Loganin Ameliorates Painful Diabetic Neuropathy by Modulating Oxidative Stress, Inflammation and Insulin Sensitivity in Streptozotocin-Nicotinamide-Induced Diabetic Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Painful Diabetic Neuropathy Rat Model and Experimental Design
2.4. SH-SY5Y Cell Culture and Treatment
2.5. Pain Sensitivity Assay
2.6. Determination of Insulin and Calculation of HOMA-IR
2.7. ELISA for Cytokines and Oxidative Stress Biomarkers
2.8. Western Blot Analysis
2.9. Immunofluorescence
2.10. RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR (qRT-PCR)
2.11. Measurement of Intracellular ROS
2.12. Cell Viability Assays
2.13. Statistical Analysis
3. Results
3.1. Loganin Ameliorated Hyperglycemia, Pain Behaviors and Insulin Resistance in STZ-NA Injected Rats
3.2. Loganin Decreased CaV3.2 T-type Calcium Channels, CGRP Expression and Glial Activation in the Spinal Dorsal Horn of PDN Rats
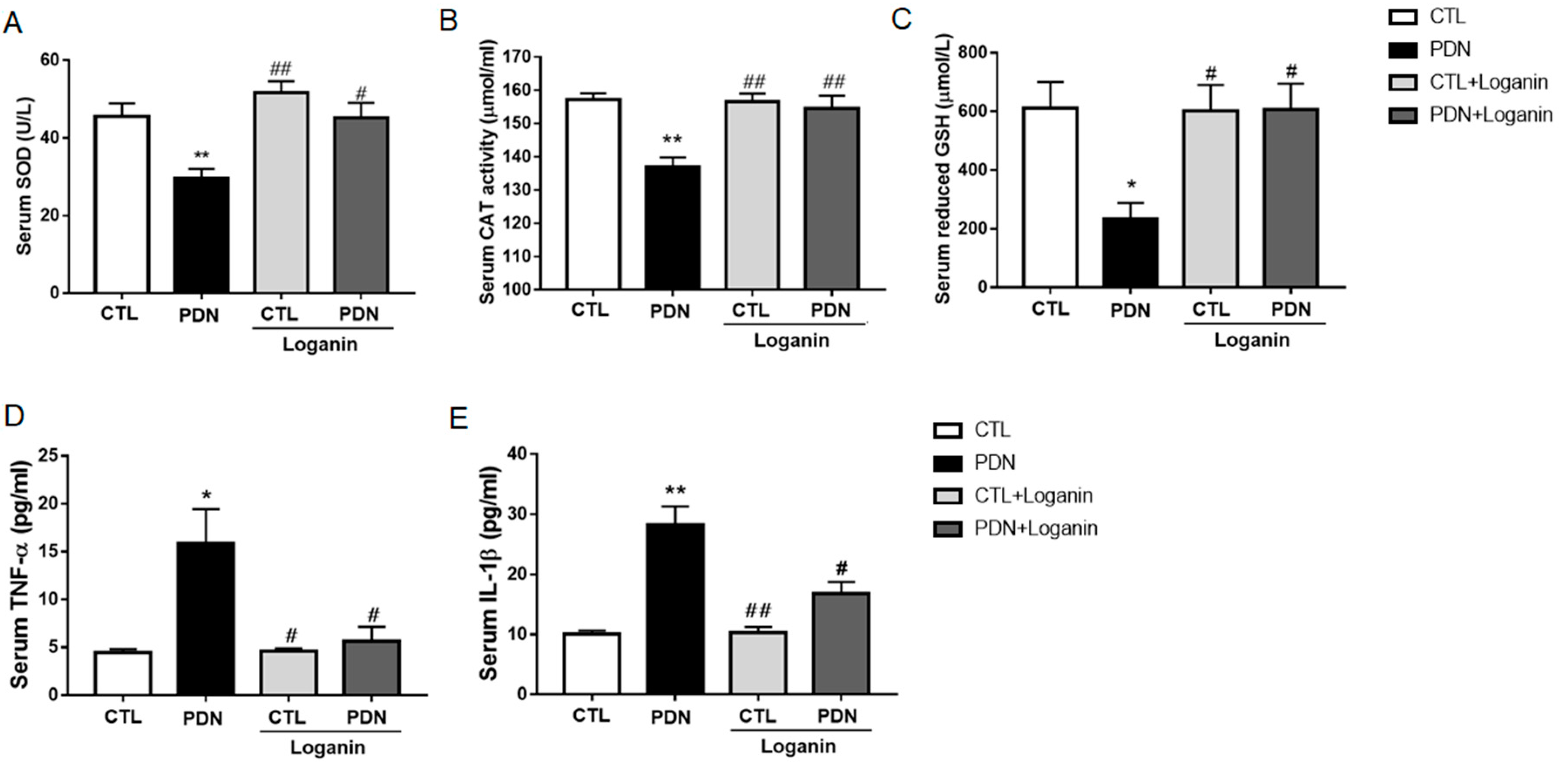
3.3. Loganin Suppressed Oxidative Stress and Proinflammatory Factors in the Serum of PDN Rats
3.4. Loganin Diminished Inflammatory Mediators by Inhibiting NF-κB Activation in the Spinal Cord Tissue of PDN Rats
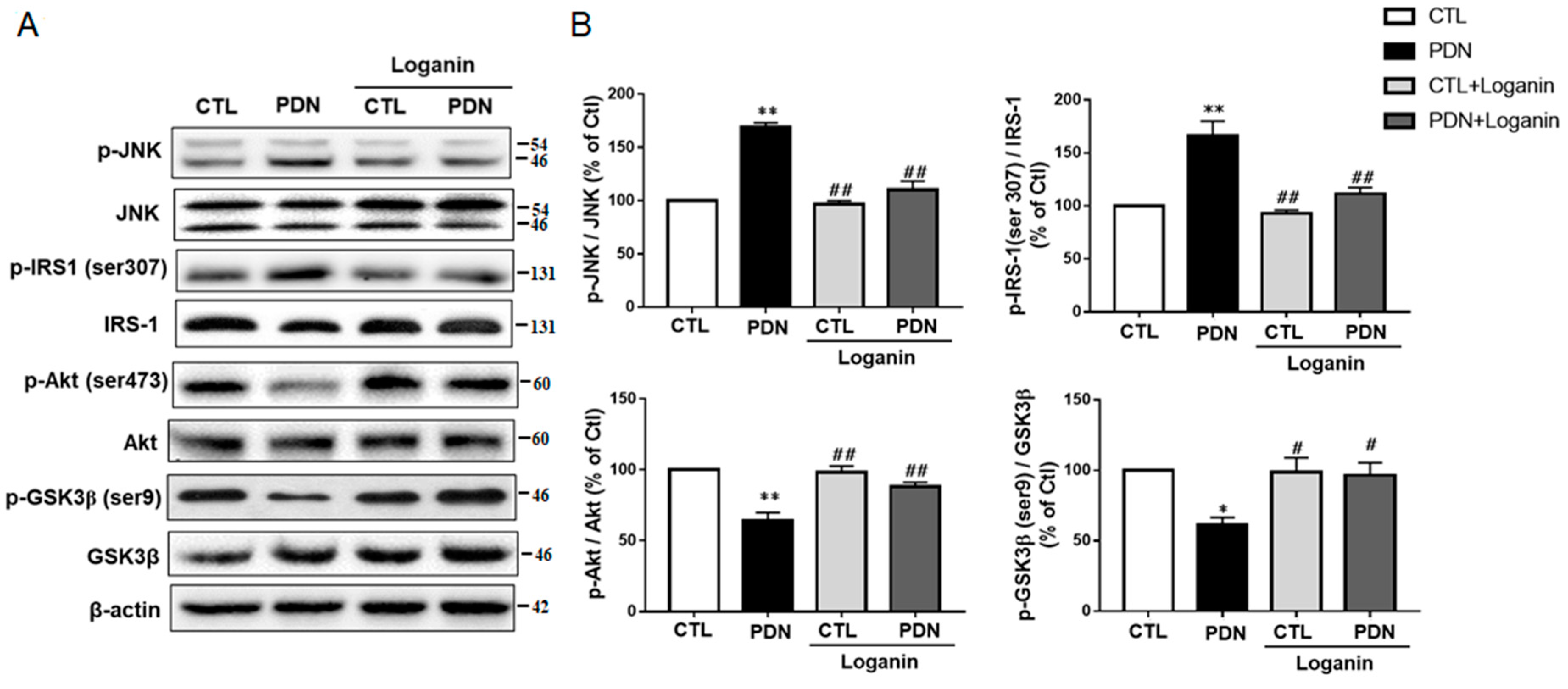
3.5. Loganin Regulated the JNK-IRS1-AKT-GSK3β Insulin Resistance Pathway in PDN Rat Spinal Cord Tissue
3.6. Loganin Had Antioxidant and Anti-Inflammatory Effects in SH-SY5Y Cells Exposed to High Glucose
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [Green Version]
- Aleidan, F.A.S.; Ahmad, B.A.; Alotaibi, F.A.; Aleesa, D.H.; Alhefdhi, N.A.; Badri, M.; Abdel Gader, A.G. Prevalence and Risk Factors for Diabetic Peripheral Neuropathy Among Saudi Hospitalized Diabetic Patients: A Nested Case-Control Study. Int. J. Gen. Med. 2020, 13, 881–889. [Google Scholar] [CrossRef]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.D. Challenges of neuropathic pain: Focus on diabetic neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [Green Version]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Primers 2019, 5, 41. [Google Scholar] [CrossRef]
