Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy
Abstract
1. Introduction
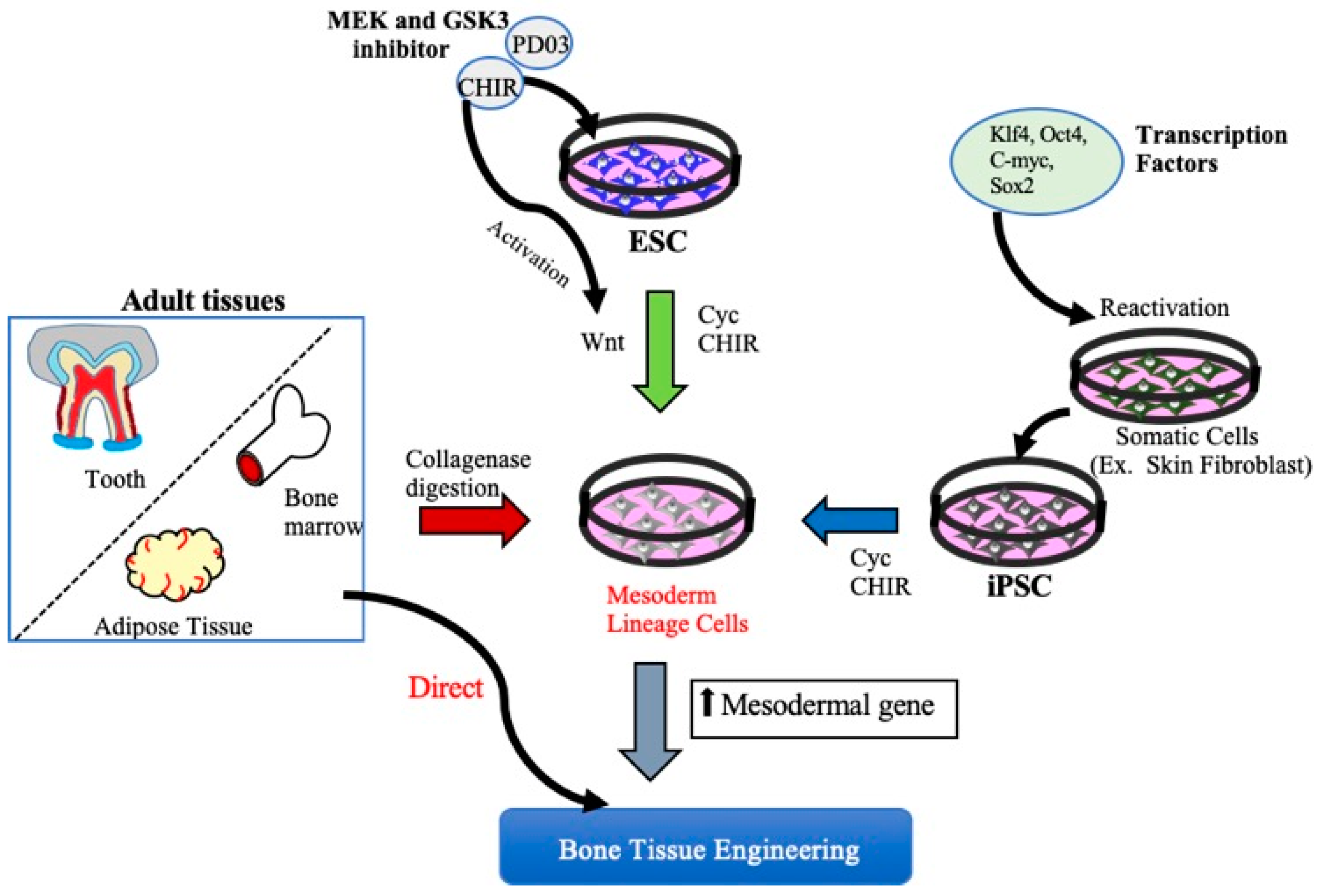
2. MSCs Derived from Embryonic Stem Cells and Induced Pluripotent Stem Cells for Bone Tissue Regeneration
2.1. ESCs and Bone Regeneration
2.2. Induced Pluripotent Stem Cells and Bone Regeneration
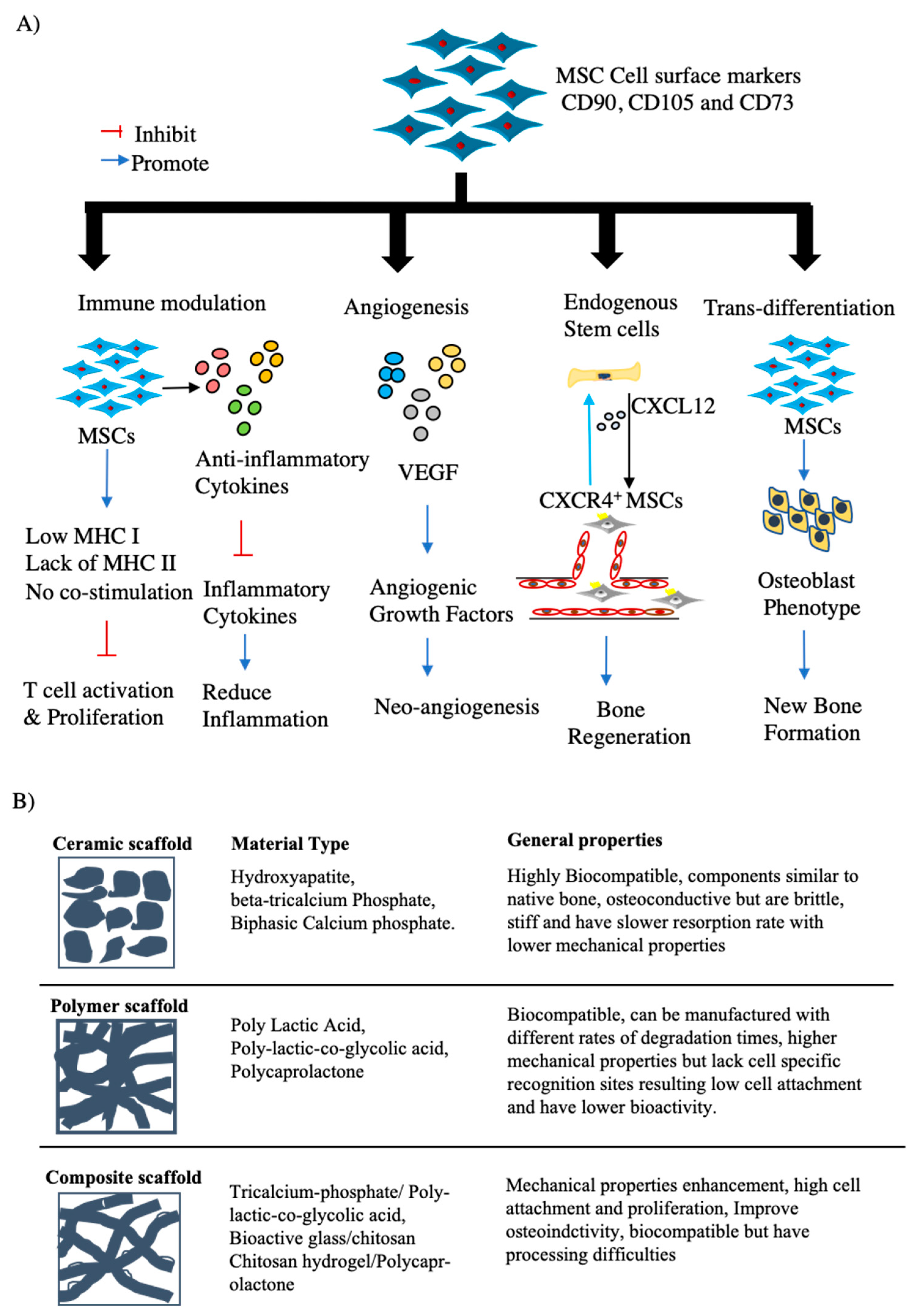
3. MSCs for Bone Regeneration
3.1. Characterization of MSCs
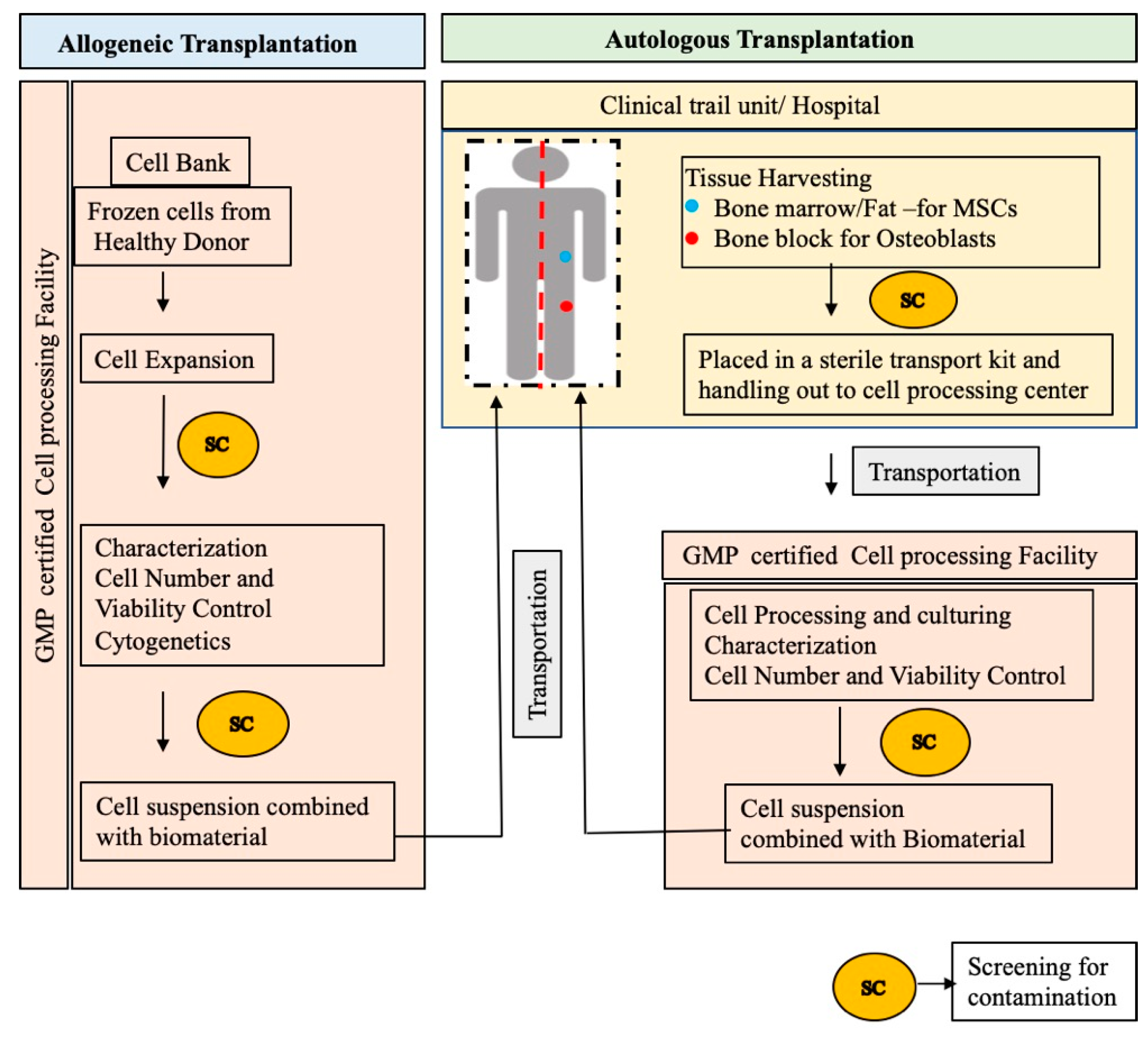
3.2. Clinical Translation of MSC-Based Bone Regeneration
3.2.1. BTE Scaffolds
3.2.2. Preclinical Studies of BTE in a Large Animal Model Using MSC/Scaffold Combinations
3.2.3. Gene Therapy for Bone Regeneration
3.2.4. Clinical Trials of MSCs for BTE
| Author | Type and Size of Defect | Transplant Groups | Origin of Cell Source | Pre-Transplant Incubation | Outcome |
|---|---|---|---|---|---|
| Dilogo et al., 2020 [85] | Nonunion fractures of Humerus/tibia with critical size bone defects | Combination of HA Bongros®-HA, Daewoong), BMP2, UC-MSCs with demineralized bone matrix | Allogeneic Umbilical Cord MSCs (UC-MSCs) | None | Allogeneic UC-MSCs can be used safely to treat the critical sized bone defects of long bones. |
| Dilogo et al., 2019 [88] | Humerus, Tibia and Femur Critical sized defects | Combination of HA granules (Bongros®-HA, Bioalpha, Seungnam, Korea), BMP2 and BMMSCs mixed with Plasma solution. | Autologous Bone marrow harvested from posterior Iliac crestal bone | None | Dramatic improvement of bone regeneration compared to preoperative radiographs. |
| Gjerde et al., 2018 [90] | Severe mandibular ridge resorption. | Expanded, autologous MSCs with biphasic calcium phosphate (MBCP+TM; Biomatlante, France) | Bone marrow cells from the posterior iliac crest | None | MSCs successfully induce significant new bone formation |
| Baba et al., 2016 [91] | Intrabony Periodontal defect. Probing depth >4 mm | The mixture of BMMSCs and PRP, combined with human thrombin dissolved in 10% calcium chloride perfused in a 3D woven-fabric composed of poly-L-lactic acid resin fibers (MSCs/PRP-3D woven Fabric) | Autologous Bone marrow harvested from posterior Iliac crestal bone | Induced under Osteogenic Medium | BMMSCs/PRP-3D woven Fabric constructs showed efficient regeneration of the periodontal tissue including alveolar bone. |
| Morrison et al., 2018 [86] | Cranial defects with less than 80 mm diameter | Allogeneic mesenchymal stromal cells (MSCs) on a ceramic carrier (ChronOS granules, synthes, and polymer scaffold, | Allogenic BMMSCs from 18–25 years aged donors | None | Allogeneic MSCs can be safely used for bone regeneration. |
| Kaigler et al., 2015 [89] | Severe Bone Atrophy of upper Jaw | Combination of BMMSCs and β-TCP (Cerasorb, Curasan AG, Germany) | Autologous Bone marrow harvested from posterior Iliac crestal bone | None | Higher density of regenerated bone with MSCs+ β-TCP group was observed than control group. |
| Marcacci et al., 2007 [87] | Humerus, Tibia and ulnar Critical sized defects | Combination of invitro expanded BMMSCs seeded with porous hydroxy apatite scaffolds (Finblock, FinCeramica Srl, Faenza, Italy) | Autologous Bone marrow harvested from posterior Iliac crestal bone | None | Significant healing of the CSDs. Attained long term durability of bone regeneration. |
| Bajada et al., 2007 [93] | Tibial non-union | Combination of invitro expanded BMMSCs seeded with calcium sulphate pellets (Stimulan, Biocomposites Ltd., Keele, United Kingdom) | Autologous Bone marrow harvested from posterior Iliac crestal bone | None | Clinical and radiological healing of nonunion was observed |
| Morishita et al., 2006 [94] | Tibial/femur massive defects | Attachment of invitro expanded BMMSCs-HA granules | Autologous Bone marrow harvested from posterior Iliac crestal bone | Induced under Osteogenic Medium | Good integration of BMMSCs-HA constructs to the host bone and increased radiographic density of the defect area. |
| NCT Number | Brief Title | Phase | Conditions | Interventions |
|---|---|---|---|---|
| NCT04297813 | Efficacy in Alveolar Bone Regeneration With Autologous MSCs and Biomaterial in Comparison to Autologous Bone Grafting | Phase I | • Alveolar Bone Atrophy | Autologous MSCs and a biomaterial, biphasic Calcium Phosphate (BCP). |
| NCT03325504 | A Comparative Study of 2 Doses of BM Autologous H- MSC+Biomaterial vs. Iliac Crest AutoGraft for Bone Healing in Non-Union | Phase III | • Non Union Fracture | Culture-expanded autologous BMMSC combined with biphasic calcium phosphate (BCP) biomaterial granules |
