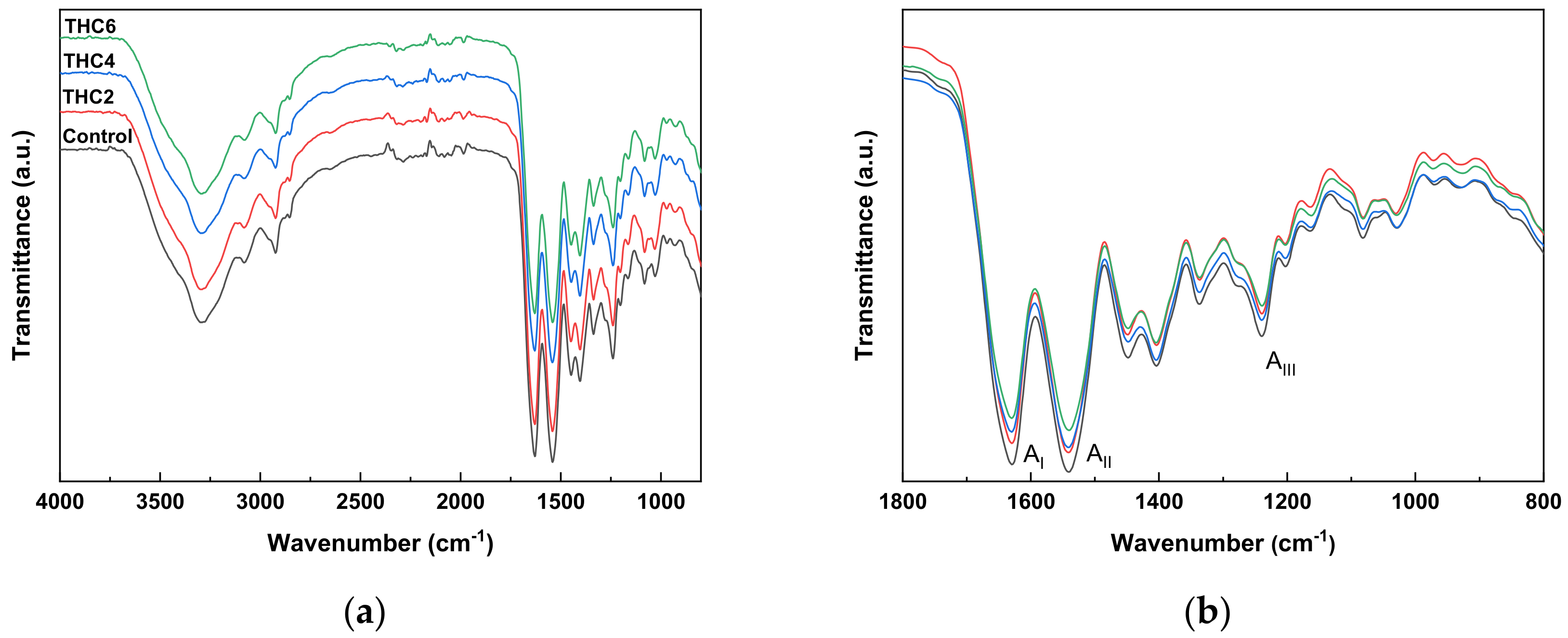
2.5. Fourier Transform Infrared (FTIR) Spectroscopy
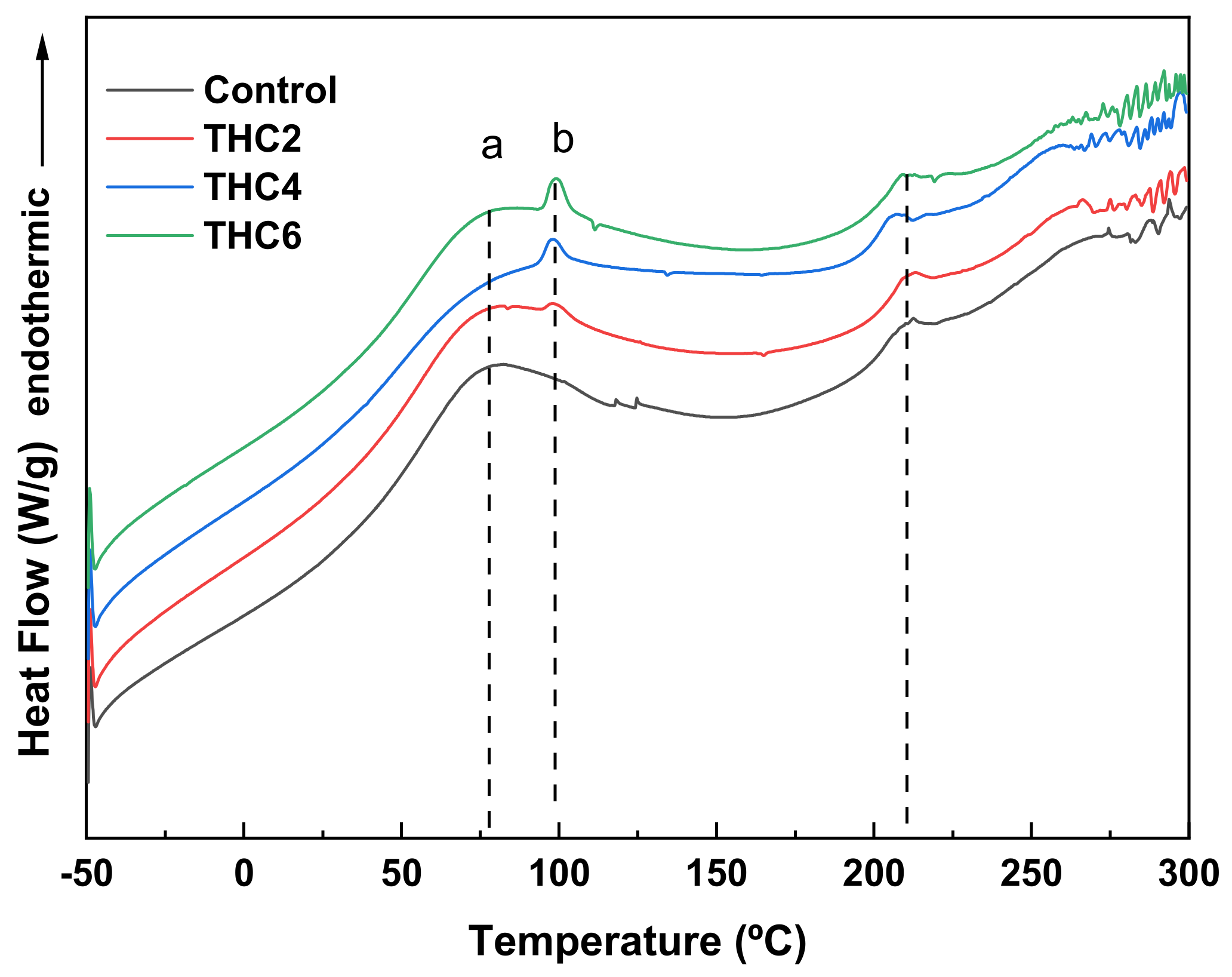
2.6. Differential Scanning Calorimetry (DSC)
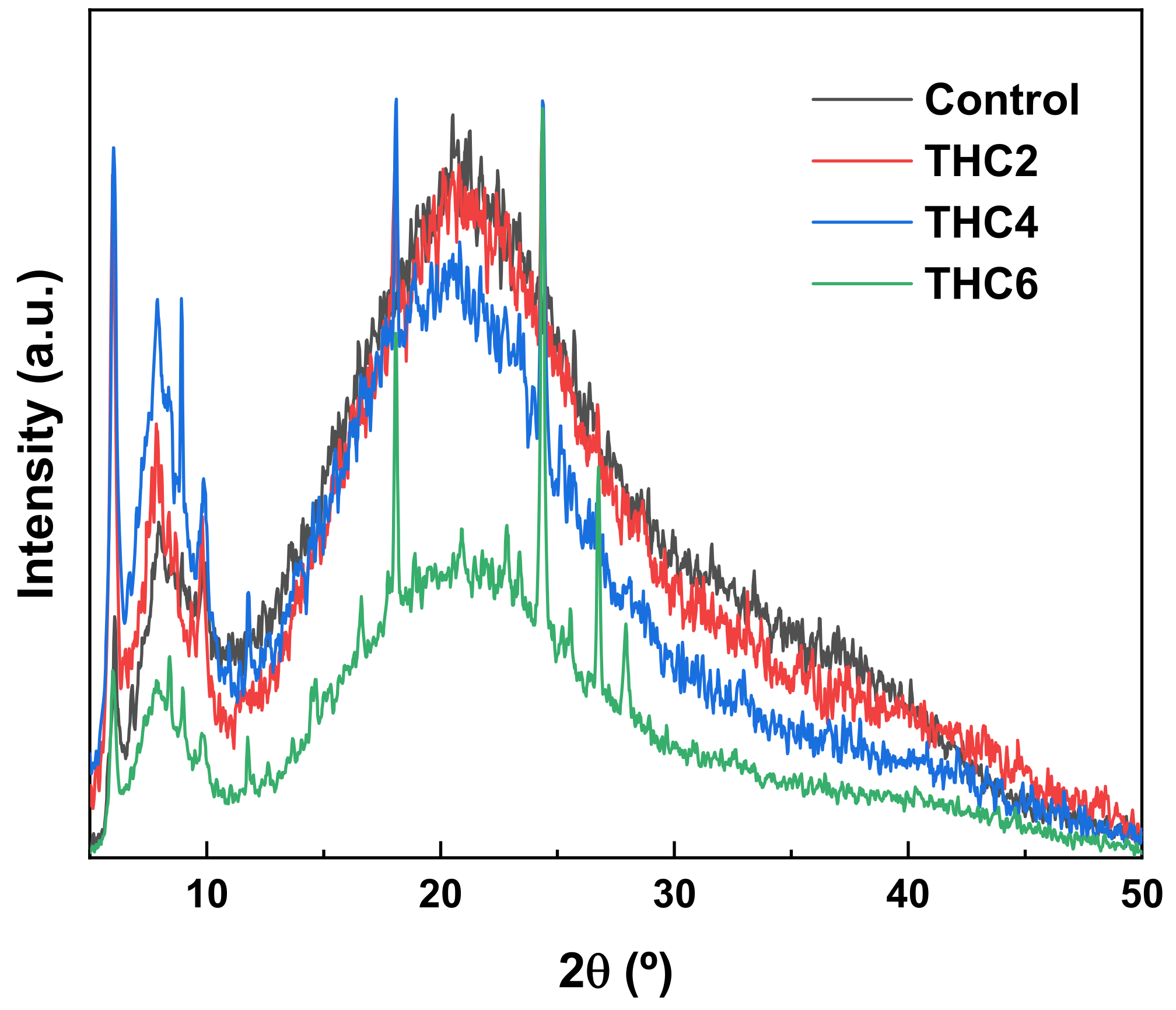
2.7. X-ray Diffraction (XRD)
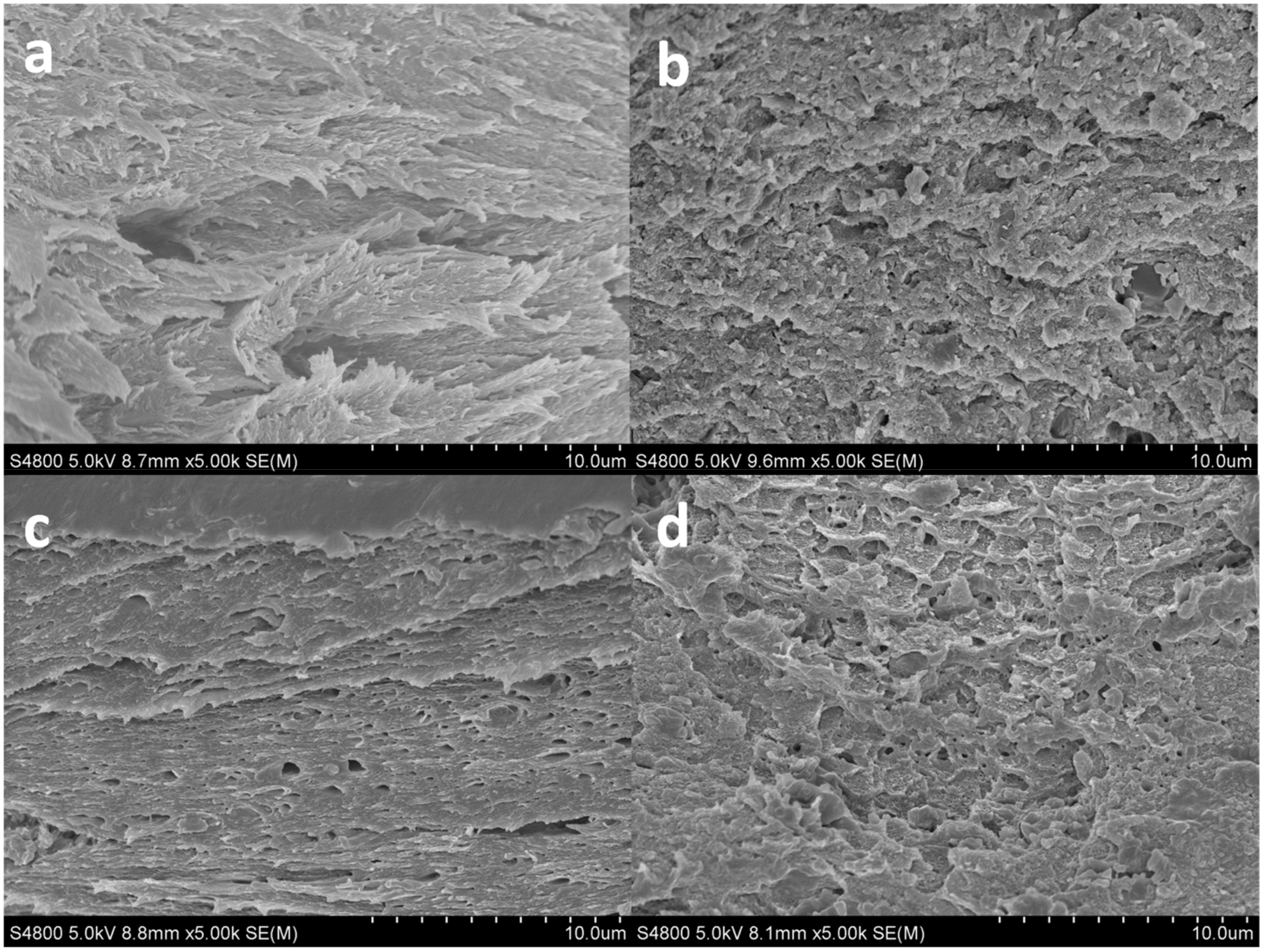
2.8. Scanning Electron Microscopy (SEM)
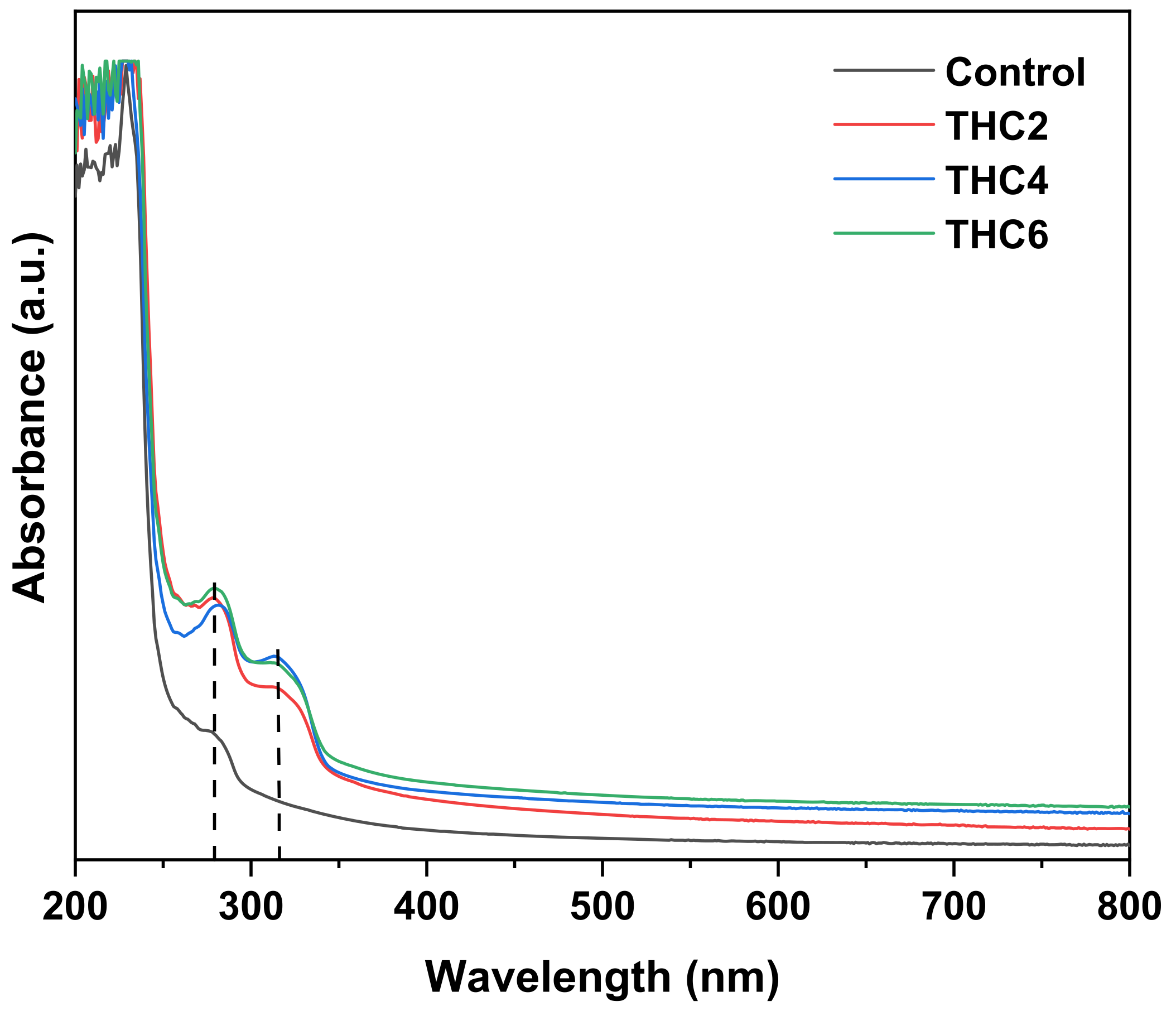
2.9. Ultraviolet-Visible (UV–Vis) Spectroscopy and THC Release
2.10. Water Uptake (WU)
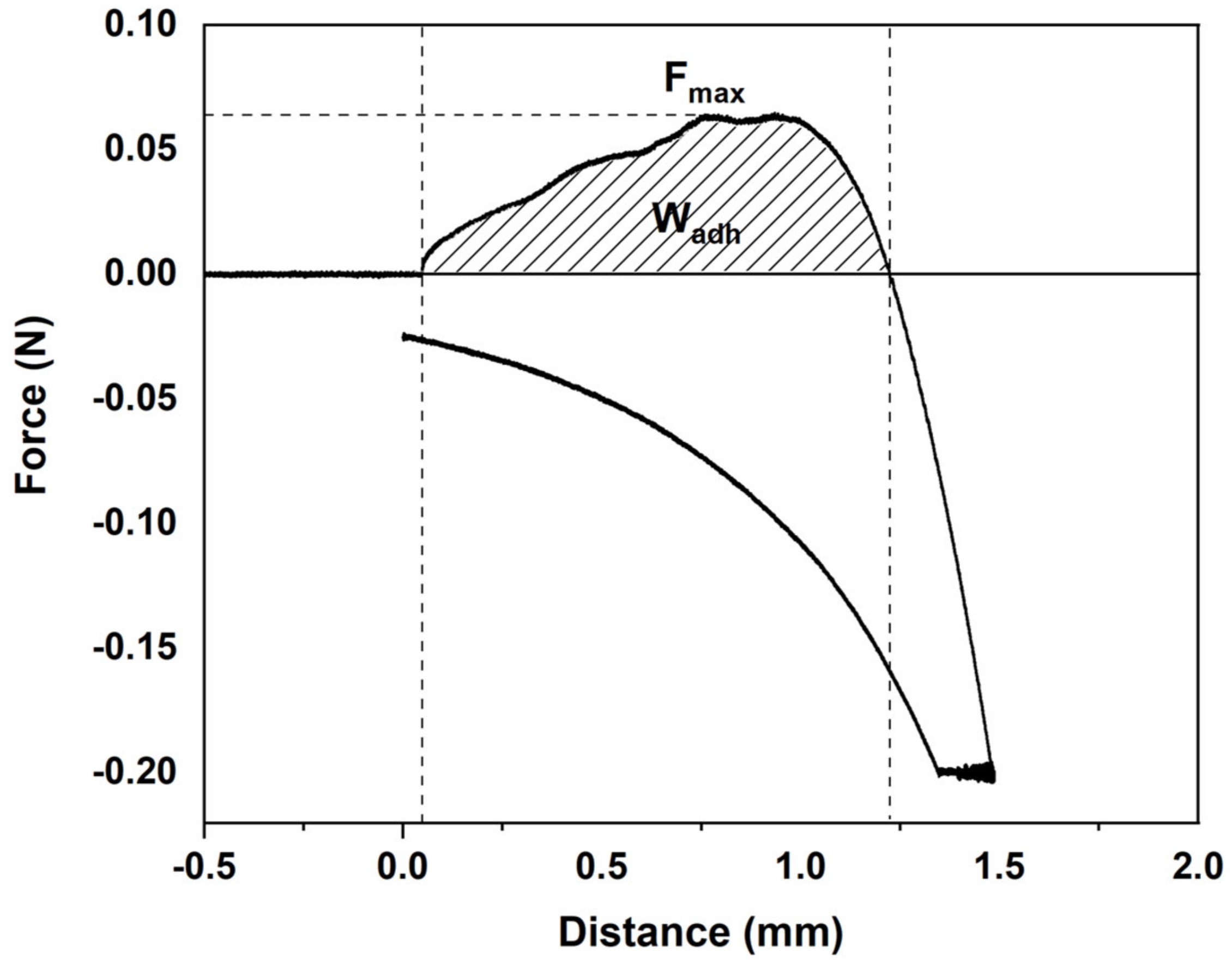
2.11. In Vitro Mucoadhesion Study
2.12. Statistical Analysis
3. Results and Discussion
3.1. Rheological Properties
3.2. Physicochemical and Thermal Properties
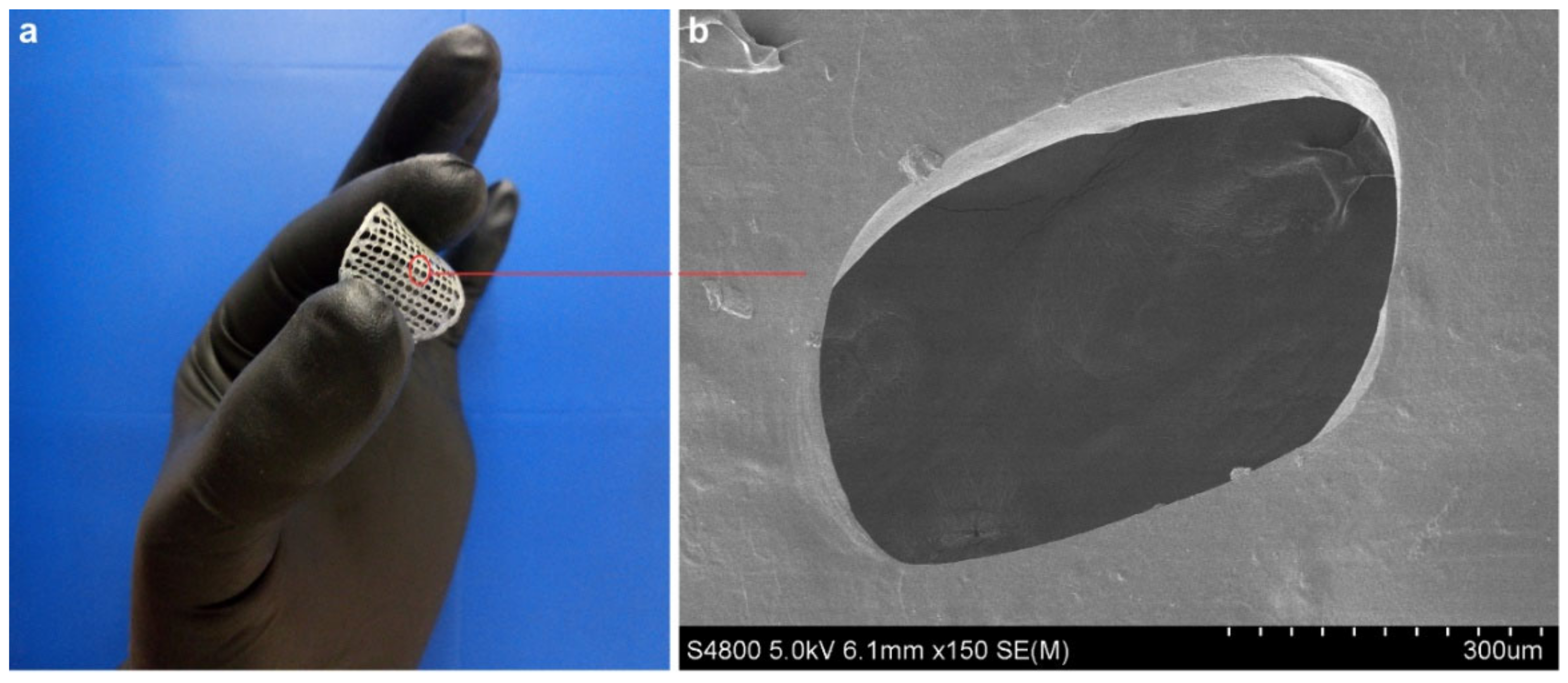
3.3. Morphological and Barrier Properties
3.4. Water Uptake (WU) and THC Release
3.5. Mucoadhesive Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 19. [Google Scholar] [CrossRef]
- Bao, G. Biofabrication in Tissue Engineering. In Racing for the Surface; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 289–312. [Google Scholar] [CrossRef]
- Saptarshi, S.M.; Zhou, D.C. Basics of 3D Printing: Engineering Aspect. In 3D Printing in Orthopaedic Surgery; Dipola, M., Wodajo, F.M., Eds.; Elsevier: Berlin/Heidelberg, Germany, 2019; pp. 17–30. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Moghaddam, K.M.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.P.K.; Burke, M.; Carter, B.M.; Davis, S.A.; Perriman, A.W. 3D Bioprinting using a template porous bioink. Adv. Healthc. Mater. 2016, 5, 1724–1730. [Google Scholar] [CrossRef] [Green Version]
- Chou, P.Y.; Chou, Y.C.; Lai, L.H.; Lin, Y.T.; Lu, C.J.; Liu, S.J. Fabrication of drug-eluting nano-hydroxylapatite filled polycaprolactone nanocomposites using solution-extrusion 3D printing technique. Polymers 2021, 13, 318. [Google Scholar] [CrossRef] [PubMed]
