Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model
Abstract
1. Introduction
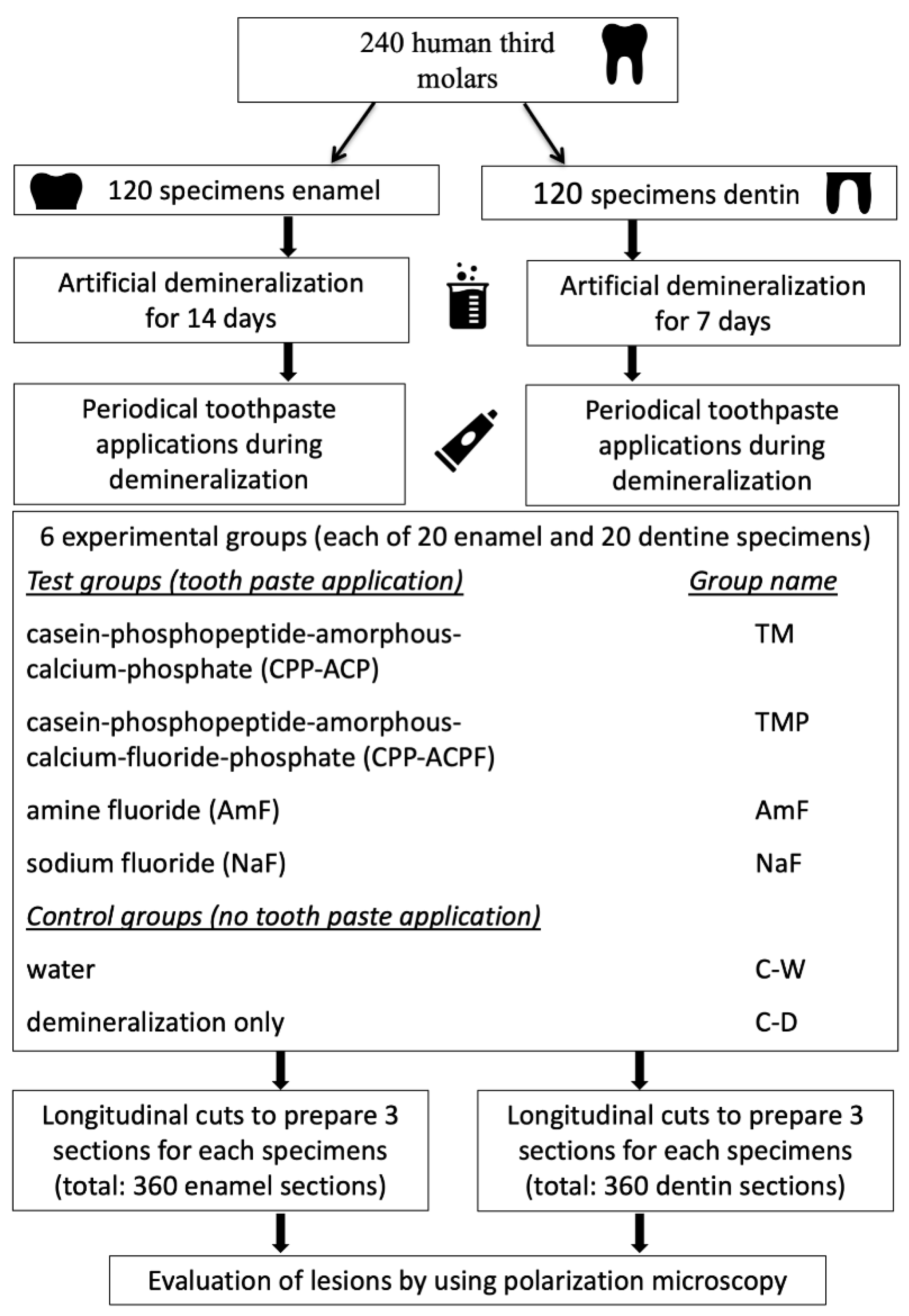
2. Materials and Methods
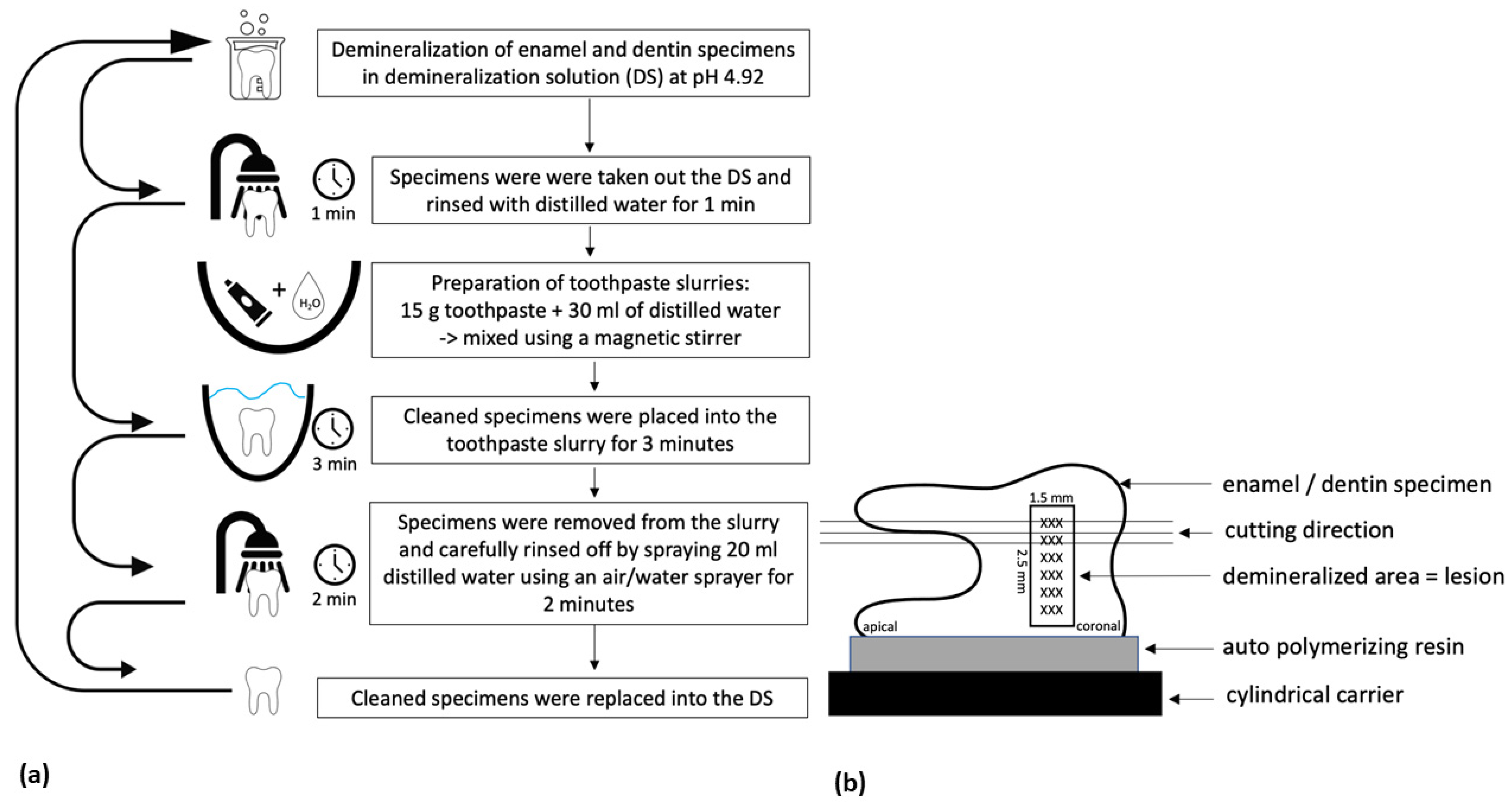
2.1. Experimental Demineralization
2.2. Experimental Groups
2.3. Application of Test Agents
2.4. Section Preparation
2.5. Polarized Light Microscopy
2.6. Statistical Analysis
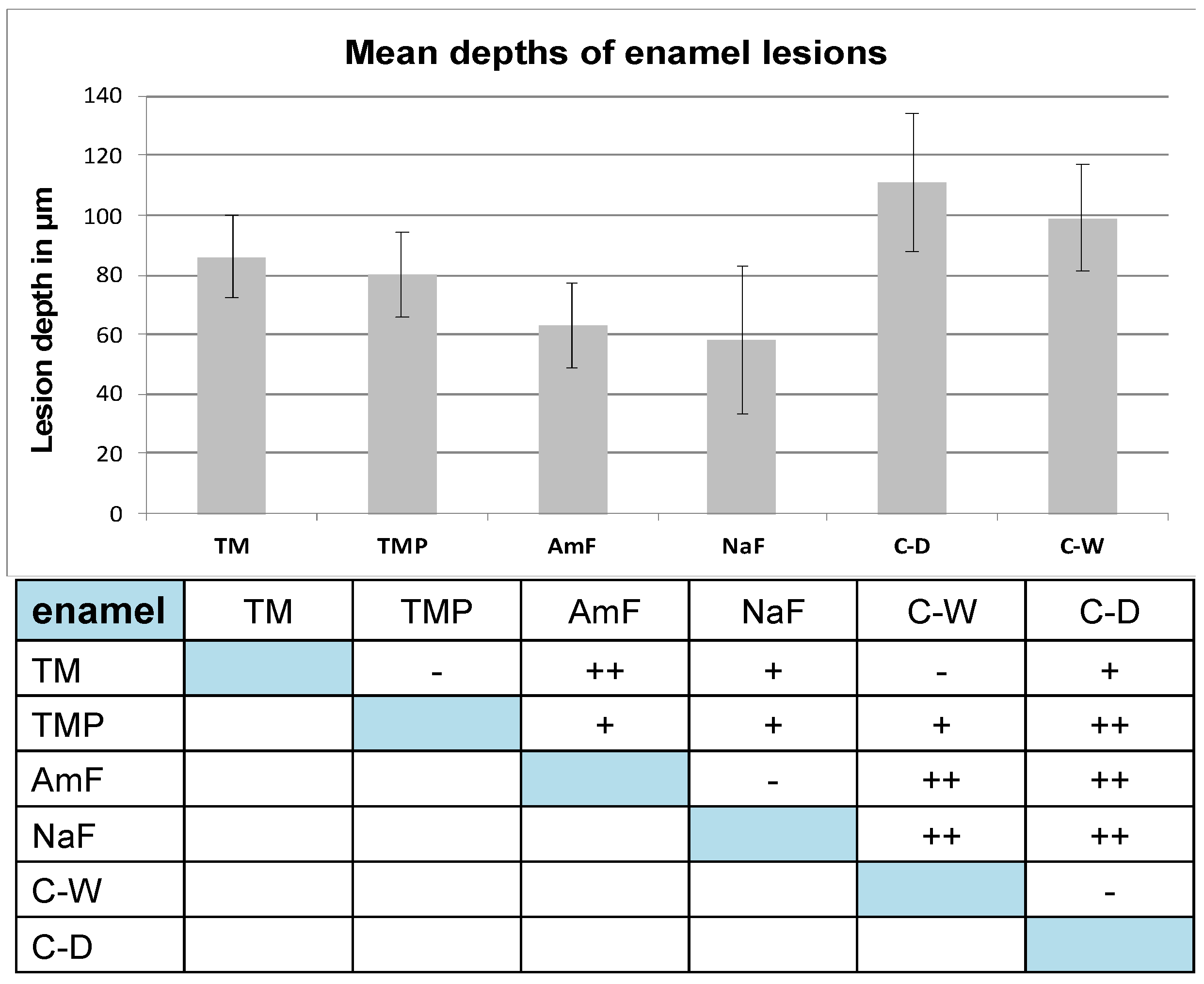
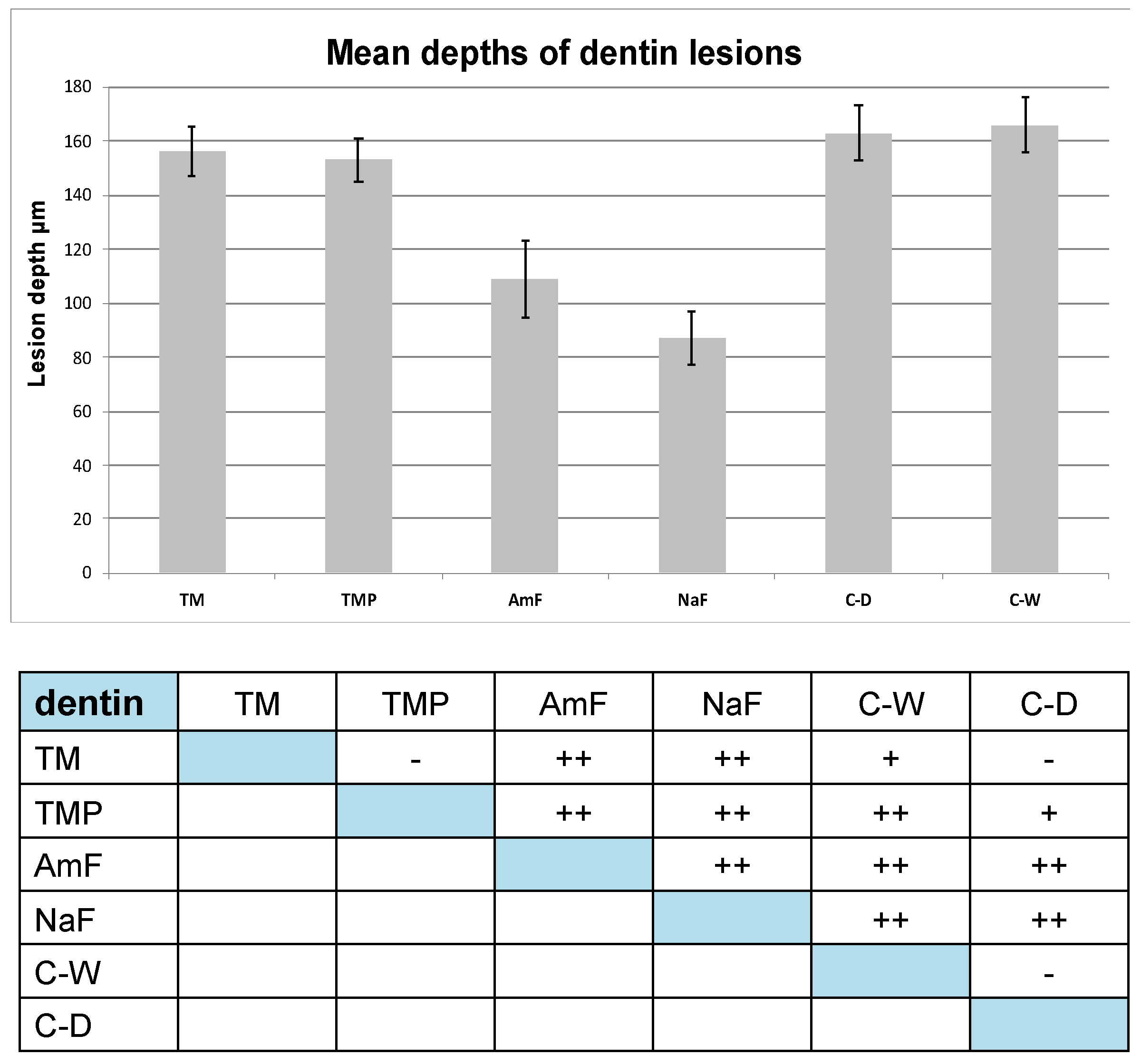
3. Results
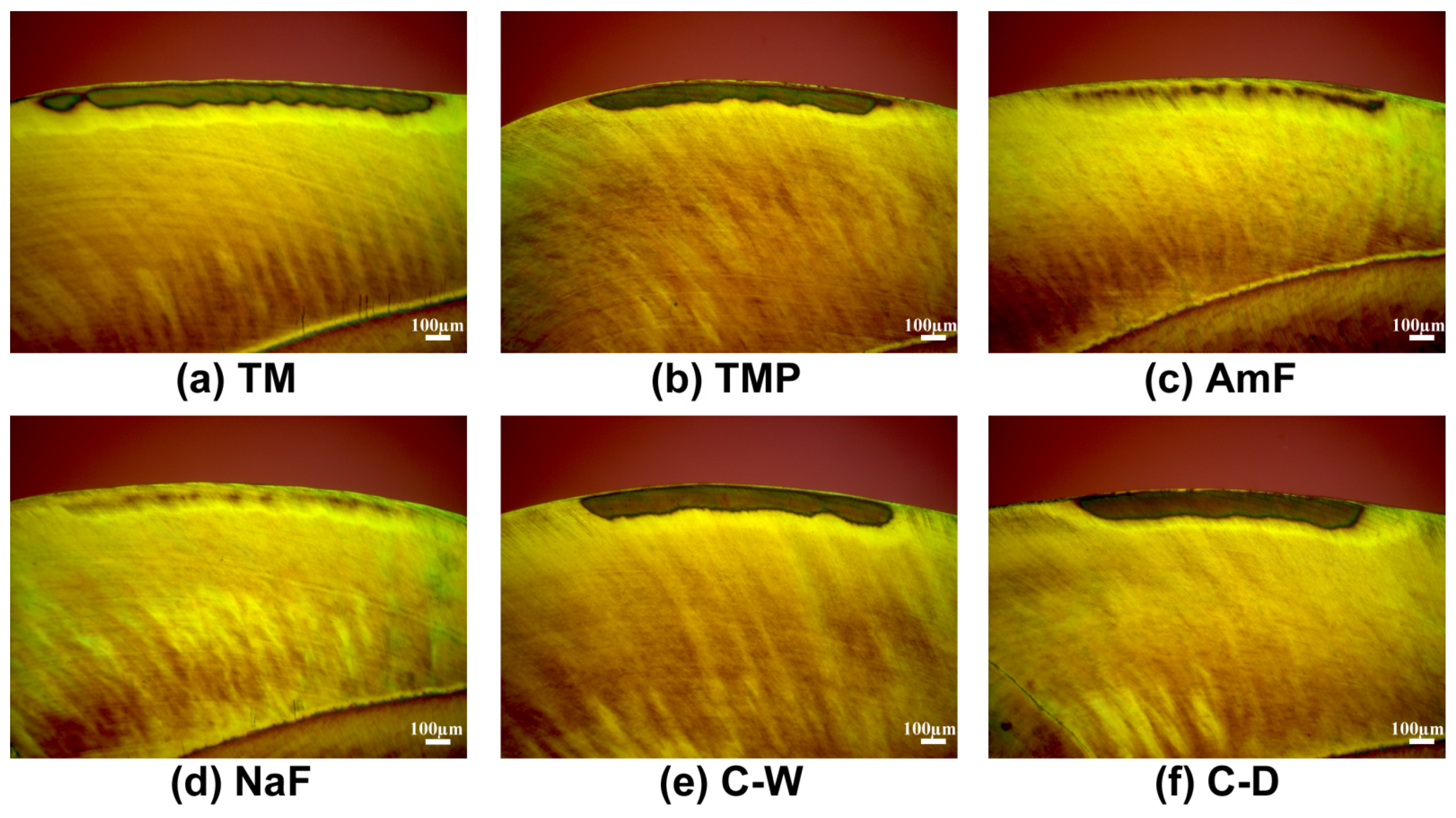
3.1. Enamel Lesions
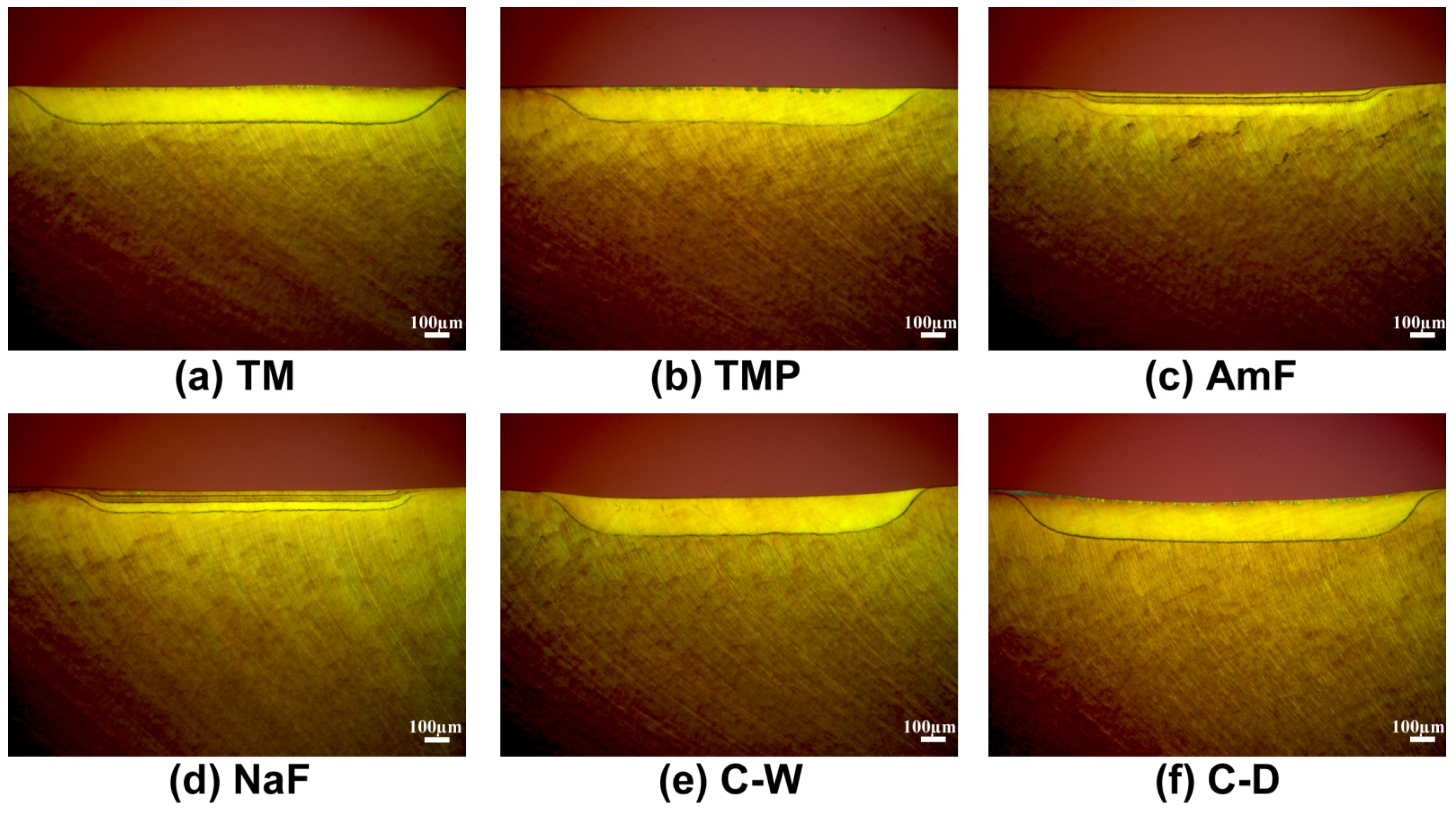
3.2. Dentin Lesions
3.3. Morphological Evaluation of the Lesions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beyer, M.; Reichert, J.; Bossert, J.; Sigusch, B.W.; Watts, D.C.; Jandt, K.D. Acids with an equivalent taste lead to different erosion of human dental enamel. Dent. Mater. 2011, 27, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Fouquet, V.; Attal, J.P.; Dursun, E. Commercially Available Fluoride-Releasing Restorative Materials: A Review and a Proposal for Classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Lussi, A. Erosive tooth wear-a multifactorial condition of growing concern and increasing knowledge. Monogr. Oral Sci. 2006, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Reichert, J.; Sigusch, B.W.; Watts, D.C.; Jandt, K.D. Morphology and structure of polymer layers protecting dental enamel against erosion. Dent. Mater. 