Insight into Seasonal Change of Phytochemicals, Antioxidant, and Anti-Aging Activities of Root Bark of Paeonia suffruticosa (Cortex Moutan) Combined with Multivariate Statistical Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Total Phenolic Content
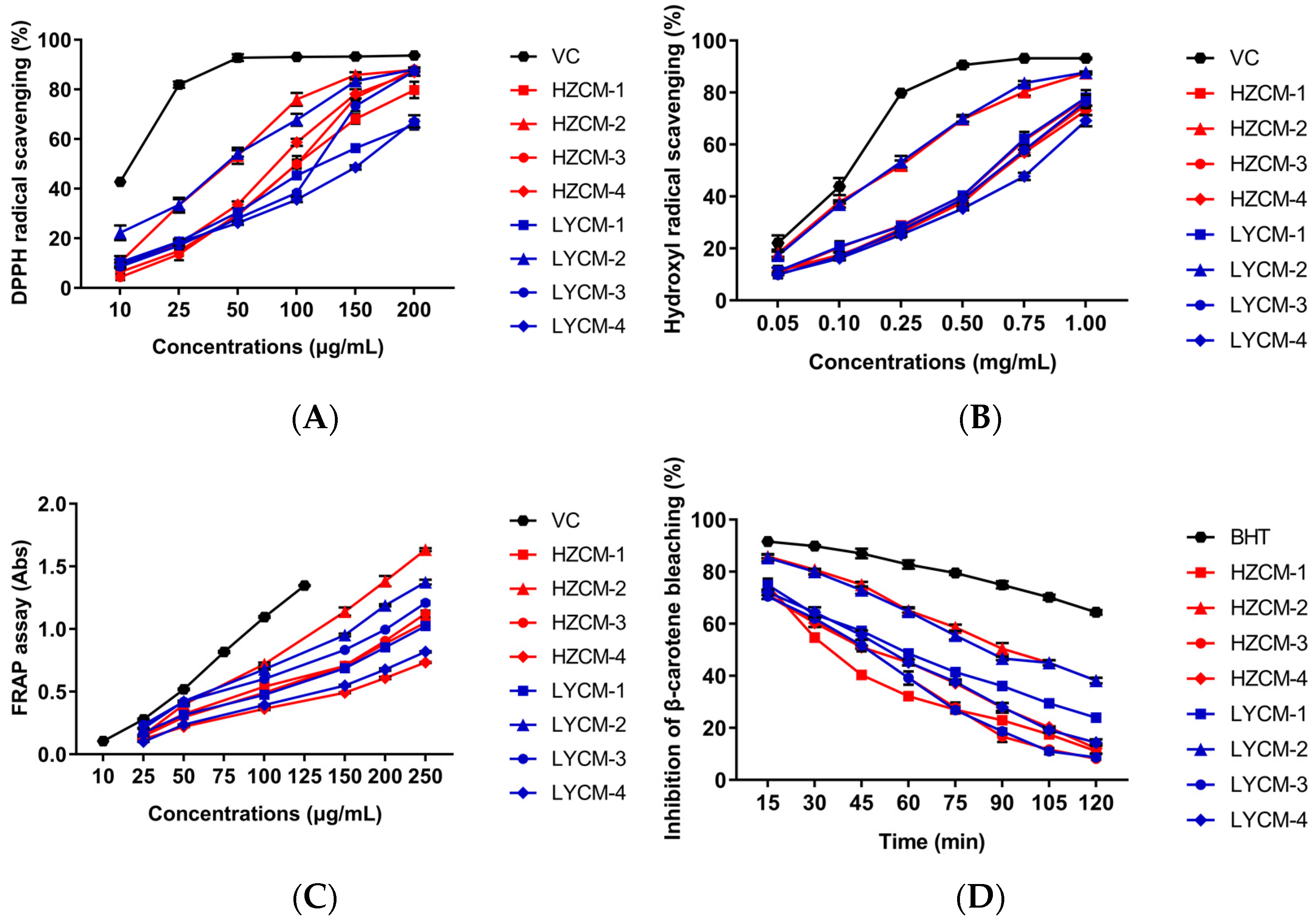
2.2. Antioxidant Activity In Vitro
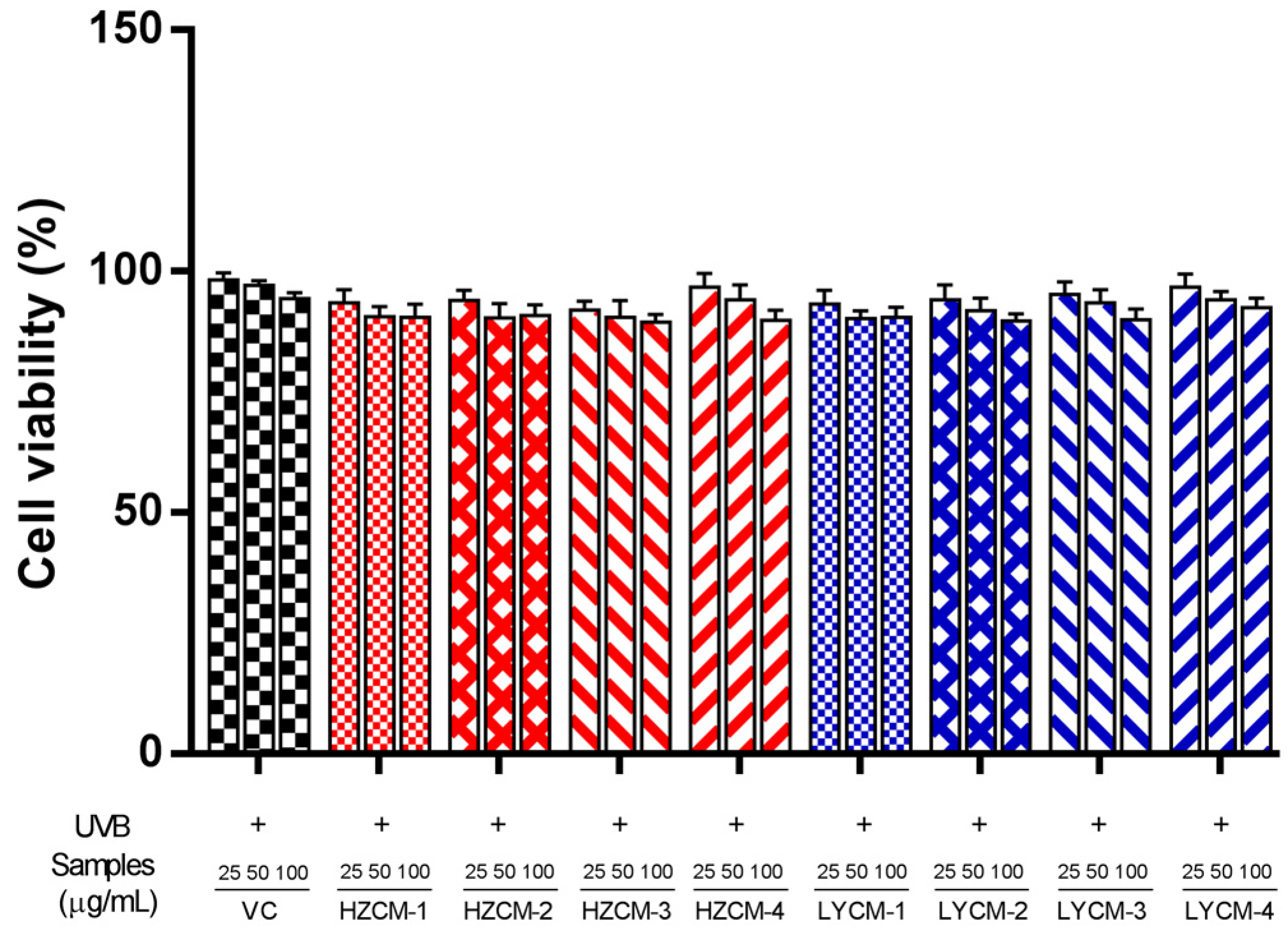
2.3. Effects of CM Extracts on Cell Viability, SOD, GSH-Px and MDA
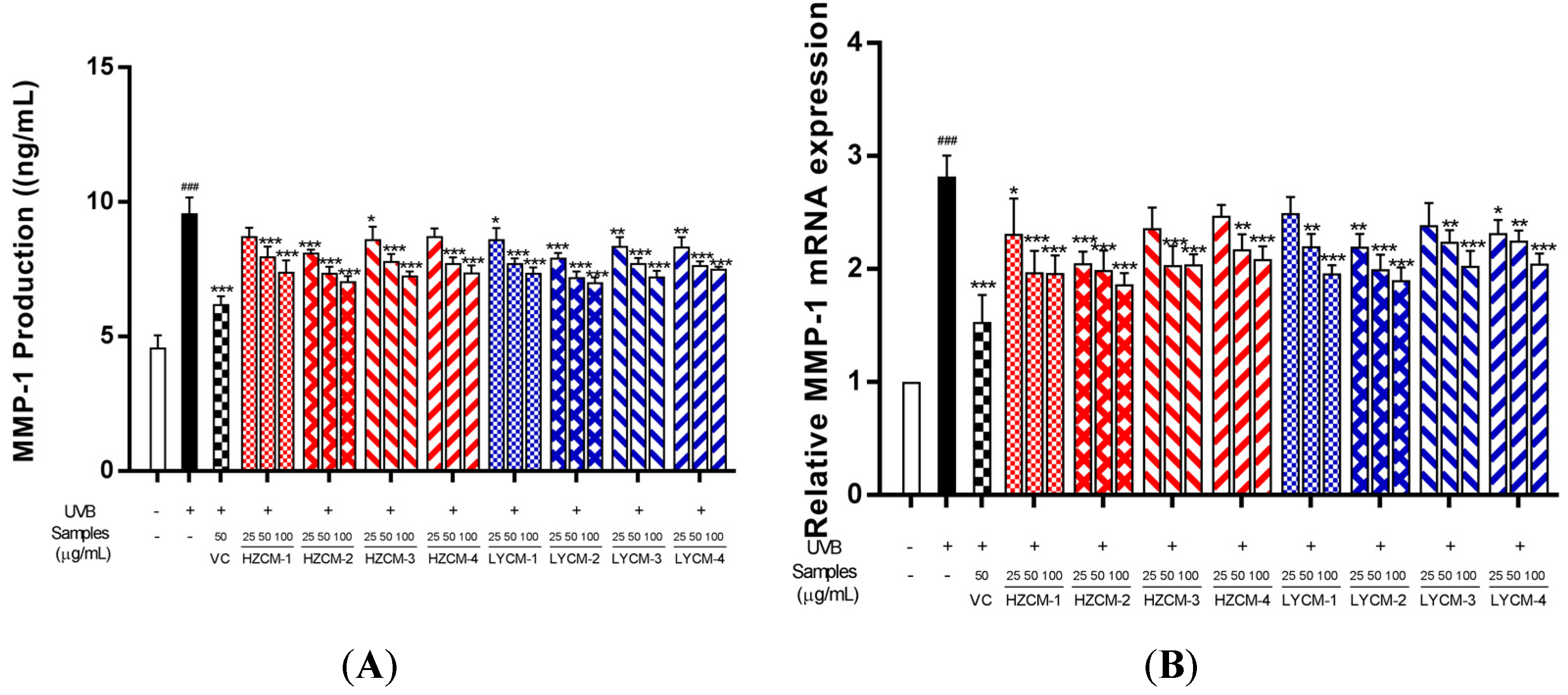
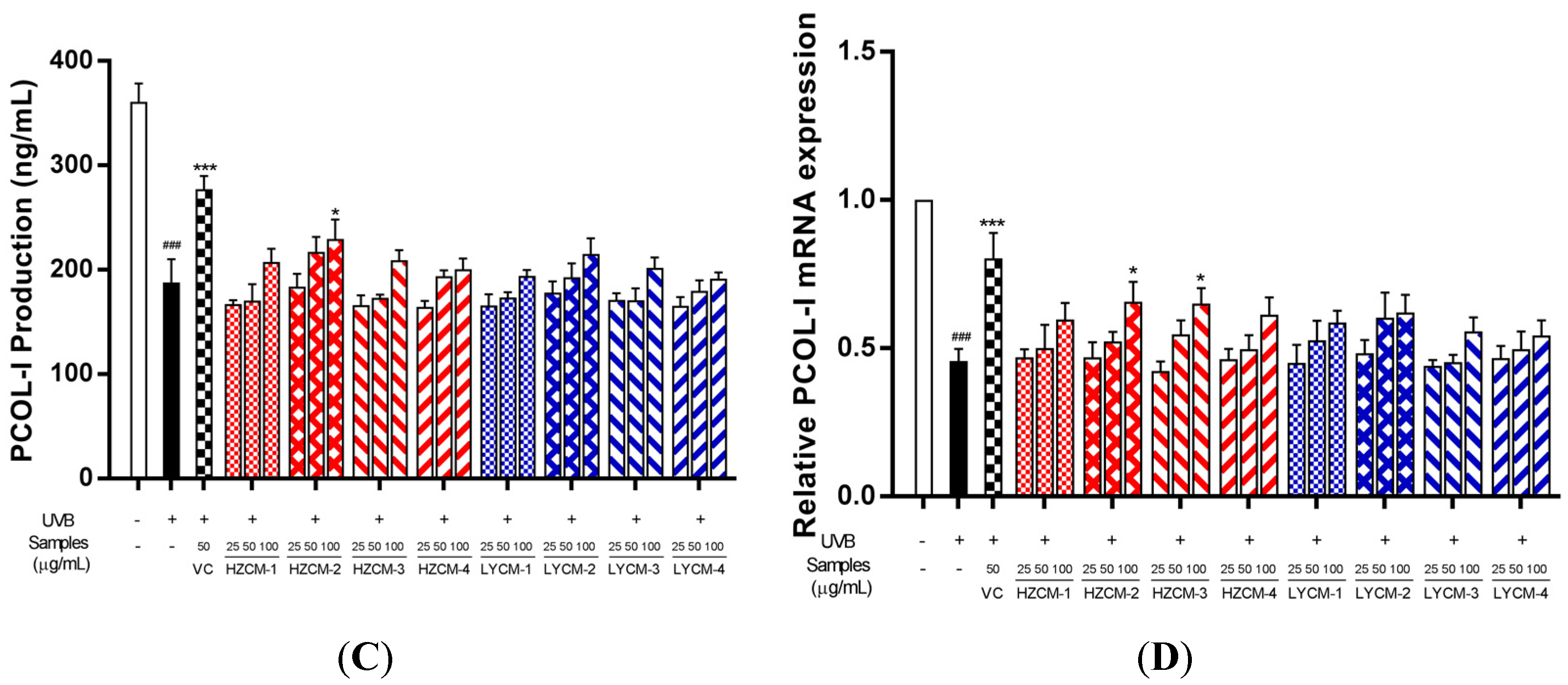
2.4. Effects on MMP-1 and PCOL-I
2.5. Chemical Compositions of CM Extracts
2.6. Determination of Phytochemicals in CM Extracts
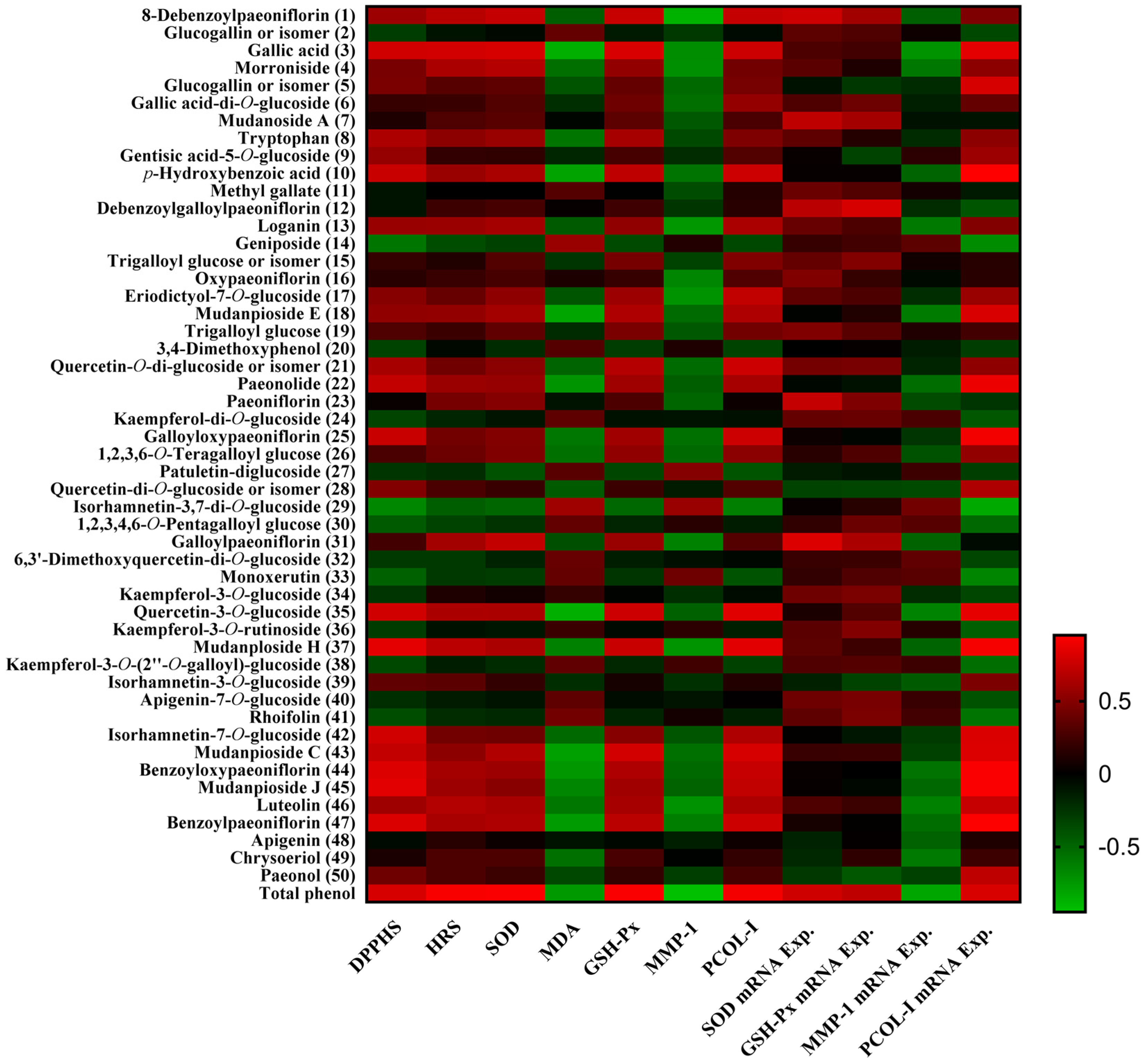
2.7. Pearson’s Correlation Analysis between Phytochemicals and Bioactivities
3. Materials and Methods
3.1. Plant Materials
3.2. Chemicals and Reagents
3.3. Sample Preparation
3.4. Total Phenolic Content Assay
3.5. Antioxidant Assay
3.5.1. DPPH Radical Scavenging Activity Assay
3.5.2. Hydroxyl Radical Scavenging Activity Assay
3.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5.4. Inhibition of β-Carotene Bleaching Assay
3.6. Cell Culture
3.7. Cell Viability
3.8. UVB Irradiation and Sample Treatment
3.9. Measurement of SOD, GSH-Px, MDA, MMP-1, and PCOL-I
3.10. Real-Time PCR Assay
3.11. UFLC-Q-TOF-MS Analysis
3.11.1. System and Conditions
3.11.2. Establishment of Tentative Peak Assignment
3.12. HPLC-DAD Analysis
3.13. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Han, C.V.; Bhat, R. In vitro control of food-borne pathogenic bacteria by essential oils and solvent extracts of underutilized flower buds of Paeonia suffruticosa (Andr.). Ind. Crop. Prod. 2014, 54, 203–208. [Google Scholar] [CrossRef]
- Li, P.; Shen, J.; Wang, Z.; Liu, S.; Liu, Q.; Li, Y.; He, C.; Xiao, P. Genus Paeonia: A comprehensive review on traditional uses, phytochemistry, pharmacological activities, clinical application, and toxicology. J. Ethnopharmacol. 2020, 269, 113708. [Google Scholar] [CrossRef]
- Pan, Y.; Gao, Z.; Huang, X.-Y.; Chen, J.-J.; Geng, C.-A. Chemical and biological comparison of different parts of Paeonia suffruticosa (Mudan) based on LCMS-IT-TOF and multi-evaluation in vitro. Ind. Crop. Prod. 2019, 144, 112028. [Google Scholar] [CrossRef]
