Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress
Abstract
:1. Introduction
2. Plant Abiotic Stresses and Reactive Oxygen Species
2.1. ROS Generation under Abiotic Stresses
2.2. Generation of ROS and Its Consequence
2.3. Antioxidant Defense System in Plants
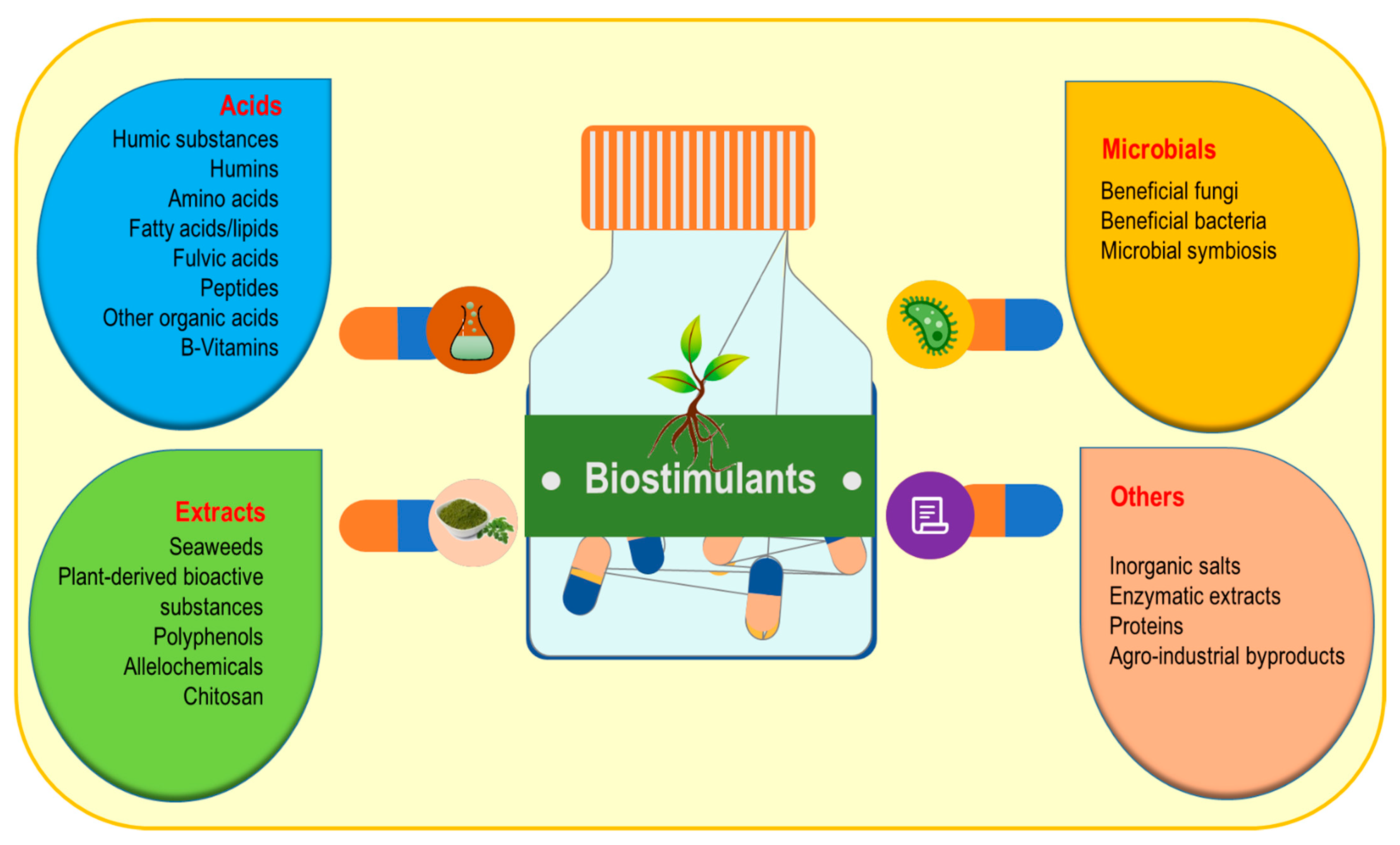
3. Biostimulants: Types, Mode of Action and Methods of Applications
3.1. Microbial Biostimulants
3.2. Acids
3.3. Extract-Type Products
3.4. Other Biostimulants
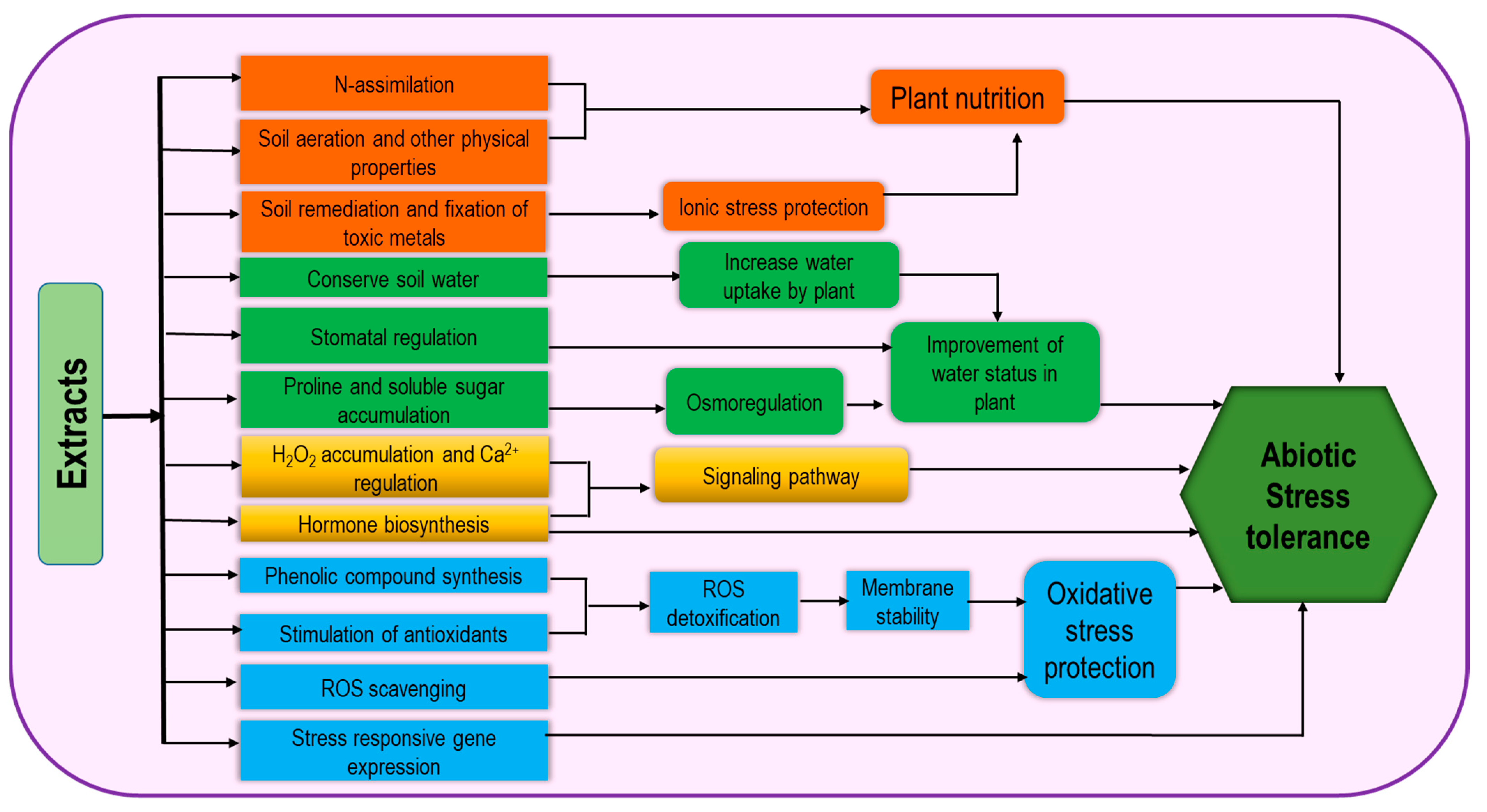
4. Biostimulants for the Regulation of ROS under Abiotic Stresses
4.1. Drought
4.2. Salinity
| Crop Species | Salinity Levels and Duration | Biostimulant Type and Dose | Antioxidant Defense and ROS Regulatory Effects | Reference |
|---|---|---|---|---|
| Triticum aestivum L. cv. Sakha 93 | 9.10 dS m−1 NaCl; 30 d after sowing (DAS) to 50 DAS | Fresh MLE (3%) and GSH (1 mM) | Increased endogenous GSH and AsA levels. Stabilized membrane integrity Decreased EL. Prevented chl breakdown. | [107] |
| Vigna unguiculata | Seawater, 3.5 and 7 dS m−1; vegetative stage | Foeniculum vulgare (FSE) and Ammi seed extracts | Decreased EL, MDA, H2O2, and O2•− Improved membrane stability index (MSI). | [112] |
| Dracocephalum moldavica L. | 50–100 mM NaCl | Fe2O3 nanoparticle; 30, 60, and 90 ppm | Increased total phenolic, flavonoid and anthocyanin contents. Improved the activities of guaiacol peroxidase, APX, CAT and GR. | [113] |
| Cucumis sativus L. | 50 mmol L−1 NaCl, at vegetative stage | ALA, 25 mg L−1 | Decreased H2O2 and MDA levels Increased AsA/DHA, GSH/GSSG. Increased ascorbic acid oxidase (AAO), APX, MDHAR and DHAR activity. Augmenting the AsA/GSH pathway exogenous ALA diminished the H2O2 level. | [110] |
| Solanum lycopersicum L. cv. Pusa Ruby | 150 mM NaCl; at 10-d-old seedlings for 5 d | Vanillic acid (40 and 50 μM | Upregulation of AsA and GSH level. Improvement of APX, MDHAR, DHAR and GR activity. Downregulated ROS generation. Decreased LOX activity and membrane injury. | [111] |
| Brassica napus L. | 1.5 dS m−1, 5 dS m−1 and 10 dS m−1 NaCl; throughout the growing period | Ca-fortified composted animal manure (Ca-FCM; 1, 2 and 3%) | Modulation of SOD, APX, CAT, GPX, GR and GST activities. Decreased EL and chl breakdown. | [114] |
| Chenopodium quinoa | Saline soil, 20 dS m−1, throughout the growing period | Burkholderia phytofirmans PsJN (CFU = 109) and biochar (1% w/w) | MDA and O2•− content decreased. Modulated SOD, APX, GR, GPX and GST activity. Modulated the GSH, GSSG and GSH/GSSG. Improvement of relative membrane permeability and membrane stability index. | [115] |
| Catharanthus roseus | 150 mM NaCl, vegetative stage | Chitosan nanoparticles (CSNPs, 1%) | Impeded chl diminution. Stimulated CAT, APX and GR activity Lessened MDA level and H2O2 production. | [108] |
| Arachis hypogaea L. | 2.5, 5, 7.5, 10, 12.5, and 15% NaCl; 72 h | Endophytes like Bacillus firmus J22N and Bacillus sp. REN51N | Increased activity of SOD, GR, CAT and APX. Decreased H2O2. | [116] |
| Phoenix dactylifera cv. Boufeggous | 240 mM NaCl; 5 months after germination, 2 weeks | AMF and/or compost | Pro and soluble sugar regulation. Improved SOD, APX and CAT activities. Reduced H2O2 content and lipid peroxidation. Checked chl degradation. | [109] |
| Vigna radiata | 150 mM NaCl; After 5 d of spore suspension application NaCl was added up to 35 d | Aspergillus awamori (EWF) | Pro, polyphenols, flavonoids and tannin accumulation increased. CAT and APX activity enhanced. Lipid peroxidation reduced. | [117] |
4.3. High Temperature
4.4. Low Temperature
| Crop Species | Level of Stress and Duration | Biostimulants and Dose | Beneficial Effects | Reference |
|---|---|---|---|---|
| Glycine max L. | 35 °C, 2 h each for 2 d | FA, 2.0 mg L−1 | Increased RWC and activity of SOD, APX and GST. Reduced oxidative damage, H2O2 and MDA content. | [137] |
| Spinacia oleracea | 30 °C, 6 h | SWE, 0.15, 0.30, 0.60 and 1.2% | Reduced MDA and H2O2 contents. | [130] |
| Triticum aestivum | 40 °C, 12 h | PGPRs strains of Ochrobactrum pseudogrignonense and Bacillus safensis | Improved cell viability, SOD, POX, CAT, APX and GR activity. Reduced EL, H2O2, O2•− and membrane damage. | [122] |
| Triticum aestivum | 37–40 °C, 95 d | PGPRs strains (Pseudomonas putida; AKMP7) | Reduced membrane damage and ROS generation. Increased SOD, APX and CAT activity. Improved Pro and sugar content. | [57] |
| Lycopersicon esculentum Mill. | 38 °C, 7 d | PGPRs strains (Agrobacterium tumefaciens) | Reduced EL and lipid peroxidation. Increased SOD, CAT, POD and APX activity. | [138] |
| Solanum lycopersicum L. landraces E17, E36, E107, PDVIT | Elevated temperature (up to 42 °C) for whole growing period | CycoFlow (sugarcane molasses with yeast extract), 400 mL plant−1 | Increased the content of reduced AsA and total AsA. Reduced the hydrophilic antioxidant activity and enhanced the lipophilic antioxidant activity. | [139] |
| Coriandrum sativum L. | 6 °C, 6 d | Asahi SL (synthetic) and Goëmar Goteo (Agrobacterium nodosum) as 0.1%, foliar spray | Reduced the content of MDA and H2O2 content as well as the EL. Increased total antioxidant activity, total phenolic content. | [135] |
| Oryza sativa L. | 10 °C, 21 d | Biochar, 1, 3, 5, 7 and 10% | Increased soluble sugar content, antioxidant activity, SOD and POD activity. Reduced lipid peroxidation. | [134] |
| Solanum melongena L. cv. Yalda | 5 °C, 7 d | AMF (Funneliformis mosseae, Claroideoglomus etunicatum, Rhizophagus irregularis, and Diversispora versiformis) | Enhanced SOD, CAT, APX, PAL and POD activity. Increased carbohydrate, soluble sugar and free phenolics content. Reduced membrane damages, EL and H2O2 content. | [131] |
