Cytokinin-Based Tissue Cultures for Stable Medicinal Plant Production: Regeneration and Phytochemical Profiling of Salvia bulleyana Shoots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Optimization of Shoot Regeneration
2.3. In Vitro Propagation
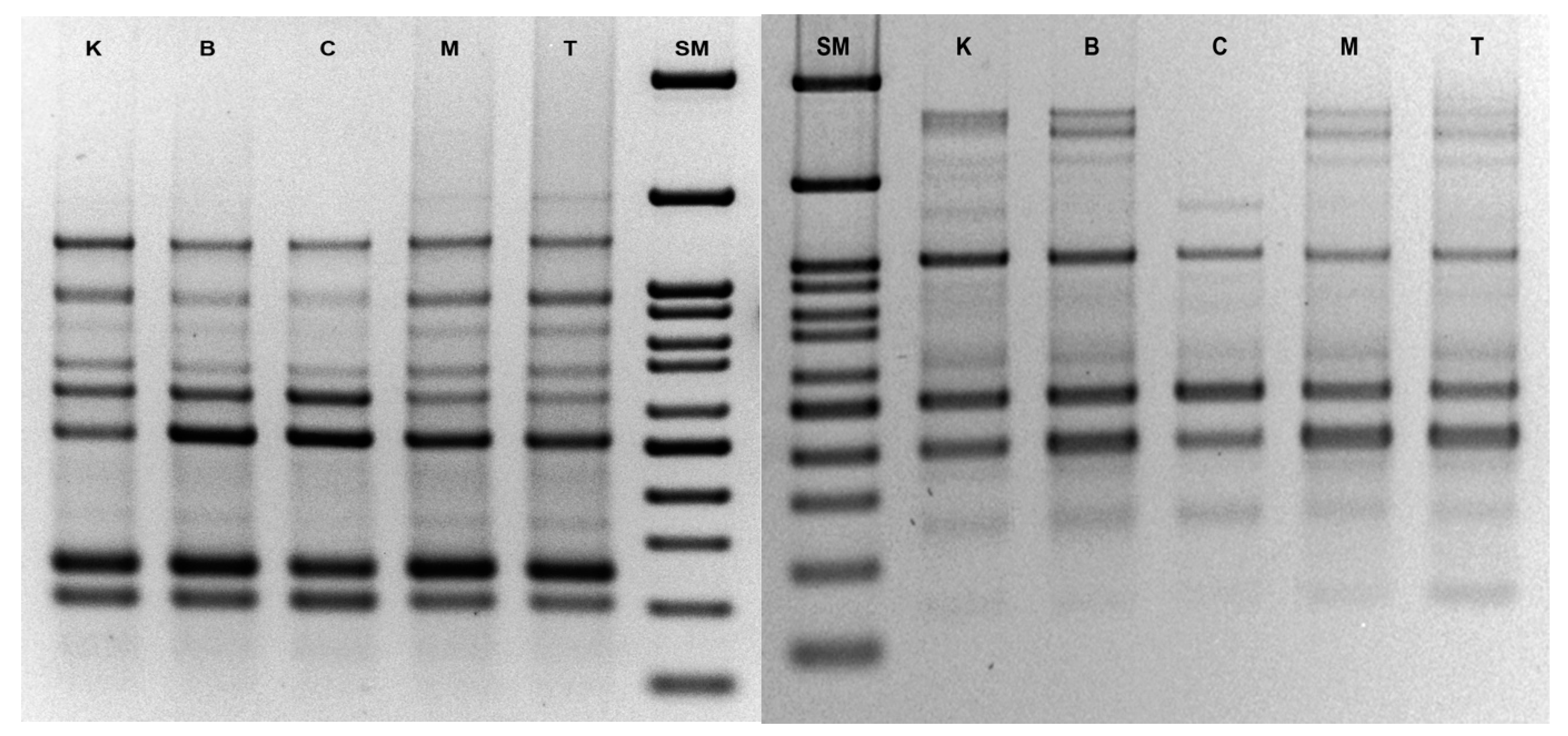
2.4. Genetic Analysis
2.4.1. DNA Extraction
2.4.2. ISSR-PCR
2.4.3. Molecular Data Analysis Regeneration and Proliferation
2.5. Identification and Accumulation of Polyphenolic Compound
2.6. Statistical Analysis
3. Results and Discussion
3.1. Shoot Organogenesis and Callus Induction
3.2. Morphological Characterization of Obtained Shoot Lines and Their Proliferation and Growth Potential
3.3. Analysis of Genetic Stability by ISSR
3.4. Polyphenol Profiling and Their Accumulation in Different Culture Lines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Green (cell) factories for advanced production of plant secondary metabolites. Crit. Rev. Biotechnol. 2020, 40, 443–458. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [Green Version]
- Hnatuszko-Konka, K.; Gerszberg, A.; Weremczuk-Jeżyna, I.; Grzegorczyk-Karolak, I. Cytokinin Signaling and de novo Shoot Organogenesis. Genes 2021, 12, 265. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Mattson, N.S.; Jeong, B.R. Efficiency of shoot regeneration from leaf, stem, petiole and petal explants of six cultivars of Chrysanthemum morifolium. Plant Cell Tissue Organ Cult. (PCTOC) 2011, 107, 295–304. [Google Scholar] [CrossRef]
- Ganeshan, S.; Caswell, K.L.; Kartha, K.K.; Chibbar, R.N. Shoot Regeneration and Proliferation In Transgenic Plants and Crops; Marcel Dekker, Inc.: New York, NY, USA, 2002; pp. 69–84. [Google Scholar]
- Li, M.H.; Chen, J.M.; Peng, Y.; Xiao, P.G. Distribution of phenolic acids in Chinese Salvia plants. World Sci. Technol. 2008, 10, 46–52. [Google Scholar] [CrossRef]
- Xu, J.; Wei, K.; Zhang, G.; Lei, L.; Yang, D.; Wang, W.; Han, Q.; Xia, Y.; Bi, Y.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical Profile and Antioxidant Activity of Aerial and Underground Parts of Salvia bulleyana Diels. Plants. Metabolites 2020, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of hairy root cultures of Salvia bulleyana Diels for production of polyphenolic compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 1987, 19, 11–15. [Google Scholar]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar] [CrossRef]