| NCT02803177 | Cell Therapy by Autologous BMC for Large Bone Defect Repair | Phase II | • Humerus Fracture Displaced Proximal | Autologous Bone Marrow-derived Mononuclear Cells (BMC) seeded onto ß-TCP |
| NCT02307435 | Allogenic Mesenchymal Stem Cell for Bone Defect or Non Union Fracture | Early Phase I | • Non Union Fracture, Metaphyseal Fibrous Defect | Allogeneic MSCs from umbilical cord/bone marrow/adipose combined and HA-CaSo4 |
| NCT02153372 | Cell Therapy by Bone Marrow- derived Mononuclear Cells (BMC) for Large Bone Defect Repair: Phase-I Clinical Trial | Phase I | • Humerus Fracture Displaced Proximal | Autologous Bone Marrow-derived Mononuclear Cells (BMC) seeded onto ß-TCP |
| NCT01958502 | Evaluation the Treatment of Nonunion of Long Bone Fracture of Lower Extremities (Femur and Tibia) Using Mononuclear Stem Cells from the Iliac Wing Within a 3-D Tissue Engineered Scaffold | Phase II | • Nonunion of Fracture | BMMSCs with BMP2 within a 3-D tissue engineered collagen scaffold |
| NCT01842477 | Evaluation of Efficacy and Safety of Autologous MSCs Combined to Biomaterials to Enhance Bone Healing | Phase I/II | • Delayed Union After Fracture of Humerus, Tibial or Femur | BMMScs mixed with biphasic calciulm granules |
| NCT00250302 | Autologous Implantation of Mesenchymal Stem Cells for the Treatment of Distal Tibial Fractures | Phase I/II | • Tibial Fracture | BMMSCs loaded onto a carrier and implanted locally at the defect site |
| NCT00557635 | Osseous Setting Improvement With Co-implantation of Osseous Matrix and Mesenchymal Progenitors Cells From Autologous Bone Marrow | Phase II | • Tibia or Femur Pseudo-arthrosis | Injection of an osseous matrix (osteopure) combined with MSC progenitors from autologous bone marrow. |
| NCT02177565 | Autologous Stem Cell Therapy for Fracture Non-union Healing | Not available | • Non-union of Fractures | Autologous BMSCs combined with carrier material |
| NCT01435434 | Mononucleotide Autologous Stem Cells and Demineralized Bone Matrix in the Treatment of Non Union/Delayed Fractures | Not available | • Non Union/Delayed Fractures | Injection of Autologous Stem Cells and Demineralized Bone Matrix |
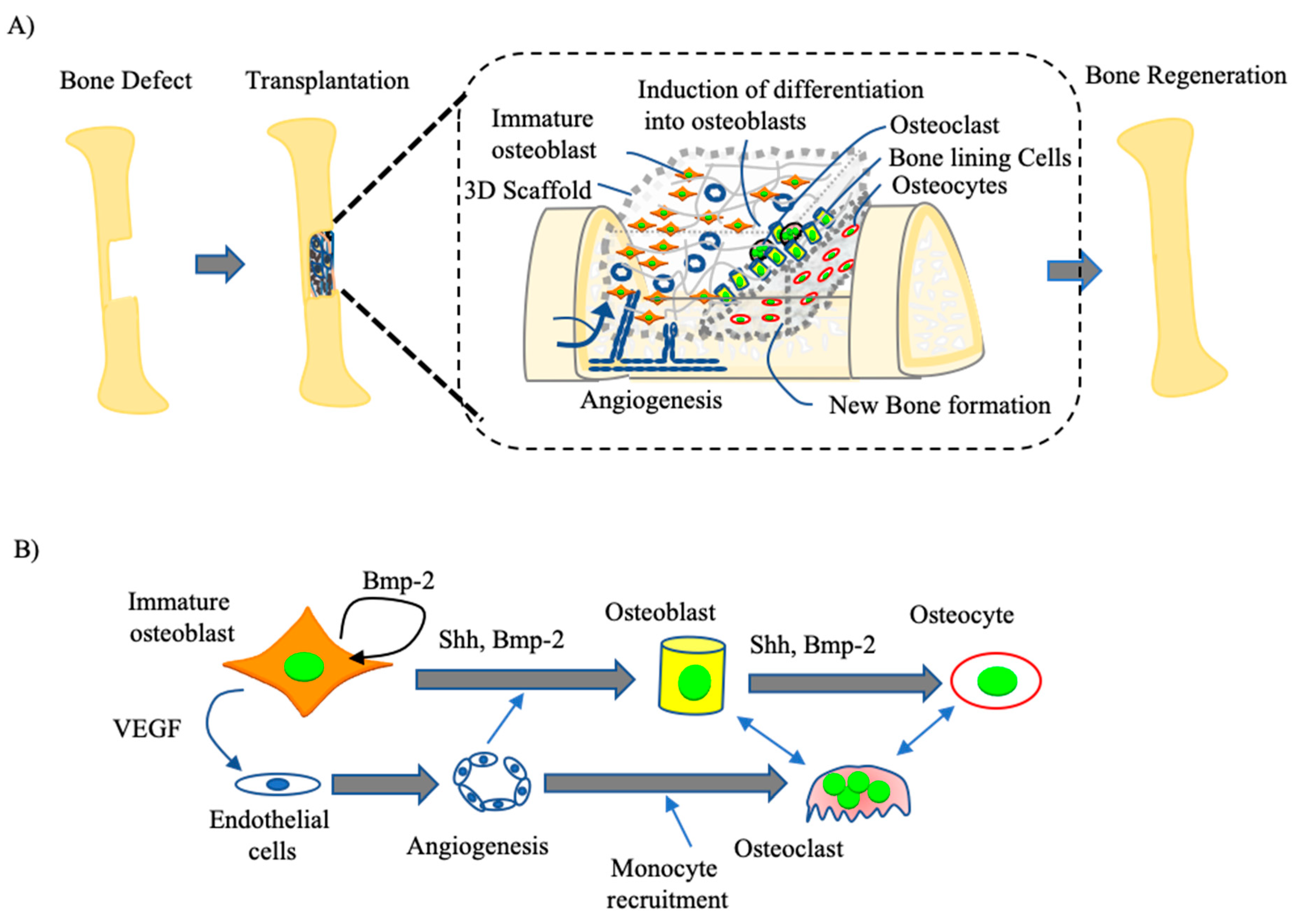
4. Osteoblast-Based Bone Tissue Regeneration
4.1. Development of Immature Osteoblast-Based BTE
4.2. Clinical Trial of Osteoblasts for BTE
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nauth, A.; Schemitsch, E.; Norris, B.; Nollin, Z.; Watson, J.T. Critical-Size Bone Defects: Is There a Consensus for Diagnosis and Treatment? J. Orthop. Trauma 2018, 32, S7–S11. [Google Scholar] [CrossRef] [PubMed]
- Roddy, E.; DeBaun, M.R.; Daoud-Gray, A.; Yang, Y.P.; Gardner, M.J. Treatment of critical-sized bone defects: Clinical and tissue engineering perspectives. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 351–362. [Google Scholar] [CrossRef]
- Yuan, H.; Xing, K.; Hsu, H.-Y. Trinity of Three-Dimensional (3D) Scaffold, Vibration, and 3D Printing on Cell Culture Application: A Systematic Review and Indicating Future Direction. Bioengineering 2018, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Bellido, T. Osteocytic signalling pathways as therapeutic targets for bone fragility. Nat. Rev. Endocrinol. 2016, 12, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, X.; Qi, X.; Jiang, C. Osteoinduction and proliferation of bone-marrow stromal cells in three-dimensional poly (ε-caprolactone)/ hydroxyapatite/collagen scaffolds. J. Transl. Med. 2015, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Bhumiratana, S.; Bernhard, J.C.; Alfi, D.M.; Yeager, K.; Eton, R.E.; Bova, J.; Shah, F.; Gimble, J.M.; Lopez, M.J.; Eisig, S.B.; et al. Tissue-engineered autologous grafts for facial bone reconstruction. Sci. Transl. Med. 2016, 8, 343ra83. [Google Scholar] [CrossRef]
- Chamieh, F.; Collignon, A.-M.; Coyac, B.R.; Lesieur, J.; Ribes, S.; Sadoine, J.; Llorens, A.; Nicoletti, A.; Letourneur, D.; Colombier, M.-L.; et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci. Rep. 2016, 6, 38814. [Google Scholar] [CrossRef] [PubMed]
- Haj, J.; Haj Khalil, T.; Falah, M.; Zussman, E.; Srouji, S. An ECM-Mimicking, Mesenchymal Stem Cell-Embedded Hybrid Scaffold for Bone Regeneration. BioMed Res. Int. 2017, 2017, 1–12. [Google Scholar] [CrossRef]