| Elymus nutans | 5 °C, 5 d | AMF (Glomas mosseae) | Decreased oxidative damage, EL, H2O2 and O2•−. Increased SOD, CAT, APX and GR activity. Improved antioxidant components such as GSH and soluble sugar content. | [132] |
| Citrullus lanatus Thunb. cvs. Crimson Sweet and Charleston Gray | 4 °C, 36 h | AMF (Glomus intraradices) | Lowered the EL, MDA and H2O2 contents. Enhanced POX activities. | [140] |
| Lolium perenne L. | 4.2 °C (average), 10 d | AMF (Glomas intraradices) | Increased activities of SOD, POD and CAT. Reduced MDA content. | [141] |
| Lolium perenne L. | 4.2 °C (average), 10 d | Biochar, 4% | Increased activities POD and CAT but declined SOD activity and MDA content. | [141] |
| Hordeum vulgare L. cvs. Abida and Nik | 5 °C, 21 d | AMF (Rhizophagus irregularis) | Reduced membrane leakage, MDA and H2O2 contents. Upregulated SOD, CAT and POD activity. | [142] |
| Camellia sinensis L. O. Kuntze cv. Anji Baicha | −4 and −8 °C, 24 h | Chitosan oligosaccharide (COS) solution, 1.25 mL L−1 | Enhanced SOD and POD activity. | [143] |
4.5. Metal/Metalloid Toxicity
| Crop Species | Metal/Metalloid Dose and Duration | Biostimulant Type and Dose | ROS Regulatory Effects of Biostimulants Used | Reference |
|---|---|---|---|---|
| Oryza sativa L. cv. BRRI dhan29 | Cd (0.25 and 0.5 mM CdCl2), 3 d | Ca (2.5 mM CaCl2), co-treatment | MDA and H2O2 contents, and LOX activity were reduced. Increased contents of DHA and GSSG were diminished by Ca, which was vice-versa for AsA. Enhancement in MDHAR, DHAR, GR and SOD activities. | [161] |
| Oryza sativa L. cv. BRRI dhan29 | Cd (0.3 mM CdCl2), 3 d | Mn (0.3 mM MnSO4), co-treatment | MDA, H2O2 contents and LOX activity were reduced. Increased AsA and decreased DHA contents. Increased DHAR and CAT activities. Enhanced SOD and MDHAR activities. | [162] |
| Brassica juncea L. cv. BARI Sharisha-11 | Cd stress (0.5 and 1.0 mM CdCl2), 3 d | Citric acid (0.5 and 1.0 mM), co-treatment | MDA, H2O2 contents and LOX activity decreased. AsA and GSH contents increased but DHA and GSSG contents decreased. SOD, CAT, DHAR, MDHAR, and GR activities upregulated. | [148] |
| Brassica. juncea L. cv. BARI Sharisha-11 | Cd stress (0.5 and 1.0 mM CdCl2), 3 d | EDTA (0.5 mM), co-treatment | 26, 26, and 28% reduction in TBARS, H2O2 contents and LOX activity, respectively in 1.0 mM Cd-stressed seedlings compared to Cd-stressed seedlings alone. AsA content was restored but DHA and GSSG contents reduced, while GSH level further increased. AsA-GSH pathway enzyme activities increased along with SOD, CAT and GPX activities. | [149] |
| Lactuca sativa L. | Cd (20 μM), 14 d | FA (0.5 g L−1), foliar application | EL, MDA, H2O2 and O2•− contents were reduced. Reduced SOD and POD activities and increased CAT and APX activities. | [150] |
| Lepidium sativum cv. Helen | Cd (100 and 200 mg kg−1 soil) | HA + FA (3500, 5250 and 7000 mg L−1), soil drenching | Minimized MDA and H2O2 contents. Differential changes in the data of CAT, POD and SOD activities were reported. | [151] |
| Brassica chinensis L. | Cd (5 and 10 mg kg−1 soil), 30 d | Biochar (2.5 and 5%) | Efficient reduction in MDA and H2O2 contents were documented. GSH content and POD, SOD, APX, CAT activities increased while GR activity was decreased. | [154] |
| Spinacia oleracea | Cd (25, 50 and 100 mg kg−1 soil), 52 d | Biochar (3 and 5%) | The contents of MDA and AsA were reduced. | [153] |
| Arabidopsis thaliana | Cd (10, 50, 100 mg kg−1 soil) or Pb (100, 500, 1000 mg kg−1 soil), 35 d | Mucor circinelloides (MC) or Trichoderma asperellum (TA) | Increased activities of SOD and CAT. | [158] |
| Zea mays | Cd (1 or 5 mg kg−1 soil), 70 d | AMF (Rhizophagus intraradices and Glomas versiforme) (5%) | Induced higher GSH and phytochelatins production. | [157] |
| Brassica juncea L. cv. BARI Sharisha-11 | Cr (0.15 and 0.3 mM K2CrO4), 5 d | GABA (125 μM), co-treatment | Reductions in MDA, H2O2 contents and LOX activity were observed. AsA and GSH contents increased but DHA and GSSG contents decreased. Activities of antioxidant enzymes measured were upregulated, except for APX at severe stress. | [159] |
| Brassica juncea L. cv. BARI Sharisha-11 | Cr (0.15 and 0.3 mM K2CrO4), 5 d | Maleic acid (0.25 mM), co-treatment | MDA, H2O2 contents and LOX activity were reduced. AsA and GSH contents increased but DHA and GSSG contents decreased. Activities of antioxidative enzymes measured were upregulated. | [147] |
| Triticum aestivum cv. Lasani 2008 | Cr (0.25 and 0.5 mM K2Cr2O7), 90 d | FA (1.5 mg L−1), foliar spray | Upregulation of CAT and APX activities in both shoot and root were observed. | [152] |
| Oryza sativa L. cv. BRRI dhan29 | As (0.5 and 1 mM Na2HAsO4), 5 d | Ca (10 mM CaCl2), co-treatment | MDA and H2O2 contents decreased by 27 and 13%, respectively by Ca supplementation in 1 mM As-stressed seedlings. Modulated AsA, DHA, GSH and GSSG level. Activities of SOD, CAT, APX and MDHAR increased. | [163] |
| Medicago sativa | Cu contaminated soil, 90 d | Paenibacillu smucilaginosus and Sinorhizobium meliloti co-inoculation | Reduced the MDA, H2O2 and O2•− contents. Lower SOD, CAT and APX activities were recorded. | [160] |
| Allium cepa L. | Cu (50, 100 or 250 µM CuSO4·5H2O), 8 d | Trichoderma asperellum inoculation | Decreased MDA content. | [159] |
4.6. Waterlogging/Flooding
5. Limitations of Using Biostimulants
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020. Transforming Food System for Affordable Healthy Diets; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Gerland, P.; Raftery, A.E.; Ševčíková, H.; Li, N.; Gu, D.; Spoorenberg, T.; Alkema, L.; Fosdick, B.K.; Chunn, J.; Lalic, N.; et al. World population stabilization unlikely this century. Science 2014, 346, 234–237. [Google Scholar] [CrossRef] [Green Version]