- Wang, Z.; He, C.; Peng, Y.; Chen, F.; Xiao, P. Origins, Phytochemistry, Pharmacology, Analytical Methods and Safety of Cortex Moutan (Paeonia suffruticosa Andrew): A Systematic Review. Molecules 2017, 22, 946. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Shirakawa, A.; Shi, Y.; Yu, X.; Tamura, T.; Shibahara, N.; Yoshimatsu, K.; Komatsu, K. Impact of different post-harvest processing methods on the chemical compositions of peony root. J. Nat. Med. 2018, 72, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.W.; Piao, M.J.; Kim, K.C.; Zheng, J.; Cha, J.W.; Hyun, J.W. 6’-O-Galloylpaeoniflorin Protects Human Keratinocytes Against Oxidative Stress-Induced Cell Damage. Biomol. Ther. 2013, 21, 349–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamal, J.; Mustafa, M.R.; Wong, P.-F. Paeonol protects against premature senescence in endothelial cells by modulating Sirtuin 1 pathway. J. Ethnopharmacol. 2014, 154, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-H.; Liu, S.; Xu, S.-Y.; Chen, L.; Shan, Y.-H.; Wei, W.; Liang, W.-Q.; Gao, J.-Q. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomedicine 2013, 20, 1082–1087. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, M.; Xie, X.; Di, T.; Zhao, J.; Lin, Y.; Xu, X.; Li, N.; Zhai, Y.; Wang, Y.; et al. Paeonol ameliorates imiquimod-induced psoriasis-like skin lesions in BALB/c mice by inhibiting the maturation and activation of dendritic cells. Int. J. Mol. Med. 2017, 39, 1101–1110. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Lechtenberg, M.; Sendker, J.; Petereit, F.; Deters, A.; Hensel, A. Wound-healing plants from TCM: In vitro investi-gations on selected TCM plants and their influence on human dermal fibroblasts and keratinocytes. Fitoterapia 2013, 84, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020; Volume 1, p. 179. [Google Scholar]
- Xiao, C.; Wu, M.; Chen, Y.; Zhang, Y.; Zhao, X.; Zheng, X. Revealing Metabolomic Variations in Cortex Moutan from Different Root Parts using HPLC-MS method. Phytochem. Anal. 2014, 26, 86–93. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, L.; Huang, Y.; Mo, M.; Wang, Q.; Guo, C.; Liang, W. Study on comprehensive evaluation of Cortex Moutan Radicis quality in different harvesting periods. World Sci. Technol. Mod. Tradit. Chin. Med. Mater. Med. 2019, 21, 240–247. [Google Scholar]