- Kjærgaard, K.; Dreyer, C.H.; Ditzel, N.; Andreasen, C.M.; Chen, L.; Sheikh, S.P.; Overgaard, S.; Ding, M. Bone Formation by Sheep Stem Cells in an Ectopic Mouse Model: Comparison of Adipose, and Bone Marrow Derived Cells and Identification of Donor-Derived Bone by Antibody Staining. Stem Cells Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Nie, L.; Yang, X.; Duan, L.; Huang, E.; Pengfei, Z.; Luo, W.; Zhang, Y.; Zeng, X.; Qiu, Y.; Cai, T.; et al. The healing of alveolar bone defects with novel bio-implants composed of Ad-BMP9-transfected rDFCs and CHA scaffolds. Sci. Rep. 2017, 7, 6373. [Google Scholar] [CrossRef]
- Lin, Y.; Umebayashi, M.; Abdallah, M.-N.; Dong, G.; Roskies, M.G.; Zhao, Y.F.; Murshed, M.; Zhang, Z.; Tran, S.D. Combination of polyetherketoneketone scaffold and human mesenchymal stem cells from temporomandibular joint synovial fluid enhances bone regeneration. Sci. Rep. 2019, 9, 472. [Google Scholar] [CrossRef]
- Medhat, D.; Rodríguez, C.I.; Infante, A. Immunomodulatory Effects of MSCs in Bone Healing. Int. J. Mol. Sci. 2019, 20, 5467. [Google Scholar] [CrossRef]
- Jayakumar, P.; Di Silvio, L. Osteoblasts in bone tissue engineering. Proc. Inst. Mech. Eng. 2010, 224, 1415–1440. [Google Scholar] [CrossRef] [PubMed]
- Aino, M.; Nishida, E.; Fujieda, Y.; Orimoto, A.; Mitani, A.; Noguchi, T.; Makino, H.; Murakami, S.; Umezawa, A.; Yoneda, T.; et al. Isolation and characterization of the human immature osteoblast culture system from the alveolar bones of aged donors for bone regeneration therapy. Expert Opin. Biol. Ther. 2014, 14, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Zhu, B.; Xu, Q.; Ding, Y.; Jin, Y. Composite cell sheet for periodontal regeneration: Crosstalk between different types of MSCs in cell sheet facilitates complex periodontal-like tissue regeneration. Stem Cell Res. Ther. 2016, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.Y.; Kwon, J.S.; Park, S.H.; Park, J.H.; Jang, S.H.; Yin, X.Y.; Yun, J.-H.; Kim, J.H.; Min, B.H.; Lee, J.H.; et al. A computer-designed scaffold for bone regeneration within cranial defect using human dental pulp stem cells. Sci. Rep. 2015, 5, 12721. [Google Scholar] [CrossRef] [PubMed]
- Marolt Presen, D.; Traweger, A.; Gimona, M.; Redl, H. Mesenchymal Stromal Cell-Based Bone Regeneration Therapies: From Cell Transplantation and Tissue Engineering to Therapeutic Secretomes and Extracellular Vesicles. Front. Bioeng. Biotechnol. 2019, 7, 352. [Google Scholar] [CrossRef] [PubMed]
- Kanke, K.; Masaki, H.; Saito, T.; Komiyama, Y.; Hojo, H.; Nakauchi, H.; Lichtler, A.C.; Takato, T.; Chung, U.; Ohba, S. Stepwise Differentiation of Pluripotent Stem Cells into Osteoblasts Using Four Small Molecules under Serum-free and Feeder-free Conditions. Stem Cell Rep. 2014, 2, 751–760. [Google Scholar] [CrossRef]
- Marolt, D.; Campos, I.M.; Bhumiratana, S.; Koren, A.; Petridis, P.; Zhang, G.; Spitalnik, P.F.; Grayson, W.L.; Vunjak-Novakovic, G. Engineering bone tissue from human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2012, 109, 8705–8709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Kimura, T.; Swami, S.; Wu, J.Y. Pluripotent stem cells as a source of osteoblasts for bone tissue regeneration. Biomaterials 2019, 196, 31–45. [Google Scholar] [CrossRef]
- Deng, P.; Yu, Y.; Hong, C.; Wang, C.-Y. Growth differentiation factor 6, a repressive target of EZH2, promotes the commitment of human embryonic stem cells to mesenchymal stem cells. Bone Res. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Jukes, J.M.; Both, S.K.; Leusink, A.; Sterk, L.M.T.; van Blitterswijk, C.A.; de Boer, J. Endochondral bone tissue engineering using embryonic stem cells. Proc. Natl. Acad. Sci. USA 2008, 105, 6840–6845. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone Tissue Engineering: Recent Advances and Challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Bilousova, G.; Jun, D.H.; King, K.B.; De Langhe, S.; Chick, W.S.; Torchia, E.C.; Chow, K.S.; Klemm, D.J.; Roop, D.R.; Majka, S.M. Osteoblasts Derived from Induced Pluripotent Stem Cells form Calcified Structures in Scaffolds Both In Vitro and In Vivo. Stem Cells 2011, 29, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Kayashima, H.; Sasaki, J.-I.; Miura, J.; Kamano, Y.; Kosaka, Y.; Imazato, S.; Yatani, H.; Matsumoto, T.; Egusa, H. Scaffold-Free Fabrication of Osteoinductive Cellular Constructs Using Mouse Gingiva-Derived Induced Pluripotent Stem Cells. Stem Cells Int. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Jungbluth, P.; Spitzhorn, L.-S.; Grassmann, J.; Tanner, S.; Latz, D.; Rahman, M.S.; Bohndorf, M.; Wruck, W.; Sager, M.; Grotheer, V.; et al. Human iPSC-derived iMSCs improve bone regeneration in mini-pigs. Bone Res. 2019, 7, 32. [Google Scholar] [CrossRef]
