Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals?
Abstract
1. Introduction
2. The Loss of RAM and TZ Does Not Affect Plant Growth and Response under Mechanical Stresses
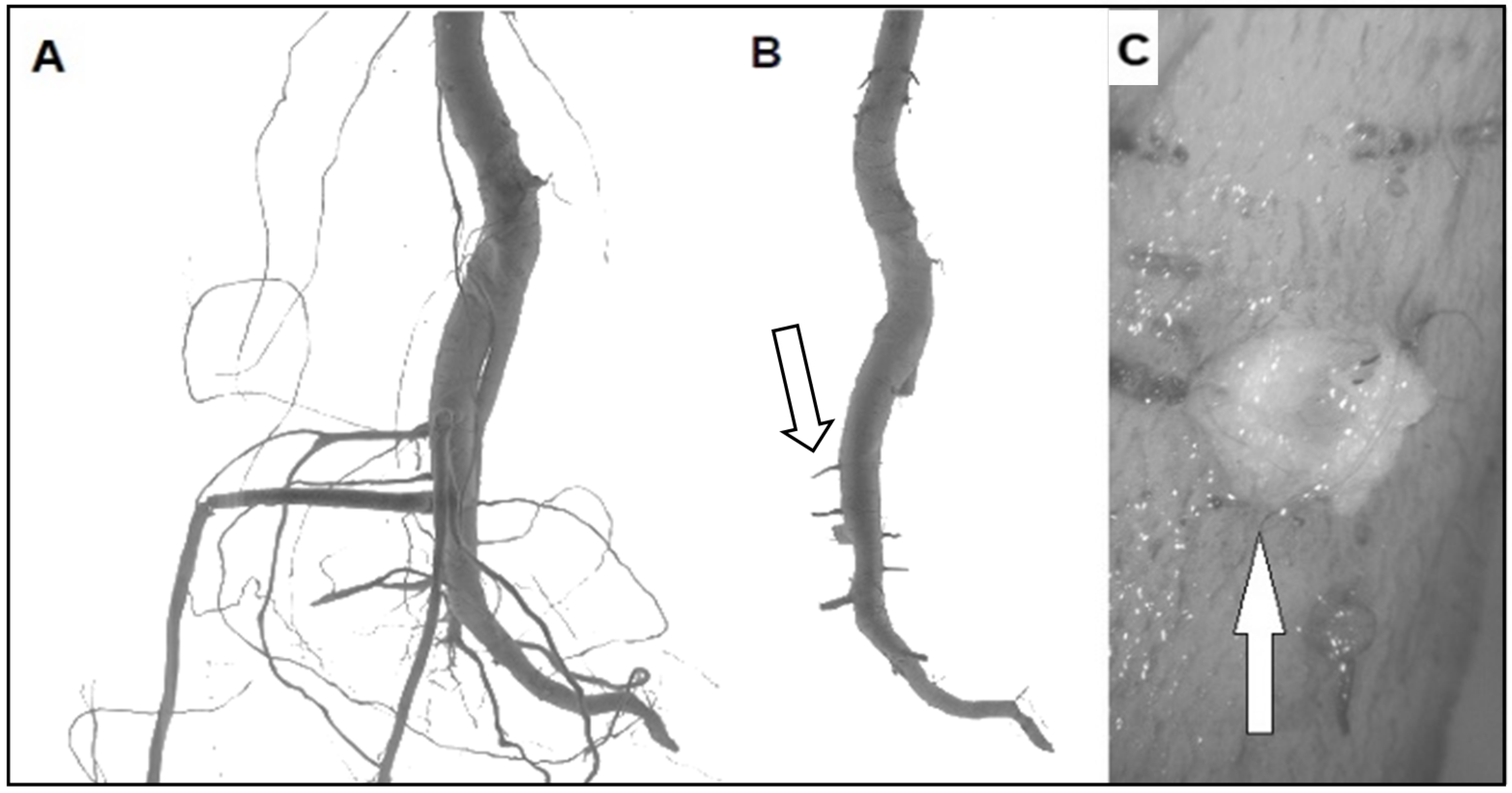
2.1. Pruning and Bending Treatments
2.2. Detection and Transduction of Mechanical Stresses
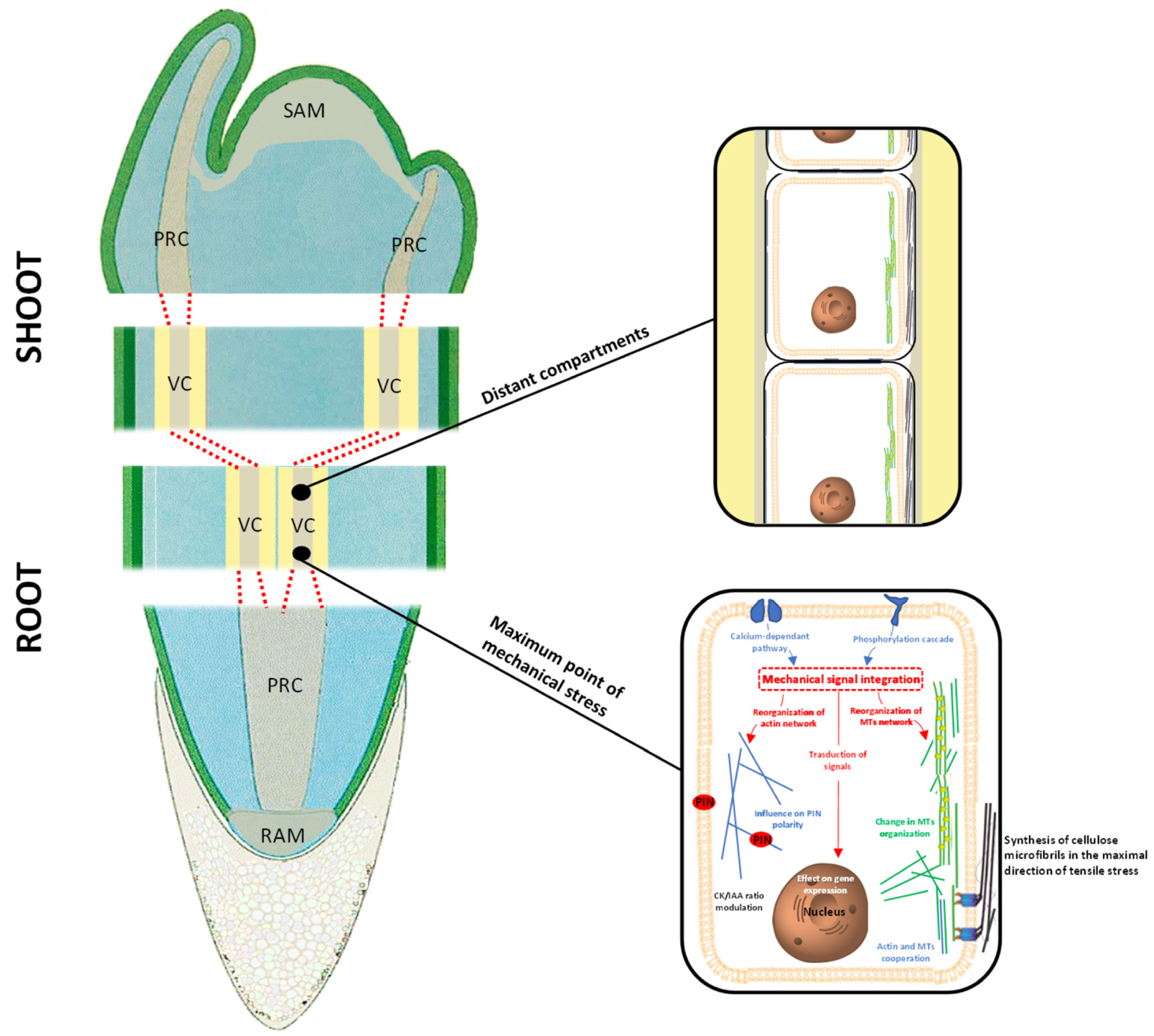
3. Meristematic Connectome and the Coordination of Plant Response to Mechanical Stresses
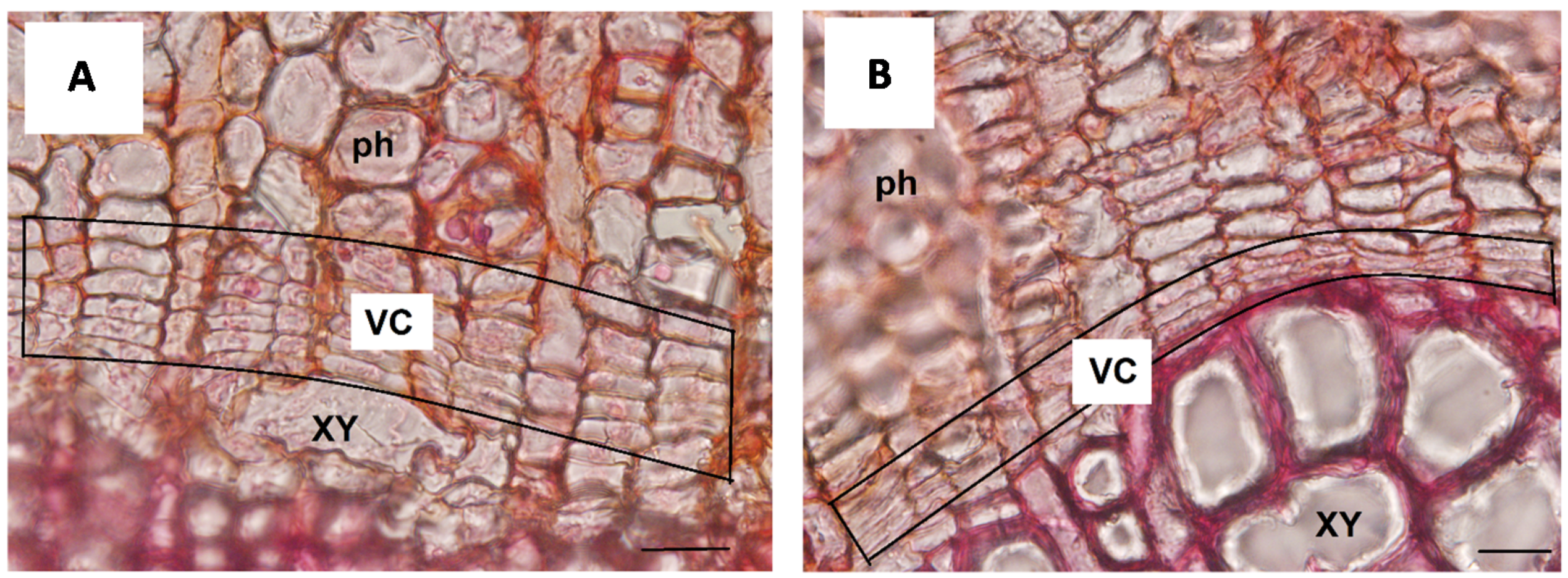
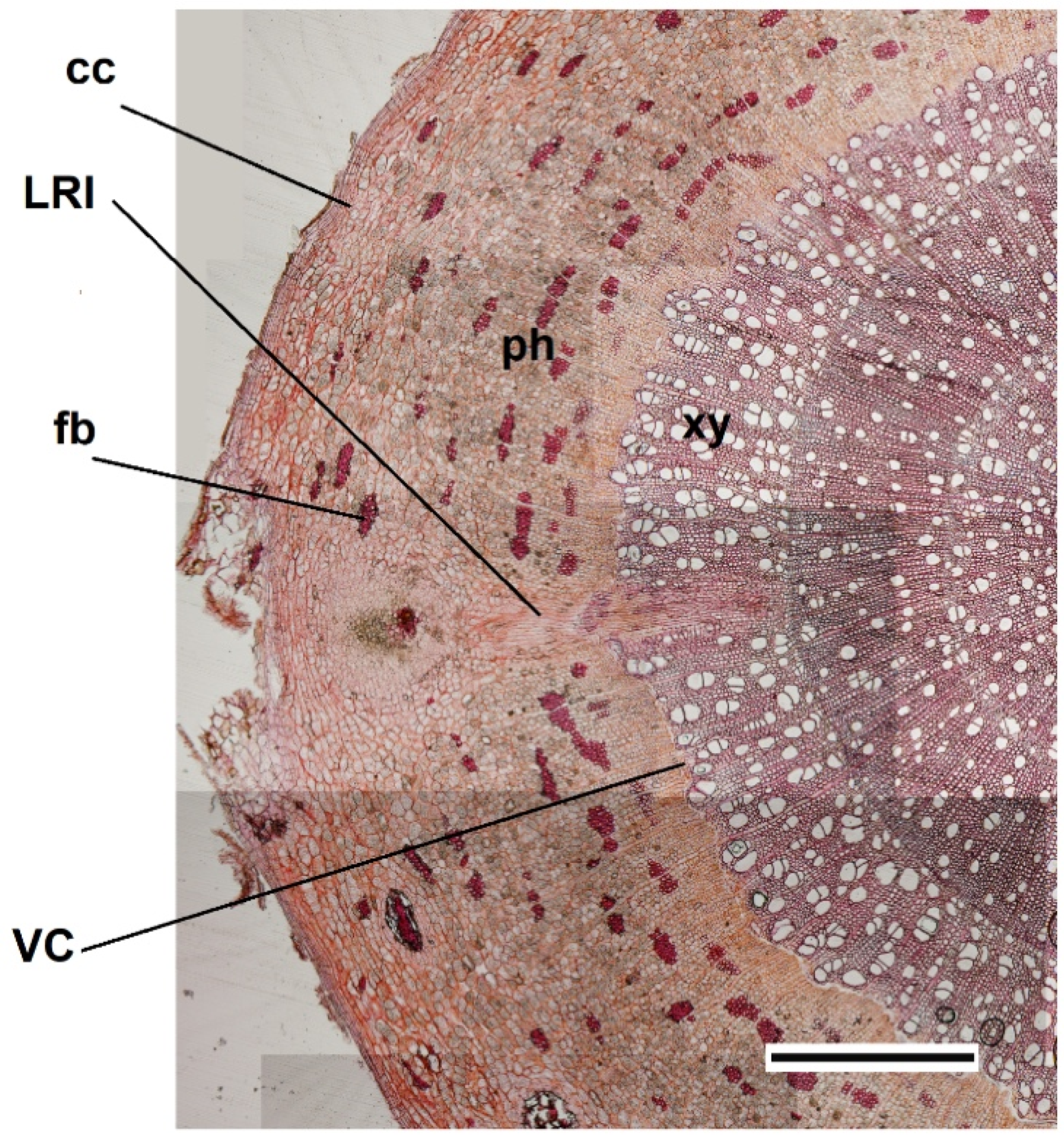
3.1. Morphological and Functional Similarities Present in the “Meristematic Connectome”
3.2. Crosstalk between Different Components of the “Meristematic Connectome”
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alabadí, D.; Blázquez, M.A. Molecular interactions between light and hormone signaling to control plant growth. Plant Mol. Biol. 2009, 69, 409–417. [Google Scholar] [CrossRef]
- Alpi, A.; Amrhein, N.; Bertl, A.; Blatt, M.R.; Blumwald, E.; Cervone, F.; Dainty, J.; De Michelis, M.I.; Epstain, E.; Galston, A.W.; et al. Plant neurobiology: No brain, no gain? Trends Plant Sci. 2007, 12, 135–136. [Google Scholar] [CrossRef]
- Mulligan, R.M.; Chory, J.; Ecker, J.R. Signaling in plants. Proc. Natl. Acad. Sci. USA 1997, 94, 2793–2795. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Draguhn, A.; Taiz, L. Plant “intelligence” changes nothing. EMBO Rep. 2020, 21, e50395. [Google Scholar] [CrossRef] [PubMed]
- Trewavas, A. Aspects of Plant Intelligence. Ann. Bot. 2003, 92, 1–20. [Google Scholar] [CrossRef]
- Wu, J.; Baldwin, I.T.; Wu, J. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009, 32, 1161–1174. [Google Scholar] [CrossRef]
- Baluska, F.; Mancuso, S. Plants, climate and humans. EMBO Rep. 2020, 21, e5. [Google Scholar] [CrossRef]
- Baluska, F.; Mancuso, S.; Volkmann, D.; Barlow, P. Root apices as plant command centres: The unique “brain-like” status of the root apex transition zone. Biology 2004, 59, 7–19. [Google Scholar]
- Brenner, E.D.; Stahlberg, R.; Mancuso, S.; Vivanco, J.; Baluska, F.; Van Volkenburgh, E. Plant neurobiology: An integrated view of plant signalling. Trends Plant Sci. 2006, 11, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Baluska, F.; Mancuso, S. Ion chnnels in plants: From bioelectricity, via signalling, to behavioral actions. Plant Signal. Behav. 2013, 8, e23009. [Google Scholar] [CrossRef]
- Masi, E.; Ciszak, M. Electrical spiking in bacterial biofilms. J. R. Soc. Interface 2014, 12, 20141036. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S.; Marras, A.M.; Volker, M.; Baluška, F. Non-invasive and continuous recordings of auxin fluxes in intact root apex with a carbon-nano tube-modified and self-referencing microelectrode. Anal. Biochem. 2005, 341, 344–351. [Google Scholar] [CrossRef]