- Zhang, Y.; Li, Y.; Chen, X.; Meng, Z.; Guo, S. Combined metabolome and transcriptome analyses reveal the effects of mycor-rhizal fungus Ceratobasidium sp. AR2 on the flavonoid accumulation in Anoectochilus roxburghii during different growth stages. Int. J. Mol. Sci. 2020, 21, 564. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, J.B.; Egipto, R.; Laureano, O.; De Castro, R.; Pereira, G.E.; Ricardo-Da-Silva, J.M. Chemical characteristics of grapes cv. Syrah (Vitis vinifera L.) grown in the tropical semiarid region of Brazil (Pernambuco state): Influence of rootstock and harvest season. J. Sci. Food Agric. 2019, 99, 5050–5063. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.D.M.; Terto, M.C.; Santos, S.G.D.; da Silva, M.S.; Tavares, J.F. Seasonal Variations of Polyphenols Content, Sun Protection Factor and Antioxidant Activity of Two Lamiaceae Species. Pharmaceutics 2021, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Q.; Yang, Q.; Yan, X.; Feng, S.; Wang, Z. Comparison of Anthraquinones, Iridoid Glycosides and Triterpe-noids in Morinda officinalis and Morinda citrifolia Using UPLC/Q-TOF-MS and Multivariate Statistical Analysis. Molecules 2020, 25, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, G.; Li, Y.; Wei, W.; Li, J.; Li, H.; Huang, Y.; Guo, D.-A. Metabolomics Combined with Multivariate Statistical Analysis for Screening of Chemical Markers between Gentiana scabra and Gentiana rigescens. Molecules 2020, 25, 1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Lin, H.; Tan, J.; Wang, C.; Wang, H.; Wu, F.; Dong, Q.; Liu, Y.; Li, P.; Liu, J. UPLC-QTOF/MS-Based Nontargeted Metabolomic Analysis of Mountain- and Garden-Cultivated Ginseng of Different Ages in Northeast China. Molecules 2018, 24, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.; Dong, Y.; Liu, X.; Wan, Y.; Gu, T.; Zhou, X.; Liu, M. Comparison of chemical compositions, antioxidant, and an-ti-photoaging activities of Paeonia suffruticosa flowers at different flowering stages. Antioxidants 2019, 8, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Xue, J.; Xue, Y.; Liu, R.; Ren, X.; Wang, S.; Zhang, X. Transcriptome sequencing and identification of key callus browning-related genes from petiole callus of tree peony (Paeonia suffruticosa cv. Kao) cultured on media with three browning inhibitors. Plant. Physiol. Biochem. 2020, 149, 36–49. [Google Scholar] [CrossRef]