- Hebert, H.L.; Veluchamy, A.; Torrance, N.; Smith, B.H. Risk factors for neuropathic pain in diabetes mellitus. Pain 2017, 158, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 32 (Suppl. S1), S62–S67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mert, T.; Sahin, E.; Yaman, S.; Sahin, M. Effects of immune cell-targeted treatments result from the suppression of neuronal oxidative stress and inflammation in experimental diabetic rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2020, 393, 1293–1302. [Google Scholar] [CrossRef] [PubMed]
- Sifuentes-Franco, S.; Pacheco-Moises, F.P.; Rodriguez-Carrizalez, A.D.; Miranda-Diaz, A.G. The Role of Oxidative Stress, Mitochondrial Function, and Autophagy in Diabetic Polyneuropathy. J. Diabetes Res. 2017, 2017, 1673081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Xu, L.; Chen, W.; Yu, X.; Shen, L.; Huang, Y. Pyridoxamine alleviates mechanical allodynia by suppressing the spinal receptor for advanced glycation end product-nuclear factor-kappaB/extracellular signal-regulated kinase signaling pathway in diabetic rats. Mol. Pain 2020, 16, 1744806920917251. [Google Scholar] [CrossRef] [PubMed]
- Khuankaew, C.; Sawaddiruk, P.; Surinkaew, P.; Chattipakorn, N.; Chattipakorn, S.C. Possible roles of mitochondrial dysfunction in neuropathy. Int. J. Neurosci. 2020, 131, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.; Sharma, N.; Pasnoor, M.; Kluding, P.M. Painful Diabetic Peripheral Neuropathy: Presentations, Mechanisms, and Exercise Therapy. J. Diabetes Metab. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.; Feldman, E.L. Insulin resistance in the nervous system. Trends Endocrinol. Metab. 2012, 23, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Garcia, G.; Gutierrez-Lara, E.J.; Centurion, D.; Granados-Soto, V.; Murbartian, J. Fructose-Induced Insulin Resistance as a Model of Neuropathic Pain in Rats. Neuroscience 2019, 404, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Ponirakis, G.; Abdul-Ghani, M.A.; Jayyousi, A.; Zirie, M.A.; Qazi, M.; Almuhannadi, H.; Petropoulos, I.N.; Khan, A.; Gad, H.; Migahid, O.; et al. Painful diabetic neuropathy is associated with increased nerve regeneration in patients with type 2 diabetes undergoing intensive glycemic control. J. Diabetes Investig. 