- Santos-Gomes, P.C.; Fernandes-Ferreira, M. Essential Oils Produced by in Vitro Shoots of Sage (Salvia officinalis L.). J. Agric. Food Chem. 2003, 51, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, S.; Amo-Marco, J.B. In vitro propagation of two spanish endemic species of Salvia throught bud proliferation. In Vitro Cell. Dev. Biol.-Plant 2000, 36, 225–229. [Google Scholar] [CrossRef]
- Skała, E.; Wysokińska, H.A.L.I.N.A. In vitro regeneration of Salvia nemorosa L. from shoot tips and leaf explants. In Vitro Cell. Dev. Biol.-Plant 2004, 40, 596–602. [Google Scholar] [CrossRef]
- Tsai, K.L.; Chen, E.G.; Chen, J.T. Thidiazuron-induced efficient propagation of Salvia miltiorrhiza through in vitro organo-genesis and medicinal constituents of regenerated plants. Acta Physiol. Plant. 2016, 38, 29. [Google Scholar] [CrossRef]
- Mithila, J.; Hall, J.; Victor, J.; Saxena, P. Thidiazuron induces shoot organogenesis at low concentrations and somatic embryogenesis at high concentrations on leaf and petiole explants of African violet (Saintpaulia ionantha Wendl.). Plant Cell Rep. 2003, 21, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.T.P.; Ozaki, Y.; Okubo, H. Callus induction and plantlet regeneration in ornamental Alocasia micholitziana. Plant Cell Tissue Organ Cult. (PCTOC) 2003, 73, 285–289. [Google Scholar] [CrossRef]
- Shen, X.; Kane, M.E.; Chen, J. Effects of genotype, explant source, and plant growth regulators on indirect shoot organogene-sis in Dieffenbachia cultivars. In Vitro Cell. Dev. Biol.-Plant 2008, 44, 282–288. [Google Scholar] [CrossRef]
- Fracaro, F.; Echeverrigaray, S. Micropropagation of Cunila galioides, a popular medicinal plant of south Brazil. Plant Cell Tissue Organ Cult. 2001, 64, 1–4. [Google Scholar] [CrossRef]
- Ashraf, M.F.; Aziz, M.A.; Kemat, N.; Ismail, I. Effect of cytokinin types, concentrations and their interactions on in vitro shoot regeneration of Chlorophytum borivilianum Sant. & Fernandez. Electron. J. Biotechnol. 2014, 17, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.-L.; Fan, Z.-B.; Liu, Z.-Q.; Qiu, X.-H.; Jiang, Y.-H. Comparison of Phytochemical and Antioxidant Activities in Micropropagated and Seed-derived Salvia miltiorrhiza Plants. HortScience 2018, 53, 1038–1044. [Google Scholar] [CrossRef] [Green Version]
- Shriram, V.; Kumar, V.; Shitole, M.G. Indirect organogenesis and plant regeneration in Helicteres isora L., an important medicinal plant. In Vitro Cell. Dev. Biol.-Plant 2008, 44, 186–193. [Google Scholar] [CrossRef]
- Ma, G.; Da Silva, J.A.T.; Lu, J.; Zhang, X.; Zhao, J. Shoot organogenesis and plant regeneration in Metabriggsia ovalifolia. Plant Cell Tissue Organ Cult. 2010, 105, 355–361. [Google Scholar] [CrossRef]