- Mandim, F.; Petropoulos, S.A.; Giannoulis, K.D.; Dias, M.I.; Fernandes, Â.; Pinela, J.; Kostic, M.; Soković, M.; Barros, L.; Santos-Buelga, C.; et al. Seasonal variation of bioactive properties and phenolic composition of Cynara cardunculus var. altilis. Food Res. Int. 2020, 134, 109281. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Wang, Z.; He, S.; He, D.; Liu, Y.; Fu, Z. Research on the relationship between phenolic acids and rooting of tree peony (Paeonia suffruticosa) plantlets in vitro. Sci. Hortic. 2017, 224, 53–60. [Google Scholar] [CrossRef]
- Gori, A.; Nascimento, L.B.; Ferrini, F.; Centritto, M.; Brunetti, C. Seasonal and Diurnal Variation in Leaf Phenolics of Three Medicinal Mediterranean Wild Species: What Is the Best Harvesting Moment to Obtain the Richest and the Most Antioxidant Extracts? Molecules 2020, 25, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, M.M.; Khatoon, S.; Rastogi, S.; Rawat, A.K.S. Determination of flavonoids, polyphenols and antioxidant activity of Tephrosia purpurea: A seasonal study. J. Integr. Med. 2016, 14, 447–455. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Measurement of antioxidant activity. J. Funct. Foods 2015, 18, 757–781. [Google Scholar] [CrossRef]
- Hemerková, P.; Vališ, M. Role of Oxidative Stress in the Pathogenesis of Amyotrophic Lateral Sclerosis: Antioxidant Metal-loenzymes and Therapeutic Strategies. Biomolecules 2021, 11, 437. [Google Scholar] [CrossRef] [PubMed]
- Griess, B.; Tom, E.; Domann, F.; Teoh-Fitzgerald, M. Extracellular superoxide dismutase and its role in cancer. Free. Radic. Biol. Med. 2017, 112, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Adwas, A.A.; Elsayed, A.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar] [CrossRef]
- Zińczuk, J.; Maciejczyk, M.; Zaręba, K.; Romaniuk, W.; Markowski, A.; Kędra, B.; Zalewska, A.; Pryczynicz, A.; Ma-towicka-Karna, J.; Guzińska-Ustymowicz, K. Antioxidant Barrier, Redox Status, and Oxidative Damage to Biomolecules in Patients with Colorectal Cancer. Can Malondialdehyde and Catalase Be Markers of Colorectal Cancer Advancement? Biomolecules 2019, 9, 637. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.A.; Ngo, H.; Hwang, E.; Park, B.; Yi, T.-H. Protective effects of Carica papaya leaf against skin photodamage by blocking production of matrix metalloproteinases and collagen degradation in UVB-irradiated normal human dermal fibroblasts. South. Afr. J. Bot. 2020, 131, 398–405. [Google Scholar] [CrossRef]