2021, 12, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.N.; Lee, K.O.; Jeong, J.; Park, H.J.; Kim, S.M.; Shin, H.Y.; Hong, J.M.; Ahn, C.W.; Choi, Y.C. The role of insulin resistance in diabetic neuropathy in Koreans with type 2 diabetes mellitus: A 6-year follow-up study. Yonsei Med. J. 2014, 55, 700–708. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Lee, H.; Kwon, C.Y.; Kim, G.Y.; Jeong, J.W.; Kim, S.O.; Choi, S.H.; Jeong, S.J.; Noh, J.S.; Choi, Y.H. Loganin Inhibits Lipopolysaccharide-Induced Inflammation and Oxidative Response through the Activation of the Nrf2/HO-1 Signaling Pathway in RAW264.7 Macrophages. Biol. Pharm. Bull. 2021, 44, 875–883. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Jiang, M.; Fu, Y.; Zhu, Y.; Jiao, N.; Liu, L.; Du, Q.; Wu, H.; Xu, H.; et al. Loganin and catalpol exert cooperative ameliorating effects on podocyte apoptosis upon diabetic nephropathy by targeting AGEs-RAGE signaling. Life Sci. 2020, 252, 117653. [Google Scholar] [CrossRef]
- Mo, F.F.; Liu, H.X.; Zhang, Y.; Hua, J.; Zhao, D.D.; An, T.; Zhang, D.W.; Tian, T.; Gao, S.H. Anti-diabetic effect of loganin by inhibiting FOXO1 nuclear translocation via PI3K/Akt signaling pathway in INS-1 cell. Iran J. Basic Med. Sci. 2019, 22, 262–266. [Google Scholar] [CrossRef]
- Rajabi, M.; Mohaddes, G.; Farajdokht, F.; Nayebi Rad, S.; Mesgari, M.; Babri, S. Impact of loganin on pro-inflammatory cytokines and depression- and anxiety-like behaviors in male diabetic rats. Physiol. Int. 2018, 105, 116–126. [Google Scholar] [CrossRef]