- Fróna, D.; Szenderák, J.; Harangi-Rákos, M. The challenge of feeding the world. Sustainability 2019, 11, 5816. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.K.; Ramankutty, N.; Mueller, N.D.; West, P.C.; Foley, J.A. Recent patterns of crop yield growth and stagnation. Nat. Commun. 2012, 3, 1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobell, D.B.; Cassman, K.G.; Field, C.B. Crop yield gaps: Their importance, magnitudes, and causes. Annu. Rev. Environ. Resour. 2009, 34, 179–204. [Google Scholar] [CrossRef] [Green Version]
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Sachdev, S.; Ansari, S.A.; Ansari, M.I.; Fujita, M.; Hasanuzzaman, M. Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 2021, 10, 277. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [Green Version]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef]
- Hossain, M.S.; Dietz, K.-J. Tuning of redox regulatory mechanisms, reactive oxygen species and redox homeostasis under salinity stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993, 125, 27–58. [Google Scholar] [CrossRef]
- Biehler, K.; Fock, H. Evidence for the contribution of the Mehler-Peroxidase reaction in dissipating excess electrons in drought-stressed wheat. Plant Physiol. 1996, 112, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Geilfus, C.-M.; Mithöfer, A.; Ludwig-Müller, J.; Zörb, C.; Muehling, K.H. Chloride-inducible transient apoplastic alkalinizations induce stomata closure by controlling abscisic acid distribution between leaf apoplast and guard cells in salt-stressed Vicia faba. New Phytol. 2015, 208, 803–816. [Google Scholar] [CrossRef]
- Osmond, C.B.; Foyer, C.H.; Bock, G.; Wingler, A.; Lea, P.J.; Quick, W.P.; Leegood, R.C. Photorespiration: Metabolic pathways and their role in stress protection. Philos. Trans. R. Soc. Lond. Ser. B 2000, 355, 1517–1529. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, S.; Liu, Y.; Ma, C. Redox regulated peroxisome homeostasis. Redox Biol. 2015, 4, 104–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.H.; Song, X.S.; Shi, K.; Xia, X.J.; Zhou, Y.H.; Yu, J.Q. Changes in electron transport, superoxide dismutase and ascorbate peroxidase isoenzymes in chloroplasts and mitochondria of cucumber leaves as influenced by chilling. Photosynthetica 2008, 46, 581. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Van Aken, O.; Schwarzländer, M.; Belt, K.; Millar, A.H. The roles of mitochondrial reactive oxygen species in cellular signaling and stress response in plants. Plant Physiol. 2016, 171, 1551–1559. [Google Scholar] [CrossRef] [Green Version]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Mailloux, R.J. An update on mitochondrial reactive oxygen species production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef] [PubMed]
- Dat, J.; Vandenabeele, S.; Vranová, E.; Van Montagu, M.; Inzé, D.; Van Breusegem, F. Dual action of the active oxygen species during plant stress responses. CMLS Cell. Mol. Life Sci. 2000, 57, 779–795. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.H.C. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, Z.-M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Pei, Z.-M.; Mori, I.C.; Schroeder, J. Abscisic acid activation of plasma membrane Ca2+ channels in guard cells requires cytosolic NAD(P)H and Is differentially disrupted upstream and downstream of reactive oxygen species production in abi1-1 and abi2-1 protein phosphatase 2C mutants. Plant Cell 2001, 13, 2513–2523. [Google Scholar] [CrossRef] [Green Version]
- Møller, I.M.; Rogowska-Wrzesinska, A.; Rao, R.S.P. Protein carbonylation and metal-catalyzed protein oxidation in a cellular perspective. J. Proteom. Redox Proteom. 2011, 74, 2228–2242. [Google Scholar] [CrossRef]
- Anjum, N.A.; Sofo, A.; Scopa, A.; Roychoudhury, A.; Gill, S.S.; Iqbal, M.; Lukatkin, A.S.; Pereira, E.; Duarte, A.C.; Ahmad, I. Lipids and proteins-major targets of oxidative modifications in abiotic stressed plants. Environ. Sci. Pollut. Res. 2015, 22, 4099–4121. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Møller, I.M. Oxidation of proteins in plants-mechanisms and consequences. In Advances in Botanical Research; Academic Press: New York, NY, USA, 2009; pp. 1–23. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef] [Green Version]
- Hollósy, F. Effects of ultraviolet radiation on plant cells. Micron 2002, 33, 179–197. [Google Scholar] [CrossRef]
- Hameed, A.; Bibi, N.; Akhter, J.; Iqbal, N. Differential changes in antioxidants, proteases, and lipid peroxidation in flag leaves of wheat genotypes under different levels of water deficit conditions. Plant Physiol. Biochem. 2011, 49, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyer, C.H.; Ruban, A.V.; Noctor, G. Viewing oxidative stress through the lens of oxidative signalling rather than damage. Biochem. J. 2017, 474, 877–883. [Google Scholar] [CrossRef] [Green Version]
- Montoro, P.; Braca, A.; Pizza, C.; De Tommasi, N. Structure-antioxidant activity relationships of flavonoids isolated from different plant species. Food Chem. 2005, 92, 349–355. [Google Scholar] [CrossRef]
- Kibria, M.G.; Hossain, M.; Murata, Y.; Hoque, M.A. Antioxidant defense mechanisms of salinity tolerance in rice genotypes. Rice Sci. 2017, 24, 155–162. [Google Scholar] [CrossRef]
- Duan, Y.; Santiago, F.E.M.; dos Reis, A.R.; de Figueiredo, M.A.; Zhou, S.; Thannhauser, T.W.; Li, L. Genotypic variation of flavonols and antioxidant capacity in broccoli. Food Chem. 2021, 338, 127997. [Google Scholar] [CrossRef]