- Zhao, X.; Psarianos, P.; Ghoraie, L.S.; Yip, K.; Goldstein, D.; Gilbert, R.; Witterick, I.; Pang, H.; Hussain, A.; Lee, J.H.; et al. Metabolic regulation of dermal fibroblasts contributes to skin extracellular matrix homeostasis and fibrosis. Nat. Metab. 2019, 1, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Chien, A.L. Photoaging: A Review of Current Literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imokawa, G.; Ishida, K. Biological Mechanisms Underlying the Ultraviolet Radiation-Induced Formation of Skin Wrinkling and Sagging I: Reduced Skin Elasticity, Highly Associated with Enhanced Dermal Elastase Activity, Triggers Wrinkling and Sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.I.; Lee, J.; Zee, O.P.; Chung, J.H. Triterpenoid fromTiarella polyphylla, regulation of type 1 procollagen and MMP-1 in ultraviolet irradiation of cultured old age human dermal fibroblasts. Arch. Pharmacal Res. 2004, 27, 1060–1064. [Google Scholar] [CrossRef]
- Chaikul, P.; Sripisut, T.; Chanpirom, S.; Ditthawutthikul, N. Anti-skin aging activities of green tea (Camelliasinensis (L) Kuntze) in B16F10 melanoma cells and human skin fibroblasts. Eur. J. Integr. Med. 2020, 40, 101212. [Google Scholar] [CrossRef]
- Papaharalambus, C.A.; Griendling, K.K. Basic Mechanisms of Oxidative Stress and Reactive Oxygen Species in Cardiovascular Injury. Trends Cardiovasc. Med. 2007, 17, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Xu, M.; Wang, H.; Wang, E.; Li, Y.; Wang, L.; Gao, J.; Zhang, J.; Yuan, X.; Zhang, H. Analysis of the transcriptome and related physiological indicators of tree peony (Paeonia suffruticosa Andr.) plantlets before and after rooting in vitro. Plant. Cell Tissue Organ. Cult. PCTOC 2021, 1–15. [Google Scholar] [CrossRef]
- Wu, M.; Xiao, C.; Zhang, H.; Zhang, Y.; Yu, J.; Zheng, X. Quality evaluation of Moutan Cortex at different growth stages. Chin. Tradit. Herb. Drugs 2014, 45, 2987–2992. [Google Scholar]
- Xia, Y.Q.; Zhu, M.M.; Li, R.; Yang, L.C.; Gao, S.; Cao, S.M.; Feng, L.; Jia, X.B. Structural characteristics of pharmacodynamical components in genuine Moutan Cortex. Zhongguo Zhong Yao Za Zhi 2020, 45, 3351–3359. [Google Scholar] [PubMed]
- Wang, L.; Luo, Y.; Wu, Y.; Wu, Z. Impact of fermentation degree on phenolic compositions and bioactivities during the fer-mentation of guava leaves with Monascus anka and Bacillus sp. J. Funct. Foods 2018, 41, 183–190. [Google Scholar] [CrossRef]