- Chu, L.W.; Cheng, K.I.; Chen, J.Y.; Cheng, Y.C.; Chang, Y.C.; Yeh, J.L.; Hsu, J.H.; Dai, Z.K.; Wu, B.N. Loganin prevents chronic constriction injury-provoked neuropathic pain by reducing TNF-alpha/IL-1beta-mediated NF-kappaB activation and Schwann cell demyelination. Phytomedicine 2020, 67, 153166. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Chu, L.W.; Chen, J.Y.; Hsieh, S.L.; Chang, Y.C.; Dai, Z.K.; Wu, B.N. Loganin Attenuates High Glucose-Induced Schwann Cells Pyroptosis by Inhibiting ROS Generation and NLRP3 Inflammasome Activation. Cells 2020, 9, 1948. [Google Scholar] [CrossRef]
- Shen, K.P.; Lin, H.L.; Yen, H.W.; Hsieh, S.L.; An, L.M.; Wu, B.N. Eugenosedin-A improves glucose metabolism and inhibits MAPKs expression in streptozotocin/nicotinamide-induced diabetic rats. Kaohsiung J. Med. Sci. 2018, 34, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Lu, J.H.; Xie, C.S.; Shen, Y.J.; Wang, J.W.; Ye, X.Y.; Zhang, M.B.; Jia, G.L.; Tao, Y.X.; Li, J.; et al. Caveolin-1 in spinal cord modulates type-2 diabetic neuropathic pain through the Rac1/NOX2/NR2B signaling pathway. Am. J. Transl. Res. 2020, 12, 1714–1727. [Google Scholar] [CrossRef]
- Jiang, W.L.; Zhang, S.P.; Hou, J.; Zhu, H.B. Effect of loganin on experimental diabetic nephropathy. Phytomedicine 2012, 19, 217–222. [Google Scholar] [CrossRef]
- Chao, P.C.; Li, Y.; Chang, C.H.; Shieh, J.P.; Cheng, J.T.; Cheng, K.C. Investigation of insulin resistance in the popularly used four rat models of type-2 diabetes. Biomed. Pharmacother. 2018, 101, 155–161. [Google Scholar] [CrossRef]
- Mao, C.F.; Sudirman, S.; Lee, C.C.; Tsou, D.; Kong, Z.L. Echinacea purpurea Ethanol Extract Improves Male Reproductive Dysfunction With Streptozotocin-Nicotinamide-Induced Diabetic Rats. Front. Vet. Sci. 2021, 8, 651286. [Google Scholar] [CrossRef] [PubMed]
- Jacus, M.O.; Uebele, V.N.; Renger, J.J.; Todorovic, S.M. Presynaptic Cav3.2 channels regulate excitatory neurotransmission in nociceptive dorsal horn neurons. J. Neurosci. 2012, 32, 9374–9382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciarlo, L.; Marzoli, F.; Minosi, P.; Matarrese, P.; Pieretti, S. Ammonium Glycyrrhizinate Prevents Apoptosis and Mitochondrial Dysfunction Induced by High Glucose in SH-SY5Y Cell Line and Counteracts Neuropathic Pain in Streptozotocin-Induced Diabetic Mice. Biomedicines 2021, 9, 608. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [Green Version]