2012, 28, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Reise, M.; Gottschaldt, M.; Matz, C.; Völpel, A.; Jandt, K.D.; Schubert, U.S.; Sigusch, B.W. Antibacterial effect of silver (I) carbohydrate complexes on oral pathogenic key species in vitro. BMC Oral Health 2016, 16, 42. [Google Scholar] [CrossRef]
- Yu, O.Y.; Mei, M.L.; Zhao, I.S.; Lo, E.C.; Chu, C.H. Effects of Fluoride on Two Chemical Models of Enamel Demineralization. Materials 2017, 10, 1245. [Google Scholar] [CrossRef]
- Göstemeyer, G.; Schulze, F.; Paris, S.; Schwendicke, F. Arrest of Root Carious Lesions via Sodium Fluoride, Chlorhexidine and Silver Diamine Fluoride In Vitro. Materials 2017, 11, 9. [Google Scholar] [CrossRef]
- Ekambaram, M.; Mohd Said, S.N.B.; Yiu, C.K.Y. A Review of Enamel Remineralisation Potential of Calcium- and Phosphate-based Remineralisation Systems. Oral Health Prev. Dent. 2017, 15, 415–420. [Google Scholar] [CrossRef]
- Schweigert, B.W.S.; Shaw, J.H. Dental caries in the cotton rat; the effect of the amount of protein, fat and carbohydrate in the diet on the incidence and extent of carious lesions. J. Nutr. 1946, 31, 439–447. [Google Scholar] [CrossRef]
- Reynolds, E.C. Calcium phosphate-based remineralization systems: Scientific evidence? Aust. Dent. J. 2008, 53, 268–273. [Google Scholar] [CrossRef]