- Asnaashari, M.; Farhoosh, R.; Sharif, A. Antioxidant activity of gallic acid and methyl gallate in triacylglycerols of Kilka fish oil and its oil-in-water emulsion. Food Chem. 2014, 159, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Ngoc, T.M.; Hung, T.M.; Thuong, P.T.; Kim, J.-C.; Choi, J.S.; Bae, K.; Hattori, M.; Choi, C.-S.; Lee, J.S.; Min, B.-S. Antioxidative Activities of Galloyl Glucopyranosides from the Stem-Bark of Juglans mandshurica. Biosci. Biotechnol. Biochem. 2008, 72, 2158–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Ohta, T.; Kawaguchi, A.; Yoshikawa, M. Bioactive Constituents of Chinese Natural Medicines. VI. Moutan Cortex. (2): Structures and Radical Scavenging Effects of Suffruticosides A, B, C, D, and E and Galloyl-oxypaeoniflorin. Chem. Pharm. Bull. 2001, 49, 69–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, G.; Park, E.K.; Joo, J.H.; Lee, B.H.; Choi, B.W.; Jung, D.S.; Lee, N.H. A new antioxidant monoterpene glycoside, al-pha-benzoyloxypaeoniflorin from Paeonia suffruticosa. Arch. Pharm. Res. 2001, 24, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Lu, Y.; Wang, H.; Feng, Y.; Jiang, S.; Gao, X.-H.; Qi, R.; Wu, Y.; Chen, H.-D. Paeoniflorin Resists H2O2-Induced Oxidative Stress in Melanocytes by JNK/Nrf2/HO-1 Pathway. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-M.; Hong, J.-H. Antioxidant Effects of Bioactive Compounds Isolated from Pressurized Steam-Treated Corni Fructus and Their Protective Effects Against UVB-Irradiated HS68 Cells. J. Med. Food 2018, 21, 1165–1172. [Google Scholar] [CrossRef]
- Choi, E.M.; Lee, Y.S. Luteolin suppresses IL-1beta-induced cytokines and MMPs production via p38 MAPK, JNK, NF-kappaB and AP-1 activation in human synovial sarcoma cell line, SW982. Food Chem. Toxicol. 2010, 48, 2607–2611. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.-W.; Yi, T.H. Gallic Acid Regulates Skin Photoaging in UVB-exposed Fibroblast and Hairless Mice. Phytotherapy Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Nazaruk, J.; Galicka, A. The Influence of Selected Flavonoids from the Leaves of Cirsium palustre (L.) Scop. on Collagen Expression in Human Skin Fibroblasts. Phytotherapy Res. 2014, 28, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- He, J.-Y.; Zhang, Y.-H.; Ma, N.; Zhang, X.-L.; Liu, M.-H.; Fu, W.-M. Comparative analysis of multiple ingredients in Rosa roxburghii and R. sterilis fruits and their antioxidant activities. J. Funct. Foods 2016, 27, 29–41. [Google Scholar] [CrossRef]