- Lee, M.-O.; Moon, S.H.; Jeong, H.-C.; Yi, J.-Y.; Lee, T.-H.; Shim, S.H.; Rhee, Y.-H.; Lee, S.-H.; Oh, S.-J.; Lee, M.-Y. Inhibition of pluripotent stem cell-derived teratoma formation by small molecules. Proc. Natl. Acad. Sci. USA 2013, 110, E3281–E3290. [Google Scholar] [CrossRef] [PubMed]
- Gorecka, J.; Kostiuk, V.; Fereydooni, A.; Gonzalez, L.; Luo, J.; Dash, B.; Isaji, T.; Ono, S.; Liu, S.; Lee, S.R.; et al. The potential and limitations of induced pluripotent stem cells to achieve wound healing. Stem Cell Res. Ther. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Morikawa, S.; Mabuchi, Y.; Niibe, K.; Suzuki, S.; Nagoshi, N.; Sunabori, T.; Shimmura, S.; Nagai, Y.; Nakagawa, T.; Okano, H.; et al. Development of mesenchymal stem cells partially originate from the neural crest. Biochem. Biophys. Res. Commun. 2009, 379, 1114–1119. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells and the NO pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 2695–2696. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult Mesenchymal Stem Cells: When, Where, and How. Stem Cells Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Sousa, B.R.; Parreira, R.C.; Fonseca, E.A.; Amaya, M.J.; Tonelli, F.M.; Lacerda, S.M.; Lalwani, P.; Santos, A.K.; Gomes, K.N.; Ulrich, H.; et al. Human adult stem cells from diverse origins: An overview from multiparametric immunophenotyping to clinical applications. Cytometry A 2014, 85, 43–77. [Google Scholar] [CrossRef]
- Mushahary, D.; Spittler, A.; Kasper, C.; Weber, V.; Charwat, V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A 2018, 93, 19–31. [Google Scholar] [CrossRef]
- Nugraha, A.P.; Rantam, F.A.; Narmada, I.B.; Ernawati, D.S.; Ihsan, I.S. Gingival-Derived Mesenchymal Stem Cell from Rabbit (Oryctolagus cuniculus): Isolation, Culture, and Characterization. Eur. J. Dent. 2021, 15, 332–339. [Google Scholar]
- Harkness, L.; Zaher, W.; Ditzel, N.; Isa, A.; Kassem, M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res. Ther. 2016, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- De Ugarte, D.A.; Alfonso, Z.; Zuk, P.A.; Elbarbary, A.; Zhu, M.; Ashjian, P.; Benhaim, P.; Hedrick, M.H.; Fraser, J.K. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 2003, 89, 267–270. [Google Scholar] [CrossRef]
- Alvarez, R.; Lee, H.-L.; Wang, C.-Y.; Hong, C. Characterization of the osteogenic potential of mesenchymal stem cells from human periodontal ligament based on cell surface markers. Int. J. Oral Sci. 2015, 7, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Vasanthan, J.; Gurusamy, N.; Rajasingh, S.; Sigamani, V.; Kirankumar, S.; Thomas, E.L.; Rajasingh, J. Role of Human Mesenchymal Stem Cells in Regenerative Therapy. Cells 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Chaparro, O.; Linero, I. Regenerative Medicine: A New Paradigm in Bone Regeneration. In Advanced Techniques in Bone Regeneration; Zorzi, A.R., de Miranda, J.B., Eds.; InTech: London, UK, 2016. [Google Scholar]
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Tang, X.; Gohil, S.V.; Laurencin, C.T. Biomaterials for bone regenerative engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Ko, H.; Kwon, I.K.; Shin, K. Extracellular matrix revisited: Roles in tissue engineering. Int. Neurourol. J. 2016, 20, S23. [Google Scholar] [CrossRef] [PubMed]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of Scaffolds for Bone-Tissue Regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Luo, D.; Liu, Y. Effect of the nano/microscale structure of biomaterial scaffolds on bone regeneration. Int. J. Oral Sci. 2020, 12, 6. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 1–33. [Google Scholar] [CrossRef]
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.; Malfatti, C.; Pranke, P. Biomaterials for bone regeneration: An orthopedic and dentistry overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar] [CrossRef]
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 2062. [Google Scholar] [CrossRef]
- Amin, A.M.M.; Ewais, E.M.M. Bioceramic Scaffolds. In Scaffolds in Tissue Engineering Materials, Technologies and Clinical Applications; Baino, F., Ed.; InTech: London, UK, 2017. [Google Scholar]
- Marques, A.; Miranda, G.; Silva, F.; Pinto, P.; Carvalho, Ó. Review on current limits and potentialities of technologies for biomedical ceramic scaffolds production. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 377–393. [Google Scholar] [CrossRef]
- Lobo, S.E.; Livingston Arinzeh, T. Biphasic Calcium Phosphate Ceramics for Bone Regeneration and Tissue Engineering Applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef]