- Mancuso, S.; Marras, A.M.; Mugnai, S.; Schlicht, M.; Zarsky, V.; Li, G.; Song, L.; Xue, H.-W.; Baluska, F. Phospholipase Dζ2 drives vesicular secretion of auxin for its polar cell–cell transport in the transition zone of the root apex. Plant Signal. Behav. 2007, 2, 240–244. [Google Scholar] [CrossRef]
- Schlicht, M.; Strnad, M.; Scanlon, M.J.; Mancuso, S.; Hochholdinger, F.; Palme, K.; Volkmann, D.; Menzel, D.; Baluska, F. Auxin immunolocalization implicates vesicular neurotransmitter-like mode of polar auxin transport in root apices. Plant Signal. Behav. 2006, 1, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Mugnai, S.; Azzarello, E.; Baluška, F.; Mancuso, S. Local root apex hypoxia induces NO-mediated hypoxic acclimation of the entire root. Plant Cell Physiol. 2012, 53, 912–920. [Google Scholar] [CrossRef]
- Baesso, B.; Chiatante, D.; Terzaghi, M.; Zenga, D.; Nieminen, K.; Mahonen, A.P.; Siligato, R.; Heliarutta, Y.; Scippa, G.S.; Montagnoli, A. Transcription factors PRE3 and WOX11 are involved in the formation of new lateral roots from secondary growth taproot in A. thaliana. Plant Biol. 2018, 20, 426–432. [Google Scholar] [CrossRef]
- Baesso, B.; Terzaghi, M.; Chiatante, D.; Scippa, G.S.; Montagnoli, A. WOX genes expression during the formation of new lateral roots from secondary structures in Populus nigra (L.) taproot. Nat. Sci. Rep. 2020, 10, 18890. [Google Scholar] [CrossRef] [PubMed]
- Chiatante, D.; Beltotto, M.; Onelli, E.; Di Iorio, A.; Montagnoli, A.; Scippa, S.G. New branch roots produced by vascular cambium derivatives in woody parental roots of Populus nigra L. Plant Biosyst. 2010, 144, 420–433. [Google Scholar] [CrossRef]
- Chiatante, D.; Rost, T.; Bryant, J.; Scippa, G.S. Regulatory networks controlling the development of the root system and the formation of lateral roots: A comparative analysis of the roles of pericycle and vascular cambium. Ann. Bot. 2018, 122, 697–710. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Scippa, S.G.; Laserre, B.; Dumroese, K. Ongoing modification to root system architecture of Pinus ponderosa growing on a sloped site revealed by tree-ring analysis. Dendrochronologia 2019, 58, 125650. [Google Scholar] [CrossRef]
- Kajala, K.; Gouran, M.; Shaar-Moshe, L.; Mason, G.A.; Rodriguez-Medina, J.; Kawa, D.; Pauluzzi, G.; Reynoso, M.; Canto-Pastor, A.; Manzano, C.; et al. Innovation, conservation, and repurposing of gene function in root cell type development. Cell 2021, 184, 3333–3348.e19. [Google Scholar] [CrossRef]
