Central Nervous System Tissue Regeneration after Intracerebral Hemorrhage: The Next Frontier
Abstract
:1. Introduction
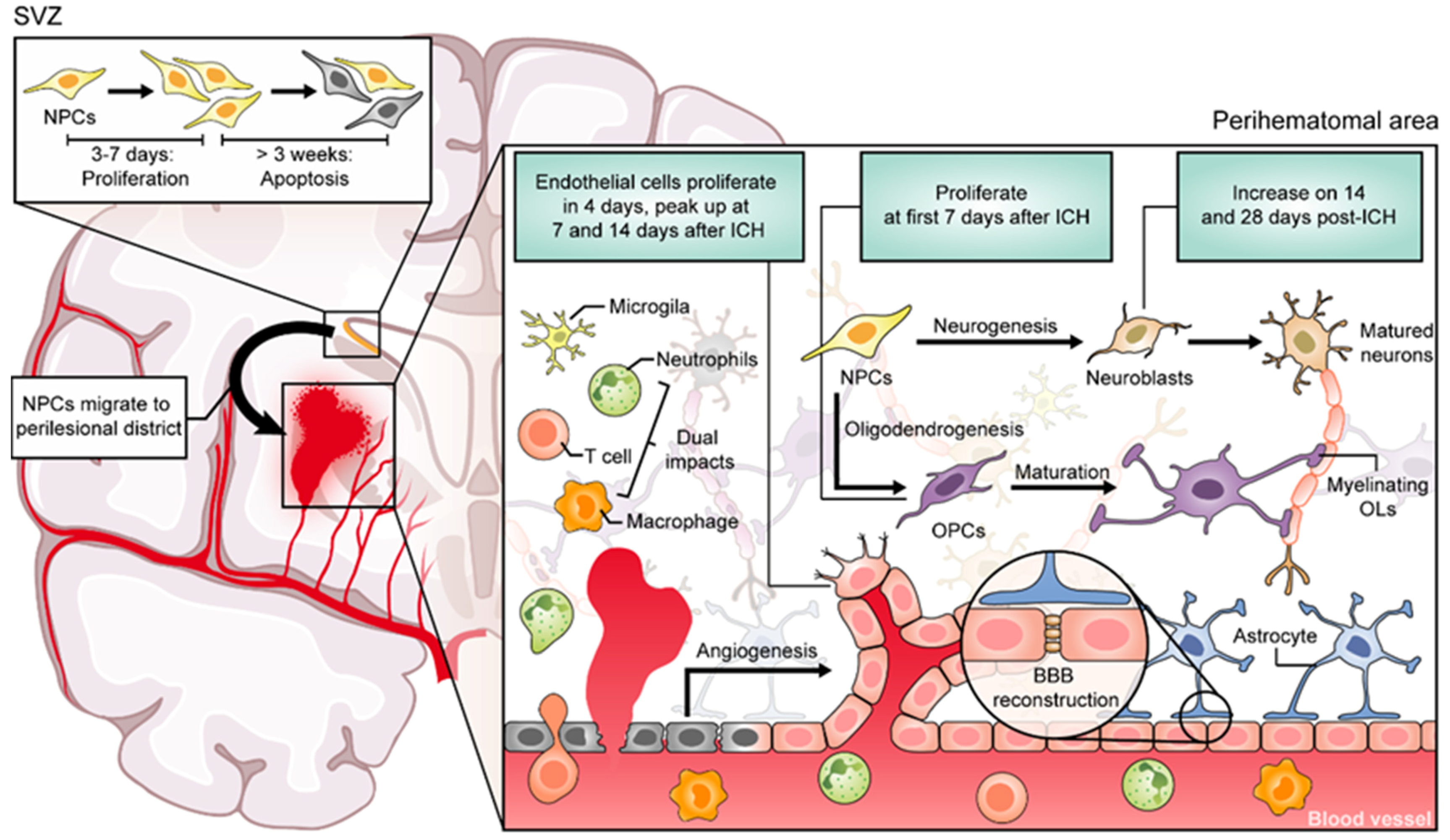
2. Regenerative Processes Occur after ICH in Animal Models
3. Evidence of CNS Regeneration in Patients with ICH
4. Neuroinflammation may Mediate Regenerative Processes after ICH: Guides from Experimental ICH
4.1. Microglia
4.2. Astrocytes
4.3. Leukocytes
5. Molecular Mechanisms Affecting Post-ICH Tissue Regeneration
6. Strategies to Promote Neural Regeneration after ICH
6.1. Medications
6.2. Stem Cell Therapy
6.3. Biomaterials and Nanoparticles
6.4. Rehabilitation Training
7. Conclusions and the Future
Author Contributions
Funding
Conflicts of Interest
References
- Cordonnier, C.; Demchuk, A.; Ziai, W.; Anderson, C.S. Intracerebral haemorrhage: Current approaches to acute management. Lancet 2018, 392, 1257–1268. [Google Scholar] [CrossRef]
- van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Kang, D.W. Intracerebral Hemorrhage: Large Disease Burden but Less Therapeutic Progress. J. Stroke 2017, 19, 1–2. [Google Scholar] [CrossRef] [Green Version]
- An, S.J.; Kim, T.J.; Yoon, B.W. Epidemiology, Risk Factors, and Clinical Features of Intracerebral Hemorrhage: An Update. J. Stroke 2017, 19, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Xia, Z.; Wu, X.; Li, J.; Liu, Z.; Chen, F.; Zhang, L.; Zhang, H.; Wan, X.; Cheng, Q. Minimally Invasive Surgery is Superior to Conventional Craniotomy in Patients with Spontaneous Supratentorial Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. World Neurosurg. 2018, 115, 266–273. [Google Scholar] [CrossRef]
- Scaggiante, J.; Zhang, X.; Mocco, J.; Kellner, C.P. Minimally Invasive Surgery for Intracerebral Hemorrhage. Stroke 2018, 49, 2612–2620. [Google Scholar] [CrossRef]
- Hanley, D.F.; Thompson, R.E.; Rosenblum, M.; Yenokyan, G.; Lane, K.; McBee, N.; Mayo, S.W.; Bistran-Hall, A.J.; Gandhi, D.; Mould, W.A.; et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet 2019, 393, 1021–1032. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Yong, V.W. Neuroinflammation in intracerebral haemorrhage: Immunotherapies with potential for translation. Lancet Neurol. 2020, 19, 1023–1032. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, B. Axon plasticity in the mammalian central nervous system after injury. Trends Neurosci. 2014, 37, 583–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emery, D.L.; Royo, N.C.; Fischer, I.; Saatman, K.E.; McIntosh, T.K. Plasticity following injury to the adult central nervous system: Is recapitulation of a developmental state worth promoting? J. Neurotrauma 2003, 20, 1271–1292. [Google Scholar] [CrossRef] [PubMed]
- Taupin, P. Adult neurogenesis and neuroplasticity. Restor. Neurol. Neurosci. 2006, 24, 9–15. [Google Scholar] [PubMed]