- Saha, S.; Adhikari, S.; Dey, T.; Ghosh, P. RAPD and ISSR based evaluation of genetic stability of micropropagated plantlets of Morus alba L. variety S-1. Meta Gene 2016, 7, 7–15. [Google Scholar] [CrossRef]
- Parveen, S.; Shahzad, A. Thiadiazuron induced high frequency shoot regeneration in Cassia sophera L. via cotyledonary node explants. Physiol. Mol. Biol. Plants 2010, 16, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Lata, H.; Chandra, S.; Wang, Y.-H.; Raman, V.; Khan, I.A. TDZ-Induced High Frequency Plant Regeneration through Direct Shoot Organogenesis in Stevia rebaudiana Bertoni: An Important Medicinal Plant and a Natural Sweetener. Am. J. Plant Sci. 2013, 4, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Wei, Y.; Zhai, Y.; Ouyang, K.; Chen, X.; Bai, L. High frequency regeneration of plants via callus-mediated organ-ogenesis from cotyledon and hypocotyl cultures in a multipurpose tropical tree (Neolamarkia cadamba). Sci. Rep. 2020, 10, 4558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, M.J.; Krishnaraj, S.; Saxena, P.K. Morphological and Physiological Changes during Thidiazuron-Induced Somatic Embryogenesis in Geranium (Pelargonium x hortorum Bailey) Hypocotyl Cultures. Int. J. Plant Sci. 1996, 157, 440–446. [Google Scholar] [CrossRef]
- Capelle, S.C.; Mok, D.W.S.; Kirchner, S.C.; Mok, M.C. Effects of thidiazuron on cytokinin autonomy and the metabolism of N6-(Δ2-Isopentyl) [8-14C] adenosine in callus tissues of Phaseolus lunatus L. J. Plant Physiol. 1983, 73, 796–802. [Google Scholar] [CrossRef] [Green Version]
- Makunga, N.P.; Van Staden, J. An efficient system for the production of clonal plantlets of the medicinally important aro-matic plant: Salvia africana-lutea L. Plant Cell Tiss. Organ Cult. 2008, 92, 63–72. [Google Scholar] [CrossRef]
- Petrova, M.; Nikolova, M.; Dimitrova, L.; Zayova, E. Micropropagation and evaluation of flavonoid content and antioxidant activity of Salvia officinalis L. Genet. Plant Physiol. 2015, 5, 48–60. [Google Scholar]
- Guo, W.L.; Gong, L.; Ding, Z.F.; Li, Y.D.; Li, F.X.; Zhao, S.P.; Liu, B. Genomic instability in phenotypically normal regenerants of medicinal plant Codonopsis lanceolata Benth. et Hook. f., as revealed by ISSR and RAPD markers. Plant Cell Rep. 2006, 25, 896–906. [Google Scholar] [CrossRef]
- Hu, J.; Gao, X.; Liu, J.; Xie, C.; Li, J. Plant regeneration from petiole callus of Amorphophallus albus and analysis of somaclonal variation of regenerated plants by RAPD and ISSR markers. Bot. Stud. 2008, 49, 189–197. [Google Scholar] [CrossRef]
- Powell, W.; Machray, G.C.; Provan, J. Polymorphism revealed by simple sequence repeats. Trends Plant Sci. 1996, 1, 215–222. [Google Scholar] [CrossRef]