- Tavares, M.T.; Gaspar, V.M.; Monteiro, M.; Farinha, J.P.S.; Baleizão, C.; Mano, J.F. GelMA/bioactive silica nanocomposite bioinks for stem cell osteogenic differentiation. Biofabrication 2021, 13, 035012. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Bhatnagar, R.S.; Li, S.; Oreffo, R.O.C. Biomimetic collagen scaffolds for human bone cell growth and differentiation. Tissue Eng. 2004, 10, 1148–1159. [Google Scholar] [CrossRef]
- Hu, K.; Hu, M.; Xiao, Y.; Cui, Y.; Yan, J.; Yang, G.; Zhang, F.; Lin, G.; Yi, H.; Han, L.; et al. Preparation recombination human-like collagen/fibroin scaffold and promoting the cell compatibility with osteoblasts. J. Biomed. Mater. Res. Part A 2020, 108, 346–353. [Google Scholar] [CrossRef]
- Sorkio, A.E.; Vuorimaa-Laukkanen, E.P.; Hakola, H.M.; Liang, H.; Ujula, T.A.; Valle-Delgado, J.J.; Österberg, M.; Yliperttula, M.L.; Skottman, H. Biomimetic collagen I and IV double layer Langmuir-Schaefer films as microenvironment for human pluripotent stem cell derived retinal pigment epithelial cells. Biomaterials 2015, 51, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Du, T.; Ruan, C.; Niu, X. Bioinspired mineralized collagen scaffolds for bone tissue engineering. Bioact. Mater. 2021, 6, 1491–1511. [Google Scholar] [CrossRef]
- Ma, C.; Wang, H.; Chi, Y.; Wang, Y.; Jiang, L.; Xu, N.; Wu, Q.; Feng, Q.; Sun, X. Preparation of oriented collagen fiber scaffolds and its application in bone tissue engineering. Appl. Mater. Today 2021, 22, 100902. [Google Scholar] [CrossRef]
- Włodarczyk-Biegun, M.K.; del Campo, A. 3D bioprinting of structural proteins. Biomaterials 2017, 134, 180–201. [Google Scholar] [CrossRef]
- Lee, J.M.; Suen, S.K.Q.; Ng, W.L.; Ma, W.C.; Yeong, W.Y. Bioprinting of collagen: Considerations, potentials and application, Macromol. Biosci. 2021, 21, 2000280. [Google Scholar] [CrossRef]