- Reynolds, E.C. Casein phosphopeptide-amorphous calcium phosphate: The scientific evidence. Adv. Dent. Res. 2009, 21, 25–29. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Cain, C.J.; Webber, F.L.; Black, C.L.; Riley, P.F.; Johnson, I.H.; Perich, J.W. Anticariogenicity of calcium phosphate complexes of tryptic casein phosphopeptides in the rat. J. Dent. Res. 1995, 74, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Ceci, M.; Mirando, M.; Beltrami, R.; Chiesa, M.; Poggio, C. Protective effect of casein phosphopeptide-amorphous calcium phosphate on enamel erosion: Atomic force microscopy studies. Scanning 2015, 37, 327–334. [Google Scholar] [CrossRef]
- Palaniswamy, U.K.; Prashar, N.; Kaushik, M.; Lakkam, S.R.; Arya, S.; Pebbeti, S. A comparative evaluation of remineralizing ability of bioactive glass and amorphous calcium phosphate casein phosphopeptide on early enamel lesion. Dent. Res. J. 2016, 13, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Raphael, S.; Blinkhorn, A. Is there a place for Tooth Mousse in the prevention and treatment of early dental caries? A systematic review. BMC Oral Health 2015, 15, 113. [Google Scholar] [CrossRef]
- Reynolds, E.C. Anticariogenic complexes of amorphous calcium phosphate stabilized by casein phosphopeptides: A review. Spec. Care Dent. 1998, 18, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ametani, A.; Kaminogawa, S.; Shimizu, M.; Yamauchi, K. Rapid screening of antigenically reactive fragments of alpha s1-casein using HPLC and ELISA. J. Biochem. 1987, 102, 421–425. [Google Scholar] [CrossRef]