- Place, E.S.; George, J.H.; Williams, C.K.; Stevens, M.M. Synthetic polymer scaffolds for tissue engineering. Chem. Soc. Rev. 2009, 38, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Yu, L.; Rowe, D.W.; Perera, I.P.; Zhang, J.; Suib, S.L.; Xin, X.; Wei, M. Intrafibrillar Mineralized Collagen–Hydroxyapatite-Based Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2020, 12, 18235–18249. [Google Scholar] [CrossRef]
- Roosa, S.M.; Kemppainen, J.M.; Moffitt, E.N.; Krebsbach, P.H.; Hollister, S.J. The pore size of polycaprolactone scaffolds has limited influence on bone regeneration in an in vivo model. J. Biomed. Mater. Res. A 2010, 92A, 359–368. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef]
- Whang, K.; Healy, K.E.; Elenz, D.R.; Nam, E.K.; Tsai, D.C.; Thomas, C.H.; Nuber, G.W.; Glorieux, F.H.; Travers, R.; Sprague, S.M. Engineering Bone Regeneration with Bioabsorbable Scaffolds with Novel Microarchitecture. Tissue Eng. 1999, 5, 35–51. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.; Wang, X.; Wu, T.; Lee, Y.; Kim, S.; Miguez, P.; Ko, C. Effect of pore size in bone regeneration using polydopamine-laced hydroxyapatite collagen calcium silicate scaffolds fabricated by 3D mould printing technology. Orthod. Craniofac. Res. 2019, 22, 127–133. [Google Scholar] [CrossRef]
- Cheng, M.; Wahafu, T.; Jiang, G.; Liu, W.; Qiao, Y.; Peng, X.; Cheng, T.; Zhang, X.; He, G.; Liu, X. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Sci. Rep. 2016, 6, 24134. [Google Scholar] [CrossRef]
- Moncal, K.K.; Aydin RS, T.; Abu-Laban, M.; Heo, D.N.; Rizk, E.; Tucker, S.M.; Lewis, G.S.; Hayes, D.; Ozbolat, I.T. Collagen-infilled 3D printed scaffolds loaded with miR-148b-transfected bone marrow stem cells improve calvarial bone regeneration in rats. Mater. Sci. Eng. C 2019, 105, 110128. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, S.-J.; Zhao, X.-R.; Zhu, Y.-F.; Yu, J.-K. 3D- Printed Poly(ε-caprolactone) Scaffold Integrated with Cell-laden Chitosan Hydrogels for Bone Tissue Engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef]
- Wang, L.; Xu, W.; Chen, Y.; Wang, J. Alveolar bone repair of rhesus monkeys by using BMP-2 gene and mesenchymal stem cells loaded three-dimensional printed bioglass scaffold. Sci. Rep. 2019, 9, 18175. [Google Scholar] [CrossRef]
- Hsieh, M.-K.; Wu, C.-J.; Su, X.-C.; Chen, Y.-C.; Tsai, T.-T.; Niu, C.-C.; Lai, P.-L.; Wu, S.-C. Bone regeneration in Ds-Red pig calvarial defect using allogenic transplantation of EGFP-pMSCs—A comparison of host cells and seeding cells in the scaffold ed G Papaccio. PLoS ONE 2019, 14, e0215499. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.; Heinayati, A.; Bao, D.; Liu, H.; Ding, X.; Tong, X.; Wang, L.; Wang, B.; Qin, H. Small molecule inhibitor of TGF-β signaling enables robust osteogenesis of autologous GMSCs to successfully repair minipig severe maxillofacial bone defects. Stem Cell Res. Ther. 2019, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Shi, Z.; Xu, H.H.K.; Yang, B.; Weir, M.D.; Li, G.; Song, Y.; Wang, J.; Hu, K.; Wang, P.; et al. Bone regeneration in minipigs via calcium phosphate cement scaffold delivering autologous bone marrow mesenchymal stem cells and platelet-rich plasma. J. Tissue Eng. Regen. Med. 2018, 12, e937–e948. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yan, B.; Cui, Z.; Cui, S.; Zhang, T.; Wang, X.; Liu, D.; Yang, R.; Jiang, N.; Zhou, Y.; et al. Bone regeneration in minipigs by intrafibrillarly-mineralized collagen loaded with autologous periodontal ligament stem cells. Sci. Rep. 2017, 7, 10519. [Google Scholar]
- Scarano, A.; Crincoli, V.; Di Benedetto, A.; Cozzolino, V.; Lorusso, F.; Podaliri Vulpiani, M.; Grano, M.; Kalemaj, Z.; Mori, G.; Grassi, F.R. Bone Regeneration Induced by Bone Porcine Block with Bone Marrow Stromal Stem Cells in a Minipig Model of Mandibular “Critical Size” Defect. Stem Cells Int. 2017, 2017, 9082869. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Wang, Y.-H.; Li, K.-C.; Sung, L.-Y.; Yeh, C.-L.; Lin, K.-J.; Yen, T.-C.; Chang, Y.-H.; Hu, Y.-C. Healing of massive segmental femoral bone defects in minipigs by allogenic ASCs engineered with FLPo/Frt-based baculovirus vectors. Biomaterials 2015, 50, 98–106. [Google Scholar] [CrossRef]
- Cao, Y.; Xiong, J.; Mei, S.; Wang, F.; Zhao, Z.; Wang, S.; Liu, Y. Aspirin promotes bone marrow mesenchymal stem cell-based calvarial bone regeneration in mini swine. Stem Cell Res. Ther. 2015, 6, 210. [Google Scholar] [CrossRef]