- Rramaswamy, R.; Mani, G.; Venkatachalam, S.; Yasam, R.V.; Rajendran, J.C.B.; Tae, J.H. Preparation and characterization of tetrahydrocurcumin-loaded cellulose acetate phthalate/polyethylene glicol electrospun nanofibers. AAPS PharmSciTech 2018, 19, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Luo, D.D.; Xie, J.H.; Xian, Y.F.; Lai, Z.Q.; Liu, Y.H.; Liu, W.H.; Chen, J.N.; Lai, X.P.; Lin, Z.X.; et al. Curmumin´s metabolites, tetrahydrocurcumin and octahydrocurcumin, possess superior anti-inflammatory effect in vitro through suppression of TAK1-NF-κB Pathway. Front. Pharmacol. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef] [Green Version]
- Manjunatha, J.R.; Bettadaiah, B.K.; Negi, P.S.; Srinivas, P. Synthesis of quinolone derivates of tetrahydrocurcumin and zingerone and evaluation of their antioxidant and antibacterial attributes. Food Chem. 2013, 136, 650–658. [Google Scholar] [CrossRef]
- Murugan, P.; Pari, L. Effect of tetrahydrocurcumin on lipod peroxidation and lipids in streptozotocin-nicotinamide-induced diabetic rats. Basic Clin. Pharmacol. Toxicol. 2006, 99, 122–127. [Google Scholar] [CrossRef]
- Mahal, A.; Wu, P.; Jiang, Z.H.; Wei, X. Schiff bases of tetrahydrocurcumin as potential anticancer agents. ChemistrySelect 2019, 4, 366–369. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Kakkar, V.; Saini, K.; Saini, M.; Kumar, M.; Narula, P.; Duggal, I. Comparison of therapeutic efficacy of nanoformulations of curcumin vs tetrahydrocurcumin in various disorders. In Nanoformulations in Human Health; Talegaonkar, S., Rai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; Volume 17, pp. 377–401. [Google Scholar] [CrossRef]
- Kakkar, V.; Kaur, I.P.; Kaur, A.P.; Saini, K.; Singh, K.K. Topical delivery of tetrahydrocurcumin nanoparticles effectively inhibits skin inflammation: In vitro and in vivo study. Drug Dev. Ind. Pharm. 2018, 44, 1701–1712. [Google Scholar] [CrossRef]