- Neuhaus, K.W.; Lussi, A. Casein phosphopeptide--amorphous calcium phosphate (CPP-ACP) and its effect on dental hard tissues. Schweiz Mon. Zahnmed 2009, 119, 110–116. [Google Scholar]
- Cross, K.J.; Huq, N.L.; Reynolds, E.C. Casein phosphopeptides in oral health--chemistry and clinical applications. Curr. Pharm. Des. 2007, 13, 793–800. [Google Scholar] [CrossRef]
- Cross, K.J.; Huq, N.L.; Stanton, D.P.; Sum, M.; Reynolds, E.C. NMR studies of a novel calcium, phosphate and fluoride delivery vehicle-alpha(S1)-casein(59-79) by stabilized amorphous calcium fluoride phosphate nanocomplexes. Biomaterials 2004, 25, 5061–5069. [Google Scholar] [CrossRef]
- Willershausen, B.; Schulz-Dobrick, B.; Gleissner, C. In vitro evaluation of enamel remineralisation by a casein phosphopeptide-amorphous calcium phosphate paste. Oral Health Prev. Dent. 2009, 7, 13–21. [Google Scholar]
- Puleio, F.; Fiorillo, L.; Gorassini, F.; Iandolo, A.; Meto, A.; D’Amico, C.; Cervino, G.; Pinizzotto, M.; Bruno, G.; Portelli, M.; et al. Systematic Review on White Spot Lesions Treatments. Eur. J. Dent. 2021. [Google Scholar] [CrossRef]
- Soares, G.G.; Magalhães, P.A.; Fonseca, A.B.M.; Tostes, M.A.; Silva, E.M.D.; Coutinho, T.C.L. Preventive Effect of CPP-ACPF Paste and Fluoride Toothpastes Against Erosion and Erosion Plus Abrasion In Vitro-A 3D Profilometric Analysis. Oral Health Prev. Dent. 2017, 15, 269–277. [Google Scholar] [CrossRef]