- Song, Z.; Li, X.; Wang, H.; Wang, J. Genetic diversity and population structure of Salvia miltiorrhiza Bge in China revealed by ISSR and SRAP. Genetica 2010, 138, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Erbano, M.; Santos, E.P.D. Genetic variability and population structure of Salvia lachnostachys: Implications for breeding and conservation programs. Int. J. Mol. Sci. 2015, 16, 7839–7850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmanan, V.; Venkataramareddy, S.R.; Neelwarne, B. Molecular analysis of genetic stability in long-term micropropagated shoots of banana using RAPD and ISSR markers. Electron. J. Biotechnol. 2007, 10, 106–113. [Google Scholar] [CrossRef]
- Rani, V.; Raina, S.N. Genetic fidelity of organized meristem-derived micropropagated plants: A critical reappraisal. In Vitro Cell. Dev. Biol.-Plant 2000, 36, 319–330. [Google Scholar] [CrossRef]
- Erişen, S.; Kurt-Gür, G.; Servi, H. In vitro propagation of Salvia sclarea L. by meta-Topolin, and assessment of genetic stability and secondary metabolite profiling of micropropagated plants. Ind. Crop. Prod. 2020, 157, 112892. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic acid—From bench to valuable applications in food industry. Trends Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kuźma, Ł.; Wysokińska, H. The use of long-term Scutellaria altissima callus cultures for shoot regeneration, production of bioactive metabolites and micropropagation. J. Med. Plants Res. 2013, 7, 3303–3313. [Google Scholar] [CrossRef]


| Primer Code | Primer Sequence 5′ → 3′ | Annealing Temperature | Number of Band for Line (Cytokinin in the Medium of Origin) | ||||
|---|---|---|---|---|---|---|---|
| Control | BAP | mT | TDZ | CPPU | |||
| UBC835 | AGAGAGAGAGAGAGAGYC * | 50 °C | 10 | 11 | 11 | 11 | 9 |
| UBC841 | GAGAGAGAGAGAGAGAYC * | 50 °C | 13 | 13 | 12 | 12 | 9 |
| ISSR-X1 | AGAGAGAGAGAGAGAG | 45 °C | 6 | 6 | 6 | 6 | 6 |
| UBC2 | GAGAGAGAGAGAGAGAT | 45 °C | 8 | 8 | 8 | 8 | 6 |
| UBC814 | CTCTCTCTCTCTCTCTA | 45 °C | 10 | 10 | 11 | 11 | 10 |
| UBC812 | GAGAGAGAGAGAGAGAA | 45 °C | 7 | 7 | 7 | 7 | 7 |
| ISSR-X2 | CTCCTCCTCCTCRC * | 45 °C | 5 | 5 | 5 | 5 | 4 |
| UBC1 | ACACACACACACACACT | 45 °C | 11 | 11 | 11 | 11 | 10 |
| UBC862 | AGCAGCAGCAGCAGCAGC | 55 °C | 2 | 2 | 2 | 2 | 2 |
| UBC808 | AGAGAGAGAGAGAGAGC | 48 °C | 16 | 16 | 16 | 16 | 14 |
| UBC809 | AGAGAGAGAGAGAGAGG | 48 °C | 9 | 9 | 10 | 10 | 8 |
| UBC840 | GAGAGAGAGAGAGAGAYT * | 48 °C | 9 | 9 | 9 | 9 | 9 |
| UBC 864 | ATGATGATGATGATGATG | 45 °C | 7 | 10 | 10 | 10 | 8 |