- Rao, A.B.; Prasad, E.; Deepthi, S.S.; Haritha, V.; Ramakrishna, S.; Madhusudan, K.; Surekha, M.V.; Rao, Y.S.R.V. Wound healing: A new perspective on glucosylated tetrahydrocurcumin. Drug Des. Dev. Ther. 2015, 9, 3579–3588. [Google Scholar] [CrossRef] [Green Version]
- Osidak, E.O.; Kozhukhov, V.I.; Osidak, M.S.; Domogatsky, S.P. Collagen as bioink for bioprinting: A comprehensive review, Int. J. Bioprinting 2020, 6, 270. [Google Scholar] [CrossRef]
- Andonegi, M.; Irastorza, A.; Izeta, A.; de la Caba, K.; Guerrero, P. Physicochemical and biological performance of aloe vera-incorporated native collagen films. Pharmaceutics 2020, 12, 1273. [Google Scholar] [CrossRef]
- Williamson, R.V. The Flow of Pseudoplastic Materials. Ind. Eng. Chem. 1929, 21, 1108–1111. [Google Scholar] [CrossRef]
- Alvarez-Castillo, E.; Oliveira, S.; Bengoechea, C.; Sousa, I.; Raymundo, A.; Guerrero, A. A rheological approach to 3D printing of plasma protein based doughs. J. Food Eng. 2021, 288, 110255. [Google Scholar] [CrossRef]
- Chaabra, R.P.; Richardson, F.J. Rheometry for non-newtonian fluids. In Non-Newtonian Flow in the Process Industries; Chaabra, R.P., Richardson, F.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 37–72. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M. Modeling and comparison of dissolution profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Corker, A.; Ng, H.C.H.; Poole, R.J.; García-Tuñón, E. 3D printing with 2D colloids: Designing rheology protocols to predict “printability” of soft-materials. Soft Matter 2019, 15, 1444–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuidem, J.M.; Rivet, C.J.; Gilbert, R.J.; Morrison, F.A. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed. Mater. Res. 2013, 102, 1063–1073. [Google Scholar] [CrossRef]
- Machado, A.A.S.; Martins, V.C.A.; Plepis, A.M.G. Thermal and rheological behavior of collagen. Chitosan blends. J. Therm. Anal. 2002, 67, 491–498. [Google Scholar] [CrossRef]