- Fernyhough, P.; Calcutt, N.A. Abnormal calcium homeostasis in peripheral neuropathies. Cell Calcium 2010, 47, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferron, L.; Koshti, S.; Zamponi, G.W. The life cycle of voltage-gated Ca(2+) channels in neurons: An update on the trafficking of neuronal calcium channels. Neuronal Signal 2021, 5, NS20200095. [Google Scholar] [CrossRef] [PubMed]
- Gomez, K.; Calderon-Rivera, A.; Sandoval, A.; Gonzalez-Ramirez, R.; Vargas-Parada, A.; Ojeda-Alonso, J.; Granados-Soto, V.; Delgado-Lezama, R.; Felix, R. Cdk5-Dependent Phosphorylation of CaV3.2 T-Type Channels: Possible Role in Nerve Ligation-Induced Neuropathic Allodynia and the Compound Action Potential in Primary Afferent C Fibers. J. Neurosci. 2020, 40, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.J.; Ma, L.X.; Jiao, C.; Kuang, H.X.; Zeng, F.; Zhou, X.Y.; Cheng, X.E.; Zhu, M.Y.; Zhang, D.Y.; Jiang, C.Y.; et al. Nerve injury elevates functional Cav3.2 channels in superficial spinal dorsal horn. Mol. Pain 2019, 15, 1744806919836569. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, T.; Kistner, K.; Joksimovic, S.L.J.; Todorovic, S.M.; Reeh, P.W.; Sauer, S.K. Painful diabetic neuropathy leads to functional CaV3.2 expression and spontaneous activity in skin nociceptors of mice. Exp. Neurol. 2021, 346, 113838. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, A.; Hwang, S.M.; Scarpa, J.; Hong, S.J.; Todorovic, S.M.; Jevtovic-Todorovic, V. CaV3.2 T-type calcium channels in peripheral sensory neurons are important for mibefradil-induced reversal of hyperalgesia and allodynia in rats with painful diabetic neuropathy. PLoS ONE 2014, 9, e91467. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.A.; Mapp, P.I.; Kelly, S. Calcitonin gene-related peptide in the joint: Contributions to pain and inflammation. Br. J. Clin. Pharmacol. 2015, 80, 965–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef] [Green Version]