| UBC 818 | CACACACACACACACAG | 48 °C | 8 | 8 | 8 | 8 | 8 |
| UBC 834 | AGAGAGAGAGAGAGAGYT * | 48 °C | 11 | 12 | 12 | 11 | 10 |
| Growth Regulators [mg/L] | Callus Formation Frequency [%] | Callus Morphology | Shoot Regeneration Frequency [%] | Mean Number of Buds/Shoots Per Explant | Bud/Shoot Ratio | |
|---|---|---|---|---|---|---|
| NAA | Cytokinin | |||||
| 0.1 | BAP 1 | 4.5 | green, hard, + | 77.3 | 5.3 ± 0.7 a (D) | 78:22 |
| 2 | 5.4 | green, hard, + | 94.6 | 5.2 ± 0.9 a (D) | 80:20 | |
| 4 | 25 | greenish, hard, + | 66.7 | 2.7 ± 0.3 c (D) | 86:14 | |
| 0.5 | 1 | 68.8 | green or greenish-white, hard, + | 40.6 | 2.4 ± 0.4 cd (D) | 87:13 |
| 2 | 64.3 | bright green, hard, +/++ | 21.4 | 3.3 ± 2.4 abc (D) | 75:25 | |
| 4 | 60 | green, +/++ | 26.7 | 3.0 ± 1.0 bcd (D) | 100:0 | |
| 0.1 | mT 1 | 32.1 | greenish, hard, + | 71.4 | 2.7 ± 0.4 c (D) | 74:26 |
| 2 | 28 | greenish, hard, + | 76.0 | 3.4 ± 0.4 ab (D) | 77:23 | |
| 4 | 48.6 | green, hard, lumpy, + | 68.4 | 2.4 ± 0.5 cd (D) | 79:18 | |
| 0.5 | 1 | 64.3 | green, hard, + | 17.9 | 1.8 ± 0.2 d (D) | 89:11 |
| 2 | 75 | green, hard, + | 42.9 | 3.3 ± 0.4 b (D) | 78:22 | |
| 4 | 75 | green, hard, +/++ | 38.9 | 3.6 ± 1.7 abcd (D) | 86:14 | |
| 0.1 | TDZ 0.2 | 100 | green, hard, ++/+++ | 27.8 | 3.3 ± 0.7 abc (I) | 82:18 |
| 0.5 | 100 | greenish-white, hard, ++/+++ | 19.4 | 2.4 ± 0.7 cd (I) | 94:6 | |
| 1 | 100 | green, hard, ++ | 13.9 | 5.2 ± 1.4 a (I) | 96:4 | |
| 0.5 | 0.2 | 100 | green, hard, ++/+++ | 11.1 | 4.5 ± 0.5 ab (I) | 100:0 |
| 0.5 | 100 | greenish-white, hard, +/++ | 8.3 | 2.6 ± 1.5 bcd (I) | 95:5 | |
| 1 | 88.9 | green, hard, +/++ | 8.3 | 2.7 ± 0.3 c (I) | 10:0 | |
| 0.1 | CPPU 1 | 86.3 | green, ++ | 13.3 | 1.8 ± 0.3 d (I) | 100:0 |
| 2 | 100 | green, soft, +++ | 16.7 | 1.6 ± 0.2 d (I) | 88:12 | |
| 4 | 100 | green, hard, ++ | 20.7 | 2.8 ± 0.5 c (I) | 82:18 | |
| 0.5 | 1 | 92.9 | green, hard, ++ | 25.0 | 2.4 ± 0.3 cd (I) | 100:0 |
| 2 | 100 | green, hard, ++ | 23.1 | 2.7 ± 0.5 c (I) | 100:0 | |
| 4 | 100 | green, hard, ++ | 18.5 | 2.2 ± 0.4 cd (I) | 100:0 | |
| Growth Parameter | Cytokinin in the Medium of Origin | ||||
|---|---|---|---|---|---|
| BAP | mT | TDZ | CPPU | Control | |
| Shoot forming buds/shoots [%] | 84 | 83 | 80 | 81 | 85 |
| Main shoot length [cm] | 1.78 ± 0.11 ab | 2.19 ± 0.11 a | 1.74 ± 0.13 ab | 1.70 ± 0.09 b | 1.82 ± 0.12 ab |
| Multiplication ratio | 2.76 ± 0.28 a | 3.00 ± 0.25 a | 2.8 ± 0.34 a | 2.7 ± 0.25 a | 2.68 ± 0.29 a |
| Buds/shoots ratio | 76:24 | 67:33 | 68:32 | 86:14 | 64:36 |
| Adventitious shoot length [cm] | 0.88 ± 0.10 ab | 1.02 ± 0.11 a | 0.76 ± 0.08 b | 0.75 ± 0.10 b | 0.85 ± 0.08 ab |