- Yang, H.; Duan, L.; Li, Q.; Tian, Z.; Li, G. Experimental and modeling investigation on the rheological behavior of collagen solution as a function of acetic acid concentration. J. Mech. Behav. Biomed. Mater. 2018, 77, 125–134. [Google Scholar] [CrossRef]
- Lille, M.; Nurmela, A.; Nordlund, E.; Metsä-Kortelainen, S.; Sozer, N. Applicability of protein and fiber-rich food materials in extrusion-based 3d printing. J. Food Eng. 2018, 220, 20–27. [Google Scholar] [CrossRef]
- Wu, T.; Gray, E.; Chen, B. A self-healing, adaptive and conductive polymer composite ink for 3D printing of gas sensors. J. Mater. Chem. C 2018, 6, 6200–6207. [Google Scholar] [CrossRef] [Green Version]
- Peralta, J.M.; Meza, B.E.; Zorrilla, S.E. Analytical solutions for the free-draining flow of a Carreau-Yasuda fluid on a vertical plate. Chem. Eng. Sci. 2017, 168, 391–402. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, M.; Devahastin, S. 3D extrusion-based printability evaluation of selected cereal grains by computational fluid dynamic simulation. J. Food Eng. 2020, 286, 110113. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D. Effect of shortenings on the rheology of gluten-free doughs: Study of chestnut flour with chia flour, olive and sunflower oils. J. Texture Stud. 2012, 43, 375–383. [Google Scholar] [CrossRef]
- Torres, M.D.; Hallmark, B.; Wilson, D.I. Effect of concentration on shear and extensional rheology of guar gum solutions. Food Hydrocoll. 2014, 40, 85–95. [Google Scholar] [CrossRef]
- Yang, F.; Guo, C.; Zhang, M.; Bhandari, B.; Liu, Y. Improving 3D printing process of lemon juice gel based on fluid flow numerical simulation. LWT-Food Sci. Technol. 2019, 102, 89–99. [Google Scholar] [CrossRef] [Green Version]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Liao, W.; Guanghua, X.; Li, Y.; Shen, X.R.; Li, C. Comparison of characteristics and fibril-forming ability of skin collagen from barramundi (Lates calcarifer) and tilapia (Oreochromis niloticus). Int. J. Biol. Macromol. 2018, 107, 549–559. [Google Scholar] [CrossRef]