- Ellis, A.; Bennett, D.L. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Pugazhenthi, S.; Zhang, Y.; Bouchard, R.; Mahaffey, G. Induction of an inflammatory loop by interleukin-1beta and tumor necrosis factor-alpha involves NF-kB and STAT-1 in differentiated human neuroprogenitor cells. PLoS ONE 2013, 8, e69585. [Google Scholar] [CrossRef] [Green Version]
- Suryavanshi, S.V.; Kulkarni, Y.A. NF-kappabeta: A Potential Target in the Management of Vascular Complications of Diabetes. Front. Pharmacol. 2017, 8, 798. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.B.; Yu, Y.B.; Wa, Q.B.; Zhou, J.W.; He, M.; Cen, Y. The role of quinazoline in ameliorating intervertebral disc degeneration by inhibiting oxidative stress and anti-inflammation via NF-kappaB/MAPKs signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2077–2086. [Google Scholar] [CrossRef]
- Zukowski, P.; Maciejczyk, M.; Matczuk, J.; Kurek, K.; Waszkiel, D.; Zendzian-Piotrowska, M.; Zalewska, A. Effect of N-Acetylcysteine on Antioxidant Defense, Oxidative Modification, and Salivary Gland Function in a Rat Model of Insulin Resistance. Oxid. Med. Cell. Longev. 2018, 2018, 6581970. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, N.; Wal, P.; Pal, R.S.; Wal, A.; Pal, Y.; Pharmacophore, D.M.J. Current review on IRS-1, JNK, NF-ΚB & m-TOR pathways in Insulin Resistance. Pharmacophores 2020, 11, 1–14. [Google Scholar]
- Alipourfard, I.; Datukishvili, N.; Mikeladze, D. TNF-alpha Downregulation Modifies Insulin Receptor Substrate 1 (IRS-1) in Metabolic Signaling of Diabetic Insulin-Resistant Hepatocytes. Mediators Inflamm. 2019, 2019, 3560819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hakeim, H.K.; Al-Kufi, S.N.; Al-Dujaili, A.H.; Maes, M. Serum Interleukin Levels and Insulin Resistance in Major Depressive Disorder. CNS Neurol. Disord. Drug Targets 2018, 17, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.F.; Herschkovitz, A.; Boura-Halfon, S.; Ronen, D.; Paz, K.; Leroith, D.; Zick, Y. Serine phosphorylation proximal to its phosphotyrosine