- Oshiro, M.; Yamaguchi, K.; Takamizawa, T.; Inage, H.; Watanabe, T.; Irokawa, A.; Ando, S.; Miyazaki, M. Effect of CPP-ACP paste on tooth mineralization: An FE-SEM study. J. Oral Sci. 2007, 49, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Rees, J.; Loyn, T.; Chadwick, B. Pronamel and tooth mousse: An initial assessment of erosion prevention in vitro. J. Dent. 2007, 35, 355–357. [Google Scholar] [CrossRef]
- Tantbirojn, D.; Huang, A.; Ericson, M.D.; Poolthong, S. Change in surface hardness of enamel by a cola drink and a CPP-ACP paste. J. Dent. 2008, 36, 74–79. [Google Scholar] [CrossRef]
- Lennon, A.M.; Pfeffer, M.; Buchalla, W.; Becker, K.; Lennon, S.; Attin, T. Effect of a casein/calcium phosphate-containing tooth cream and fluoride on enamel erosion in vitro. Caries Res. 2006, 40, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, B.; Reise, M.; Klukowska, M.; Grender, J.M.; Timm, H.; Sigusch, B.W. A randomized clinical trial comparing plaque removal efficacy of an oscillating-rotating power toothbrush to a manual toothbrush by multiple examiners. Int. J. Dent. Hyg. 2016, 14, 278–283. [Google Scholar] [CrossRef]
- Bächli, K.; Schmidlin, P.R.; Wegehaupt, F.; Paqué, F.; Ramenzoni, L.; Botter, S. Remineralization of Artificial Dentin Caries Using Dentin and Enamel Matrix Proteins. Materials 2019, 12, 2116. [Google Scholar] [CrossRef] [PubMed]