- de Campos Vidal, B.; Mello, M.L.S. Collagen type I amide I band infrared spectroscopy. Micron 2011, 42, 283–289. [Google Scholar] [CrossRef]
- Kaczmarek, B.; Sionkowska, A.; Skopinska-Wisniewska, J. Influence of glycosaminoglycans on the properties of thin films based on chitosan/collagen blends. J. Mech. Behav. Biomed. Mater. 2018, 80, 189–193. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670 K. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 80, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Schroepfer, M.; Meyer, M. DSC investigation of bovine hide collagen at varying degrees of crosslinking and humidities. Int. J. Biol. Macromol. 2017, 103, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Liu, F.; Yu, Z.; Chang, B.; Goff, H.D.; Zhong, F. Effect of aging treatment on the physicochemical properties of collagen films. Food Hydrocoll. 2019, 87, 436–447. [Google Scholar] [CrossRef]
- Valencia, G.A.; Luciano, C.G.; Lourenço, R.V.; Bittante, A.M.Q.B.; Sobral, P.J.A. Morphological and physical properties of nano-biocomposite films base on collagen loaded with laponite®. Food Packag. Shelf Life 2019, 19, 24–30. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, L.; Cai, P.; Li, P.; Zhang, M.; Sun, Z.; Sun, C.; Xu, W.; Wang, D. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. Int. J. Biol. Macromol. 2017, 105, 1602–1610. [Google Scholar] [CrossRef]
- Trivedi, M.K.; Gangwar, M.; Mondal, S.C.; Jana, S. Protective effects of tetrahydrocurcumin (THC) on fibroblast and melanoma cell lines in vitro: It´s implication for wound healing. J. Food Sci. Technol.-Mysore 2017, 54, 1137–1145. [Google Scholar] [CrossRef] [Green Version]