- Chiatante, D.; Di Iorio, A.; Sarnataro, M.; Scippa, G.S. Improving vigour assessment of pine (Pinus nigra Arnold) seedlings before their use in reforestation. Plant Biosyst. 2002, 136, 209–216. [Google Scholar] [CrossRef]
- Chiatante, D.; Scippa, G.S.; Di Iorio, A.; De Micco, V.; Sarnataro, M. Lateral root emission in woody taproots of Fraxinus ornus L. Plant Biosyst. 2007, 141, 204–213. [Google Scholar] [CrossRef]
- Trupiano, D.; Di Iorio, A.; Montagnoli, A.; Lasserre, B.; Rocco, M.P.; Grosso, A.; Scaloni, A.; Marra, M.; Chiatante, D.; Scippa, G.S. Involvement of lignin and hormones in the response of woody poplar taproots to mechanical stress. Physiol. Plant. 2012, 146, 39–52. [Google Scholar] [CrossRef]
- De Zio, E.; Montagnoli, A.; Karady, M.; Terzaghi, M.; Sferra, G.; Antoniadi, I.; Scippa, G.S.; Ljung, K.; Chiatante, D.; Trupiano, D. Reaction wood anatomical traits and hormonal profiles in poplar bent stem and root. Front. Plant Sci. 2020, 11, 590985. [Google Scholar] [CrossRef]
- Ditengou, F.A.; Teale, W.D.; Kochersperger, P.; Flittner, K.A.; Kneuper, I.; van der Graaff, E.; Nziengui, H.; Pinosa, F.; Li, X.; Nitschke, R.; et al. Mechanical induction of de novo lateral root initiation in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 18818–18823. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, M.; Grieneisen, V.A.; Hofhuis, H.; Hove, C.A.T.; Hogeweg, P.; Marée, A.F.M.; Scheres, B. Root system architecture from coupling cell shape to auxin transport. PLoS Biol. 2008, 6, e307. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.L. Mechanical Induction of Lateral Root Initiation. Ph.D. Dissertation, The Huck Institutes of the Life Sciences, The Graduate School, The Pennsylvania State University, University Park, PA, USA, 2012. [Google Scholar]
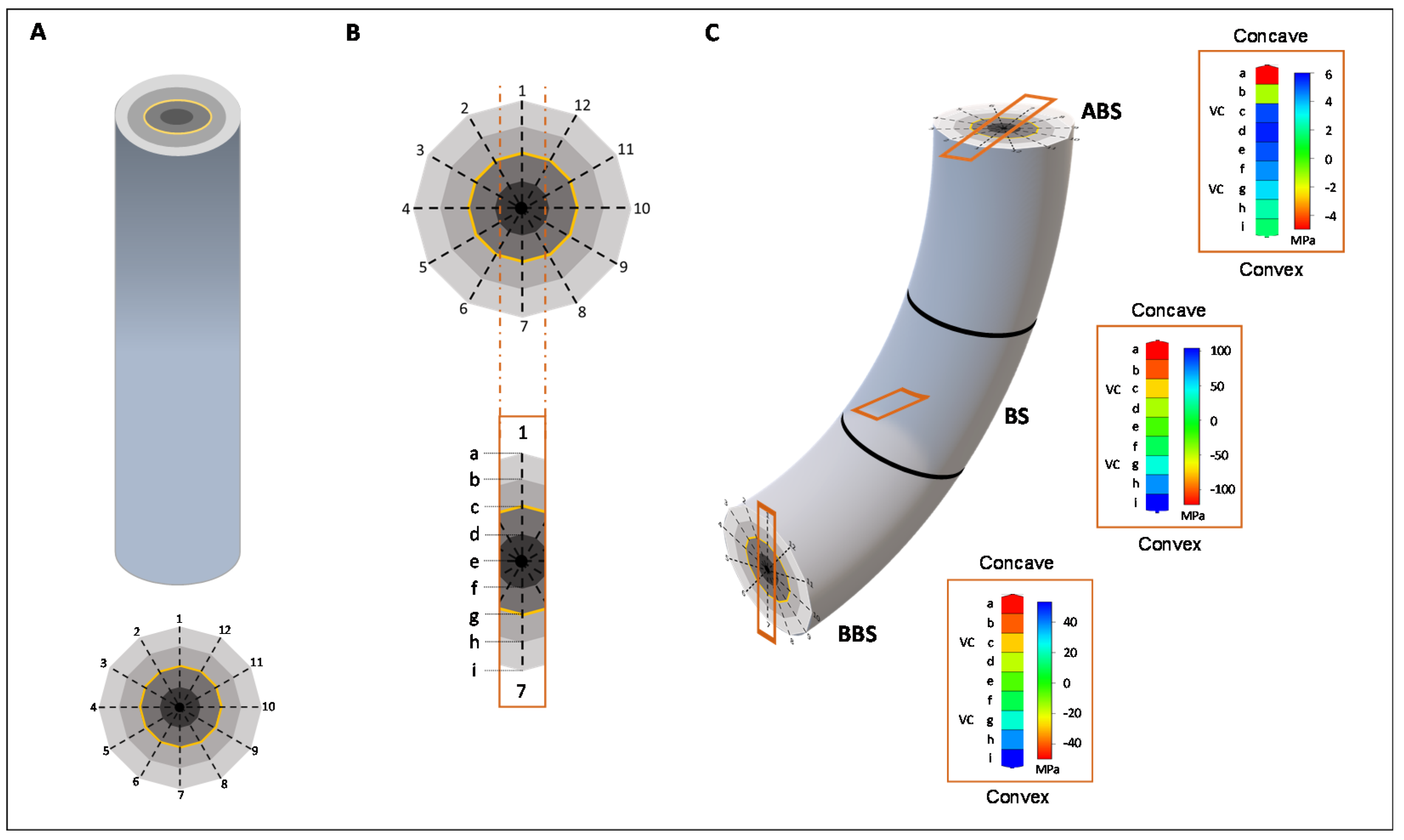
- De Zio, E.; Trupiano, D.; Montagnoli, A.; Terzaghi, M.; Chiatante, D.; Grosso, A.; Marra, M.; Scaloni, A.; Scippa, G.S. Poplar woody taproot under bending stress: The asymmetric response of the convex and concave sides. Ann. Bot. 2016, 118, 865–883. [Google Scholar] [CrossRef] [PubMed]
- Givnish, T.J. On the adaptive significance of leaf height in forest herbs. Am. Nat. 1982, 120, 353–381. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptation to sun and shade: A whole plant perspective. Aust. J. Plant Physiol. 1988, 15, 63–92. [Google Scholar] [CrossRef]
- Polzella, A.; Terzaghi, M.; Trupiano, D.; Baronti, S.; Scippa, G.S.; Chiatante, D.; Montagnoli, A. Morpho-Physiological Responses of Pisum sativum L. to Different Light-Emitting Diode (LED) Light Spectra in Combination with Biochar Amendment. Agronomy 2020, 10, 398. [Google Scholar] [CrossRef]