- Imataki, R.; Shinonaga, Y.; Nishimura, T.; Abe, Y.; Arita, K. Mechanical and Functional Properties of a Novel Apatite-Ionomer Cement for Prevention and Remineralization of Dental Caries. Materials 2019, 12, 3998. [Google Scholar] [CrossRef] [PubMed]
- Nongonierma, A.B.; Fitzgerald, R.J. Biofunctional properties of caseinophosphopeptides in the oral cavity. Caries Res. 2012, 46, 234–267. [Google Scholar] [CrossRef]
- Borges, B.C.; de Souza Borges, J.; de Araujo, L.S.; Machado, C.T.; Dos Santos, A.J.; de Assunçao Pinheiro, I.V. Update on nonsurgical, ultraconservative approaches to treat effectively non-cavitated caries lesions in permanent teeth. Eur. J. Dent. 2011, 5, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Caruana, P.C.; Mulaify, S.A.; Moazzez, R.; Bartlett, D. The effect of casein and calcium containing paste on plaque pH following a subsequent carbohydrate challenge. J. Dent. 2009, 37, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Arango-Santander, S.; Montoya, C.; Pelaez-Vargas, A.; Ossa, E.A. Chemical, structural and mechanical characterization of bovine enamel. Arch. Oral Biol. 2020, 109, 104573. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Okawa, S.; Miyakawa, O. Surface texture and composition of titanium brushed with toothpaste slurries of different pHs. Dent. Mater. 2007, 23, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Grenby, T.H.; Andrews, A.T.; Mistry, M.; Williams, R.J. Dental caries-protective agents in milk and milk products: Investigations in vitro. J. Dent. 2001, 29, 83–92. [Google Scholar] [CrossRef]