- Kezwoń, A.; Góral, I.; Wojciechowski, K. Effect of surfactants on surface active and rheological properties of type I collagen at air/water interface. Colloid Surf. B-Biointerfaces 2016, 148, 238–248. [Google Scholar] [CrossRef]
- Adriani, N.H.; Rahayu, D.U.C.; Saepudin, E. Activity of hydrogenated curcuminoid on Pd/C catalyst and its antibacterial activity against Staphylococcus aureus and Streptococcus mutans. IOP Conf. Ser. Mater. Sci. Eng. 2020, 902, 012068. [Google Scholar] [CrossRef]
- Castellan, A.; Ruggiero, R.; da Silva, L.G.; Portes, E.; Grelier, S.; Gardrat, C. Photophysics and photochemistry of tetrahydrocurcuminoids. J. Photochem. Photobiol. A-Chem. 2007, 190, 110–120. [Google Scholar] [CrossRef]
- Abubakr, N.; Lin, S.X.; Chin, X.D. Effects of drying methods on the release kinetics of vitamin B12 in calcium alginate beads. Dry. Technol. 2009, 27, 1258–1265. [Google Scholar] [CrossRef]
- Supramaniam, J.; Adnan, R.; Kaus, N.H.M.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef]
- Boddupalli, B.M.; Mohammed, Z.N.K.; Nath, R.A.; Banji, D. Mucoadhesive drug delivery system: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Thirawong, N.; Nunthanid, J.; Puttipipatkhachorn, S.; Sriamornsak, P. Mucoadhesive properties of various pectins on gastrointestinal mucosa: An in vitro evaluation using texture analyzer. Eur. J. Pharm. Biopharm. 2007, 67, 132–140. [Google Scholar] [CrossRef]
- Soe, M.T.; Chitropas, P.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. Thai glutinous rice starch modified by ball milling and its application as a mucoadhesive polymer. Carbohydr. Polym. 2020, 232, 115812. [Google Scholar] [CrossRef]
- Bassi da Silva, J.; Ferreira, S.B.D.S.; Reis, A.V.; Cook, M.T.; Bruschi, M.L. Assessing mucoadhesion in polymer gels: The effect of method type and instrument variables. Polymers 2018, 10, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