- Montagnoli, A.; Terzaghi, M.; Di Iorio, A.; Scippa, G.S.; Chiatante, D. Fine-root morphological and growth traits in a Turkey-oak stand in relation to seasonal changes in soil moisture in the Southern Apennines, Italy. Ecol. Res. 2012, 27, 1015–1025. [Google Scholar] [CrossRef]
- Montagnoli, A.; Di Iorio, A.; Terzaghi, M.; Trupiano, D.; Scippa, G.S.; Chiatante, D. Influence of soil temperature and water content on fine root seasonal growth of European beech natural forest in Southern Alps, Italy. Eur. J. For. Res. 2014, 133, 957–968. [Google Scholar] [CrossRef]
- Montagnoli, A.; Baronti, S.; Danieli, A.; Chiatante, D.; Scippa, S.G.; Terzaghi, M. Pioneer and fibrous root seasonal dynamics of Vitis vinifera L. are affected by biochar application to a low fertility soil: A rhizobox approach. Sci. Total Environ. 2021, 751, 141455. [Google Scholar] [CrossRef]
- Sachs, T.; Novoplansky, A.; Cohen, D. Plants as competing populations of redundant organs. Plant Cell Environ. 1993, 16, 765–770. [Google Scholar] [CrossRef]
- Sachs, T. How can plants choose the most promising organs? In Communication in Plants; Baluska, F., Mancuso, S., Volkmanneds, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 53–63. [Google Scholar]
- Leyser, O. The control of shoot branching: An example of plant information processing. Plant Cell Environ. 2009, 32, 694–703. [Google Scholar] [CrossRef]
- Ballarè, C.L.; Trewavas, A. Plant behaviour special issue. Plant Cell Environ. 2009, 32, 605. [Google Scholar] [PubMed]
- Yu, M.; Yuan, X.; Lu, C.; Le, S.; Kawamura, R.; Efemov, A.K.; Zhao, Z.; Kozlov, M.M.; Sheetz, M.; Bershadsky, A.; et al. mDia1 senses both force and torque during F-actin filament polymerization. Nat. Commun. 2017, 8, 1650. [Google Scholar] [CrossRef]
- Risca, V.I.; Wand, E.B.; Chaudhuri, O.; Chia, J.J.; Geissler, P.L.; Fletcher, D.A. Actin filament curvature biases branching direction. Proc. Nat. Acad. Sci. USA 2009, 109, 2913–2918. [Google Scholar] [CrossRef] [PubMed]
- Gittes, F.; Mickey, B.; Nettleton, J.; Howard, J. Flexural rigidity of microtubules and actin filaments measured from thermal fluctuations in shape. J. Cell Biol. 1993, 120, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Hamant, O.; Inoue, D.; Bouchez, D.; Dumais, J.; Mjolness, E. Are microtubules tension sensors? Nat. Commun. 2019, 10, 2360. [Google Scholar] [CrossRef]
- Fischer, K.; Schopfer, P. Physical strain-mediated microtubule reorientation in theepidermis of gravitropically or phototropically stimulatedmaize coleoptiles. Plant J. 1998, 15, 119–123. [Google Scholar] [CrossRef]