- Fan, H.; Zeng, X.; Wang, X.; Zhu, R.; Pei, G. Efficacy of prevascularization for segmental bone defect repair using β-tricalcium phosphate scaffold in rhesus monkey. Biomaterials 2014, 35, 7407–7415. [Google Scholar] [CrossRef]
- Lalande, C.; Miraux, S.; Derkaoui, S.; Mornet, S.; Bareille, R.; Fricain, J.; Franconi, J.; Le Visage, C.; Letourneur, D.; Amédée, J.; et al. Magnetic resonance imaging tracking of human adipose derived stromal cells within three-dimensional scaffolds for bone tissue engineering. Eur. Cells Mater. 2011, 21, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Brennan, M.Á.; Renaud, A.; Amiaud, J.; Rojewski, M.T.; Schrezenmeier, H.; Heymann, D.; Trichet, V.; Layrolle, P. Pre-clinical studies of bone regeneration with human bone marrow stromal cells and biphasic calcium phosphate. Stem Cell Res. Ther. 2014, 5, 114. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Sanchez, D.; Fernandez, D.; Rodríguez-Rey, J.C.; Perez-Campo, F.M. Enhancing survival, engraftment, and osteogenic potential of mesenchymal stem cells. World J. Stem Cells 2019, 11, 748–763. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- Evans, C. Gene therapy for the regeneration of bone. Injury 2011, 42, 599–604. [Google Scholar] [CrossRef][Green Version]
- Franceschi, R.T.; Yang, S.; Rutherford, R.B.; Krebsbach, P.H.; Zhao, M.; Wang, D. Gene Therapy Approaches for Bone Regeneration. Cells Tissues Organs 2004, 176, 95–108. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, J.S.; Yi, S.W.; Oh, H.J.; Kim, J.-H.; Park, K.-H. Sequential transfection of RUNX2/SP7 and ATF4 coated onto dexamethasone-loaded nanospheres enhances osteogenesis. Sci. Rep. 2018, 8, 1447. [Google Scholar] [CrossRef]
- Peterson, B.; Zhang, J.; Iglesias, R.; Kabo, M.; Hedrick, M.; Benhaim, P.; Lieberman, J.R. Healing of Critically Sized Femoral Defects, Using Genetically Modified Mesenchymal Stem Cells from Human Adipose Tissue. Tissue Eng. 2005, 11, 120–129. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, Q.; Kulbatski, I.; Yuan, Q.; Yang, S.; Shao, Z.; Wang, H.; Xiao, B.; Pan, Z.; Tang, S. Bone regeneration with active angiogenesis by basic fibroblast growth factor gene transfected mesenchymal stem cells seeded on porous β-TCP ceramic scaffolds. Biomed. Mater. 2006, 1, 93–99. [Google Scholar] [CrossRef]
- Qu, D.; Li, J.; Li, Y.; Gao, Y.; Zuo, Y.; Hsu, Y.; Hu, J. Angiogenesis and osteogenesis enhanced by bFGF ex vivo gene therapy for bone tissue engineering in reconstruction of calvarial defects. J. Biomed. Mater. Res. A 2011, 96A, 543–551. [Google Scholar] [CrossRef]
- Leng, Q.; Chen, L.; Lv, Y. RNA-based scaffolds for bone regeneration: Application and mechanisms of mRNA, miRNA and siRNA. Theranostics 2020, 10, 3190–3205. [Google Scholar] [CrossRef]
- Zhu, L.; Chuanchang, D.; Wei, L.; Yilin, C.; Jiasheng, D. Enhanced healing of goat femur-defect using BMP7 gene-modified BMSCs and load-bearing tissue-engineered bone: BMP7 Gene-modified bmscs enhance bone regeneration. J. Orthop. Res. 2010, 28, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Dilogo, I.H.; Rahmatika, D.; Pawitan, J.A.; Liem, I.K.; Kurniawati, T.; Kispa, T.; Mujadid, F. Allogeneic umbilical cord-derived mesenchymal stem cells for treating critical-sized bone defects: A translational study. Eur. J. Orthop. Surg. Traumatol. 2021, 31, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.A.; Kop, A.M.; Nilasaroya, A.; Sturm, M.; Shaw, K.; Honeybul, S. Cranial reconstruction using allogeneic mesenchymal stromal cells: A phase 1 first-in-human trial. J. Tissue Eng. Regen. Med. 2018, 12, 341–348. [Google Scholar] [CrossRef]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem Cells Associated with Macroporous Bioceramics for Long Bone Repair: 6- to 7-Year Outcome of a Pilot Clinical Study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Dilogo, I.H.; Phedy, P.; Kholinne, E.; Djaja, Y.P.; Fiolin, J.; Kusnadi, Y.; Yulisa, N.D. Autologous mesenchymal stem cell implantation, hydroxyapatite, bone morphogenetic protein-2, and internal fixation for treating critical-sized defects: A translational study. Int. Orthop. 2019, 43, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Kaigler, D.; Avila-Ortiz, G.; Travan, S.; Taut, A.D.; Padial-Molina, M.; Rudek, I.; Wang, F.; Lanis, A.; Giannobile, W.V. Bone Engineering of Maxillary Sinus Bone Deficiencies Using Enriched CD90+ Stem Cell Therapy: A Randomized Clinical Trial: Stem cell therapy for advanced maxillary bone defects. J. Bone Miner. Res. 2015, 30, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Gjerde, C.; Mustafa, K.; Hellem, S.; Rojewski, M.; Gjengedal, H.; Yassin, M.A.; Feng, X.; Skaale, S.; Berge, T.; Rosen, A.; et al. Cell therapy induced regeneration of severely atrophied mandibular bone in a clinical trial. Stem Cell Res. Ther. 2018, 9, 213. [Google Scholar] [CrossRef]