binding domain inhibits insulin receptor substrate 1 function and promotes insulin resistance. Mol. Cell Biol. 2004, 24, 9668–9681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, T.T.; Qin, Z.L.; Ren, H.; Zhao, P.; Qi, Z.T. Inhibition of IRS-1 by hepatitis C virus infection leads to insulin resistance in a PTEN-dependent manner. Virol. J. 2015, 12, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
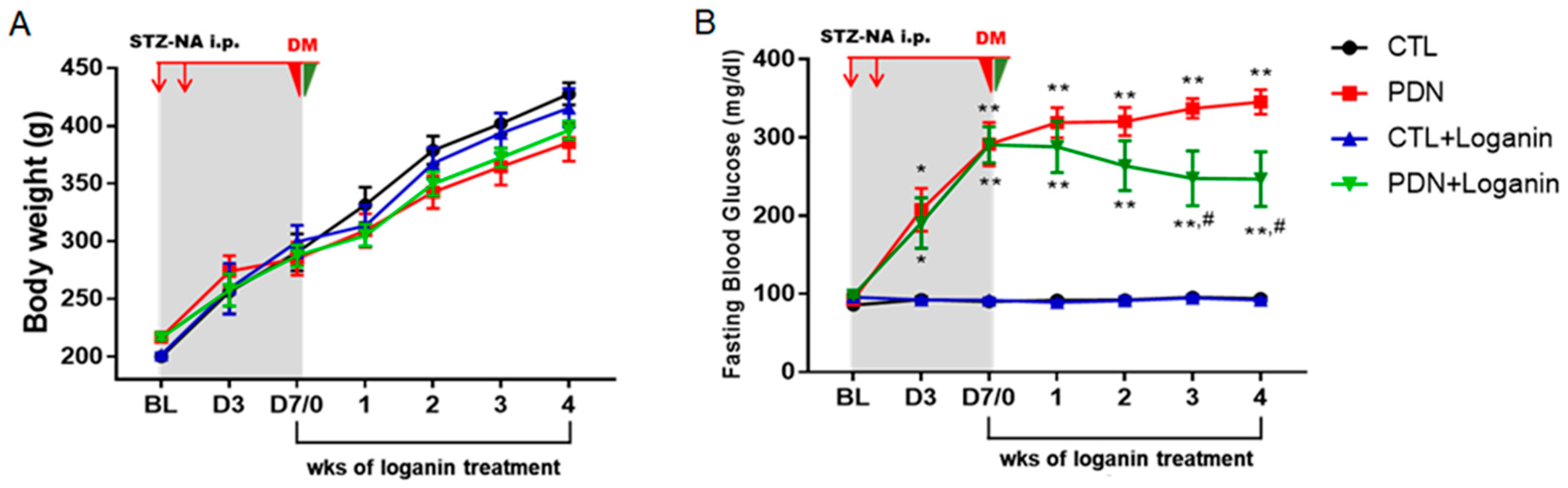





| Parameters | Groups | |||
|---|---|---|---|---|
| CTL | PDN | CTL + Loganin | PDN + Loganin | |
| Fasting blood glucose (mg/dL) | 120.5 ± 4.1 | 428.3 ± 21.0 ** | 118.3 ± 4.48 ## | 335.2 ± 41.1 **,# |
| Fasting plasma insulin (mIU/L) | 14.5 ± 0.68 | 13.1 ± 0.41 | 14.4 ± 0.75 | 13.2 ± 0.54 |
| HOMA-IR score | 3.84 ± 0.17 | 11.1 ± 0.36 ** | 4.05 ± 0.23 ## | 8.82 ± 0.8 **,# |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.-C.; Chiu, Y.-M.; Dai, Z.-K.; Wu, B.-N. Loganin Ameliorates Painful Diabetic Neuropathy by Modulating Oxidative Stress, Inflammation and Insulin Sensitivity in Streptozotocin-Nicotinamide-Induced Diabetic Rats. Cells 2021, 10, 2688. https://doi.org/10.3390/cells10102688
Cheng Y-C, Chiu Y-M, Dai Z-K, Wu B-N. Loganin Ameliorates Painful Diabetic Neuropathy by Modulating Oxidative Stress, Inflammation and Insulin Sensitivity in Streptozotocin-Nicotinamide-Induced Diabetic Rats. Cells. 2021; 10(10):2688. https://doi.org/10.3390/cells10102688
Chicago/Turabian StyleCheng, Yu-Chi, Yu-Min Chiu, Zen-Kong Dai, and Bin-Nan Wu. 2021. "Loganin Ameliorates Painful Diabetic Neuropathy by Modulating Oxidative Stress, Inflammation and Insulin Sensitivity in Streptozotocin-Nicotinamide-Induced Diabetic Rats" Cells 10, no. 10: 2688. https://doi.org/10.3390/cells10102688
APA StyleCheng, Y.-C., Chiu, Y.-M., Dai, Z.-K., & Wu, B.-N. (2021). Loganin Ameliorates Painful Diabetic Neuropathy by Modulating Oxidative Stress, Inflammation and Insulin Sensitivity in Streptozotocin-Nicotinamide-Induced Diabetic Rats. Cells, 10(10), 2688. https://doi.org/10.3390/cells10102688