- Heisler, M.G.; Hamant, O.; Krupinski, P.; Uyttewaal, M.; Ohno, C.; Jönsson, H.; Traas, J.; Meyerowitz, E.M. Alignment between PIN1 polarity and microtubule orientation in the shoot apical meristem reveals a tight coupling between morphogenesis and auxin transport. PLoS Biol. 2010, 8, e1000516. [Google Scholar] [CrossRef]
- Haswell, E.S.; Phillips, R.; Rees, D.C. Mechanosensitive channels: What can they do and how do they do it? Structure 2019, 19, 1356–1369. [Google Scholar] [CrossRef]
- Kurusu, T.; Hamada, H.; Koyano, T.; Kuchitsu, K. Intracellular localization and physiological function of a rice Ca2+-permeable channel. Plant Signal. Behav. 2012, 7, 1428–1430. [Google Scholar] [CrossRef]
- Chin, K.; De Falco, T.A.; Moeder, W.; Yoshioka, K. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol. 2013, 163, 611–624. [Google Scholar] [CrossRef]
- Morgan, A.J.; Galione, A. Two-pore channels (TPCs): Current controversies. Bioessays 2014, 36, 173–183. [Google Scholar] [CrossRef]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The evolution of calcium-based signalling in plants. Curr. Biol. 2017, 27, 667–679. [Google Scholar] [CrossRef]
- Trupiano, D.; Rocco, M.; Renzone, G.; Scaloni, A.; Viscosi, V.; Chiatante, D.; Scippa, G. The proteome of Populus nigra woody root: Response to bending. Ann. Bot. 2012, 110, 415–432. [Google Scholar] [CrossRef]
- Trupiano, D.; Rocco, M.; Renzone, G.; Scaloni, A.; Montagnoli, A.; Terzaghi, M. Poplar woody root proteome during the transition dormancy–active growth. Plant Biol. 2013, 147, 1095–1100. [Google Scholar] [CrossRef][Green Version]
- Richter, G.L.; Monshausen, G.B.; Krol, A.; Gilroy, S. Mechanical stimuli modulate lateral root organogenesis. Plant Physiol. 2009, 151, 1855–1866. [Google Scholar] [CrossRef] [PubMed]
- De Smet, I.; Tetsumura, T.; De Rybel, B.; Frey, N.F.; Laplaze, L.; Casimiro, I.; Swarup, R.; Naudts, M.; Vanneste, S.; Audenaert, D.; et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development 2007, 134, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Waidmann, S.; Rosquete, M.R.; Schöller, M.; Sarkel, E.; Lindner, H.; LaRue, T.; Petrik, I.; Dunser, K.; Martopawiro, S.; Sasidharan, R.; et al. Cytokinin functions as an asymmetric and anti-gravitropic signal in lateral roots. Nat. Commun. 2019, 10, 3540. [Google Scholar] [CrossRef]
- Waidmann, S.; Kleine-Vehn, J. Asymmetric cytokinin signaling opposes gravitropism in roots. J. Integr. Plant Biol. 2020, 62, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Trewavas, A. The foundations of plant intelligence. Interface Focus 2017, 7, 20160098. [Google Scholar] [CrossRef]