- Weyant, R.J.; Tracy, S.L.; Anselmo, T.T.; Beltrán-Aguilar, E.D.; Donly, K.J.; Frese, W.A.; Hujoel, P.P.; Iafolla, T.; Kohn, W.; Kumar, J.; et al. Topical fluoride for caries prevention: Executive summary of the updated clinical recommendations and supporting systematic review. J. Am. Dent. Assoc. 2013, 144, 1279–1291. [Google Scholar] [CrossRef]
- Bakry, A.S.; Abbassy, M.A.; Alharkan, H.F.; Basuhail, S.; Al-Ghamdi, K.; Hill, R. A Novel Fluoride Containing Bioactive Glass Paste is Capable of Re-Mineralizing Early Caries Lesions. Materials 2018, 11, 1636. [Google Scholar] [CrossRef]
- Kensche, A.; Pötschke, S.; Hannig, C.; Richter, G.; Hoth-Hannig, W.; Hannig, M. Influence of Calcium Phosphate and Apatite Containing Products on Enamel Erosion. Sci. World J. 2016, 2016, 7959273. [Google Scholar] [CrossRef]
- Hannig, M.; Balz, M. Protective properties of salivary pellicles from two different intraoral sites on enamel erosion. Caries Res. 2001, 35, 142–148. [Google Scholar] [CrossRef]
- Miyahira, K.; Coutinho, T.; Silva, E.; Pereira, A.; Tostes, M. Evaluation of CPP-ACP and Fluoride on Inhibition of Human Enamel demineralisation: Cross-sectional Hardness and MicroCT Studies. Oral Health Prev. Dent. 2017, 15, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Rehder Neto, F.C.; Maeda, F.A.; Turssi, C.P.; Serra, M.C. Potential agents to control enamel caries-like lesions. J. Dent. 2009, 37, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, E.C.; Cai, F.; Cochrane, N.J.; Shen, P.; Walker, G.D.; Morgan, M.V.; Reynolds, C. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J. Dent. Res. 2008, 87, 344–348. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Cai, F.; Shen, P.; Walker, G.D. Retention in plaque and remineralization of enamel lesions by various forms of calcium in a mouthrinse or sugar-free chewing gum. J. Dent. Res. 2003, 82, 206–211. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reise, M.; Kranz, S.; Heyder, M.; Jandt, K.D.; Sigusch, B.W. Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model. Materials 2021, 14, 5974. https://doi.org/10.3390/ma14205974
Reise M, Kranz S, Heyder M, Jandt KD, Sigusch BW. Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model. Materials. 2021; 14(20):5974. https://doi.org/10.3390/ma14205974
Chicago/Turabian StyleReise, Markus, Stefan Kranz, Markus Heyder, Klaus D. Jandt, and Bernd W. Sigusch. 2021. "Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model" Materials 14, no. 20: 5974. https://doi.org/10.3390/ma14205974
APA StyleReise, M., Kranz, S., Heyder, M., Jandt, K. D., & Sigusch, B. W. (2021). Effectiveness of Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP) Compared to Fluoride Products in an In-Vitro Demineralization Model. Materials, 14(20), 5974. https://doi.org/10.3390/ma14205974