- Baba, S.; Yamada, Y.; Komuro, A.; Yotsui, Y.; Umeda, M.; Shimuzutani, K.; Nakamura, S. Phase I/II Trial of Autologous Bone Marrow Stem Cell Transplantation with a Three-Dimensional Woven-Fabric Scaffold for Periodontitis. Stem Cells Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative Effects of Transplanted Mesenchymal Stem Cells in Fracture Healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef]
- Bajada, S.; Harrison, P.E.; Ashton, B.A.; Cassar-Pullicino, V.N.; Ashammakhi, N.; Richardson, J.B. Successful treatment of refractory tibial nonunion using calcium sulphate and bone marrow stromal cell implantation. J. Bone Jt. Surg. Br. 2007, 89-B, 1382–1386. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Honoki, K.; Ohgushi, H.; Kotobuki, N.; Matsushima, A.; Takakura, Y. Tissue Engineering Approach to the Treatment of Bone Tumors: Three Cases of Cultured Bone Grafts Derived From Patients’ Mesenchymal Stem Cells. Artif. Organs 2006, 30, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Mai, R.; Hagedorn, M.G.; Gelinsky, M.; Werner, C.; Turhani, D.; Späth, H.; Gedrange, T.; Lauer, G. Ectopic bone formation in nude rats using human osteoblasts seeded poly(3)hydroxybutyrate embroidery and hydroxyapatite-collagen tapes constructs. J. Cranio-Maxillofac. Surg. 2006, 34, 101–109. [Google Scholar] [CrossRef]
- Ortiz, M.; Escobar-Garcia, D.M.; Álvarez-Pérez, M.A.; Pozos-Guillén, A.; Grandfils, C.; Flores, H. Evaluation of the Osteoblast Behavior to PGA Textile Functionalized with RGD as a Scaffold for Bone Regeneration. J. Nanomater. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Gutiérrez-Prieto, S.J.; Perdomo-Lara, S.J.; Diaz-Peraza, J.M.; Sequeda-Castañeda, L.G. Analysis of In Vitro Osteoblast Culture on Scaffolds for Future Bone Regeneration Purposes in Dentistry. Adv. Pharmacol. Sci. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Regenerative Medicine Institute; National Centre for Biomedical Engineering Science; National University of Ireland; Galway Czekanska, E.; Stoddart, M.; Richards, R.; Hayes, J. In search of an osteoblast cell model for in vitro research. Eur. Cell. Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Borciani, G.; Montalbano, G.; Baldini, N.; Cerqueni, G.; Vitale-Brovarone, C.; Ciapetti, G. Co–culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020, 108, 22–45. [Google Scholar] [CrossRef]
- Hu, K.; Olsen, B.R. Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J. Clin. Investig. 2016, 126, 509–526. [Google Scholar] [CrossRef]
- Marolt, D.; Rode, M.; Kregar-Velikonja, N.; Jeras, M.; Knezevic, M. Primary Human Alveolar Bone Cells Isolated from Tissue Samples Acquired at Periodontal Surgeries Exhibit Sustained Proliferation and Retain Osteogenic Phenotype during In Vitro Expansion. PLoS ONE 2014, 9, e92969. [Google Scholar] [CrossRef]
- Skottke, J.; Gelinsky, M.; Bernhardt, A. In Vitro Co-culture Model of Primary Human Osteoblasts and Osteocytes in Collagen Gels. Int. J. Mol. Sci. 2019, 20, 1998. [Google Scholar] [CrossRef]
- El-Amin, S.F.; Botchwey, E.; Tuli, R.; Kofron, M.D.; Mesfin, A.; Sethuraman, S.S.; Tuan, R.; Laurencin, C.T. Human osteoblast cells: Isolation, characterization, and growth on polymers for musculoskeletal tissue engineering. J. Biomed. Mater. Res. A 2006, 76A, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, K.B.; Frost, A.; Nilsson, O.; Ljunghall, S.; Ljunggren, Ö. Three isolation techniques for primary culture of human osteoblast-like cells: A comparison. Acta Orthop. Scand. 1999, 70, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Pradel, W.; Lauer, G. Tissue-Engineered Bone Grafts for Osteoplasty in Patients with Cleft Alveolus. Ann. Anat. Anat. Anz. 2012, 194, 545–548. [Google Scholar] [CrossRef]
- Pradel, W.; Eckelt, U.; Lauer, G. Bone regeneration after enucleation of mandibular cysts: Comparing autogenous grafts from tissue-engineered bone and iliac bone. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Springer, I.N.; Nocini, P.F.; Schlegel, K.A.; Santis, D.D.; Park, J.; Warnke, P.H.; Terheyden, H.; Zimmermann, R.; Chiarini, L.; Gardner, K. Two techniques for the preparation of cell-scaffold constructs suitable for sinus augmentation: Steps into clinical application. Tissue Eng. 2006, 12, 2649–2656. [Google Scholar] [CrossRef]