- Baluska, F.; Volkmann, D.; Menzel, D. Plant synapses: Actin-based domains for cell-to-cell communication. Trends Plant Sci. 2005, 10, 106–111. [Google Scholar] [CrossRef]
- Choi, W.-G.; Hilleary, R.; Swanson, S.J.; Kim, S.-U.; Gilroy, S. Rapid long-distance electrical and calcium signalling in plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef]
- Pierik, R.; deWit, M. Shade avoidance: Phytochrome signalling and other above ground neighbour detection cues. J. Exp. Bot. 2013, 65, 2815–2824. [Google Scholar] [CrossRef]
- Aphalo, P.J.; Ballare, C.L.; Scopel, A.L. Plant–plant signalling, the shade avoidance response and competition. J. Exp. Bot. 1999, 50, 1629–1634. [Google Scholar] [CrossRef]
- Landrein, B.; Hamant, O. How mechanical stress controls microtubule behavior and morphogenesis in plants: History, experiments and revisited theories. Plant J. 2013, 75, 324–338. [Google Scholar] [CrossRef]
- Kutsher, Y.; Evenor, D.; Belausov, E.; Lapidot, M.; Reuveni, M. Leaf Plasmodesmata Respond Differently to TMV, ToBRFV and TYLCV Infection. Plants 2021, 10, 1442. [Google Scholar] [CrossRef]
- Stahl, Y.; Simon, R. Plant primary meristems: Shared functions and regulatory mechanisms. Curr. Opin. Plant Biol. 2010, 13, 53–58. [Google Scholar] [CrossRef]
- Ojolo, S.P.; Cao, S.; Priyadarshani, S.V.G.N.; Li, W.; Yan, M.; Aslam, M.; Zhao, H.; Qin, Y. Regulation of plant growth and development: A review from a chromatin remodeling perspective. Front. Plant Sci. 2018, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Jura-Morawiec, J.; Oskolski, A.; Simpson, P. Revisiting the anatomy of the monocot cambium, a novel meristem. Planta 2021, 254, 6. [Google Scholar] [CrossRef]
- Phillips, H.L.; Torrey, J.G. The ultrastructure of the quiescent center in the apex of cultured roots of Convolvulus arvensis L. Am. J. Bot. 1974, 61, 871–878. [Google Scholar] [CrossRef]
- Myskow, E.; Gola, E.M.; Tulik, M. Continuity of Procambium and Anomalous Cambium during Formation of Successive Cambia in Celosia argentea. J. Plant Growth Regul. 2019, 38, 1458–1466. [Google Scholar] [CrossRef]
- Spicer, R.; Groover, A. Evolution of development of vascular cambia and secondary growth. New Phytol. 2010, 186, 577–592. [Google Scholar] [CrossRef] [PubMed]
- Catesson, A.M. Cambial ultrastructure and biochemistry: Changes in relation to vascular tissue differentiation and the seasonal cycle. Int. J. Plant Sci. 1994, 155, 251–261. [Google Scholar] [CrossRef]
- Rao, K.S.; Dave, Y.S. Ultrastructure of active and dormant cambial cells in teak (Tectona grandis L.F). New Phytol. 1983, 93, 447–456. [Google Scholar] [CrossRef]