- Stangel, M.; Kuhlmann, T.; Matthews, P.M.; Kilpatrick, T.J. Achievements and obstacles of remyelinating therapies in multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, G.; Baer, K.; Buffo, A.; Curtis, M.A.; Faull, R.L.; Rees, M.I.; Gotz, M.; Dimou, L. Dynamic changes in myelin aberrations and oligodendrocyte generation in chronic amyloidosis in mice and men. Glia 2013, 61, 273–286. [Google Scholar] [CrossRef]
- Wang, F.; Ren, S.Y.; Chen, J.F.; Liu, K.; Li, R.X.; Li, Z.F.; Hu, B.; Niu, J.Q.; Xiao, L.; Chan, J.R.; et al. Myelin degeneration and diminished myelin renewal contribute to age-related deficits in memory. Nat. Neurosci. 2020, 23, 481–486. [Google Scholar] [CrossRef]
- Uyeda, A.; Muramatsu, R. Molecular Mechanisms of Central Nervous System Axonal Regeneration and Remyelination: A Review. Int. J. Mol. Sci. 2020, 21, 8116. [Google Scholar] [CrossRef] [PubMed]
- Reuter, H.; Vogg, M.C.; Serras, F. Repair, regenerate and reconstruct: Meeting the state-of-the-art. Development 2019, 146, dev176974. [Google Scholar] [CrossRef] [Green Version]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian central nervous system. Annu Rev. Neurosci. 2005, 28, 223–250. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Mendelow, A.D.; Hanley, D.F. Intracerebral haemorrhage. Lancet 2009, 373, 1632–1644. [Google Scholar] [CrossRef] [Green Version]
- Askenase, M.H.; Sansing, L.H. Stages of the Inflammatory Response in Pathology and Tissue Repair after Intracerebral Hemorrhage. Semin. Neurol. 2016, 36, 288–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, T.; Isobe, Y.; Aihara, N.; Furuyama, F.; Misumi, S.; Kim, T.S.; Nishino, H.; Hida, H. Increase in neurogenesis and neuroblast migration after a small intracerebral hemorrhage in rats. Neurosci. Lett. 2007, 425, 114–119. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Chen, X.; Liu, J.; Lu, H.; Yang, P.; Xiao, X.; Zhao, L.; Jiao, Q.; Zhao, B.; et al. The increased expression of metabotropic glutamate receptor 5 in subventricular zone neural progenitor cells and enhanced neurogenesis in a rat model of intracerebral hemorrhage. Neuroscience 2012, 202, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.; Zurita, M.; Bonilla, C.; Rico, M.A.; Aguayo, C.; Rodriguez, A.; Vaquero, J. Endogenous neurogenesis after intracerebral hemorrhage. Histol. Histopathol. 2012, 27, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Mino, M.; Kamii, H.; Fujimura, M.; Kondo, T.; Takasawa, S.; Okamoto, H.; Yoshimoto, T. Temporal changes of neurogenesis in the mouse hippocampus after experimental subarachnoid hemorrhage. Neurol. Res. 2003, 25, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Hostettler, I.C.; Seiffge, D.J.; Werring, D.J. Intracerebral hemorrhage: An update on diagnosis and treatment. Expert Rev. Neurother. 2019, 19, 679–694. [Google Scholar] [CrossRef]