- Dumont, S.; Rivoal, J. Consequences of oxidative stress on plant glycolytic and respiratory metabolism. Front. Plant Sci. 2019, 10, 166. [Google Scholar] [CrossRef]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The use of biostimulants for enhancing nutrient uptake. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 141–174. [Google Scholar]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, M.H.M.B.; Mohsin, S.M.; Mahmud, J.A.; Hasanuzzaman, M. Use of Biostimulants for Improving Abiotic Stress Tolerance in Brassicaceae Plants. In The Plant Family Brassicaceae; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 497–531. [Google Scholar]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascale, S.D.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2017, 82, 277–285. [Google Scholar] [CrossRef]
- Ruzzi, M.; Aroca, R. Plant growth-promoting rhizobacteria act as biostimulants in horticulture. Sci. Hortic. 2015, 196, 124–134. [Google Scholar] [CrossRef]
- Turan, M.; Yildirim, E.; Kitir, N.; Unek, C.; Nikerel, E.; Ozdemir, B.S.; Güneş, A.; Mokhtari, N.E.P. Beneficial role of plant growth-promoting bacteria in vegetable production under abiotic stress. In Microbial Strategies for Vegetable Production; Zaidi, A., Khan, M.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 151–166. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, A.S.; Grover, M.; Reddy, G.; Venkateswarlu, B. Alleviation of drought stress effects in sunflower seedlings by the exopolysaccharides producing Pseudomonas putida strain GAP-P45. Biol. Fertil. Soils 2009, 46, 17–26. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Miyakawa, T.; Fujita, Y.; Yamaguchi-Shinozaki, K.; Tanokura, M. Structure and function of abscisic acid receptors. Trends Plant Sci. 2013, 18, 259–266. [Google Scholar] [CrossRef]
- Theocharis, A.; Bordiec, S.; Fernandez, O.; Paquis, S.; Dhondt-Cordelier, S.; Baillieul, F.; Clément, C.; Barka, E.A. Burkholderia phytofirmans PsJN primes Vitis vinifera L. and confers a better tolerance to low nonfreezing temperatures. Mol. Plant Microbe Interact. 2012, 25, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Linga, V.R.; Bandi, V. Effect of inoculation with a thermotolerant plant growth promoting Pseudomonas putida strain AKMP7 on growth of wheat (Triticum spp.) under heat stress. J. Plant Interact. 2011, 6, 239–246. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, P.; Kim, K.; Krishnamoorthy, R.; Mageswari, A.; Selvakumar, G.; Sa, T. Cold stress tolerance in psychrotolerant soil bacteria and their conferred chilling resistance in tomato (Solanum lycopersicum Mill.) under low temperatures. PLoS ONE 2016, 11, e0161592. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Canellas, L.P.; Dobbss, L.B.; Oliveira, A.L.; Chagas, J.G.; Aguiar, N.O.; Rumjanek, V.M.; Novotny, E.H.; Olivares, F.L.; Spaccini, R.; Piccolo, A. Chemical properties of humic matter as related to induction of plant lateral roots. Eur. J. Soil Sci. 2012, 63, 315–324. [Google Scholar] [CrossRef]
- Çimrin, K.M.; Türkmen, Ö.; Turan, M.; Tuncer, B. Phosphorus and humic acid application alleviate salinity stress of pepper seedling. Afr. J. Biotechnol. 2010, 9, 5845–5851. [Google Scholar]
- Aydin, A.; Kant, C.; Turan, M. Humic acid application alleviates salinity stress of bean (Phaseolus vulgaris L.) plants decreasing membrane leakage. Afr. J. Agric. Res. 2012, 7, 1073–1086. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Farrell, M.; Prendergast-Miller, M.; Jones, D.L.; Hill, P.W.; Condron, L.M. Soil microbial organic nitrogen uptake is regulated by carbon availability. Soil Biol. Biochem. 2014, 77, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Schiavon, M.; Ertani, A.; Nardi, S. Effects of an alfalfa protein hydrolysate on the gene expression and activity of enzymes of the tricarboxylic acid (TCA) cycle and nitrogen metabolism in Zea mays L. J. Agric. Food Chem. 2008, 56, 11800–11808. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006, 41, 1296–1302. [Google Scholar] [CrossRef]
- Ramya, S.S.; Vijayanand, N.; Rathinavel, S. Foliar application of liquid biofertilizer of brown alga Stoechospermum marginatum on growth, biochemical and yield of Solanum melongena. Int. J. Recycl. Org. Waste Agric. 2015, 4, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Nair, P.; Kandasamy, S.; Zhang, J.; Ji, X.; Kirby, C.; Benkel, B.; Hodges, M.D.; Critchley, A.T.; Hiltz, D.; Prithiviraj, B. Transcriptional and metabolomic analysis of Ascophyllum nodosum mediated freezing tolerance in Arabidopsis thaliana. BMC Genom. 2012, 13, 643. [Google Scholar] [CrossRef] [Green Version]
- Elansary, H.O.; Yessoufou, K.; Abdel-Hamid, A.M.E.; El-Esawi, M.A.; Ali, H.M.; Elshikh, M.S. Seaweed extracts enhance salam turfgrass performance during prolonged irrigation intervals and saline shock. Front. Plant Sci. 2017, 8, 830. [Google Scholar] [CrossRef] [Green Version]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef] [Green Version]
- Ziosi, V.; Zandoli, R.; Nardo, A.D.; Biondi, S.; Antognoni, F.; Calandriello, F. Biological activity of different botanical extracts as evaluated by means of an array of in vitro and in vivo bioassays. Acta Hortic. 2012, 1009, 61–66. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Kumaresapillai, N.; Basha, R.A.; Sathish, R. Production and evaluation of chitosan from Aspergillus niger MTCC strains. Iran. J. Pharm. Res. 2011, 10, 553–558. [Google Scholar] [PubMed]
- Nwe, N.; Furuike, T.; Tamura, H. Production, properties and applications of fungal cell wall polysaccharides: Chitosan and glucan. In Chitosan for Biomaterials II. Advances in Polymer Science; Jayakumar, R., Prabaharan, M., Muzzarelli, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 187–208. [Google Scholar] [CrossRef]