- Begum, S.; Shibagaki, M.; Furusawa, O.; Nakaba, S.; Yamagishi, Y.; Yoshimoto, J.; Jin, H.-O.; Sano, Y.; Funada, R. Cold stability of microtubules in wood-forming tissues of conifers during seasons of active and dormant cambium. Planta 2012, 235, 165–179. [Google Scholar] [CrossRef]
- Chaffey, N.; Barnett, J.; Barlow, P. Endomembranes, cytoskeleton, and cell walls: Aspects of the ultrastructure vascular cambium of taproots of Aescuilus hippocastanum L. (Hippocastanaceae). Int. J. Plant Sci. 1997, 158, 97–109. [Google Scholar] [CrossRef]
- Vertosick, F.T. The Genius within. Discovering the Intelligence of Every Living Thing; Harcourt Inc.: New York, NY, USA, 2002. [Google Scholar]
- De Rybel, B.; Breda, A.S.; Weijers, D. Prenatal plumbing-vascular tissue formation in the plant embryo. Physiol. Plant. 2014, 151, 126–133. [Google Scholar] [CrossRef]
- Nieminen, K.; Blomster, T.; Heliarutta, Y.; Mahonen, A.P. Vascular Cambium Development. Arab. Book 2015, 13, e0177. [Google Scholar] [CrossRef]
- Schrader, J.; Nilsson, J.; Mellerowicz, E.; Berglund, A.; Nilsson, P.; Hertzberg, M.; Sandberg, G. A high-resolution transcript profile across the wood-forming meristem of poplar identifies potential regulators of cambial stem cell identity. Plant Cell 2004, 16, 2278–2292. [Google Scholar] [CrossRef]
- Groower, A.T.; Mansfield, S.D.; Di Fazio, S.P.; Dupper, G.; Fontana, J.R.; Millar, R.; Wang, Y. The Populus homeobox gene ARBORKNOX1 reveals overlapping mechanisms regulating the shoot apical meristem and the vascular cambium. Plant Mol. Biol. 2006, 61, 917–932. [Google Scholar] [CrossRef]
- Fukuda, H. Signaling, transcriptional regulation, and asynchronous pattern formation governing plant xylem development. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2016, 92, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Miyashima, S.; Roszak, P.; Sevilem, I.; Toyokura, K.; Blob, B.; Heo, J.-O.; Mellor, N.; Help-Rinta-Rahko, H.; Otero, S.; Smet, W.; et al. Mobile PEAR transcription factors integrate positional cues to prime cambial growth. Nature 2019, 565, 490–510. [Google Scholar] [CrossRef] [PubMed]
- Smetana, O.; Mäkilä, R.; Lyu, M.; Amiryousefi, A.; Sánchez Rodrígue, F.; Wu, M.-F.; Solé-Gil, A.; Leal Gavarrón, M.; Siligato, R.; Miyashima, S.; et al. High levels of auxin signalling define the stem-cell organizer of the vascular cambium. Nature 2019, 565, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Sanio, C. Anatomie der gemeinen Kiefer (Pinus silvestris L.) II. Entwickelungsgeschichte der Holzzellen. Jahrb. Wiss. Bot. 1873, 9, 50–128. [Google Scholar]