| Fresh weight [g] | 1.04 ± 0.07 ab | 1.19 ± 0.07 a | 1.09 ± 0.06 ab | 0.96 ± 0.07 b | 1.07 ± 0.07 ab |
| Dry weight [g] | 0.11 ± 0.009 a | 0.12 ± 0.005 a | 0.11 ± 0.006 a | 0.10 ± 0.008 a | 0.11 ± 0.007 a |
| Compound | [M–H] | Cytokinin in the Medium of Origin | ||||
|---|---|---|---|---|---|---|
| BAP | mT | TDZ | CPPU | Control | ||
| Caffeoyl-threonic acid I | 297 | 56.3 ± 6.5 ab | 47.9 ± 0.5 b | 59.4 ± 0.2 a | 34.5 ± 0.3 c | tr. |
| Caffeoyl-threonic acid II | 297 | 228.8 ± 25.3 a | 214.5 ± 5.0 a | 202.7 ± 8.7 a | 209.3 ± 0.7 a | 26.6 ± 0.3 b |
| Caffeic acid | 179 | 341.1 ± 37.3 b | 174.4 ± 10.9 d | 432.9 ± 10.9 a | 173.0 ± 1.7 d | 211.4 ± 2.2 c |
| Caffeoyl-threonic acid III | 297 | 122.6 ± 11.1 a | 89.2 ± 1.4 b | 98.3 ± 2.9 ab | 81.1 ± 0.5 c | 47.4 ± 0.3 d |
| Rosmarinic acid hexoside | 521 | 67.0 ± 7.1 a | 49.5 ± 7.9 ab | 40.6 ± 2.6 b | 34.6 ± 0.3 b | 20.7 ± 0.4 d |
| Rosmarinic acid | 359 | 8991.3 ± 7776 b | 9142.1 ± 225.5 b | 8057.5 ± 281.8 b | 15,437.3 ± 87.6 a | 8829.9 ± 52.1 b |
| Salvianolic acid K | 555 | 163.8 ± 12.2 d | 272 ± 66.5 c | 160.2 ± 10.3 d | 956.7 ± 16.3 a | 434.6 ± 6.7 b |
| Methyl rosmarinate | 373 | 92.8 ± 10.8 c | 180.4 ± 17.1 b | 115.8 ± 2.0 c | 412.4 ± 2.8 a | 191.4 ± 0.3 b |
| Salvianolic acid F isomer I | 313 | 451.5 ± 38.9 b | 136.8 ± 7.3 d | 567.6 ± 21.4 a | 269.2 ± 5.5 c | 241.4 ± 2.9 c |
| Salvianolic acid F isomer II | 313 | 364.8 ± 32.7 a | 105.7 ± 3.6 c | 348.0 ± 18.1 a | 318.6 ± 1.1 a | 191.0 ± 4.5 b |
| Total | - | 10,880.2 ± 959.4 b | 10,412.9 ± 293.3 b | 10,083.0 ± 350.9 b | 17,926.7 ± 105.1 a | 10,194.4 ± 43.9 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzegorczyk-Karolak, I.; Hnatuszko-Konka, K.; Krzemińska, M.; Olszewska, M.A.; Owczarek, A. Cytokinin-Based Tissue Cultures for Stable Medicinal Plant Production: Regeneration and Phytochemical Profiling of Salvia bulleyana Shoots. Biomolecules 2021, 11, 1513. https://doi.org/10.3390/biom11101513
Grzegorczyk-Karolak I, Hnatuszko-Konka K, Krzemińska M, Olszewska MA, Owczarek A. Cytokinin-Based Tissue Cultures for Stable Medicinal Plant Production: Regeneration and Phytochemical Profiling of Salvia bulleyana Shoots. Biomolecules. 2021; 11(10):1513. https://doi.org/10.3390/biom11101513
Chicago/Turabian StyleGrzegorczyk-Karolak, Izabela, Katarzyna Hnatuszko-Konka, Marta Krzemińska, Monika A. Olszewska, and Aleksandra Owczarek. 2021. "Cytokinin-Based Tissue Cultures for Stable Medicinal Plant Production: Regeneration and Phytochemical Profiling of Salvia bulleyana Shoots" Biomolecules 11, no. 10: 1513. https://doi.org/10.3390/biom11101513