- Muñoz, G.; Valencia, C.; Valderruten, N.; Ruiz-Durántez, E.; Zuluaga, F. Extraction of chitosan from Aspergillus niger mycelium and synthesis of hydrogels for controlled release of betahistine. React. Funct. Polym. 2015, 91–92, 1–10. [Google Scholar] [CrossRef]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Hadwiger, L.A. Multiple effects of chitosan on plant systems: Solid science orhype. Plant Sci. 2013, 208, 42–49. [Google Scholar] [CrossRef]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Povero, G.; Loreti, E.; Pucciariello, C.; Santaniello, A.; Di Tommaso, D.; Di Tommaso, G.; Kapetis, D.; Zolezzi, F.; Piaggesi, A.; Perata, P. Transcript profiling of chitosan-treated Arabidopsis seedlings. J. Plant Res. 2011, 124, 619–629. [Google Scholar] [CrossRef]
- Ferri, M.; Franceschetti, M.; Naldrett, M.J.; Saalbach, G.; Tassoni, A. Effects of Chitosan on the protein profile of grape cell culture subcellular fractions. Electrophoresis 2014, 35, 1685–1692. [Google Scholar] [CrossRef]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Geelen, D. Developing biostimulants from agro-food and industrial by-products. Front. Plant Sci. 2018, 9, 1567. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Schiavon, M.; Altissimo, A.; Franceschi, C.; Nardi, S. Phenol-containing organic substances stimulate phenylpropanoid metabolism in Zea mays. J. Plant Nutr. Soil Sci. 2011, 174, 496–503. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- García, A.C.; Santos, L.A.; Izquierdo, F.G.; Sperandio, M.V.L.; Castro, R.N.; Berbara, R.L.L. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol. Eng. 2012, 47, 203–208. [Google Scholar] [CrossRef]
- Zhao, T.; Dai, A. The magnitude and causes of global drought changes in the twenty-first century under a low–moderate emissions scenario. J. Clim. 2015, 28, 4490–4512. [Google Scholar] [CrossRef]
- Kim, W.; Iizumi, T.; Nishimori, M. Global patterns of crop production losses associated with droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 2019, 58, 1233–1244. [Google Scholar] [CrossRef]
- Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration. Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef]
- Arslan, E.; Agar, G.; Aydin, M. Humic acid as a biostimulant in improving drought tolerance in wheat: The expression patterns of drought-related genes. Plant Mol. Biol. Rep. 2021. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Nardi, S. Humic substance: Relationship between structure and activity. Deeper information suggests univocal findings. J. Geochem. Explor. 2013, 129, 57–63. [Google Scholar] [CrossRef]
- Shen, J.; Guo, M.; Wang, Y.; Yuan, X.; Wen, Y.; Song, X.; Dong, S.; Guo, P. Humic acid improves the physiological and photosynthetic characteristics of millet seedlings under drought stress. Plant Signal. Behav. 2020, 15, 1774212. [Google Scholar] [CrossRef]
- Aguiar, N.O.; Medici, L.O.; Olivares, F.L.; Dobbss, L.B.; Torres-Netto, A.; Silva, S.F.; Novotny, E.H.; Canellas, L.P. Metabolic profile and antioxidant responses during drought stress recovery in sugarcane treated with humic acids and endophytic diazotrophic bacteria. Ann. Appl. Biol. 2016, 168, 203–213. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB Plants 2018, 10, plx051. [Google Scholar] [CrossRef] [PubMed]
- Pourghasemian, N.; Moradi, R.; Naghizadeh, M.; Landberg, T. Mitigating drought stress in sesame by foliar application of salicylic acid, beeswax waste and licorice extract. Agric. Water Manag. 2020, 231, 105997. [Google Scholar] [CrossRef]
- Kaya, C.; Şenbayram, M.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.). Sci. Rep. 2020, 10, 6432. [Google Scholar] [CrossRef] [Green Version]
- Chandra, P.; Wunnava, A.; Verma, P.; Chandra, A.; Sharma, R.K. Strategies to mitigate the adverse effect of drought stress on crop plants—Influences of soil bacteria: A review. Pedosphere 2021, 31, 496–509. [Google Scholar] [CrossRef]
- Chiappero, J.; Cappellari, L.D.R.; Alderete, L.G.S.; Palermo, T.B.; Banchio, E. Plant growth promoting rhizobacteria improve the antioxidant status in Mentha piperita grown under drought stress leading to an enhancement of plant growth and total phenolic content. Ind. Crops Prod. 2019, 139, 111553. [Google Scholar] [CrossRef]
- Taha, R.S.; Alharby, H.F.; Bamagoos, A.A.; Medani, R.A.; Rady, M.M. Elevating tolerance of drought stress in Ocimum basilicum using pollen grains extract; a natural biostimulant by regulation of plant performance and antioxidant defense system. S. Afr. J. Bot. 2020, 128, 42–53. [Google Scholar] [CrossRef]
- de Vasconcelos, A.C.F.; Zhang, X.; Ervin, E.H.; Kiehl, J.D.C. Enzymatic antioxidant responses to biostimulants in maize and soybean subjected to drought. Sci. Agric. 2009, 66, 395–402. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effect on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer: New York, NY, USA, 2013; pp. 25–87. [Google Scholar] [CrossRef]
- Rehman, H.U.; Alharby, H.F.; Bamagoos, A.A.; Abdelhamid, M.T.; Rady, M.M. Sequenced application of glutathione as an antioxidant with an organic biostimulant improves physiological and metabolic adaptation to salinity in wheat. Plant Physiol. Biochem. 2021, 158, 43–52. [Google Scholar] [CrossRef]
- Hassan, F.A.S.; Ali, E.; Gaber, A.; Fetouh, M.I.; Mazrou, R. Chitosan nanoparticles effectively combat salinity stress by enhancing antioxidant activity and alkaloid biosynthesis in Catharanthus roseus (L.) G. Don. Plant Physiol. Biochem. 2021, 162, 291–300. [Google Scholar] [CrossRef]
- Ait-El-Mokhtar, M.; Baslam, M.; Ben-Laouane, R.; Anli, M.; Boutasknit, A.; Mitsui, T.; Wahbi, S.; Meddich, A. Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst. 2020, 4, 131. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, L.; Liao, W.; Dawuda, M.M.; Lyu, J.; Xie, J.; Feng, Z.; Calderón-Urrea, A.; Yu, J. Foliar application of 5-aminolevulinic acid (ALA) alleviates NaCl stress in cucumber (Cucumis sativus L.) seedlings through the enhancement of ascorbate-glutathione cycle. Sci. Hortic. 2019, 257, 108761. [Google Scholar] [CrossRef]