- Diaz-Sala, C. A Perspective on Adventitious Root Formation in Tree Species. Plants 2020, 9, 1789. [Google Scholar] [CrossRef]
- Baluska, F.; Samaj, J.; Wojtazek, P.; Volmann, D.; Menzel, D. Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol. 2003, 133, 482–491. [Google Scholar] [CrossRef]
- Volkov, A.G.; Shtessel, Y.B. Underground electronic signal transmission between plants. Commun. Integr. Biol. 2020, 13, 54–58. [Google Scholar] [CrossRef]
- Monshausen, G.B.; Bibikova, T.N.; Weisenseel, W.H.; Gilroy, S. Ca2+ regulates reactive oxygen species production and pH during mechanosensing in Arabidopsis roots. Plant Cell 2009, 21, 2341–2356. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.C.; Ghosh, R.; Bae, H. Plant acoustics: In the search of a sound mechanism for sound signaling in plants. J. Exp. Bot. 2016, 67, 4483–4494. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo-Moreno, A.; Bazihizina, N.; Azzarello, E.; Masi, E.; Tran, D.; Bouteau, F.; Baliska, F.; Mancuso, S. Root phonotropism: Early signalling events following sound perception in Arabidopsis roots. Plant Sci. 2017, 264, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Monshausen, G.B.; Haswell, E.S. A force of nature: Molecular mechanisms of mechanoperception in plants. J. Exp. Bot. 2013, 64, 4663–4680. [Google Scholar] [CrossRef] [PubMed]
- Trewavas, A.J.; Malhó, R. Signal perception and transduction: The origin of the phenotype. Plant Cell 1997, 9, 1181–1195. [Google Scholar] [CrossRef]
- Wasteneys, G.O. Progress in understanding the role of microtubules in plant cells. Curr. Opionion Plant Biol. 2004, 7, 651–660. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiatante, D.; Montagnoli, A.; Trupiano, D.; Sferra, G.; Bryant, J.; Rost, T.L.; Scippa, G.S. Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals? Cells 2021, 10, 2544. https://doi.org/10.3390/cells10102544
Chiatante D, Montagnoli A, Trupiano D, Sferra G, Bryant J, Rost TL, Scippa GS. Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals? Cells. 2021; 10(10):2544. https://doi.org/10.3390/cells10102544
Chicago/Turabian StyleChiatante, Donato, Antonio Montagnoli, Dalila Trupiano, Gabriella Sferra, John Bryant, Thomas L. Rost, and Gabriella S. Scippa. 2021. "Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals?" Cells 10, no. 10: 2544. https://doi.org/10.3390/cells10102544
APA StyleChiatante, D., Montagnoli, A., Trupiano, D., Sferra, G., Bryant, J., Rost, T. L., & Scippa, G. S. (2021). Meristematic Connectome: A Cellular Coordinator of Plant Responses to Environmental Signals? Cells, 10(10), 2544. https://doi.org/10.3390/cells10102544