- Qureshi, A.I.; Tuhrim, S.; Broderick, J.P.; Batjer, H.H.; Hondo, H.; Hanley, D.F. Spontaneous intracerebral hemorrhage. N. Engl. J. Med. 2001, 344, 1450–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feigin, V.L.; Forouzanfar, M.H.; Krishnamurthi, R.; Mensah, G.A.; Connor, M.; Bennett, D.A.; Moran, A.E.; Sacco, R.L.; Anderson, L.; Truelsen, T.; et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease Study 2010. Lancet 2014, 383, 245–254. [Google Scholar] [CrossRef]
- Hu, Y.Z.; Wang, J.W.; Luo, B.Y. Epidemiological and clinical characteristics of 266 cases of intracerebral hemorrhage in Hangzhou, China. J. Zhejiang Univ. Sci. B 2013, 14, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, J.K.; Schlichter, L.C. White matter injury in young and aged rats after intracerebral hemorrhage. Exp. Neurol. 2008, 214, 266–275. [Google Scholar] [CrossRef]
- Puig, J.; Blasco, G.; Terceno, M.; Daunis, I.E.P.; Schlaug, G.; Hernandez-Perez, M.; Cuba, V.; Carbo, G.; Serena, J.; Essig, M.; et al. Predicting Motor Outcome in Acute Intracerebral Hemorrhage. AJNR Am. J. Neuroradiol. 2019, 40, 769–775. [Google Scholar] [CrossRef]
- Plemel, J.R.; Liu, W.Q.; Yong, V.W. Remyelination therapies: A new direction and challenge in multiple sclerosis. Nat. Rev. Drug Discov. 2017, 16, 617–634. [Google Scholar] [CrossRef]
- Joseph, M.J.; Caliaperumal, J.; Schlichter, L.C. After Intracerebral Hemorrhage, Oligodendrocyte Precursors Proliferate and Differentiate Inside White-Matter Tracts in the Rat Striatum. Transl. Stroke Res. 2016, 7, 192–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keep, R.F.; Zhou, N.; Xiang, J.; Andjelkovic, A.V.; Hua, Y.; Xi, G. Vascular disruption and blood-brain barrier dysfunction in intracerebral hemorrhage. Fluids Barriers CNS 2014, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keep, R.F.; Xiang, J.; Ennis, S.R.; Andjelkovic, A.; Hua, Y.; Xi, G.; Hoff, J.T. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir. Suppl. 2008, 105, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Liu, X.J.; Zhang, Z.Q.; Zhou, H.J.; Luo, J.K.; Huang, J.F.; Yang, Q.D.; Li, X.Q. Cerebral angiogenesis after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2007, 1175, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Xie, L.; Mao, X.; Zhou, Y.; Zhan, R.; Greenberg, D.A.; Jin, K. Neurogenesis after primary intracerebral hemorrhage in adult human brain. J. Cereb. Blood Flow Metab. 2008, 28, 1460–1468. [Google Scholar] [CrossRef] [Green Version]
- Stepien, T.; Tarka, S.; Chutoranski, D.; Felczak, P.; Acewicz, A.; Wierzba-Bobrowicz, T. Neurogenesis in adult human brain after hemorrhage and ischemic stroke. Folia Neuropathol. 2018, 56, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Sgubin, D.; Aztiria, E.; Perin, A.; Longatti, P.; Leanza, G. Activation of endogenous neural stem cells in the adult human brain following subarachnoid hemorrhage. J. Neurosci. Res. 2007, 85, 1647–1655. [Google Scholar] [CrossRef]