- Liu, M.-H.; Ko, C.-H.; Ma, N.; Tan, P.-W.; Fu, W.-M.; He, J.-Y. Chemical profiles, antioxidant and anti-obesity effects of extract of Bambusa textilis McClure leaves. J. Funct. Foods 2016, 22, 533–546. [Google Scholar] [CrossRef]
- Lee, H.; Sung, J.; Kim, Y.; Jeong, H.S.; Lee, J. Protective Effects of Unsaponifiable Matter from Perilla Seed Meal on UVB-induced Damages and the Underlying Mechanisms in Human Skin Fibroblasts. Antioxidants 2019, 8, 644. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Sun, B.; Luo, X.; Zhao, M.; Zheng, F.; Sun, J.; Li, H.; Sun, X.; Huang, M. Cytoprotective effects of a tripeptide from Chinese Baijiu against AAPH-induced oxidative stress in HepG2 cells via Nrf2 signaling. RSC Adv. 2018, 8, 10898–10906. [Google Scholar] [CrossRef] [Green Version]
- Bang, J.S.; Choung, S.-Y. Inhibitory effect of oyster hydrolysate on wrinkle formation against UVB irradiation in human dermal fibroblast via MAPK/AP-1 and TGFβ/Smad pathway. J. Photochem. Photobiol. B Biol. 2020, 209, 111946. [Google Scholar] [CrossRef]


| Samples | Extraction Yields (%) | Total Phenolic Content (mg GAE/g ext.) | DPPH Radical Scavenging Activity (μg/mL) | Hydroxyl Radical Scavenging Activity (mg/mL) |
|---|---|---|---|---|
| VC | – | – | 9.81 ± 0.48 | 0.11 ± 0.004 |
| HZCM-1 | 20.33 ± 0.99 | 73.47 ± 1.01 | 88.00 ± 2.37 | 0.48 ± 0.02 |
| HZCM-2 | 21.73 ± 1.72 | 112.95 ± 3.97 | 42.70 ± 0.79 | 0.20 ± 0.006 |
| HZCM-3 | 22.21 ± 0.84 | 80.80 ± 4.48 | 79.98 ± 1.98 | 0.54 ± 0.02 |
| HZCM-4 | 21.44 ± 1.75 | 64.36 ± 2.85 | 68.47 ± 2.55 | 0.55 ± 0.02 |
| LYCM-1 | 21.13 ± 0.93 | 67.71 ± 2.09 | 112.74 ± 0.66 | 0.48 ± 0.04 |
| LYCM-2 | 22.53 ± 1.04 | 107.52 ± 3.03 | 40.38 ± 1.86 | 0.19 ± 0.01 |
| LYCM-3 | 21.67 ± 1.07 | 80.70 ± 2.95 | 85.45 ± 0.80 | 0.53 ± 0.03 |
| LYCM-4 | 22.01 ± 0.92 | 63.81 ± 3.96 | 138.36 ± 2.78 | 0.69 ± 0.04 |
| Compounds | Contents (mg/g Extracts) | |||||||
|---|---|---|---|---|---|---|---|---|
| HZCM-1 | HZCM-2 | HZCM-3 | HZCM-4 | LYCM-1 | LYCM-2 | LYCM-3 | LYCM-4 | |
| Gallic acid (3) | 11.39 ± 0.86 | 14.86 ± 1.12 | 14.51 ± 0.75 | 9.33 ± 0.71 | 10.77 ± 1.30 | 16.55 ± 1.05 | 8.40 ± 1.10 | 9.27 ± 0.95 |
| p-Hydroxybenzoic acid (10) | 2.99 ± 0.39 | 5.29 ± 0.34 | 5.93 ± 0.27 | 2.36 ± 0.23 | 2.91 ± 0.19 | 4.64 ± 0.42 | 0.73 ± 0.12 | 0.40 ± 0.04 |