| Author | Experiment Animal | Type and Size of Defect | Experimental Transplant Groups | Post-Transplant Follow up Period | Outcome |
|---|---|---|---|---|---|
| Probst et al., 2020 [50] | Mini pigs | Critical mandibular defect (3 × 1 × 2 cm) | 3D TCP-PLGA scaffold seeded with osteogenic differentiated Porcine ADSCs (pADSCs). | 12 weeks | pADSCs seeded TCP-PLGA scaffold constructs significantly improved bone regenerations compared to empty scaffold. |
| Wang et al., 2019 [64] | Rhesus Monkeys | Critical alveolar bone defect (10 × 10 × 5 mm) | 3D-Bioactive glass (BG) + BMP/chitosan (CS) + BMMSCs | 12 weeks | BMP/CS nanoparticles loaded on 3D-BG scaffold promoted bone regeneration ability in vivo, and preload of BMMSCs promote this ability further. |
| Hsieh et al., 2019 [65] | domestic Ds-Red pigs | Calvarias defect (8 mm in diameter and 2 mm in depth) | Hemostatic gelatin sponge scaffold seeded with EGFP pig BMMSCs | 1, 2, 3 and 4 weeks | Osteoid formation in the scaffolds transplanted with seeded BMMSCs was significantly higher than the control group. |
| Shi et al., 2019 [66] | Minipigs | Maxillary Intraosseous circular defects (12 mm in diameter and 5 mm in depth) | Bio-Oss/autogenous (Pig Gingival MSCs) pGMSCs (2 × 106)/SB431542 (TGF-β signalling inhibitor). | 8 weeks | pGMSCs treated with a TGF- β signaling inhibitor successfully repair minipig severe maxillofacial bone defects. |
| Qiu et al., 2018 [67] | Minipigs | Lateral femoral condyle defect (8 mm in diameter and 10 mm in depth) | Calcium phosphate cement (CPC) scaffold seeded with autologous BMMSCs plus autologous PRP (CPC-BMSC-PRP, 1 × 106 cells/scaffold) | 6 and 12 weeks | CPC scaffold co-delivered BMMSCs-PRP promoted scaffold resorption and doubled bone regeneration in large defects than control groups |
| Zhang et al., 2017 [68] | Minipigs | Non-healing full thickness cranial defects (2 cm width × 3 cm length × 0.5 cm depth) | IMC (intrafibrillarly-mineralized collagen) scaffold seeded with 1 × 106 PDLSCs cells | 12 weeks | Compared with HA, IMC-seeded PDLSCs achieved a significantly higher extent of new bone formation, with the normal architecture of natural bones and blood vessels. |
| Scarano et al., 2017 [69] | Minipigs | Critical-size circular defects (5 mm diameter; 5 mm thickness) in the mandibular body | Bone porcine block (BPB) scaffold seeded with 100 ul cell suspension of BMMSCs | 12 weeks | BPB when used as a scaffold induce bone regeneration and further benefit from the addition of BMMSCs in the tissue-engineered constructs. |
| Lin et al., 2015 [70] | Minipigs | Massive segmental bone defects (30 mm in length) at the mid-diaphysis of femora | Transduced pig ADSCs loaded onto PLGA scaffold | 2, 4, 8 and 12 weeks | ADSCs/scaffold constructs successfully healed massive segmental bone defects at the mid-diaphysis of femora in minipigs significantly than control group. |
| Cao et al., 2015 [71] | Mini pigs | Calvarial bone defects (3 cm × 1.8 cm oval defect) | BMMSCs pretreated with 75 μg/mL aspirin for 24 h seeded onto hydroxyapatite/tricalcium phosphate (HA/TCP) | 6 months | BMMSCs pretreated with aspirin have a greater capacity to repair calvarial bone defects in a mini swine model |
| Fan et al., 2014 [72] | Rhesus monkeys | Segmental tibial defects (20 mm in length) | Autologous prevascularized BMMSCs (5 × 106)-β-TCP constructs | 4, 8 and 12 weeks | Significantly higher amount of neo-vascularization and radiographic grading score in prevascularized BMMSCs-β-TCP constructs |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkataiah, V.S.; Yahata, Y.; Kitagawa, A.; Inagaki, M.; Kakiuchi, Y.; Nakano, M.; Suzuki, S.; Handa, K.; Saito, M. Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy. Cells 2021, 10, 2687. https://doi.org/10.3390/cells10102687
Venkataiah VS, Yahata Y, Kitagawa A, Inagaki M, Kakiuchi Y, Nakano M, Suzuki S, Handa K, Saito M. Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy. Cells. 2021; 10(10):2687. https://doi.org/10.3390/cells10102687
Chicago/Turabian StyleVenkataiah, Venkata Suresh, Yoshio Yahata, Akira Kitagawa, Masahiko Inagaki, Yusuke Kakiuchi, Masato Nakano, Shigeto Suzuki, Keisuke Handa, and Masahiro Saito. 2021. "Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy" Cells 10, no. 10: 2687. https://doi.org/10.3390/cells10102687
APA StyleVenkataiah, V. S., Yahata, Y., Kitagawa, A., Inagaki, M., Kakiuchi, Y., Nakano, M., Suzuki, S., Handa, K., & Saito, M. (2021). Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy. Cells, 10(10), 2687. https://doi.org/10.3390/cells10102687