- Parvin, K.; Nahar, K.; Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Mohsin, S.M.; Fujita, M. Exogenous vanillic acid enhances salt tolerance of tomato: Insight into plant antioxidant defense and glyoxalase systems. Plant Physiol. Biochem. 2020, 150, 109–120. [Google Scholar] [CrossRef]
- Desoky, E.M.; El-Maghraby, L.M.M.; Awad, A.E.; Abdo, A.I.; Rady, M.M.; Semida, W.M. Fennel and ammi seed extracts modulate antioxidant defence system and alleviate salinity stress in cowpea (Vigna unguiculata). Sci. Hortic. 2020, 272, 109576. [Google Scholar] [CrossRef]
- Moradbeygi, H.; Jamei, R.; Heidari, R.; Darvishzadeh, R. Investigating the enzymatic and non-enzymatic antioxidant defense by applying iron oxide nanoparticles in Dracocephalum moldavica L. plant under salinity stress. Sci. Hortic. 2020, 272, 109537. [Google Scholar] [CrossRef]
- Niamat, B.; Naveed, M.; Ahmad, Z.; Yaseen, M.; Ditta, A.; Mustafa, A.; Rafique, M.; Bibi, R.; Sun, N.; Xu, M. Calcium-enriched animal manure alleviates the adverse effects of salt stress on growth, physiology and nutrients homeostasis of Zea mays L. Plants 2019, 8, 480. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Ramzan, N.; Mustafa, A.; Samad, A.; Niamat, B.; Yaseen, M.; Ahmad, Z.; Hasanuzzaman, M.; Sun, N.; Shi, W.; et al. Alleviation of salinity induced oxidative stress in Chenopodium quinoa by Fe biofortification and biochar—Endophyte interaction. Agronomy 2020, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Pal, K.K.; Dey, R.; Sherathia, D.N.; Devidayal; Mangalassery, S.; Kumar, A.; Rupapara, R.B.; Mandaliya, M.; Rawal, P.; Bhadania, R.A.; et al. Alleviation of salinity stress in peanut by application of endophytic bacteria. Front. Microbiol. 2021, 12, 650771. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.; Gul, H.; Hamayun, M.; Rauf, M.; Iqbal, A.; Shah, M.; Hussain, A.; Bibi, H.; Lee, I.J. Aspergillus awamori ameliorates the physicochemical characteristics and mineral profile of mung bean under salt stress. Chem. Biol. Technol. Agric. 2021, 8, 9. [Google Scholar] [CrossRef]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.M.; Kim, K.M.; Lee, I.J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 2020, 15, e0232228. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, B.; Chakraborty, U. Plant growth promoting rhizobacteria protect wheat plants against temperature stress through antioxidant signalling and reducing chloroplast and membrane injury. J. Plant Growth Regul. 2018, 37, 1396–1412. [Google Scholar] [CrossRef]
- Srivastava, S.; Yadav, A.; Seem, K.; Mishra, S.; Chaudhary, V.; Nautiyal, C.S. Effect of high temperature on Pseudomonas putida NBRI0987 biofilm formation and expression of stress sigma factor RpoS. Curr. Microbiol. 2008, 56, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.Z.; Sandhya, V.; Grover, M.; Kishore, N.; Rao, L.V.; Venkateswarlu, B. Pseudomonas sp. strain AKM-P6 enhances tolerance of sorghum seedlings to elevated temperatures. Biol. Fertil. Soils 2009, 46, 45–55. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil 2014, 379, 337–350. [Google Scholar] [CrossRef]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol. Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Kumar, A.; Friedman, H.; Tsechansky, L.; Graber, E.R. Distinctive in-planta acclimation responses to basal growth and acute heat stress were induced in Arabidopsis by cattle manure biochar. Sci. Rep. 2021, 11, 9875. [Google Scholar] [CrossRef]
- Mathur, S.; Jajoo, A. Arbuscular mycorrhizal fungi protects maize plants from high temperature stress by regulating photosystem II heterogeneity. Ind. Crops Prod. 2019, 143, 111934. [Google Scholar] [CrossRef]
- EL Boukhari, M.E.; Barakate, M.; Bouhia, Y.; Lyamlouli, K. Trends in seaweed extract based biostimulants: Manufacturing process and beneficial effect on soil-plant systems. Plants 2020, 9, 359. [Google Scholar] [CrossRef] [Green Version]
- Anjos Neto, A.P.; Oliveira, G.R.F.; Mello, S.D.C.; Silva, M.S.D.; Gomes-Junior, F.G.; Novembre, A.D.; Azevedo, R.A. Seed priming with seaweed extract mitigate heat stress in spinach: Effect on germination, seedling growth and antioxidant capacity. Bragantia 2020, 79, 502–511. [Google Scholar] [CrossRef]
- Pasbani, B.; Salimi, A.; Aliasgharzad, N.; Hajiboland, R. Colonization with arbuscular mycorrhizal fungi mitigates cold stress through improvement of antioxidant defense and accumulation of protecting molecules in eggplants. Sci. Hortic. 2020, 272, 109575. [Google Scholar] [CrossRef]
- Chu, X.T.; Fu, J.J.; Sun, Y.F.; Xu, Y.M.; Miao, Y.J.; Xu, Y.F.; Hu, T.M. Effect of arbuscular mycorrhizal fungi inoculation on cold stress-induced oxidative damage in leaves of Elymus nutans Griseb. S. Afr. J. Bot. 2016, 104, 21–29. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Meng, J.; Liang, X.; Yang, X.; Chen, W. Organic molecules from biochar leacheates have a positive effect on rice seedling cold tolerance. Front. Plant Sci. 2017, 8, 1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pokluda, R.; Sękara, A.; Jezdinský, A.; Kalisz, A.; Neugebauerová, J.; Grabowska, A. The physiological status and stress biomarker concentration of Coriandrum sativum L. plants subjected to chilling are modified by biostimulant application. Biol. Agric. Hortic. 2016, 32, 258–268. [Google Scholar] [CrossRef]