| Oxypaeoniflorin (16) | 5.95 ± 0.47 | 8.31 ± 0.54 | 8.24 ± 0.64 | 6.23 ± 0.38 | 5.38 ± 0.28 | 9.79 ± 0.41 | 10.25 ± 0.35 | 5.11 ± 0.12 |
| Paeoniflorin (23) | 9.41 ± 1.07 | 14.23 ± 0.88 | 11.85 ± 0.55 | 10.80 ± 0.70 | 16.69 ± 0.77 | 21.01 ± 1.48 | 18.38 ± 0.71 | 19.50 ± 0.72 |
| 1,2,3,4,6-O-Pentagalloyl glucose (30) | 30.02 ± 1.26 | 34.00 ± 1.60 | 34.87 ± 1.40 | 26.44 ± 1.06 | 23.49 ± 2.03 | 28.09 ± 1.04 | 38.01 ± 1.66 | 35.49 ± 1.46 |
| Benzoyl paeoniflorin (47) | 0.47 ± 0.04 | 0.49 ± 0.02 | 0.49 ± 0.04 | 0.42 ± 0.02 | 0.35 ± 0.04 | 0.57 ± 0.05 | 0.24 ± 0.03 | 0.13 ± 0.02 |
| Paeonol (50) | 13.21 ± 1.39 | 18.11 ± 1.36 | 22.58 ± 1.63 | 16.93 ± 0.55 | 22.77 ± 2.25 | 18.13 ± 1.34 | 0.52 ± 0.04 | 0.48 ± 0.03 |
| Gene Names | Forward Primer Sequences (5′–3′) | Reverse Primer Sequences (5′–3′) |
|---|---|---|
| SOD | TGGAGATAATACAGCAGGCT | AGTCACATTGCCCAAGTCTC |
| GSH-Px | AGAAGTGCGAGGTGAACGGT | CCCACCAGGAACTTCTCAAA |
| MMP-1 | ATTCTACTGATATCGGGGCTTTGA | ATGTCCTTGGGGTATCCGTGTAG |
| COL1A1 | AGGGCCACGAAGACATC | AGATGACGTCATCGCACAACA |
| GAPDH | ACCACAGTCCATGCCATCAC | CCACCACCCTGTTGCTGTAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Liu, X.; He, J.; Liu, M. Insight into Seasonal Change of Phytochemicals, Antioxidant, and Anti-Aging Activities of Root Bark of Paeonia suffruticosa (Cortex Moutan) Combined with Multivariate Statistical Analysis. Molecules 2021, 26, 6102. https://doi.org/10.3390/molecules26206102
Yang S, Liu X, He J, Liu M. Insight into Seasonal Change of Phytochemicals, Antioxidant, and Anti-Aging Activities of Root Bark of Paeonia suffruticosa (Cortex Moutan) Combined with Multivariate Statistical Analysis. Molecules. 2021; 26(20):6102. https://doi.org/10.3390/molecules26206102
Chicago/Turabian StyleYang, Shicong, Xiaoyan Liu, Jingyu He, and Menghua Liu. 2021. "Insight into Seasonal Change of Phytochemicals, Antioxidant, and Anti-Aging Activities of Root Bark of Paeonia suffruticosa (Cortex Moutan) Combined with Multivariate Statistical Analysis" Molecules 26, no. 20: 6102. https://doi.org/10.3390/molecules26206102
APA StyleYang, S., Liu, X., He, J., & Liu, M. (2021). Insight into Seasonal Change of Phytochemicals, Antioxidant, and Anti-Aging Activities of Root Bark of Paeonia suffruticosa (Cortex Moutan) Combined with Multivariate Statistical Analysis. Molecules, 26(20), 6102. https://doi.org/10.3390/molecules26206102