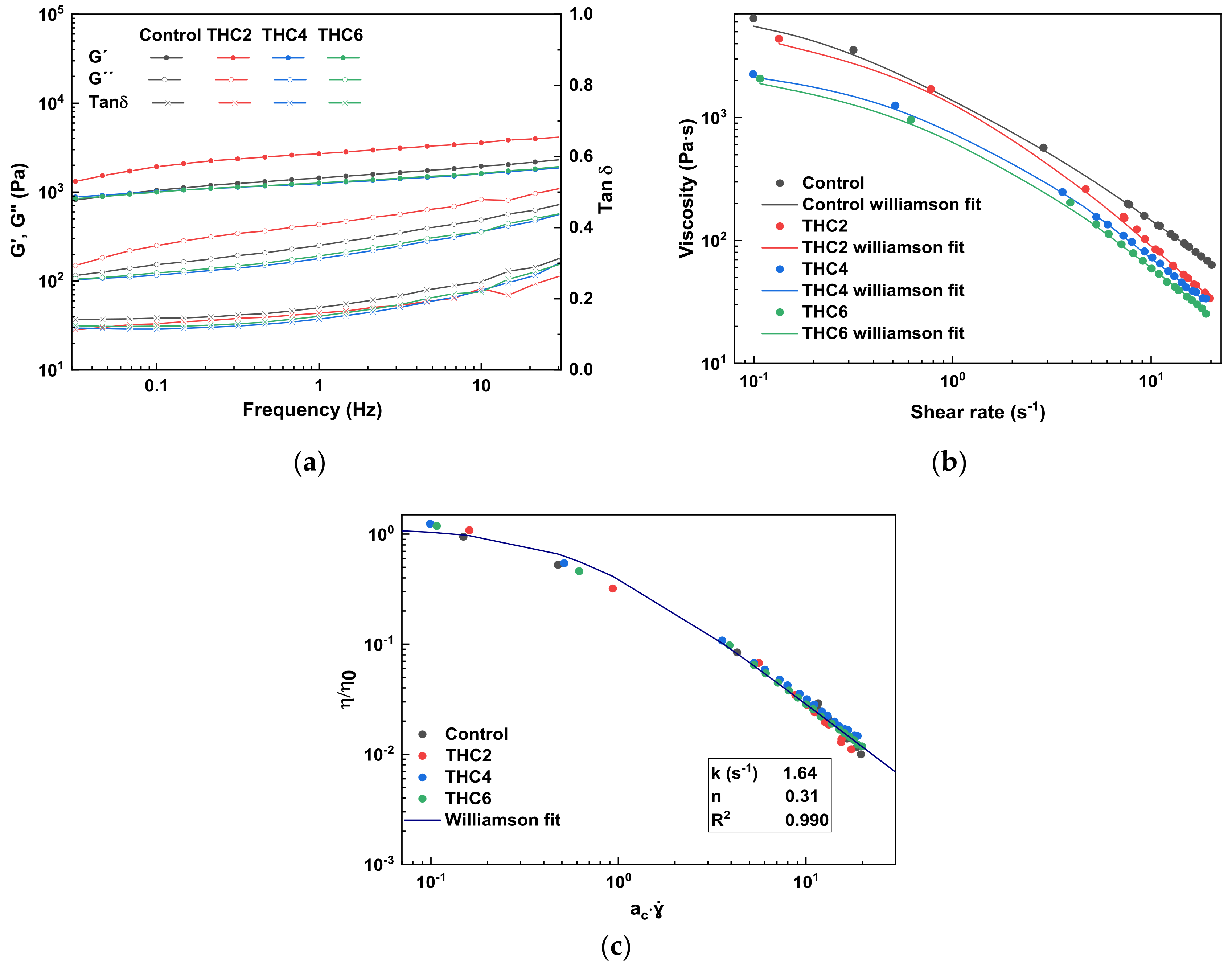


| Sample | η0 (Pa·s−1) | k (s−1) | n | R2 | ac | ɣ̇w |
|---|---|---|---|---|---|---|
| Control | 6764 | 2.71 | 0.15 | 0.996 | 1 | 69.05 |
| THC2 | 4402 | 1.51 | 0.42 | 0.989 | 0.80 | 38.43 |
| THC4 | 2298 | 1.37 | 0.31 | 0.996 | 0.67 | 44.47 |
| THC6 | 2083 | 1.57 | 0.28 | 0.997 | 0.67 | 46.94 |
| Sample | THC2 | THC4 | THC6 |
| k | 1.3227 | 1.4457 | 0.8651 |
| n | 1.0660 | 0.8947 | 0.9406 |
| R2 | 0.9913 | 0.9900 | 0.9944 |
| Film | Fmax (N) | Wadh (N·mm) |
|---|---|---|
| Control | 0.0652 ± 0.0035 a | 0.0492 ± 0.00304 a |
| THC2 | 0.0672 ± 0.0020 a | 0.0475 ± 0.00203 a |
| THC4 | 0.0681 ± 0.0020 a | 0.0494 ± 0.00303 a |
| THC6 | 0.0699 ± 0.0030 a | 0.0485 ± 0.00233 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andonegi, M.; Carranza, T.; Etxabide, A.; de la Caba, K.; Guerrero, P. 3D-Printed Mucoadhesive Collagen Scaffolds as a Local Tetrahydrocurcumin Delivery System. Pharmaceutics 2021, 13, 1697. https://doi.org/10.3390/pharmaceutics13101697
Andonegi M, Carranza T, Etxabide A, de la Caba K, Guerrero P. 3D-Printed Mucoadhesive Collagen Scaffolds as a Local Tetrahydrocurcumin Delivery System. Pharmaceutics. 2021; 13(10):1697. https://doi.org/10.3390/pharmaceutics13101697
Chicago/Turabian StyleAndonegi, Mireia, Teresa Carranza, Alaitz Etxabide, Koro de la Caba, and Pedro Guerrero. 2021. "3D-Printed Mucoadhesive Collagen Scaffolds as a Local Tetrahydrocurcumin Delivery System" Pharmaceutics 13, no. 10: 1697. https://doi.org/10.3390/pharmaceutics13101697
APA StyleAndonegi, M., Carranza, T., Etxabide, A., de la Caba, K., & Guerrero, P. (2021). 3D-Printed Mucoadhesive Collagen Scaffolds as a Local Tetrahydrocurcumin Delivery System. Pharmaceutics, 13(10), 1697. https://doi.org/10.3390/pharmaceutics13101697