- Bradacova, K.; Weber, N.F.; Morad-Talab, N.; Asim, M.; Imran, M.; Weinmann, M.; Neumann, G. Micronutrients (Zn/Mn), seaweed extracts, and plant growth-promoting bacteria as cold-stress protectants in maize. Chem. Biol. Technol. Agric. 2016, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dinler, B.S.; Gunduzer, E.; Tekinay, T. Pre-treatment of fulvic acid plays a stimulant role in protection of soybean (Glycine max L.) leaves against heat and salt stress. Acta Biol. Crac. Bot. 2016, 58, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zou, Y.; Ding, S.; Zhang, J.; Yu, X.; Cao, J.; Lu, G. Polyamine accumulation in transgenic tomato enhances the tolerance to high temperature stress. J. Integr. Plant Biol. 2009, 51, 489–499. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Mele, B.H.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Bidabadi, S.S.; Mehralian, M. Arbuscular mycorrhizal fungi inoculation to enhance chilling stress tolerance of watermelon. Gesunde Pflanzen 2020, 72, 171–179. [Google Scholar] [CrossRef]
- Yan, P.; Li, G.; Sun, H.; Zhang, Z.; Yang, R.; Sun, J. Can arbuscular mycorrhizal fungi and biochar enhance plant resistance to low-temperature stress? Agron. J. 2020, 113, 1457–1466. [Google Scholar] [CrossRef]
- Hajiboland, R.; Joudmand, A.; Aliasgharzad, N.; Tolra, R.; Poschenrieder, C. Arbuscular mycorrhizal fungi alleviate low-temperature stress and increase freezing resistance as a substitute for acclimation treatment in barley. Crop Pasture Sci. 2019, 70, 218–233. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Ou, L.; Ji, D.; Liu, T.; Lan, R.; Li, X.; Jin, L. Response to the cold stress signaling of the tea plant (Camellia sinensis) elicited by chitosan oligosaccharide. Agronomy 2020, 10, 915. [Google Scholar] [CrossRef]
- Mahmud, J.A.; Bhuyan, M.H.M.B.; Anee, T.I.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Reactive oxygen species metabolism and antioxidant defense in plants under metal/metalloid stress. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H., Eds.; Springer: Cham, Switzerland, 2019; pp. 221–257. [Google Scholar]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Annu. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Hossaina, M.S.; Fujita, M. γ-aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology 2017, 26, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Hossaina, M.S.; Fujita, M. Maleic acid assisted improvement of metal chelation and antioxidant metabolism confers chromium tolerance in Brassica juncea L. Ecotoxicol. Environ. Saf. 2017, 144, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Bhuyan, M.H.M.B.; Fujita, F. Insights into citric acid-induced cadmium tolerance and phytoremediation in Brassica juncea L.: Coordinated functions of metal chelation, antioxidant defense and glyoxalase systems. Ecotoxicol. Environ. Saf. 2018, 147, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, J.A.; Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Fujita, F. EDTA reduces cadmium toxicity in mustard (Brassica juncea L.) by enhancing metal chelation, antioxidant defense and glyoxalase systems. Acta Agrobot. 2019, 72, 1722. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxicol. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef]
- Yildirim, E.; Ekinci, M.; Turan, M.; Ağar, G.; Dursun, A.; Kul, R.; Alim, Z.; Argin, S. Humic+fulvic acid mitigated Cd adverse effects on plant growth, physiology and biochemical properties of garden cress. Sci. Rep. 2021, 11, 8040. [Google Scholar] [CrossRef]
- Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Kanwal, S.; Ali, Q.; Ibrahim, M.; Gill, R.A.; Khan, M.D. Fulvic acid mediates chromium (Cr) tolerance in wheat (Triticum aestivum L.) through lowering of Cr uptake and improved antioxidant defense system. Environ. Sci. Pollut. Res. 2015, 22, 10601–10609. [Google Scholar] [CrossRef]
- Younis, U.; Malik, S.A.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Shah, M.H.R.; Rehman, R.A.; Ahmed, N. Biochar enhances the cadmium tolerance in spinach (Spinacia oleracea) through modification of Cd uptake and physiological and biochemical attributes. Environ. Sci. Pollut. Res. 2016, 23, 21385–21394. [Google Scholar] [CrossRef]
- Kamran, M.; Malik, Z.; Parveen, A.; Xong, Y.; Abbasi, G.H.; Rafiq, M.T.; Shaaban, M.; Mustafa, A.; Bashir, S.; Rafay, M.; et al. Biochar alleviates Cd phytotoxicity by minimizing bioavailability and oxidative stress in pak choi (Brassica chinensis L.) cultivated in Cd-polluted soil. J. Environ. Manag. 2019, 250, 109500. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Rizwan, M.; Ali, S.; Zia-ur-Rehman, M.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.C.; Beiyuan, J.; Wang, L.; Tsang, D.C.; Baek, K.; Bolan, N.S.; Ok, Y.S.; Li, X.D. A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived biochar stabilization of metal-contaminated soils. Sci. Total Environ. 2018, 616, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Hu, Z.-H.; Yan, T.-X.; Lu, R.-R.; Peng, C.-L.; Li, S.-S.; Jing, Y.-X. Arbuscular mycorrhizal fungi alleviate Cd phytotoxicity by altering Cd subcellular distribution and chemical forms in Zea mays. Ecotoxicol. Environ. Saf. 2019, 171, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Yang, H.; Cui, Z. Biochemical mechanism of phytoremediation process of lead and cadmium pollution with Mucor circinelloides and Trichoderma asperellum. Ecotoxicol. Environ. Saf. 2018, 157, 21–28. [Google Scholar] [CrossRef]
- Vargas, J.T.; Rodríguez-Monroy, M.; Meyer, M.L.; Montes-Belmont, R.; Sepúlveda-Jiménez, G. Trichoderma asperellum ameliorates phytotoxic effects of copper in onion (Allium cepa L.). Environ. Exp. Bot. 2017, 136, 85–93. [Google Scholar] [CrossRef]
- Ju, W.; Liu, L.; Fang, L.; Cui, Y.; Duan, C.; Wu, H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol. Environ. Saf. 2019, 167, 218–226. [Google Scholar] [CrossRef]
- Rahman, A.; Mostofa, M.G.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz. J. Bot. 2015, 39, 393–407. [Google Scholar] [CrossRef]
- Rahman, A.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Manganese-induced cadmium stress tolerance in rice seedlings: Coordinated action of antioxidant defense, glyoxalase system and nutrient homeostasis. C. R. Biol. 2016, 339, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Mostofa, M.G.; Alam, M.M.; Nahar, K.; Hasanuzzaman, M.; Fujita, M. Calcium mitigates arsenic toxicity in rice seedlings by reducing arsenic uptake and modulating the antioxidant defense and glyoxalase systems and stress markers. BioMed Res. Int. 2015, 2015, 340812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, M.B.; Colmer, T.D. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Qi, L.; Wang, L. ALA pretreatment improves waterlogging tolerance of fig plants. PLoS ONE 2016, 11, e0147202. [Google Scholar] [CrossRef] [PubMed]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Front. Plant Sci. 2021, 11, 614971. [Google Scholar] [CrossRef]
- Faran, M.; Farooq, M.; Rehman, A.; Nawaz, A.; Saleem, M.K.; Ali, N.; Siddique, K.H.M. High intrinsic seed Zn concentration improves abiotic stress tolerance in wheat. Plant Soil 2019, 437, 195–213. [Google Scholar] [CrossRef]
- Sharma, S.H.S.; Lyons, G.; McRoberts, C.; McCall, D.; Carmichael, E.; Andrews, F.; McCormack, R. Brown seaweed species from Strangford Lough: Compositional analyses of seaweed species and biostimulant formulations by rapid instrumental methods. J. Appl. Phycol. 2012, 24, 1141–1157. [Google Scholar] [CrossRef]
- Jannin, L.; Arkoun, M.; Ourry, A.; Laîné, P.; Goux, D.; Garnica, M.; Fuentes, M.; Francisco, S.S.; Baigorri, R.; Cruz, F.; et al. Micro array analysis of humic acid effects on Brassica napus growth: Involvement of N, C and S metabolisms. Plant Soil 2012, 359, 297–319. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, J.Y.; Jung, H.I.; Lee, D.J.; Shin, D.Y.; Hyun, K.H.; Kuk, Y.I. Ameliorative effects of squash (Cucurbita moschata Duchesne ex Poiret) leaf extracts on oxidative stress. Plant Growth Regul. 2012, 67, 9–17. [Google Scholar] [CrossRef]




| Crop Species | Stress Type and Duration | Biostimulant Type and Dose | ROS Regulatory Effects of Biostimulants Used | Reference |
|---|---|---|---|---|
| Setaria italica (L.) Beauv. | Watering withdrawal at 3–5 leaf stage up to 10 days | HA, seed soaking (100 mg L−1) | Reduced the generation of O2•− and H2O2 Decreased activity of SOD and POD. | [94] |
| Saccaharum officinarum L. | After 90 days, irrigation was withheld for 21 days (up to 13% moisture content) | HA (400 mL per 9 kg Soil) | SOD, CAT and APX activities were higher in root as well as in leaves after rehydration. | [95] |
| Zea mays | Water stressed field received only 67% water of evaporation loss (at every three days as compared to no stress field which received daily 100% water of evaporation) | 1250 kg S and 37.5 kg HA ha−1 | MDA and H2O2 content decreased Increased SOD and CAT activities with reduced POD activities. | [100] |
| Glycine max | Withholding irrigation, at 14 days after planting for 75 h | 7.0 mL L−1 commercial extract of Ascophyllum nodosum | Treated plants exhibited higher free-radical scavenging activity. | [98] |
| Paspalum vaginatum | Irrigation intervals were 2 and 6 days up to 6 weeks | Foliar spray of 5- or 7 mL L−1 A. nodosum extract | Decreased DPPH antioxidant and lipid peroxidation. | [71] |
| Mentha piperita | Drought stress was imposed as 50% field capacity (mild stress, irrigation until 10 days before harvest and 35% field capacity (severe stress, irrigation until 20 days before harvest) | PGPR (Pseudomonas fluorescens and Bacillus amyloliquefaciens), 1 mL bacterial suspension per 250 g growing media | The activity of SOD and total peroxidase were enhanced. Lipid peroxidation decreased by 50 and 70% under mild and severe water stress, respectively. Antioxidant scavenging capacity increased by two folds (DPPH and AsA equivalents). | [102] |
| Ocimum basilicum L. | 50% soil water holding capacity was maintained for the whole growing season | Foliar application of palm pollen grain extract 1.0 g L−1 at 30, 45 and 60 days after transplanting | Activities of SOD, CAT and guaiacol peroxidase increased. AsA and GSH contents increased. | [103] |
| Zea mays and Glycine max | Near to permanent wilting point (−1.5 MPa) after 10 weeks of growth | Mixture of nutrients, HA and FA (25 to 300 L ha−1) | SOD, CAT and APX activities increased. | [104] |
| Crop Species | Waterlogging Duration | Biostimulant Type and Dose | ROS Regulatory Effects of Biostimulants Used | Reference |
|---|---|---|---|---|
| Ficus carica L. cv. Masui Dauphine | 6 d | ALA (5 mg L−1) pretreatment | Leaf O2•− production decreased by 62%. MDA contents were reduced. Enhanced SOD and POD activities. | [165] |
| Triticum aestivum L. | 5 d | Trichoderma asperellum (strain MAP1) inoculums | Minimized the contents of MDA, H2O2, and EL. GSH content and activity of SOD and POD decreased. | [166] |
| Triticum aestivum L. Faisalabad-2008 | 7 d | Three Zn levels in seed: high (49 mg), medium (42 mg) and low (35 mg) kg−1 grain | Accumulation of MDA and antioxidant activity declined with the increase in intrinsic seed Zn levels. | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells 2021, 10, 2537. https://doi.org/10.3390/cells10102537
Hasanuzzaman M, Parvin K, Bardhan K, Nahar K, Anee TI, Masud AAC, Fotopoulos V. Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells. 2021; 10(10):2537. https://doi.org/10.3390/cells10102537
Chicago/Turabian StyleHasanuzzaman, Mirza, Khursheda Parvin, Kirti Bardhan, Kamrun Nahar, Taufika Islam Anee, Abdul Awal Chowdhury Masud, and Vasileios Fotopoulos. 2021. "Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress" Cells 10, no. 10: 2537. https://doi.org/10.3390/cells10102537
APA StyleHasanuzzaman, M., Parvin, K., Bardhan, K., Nahar, K., Anee, T. I., Masud, A. A. C., & Fotopoulos, V. (2021). Biostimulants for the Regulation of Reactive Oxygen Species Metabolism in Plants under Abiotic Stress. Cells, 10(10), 2537. https://doi.org/10.3390/cells10102537