- Bai, Q.; Xue, M.; Yong, V.W. Microglia and macrophage phenotypes in intracerebral haemorrhage injury: Therapeutic opportunities. Brain 2020, 143, 1297–1314. [Google Scholar] [CrossRef]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J. Stroke 2020, 22, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Emsley, H.C.; Tyrrell, P.J. Inflammation and infection in clinical stroke. J. Cereb. Blood Flow Metab. 2002, 22, 1399–1419. [Google Scholar] [CrossRef]
- Shtaya, A.; Bridges, L.R.; Esiri, M.M.; Lam-Wong, J.; Nicoll, J.A.R.; Boche, D.; Hainsworth, A.H. Rapid neuroinflammatory changes in human acute intracerebral hemorrhage. Ann. Clin. Transl. Neurol. 2019, 6, 1465–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Dore, S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain 2007, 130, 1643–1652. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Del Bigio, M.R. Intracerebral injection of autologous whole blood in rats: Time course of inflammation and cell death. Neurosci. Lett. 2000, 283, 230–232. [Google Scholar] [CrossRef]
- Xue, M.; Del Bigio, M.R. Immune pre-activation exacerbates hemorrhagic brain injury in immature mouse brain. J. Neuroimmunol. 2005, 165, 75–82. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, L.; Sherchan, P.; Ding, Y.; Yu, J.; Nowrangi, D.; Tang, J.; Xia, Y.; Zhang, J.H. Activation of melanocortin receptor 4 with RO27-3225 attenuates neuroinflammation through AMPK/JNK/p38 MAPK pathway after intracerebral hemorrhage in mice. J. Neuroinflammation 2018, 15, 106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Huang, Q.; Su, Y.; Zhang, Y.; Wang, G.; Li, F. NF-kappaB activation and cell death after intracerebral hemorrhage in patients. Neurol. Sci. 2014, 35, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Z.; Ren, H.; Jin, W.N.; Wood, K.; Liu, Q.; Sheth, K.N.; Shi, F.D. Colony stimulating factor 1 receptor inhibition eliminates microglia and attenuates brain injury after intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2017, 37, 2383–2395. [Google Scholar] [CrossRef] [Green Version]
- Yong, H.Y.F.; Rawji, K.S.; Ghorbani, S.; Xue, M.; Yong, V.W. The benefits of neuroinflammation for the repair of the injured central nervous system. Cell Mol. Immunol. 2019, 16, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.F.; Miron, V.E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 2019, 15, 447–458. [Google Scholar] [CrossRef]
- Yang, J.; Ding, S.; Huang, W.; Hu, J.; Huang, S.; Zhang, Y.; Zhuge, Q. Interleukin-4 Ameliorates the Functional Recovery of Intracerebral Hemorrhage Through the Alternative Activation of Microglia/Macrophage. Front. Neurosci. 2016, 10, 61. [Google Scholar] [CrossRef]
- Chang, C.F.; Wan, J.; Li, Q.; Renfroe, S.C.; Heller, N.M.; Wang, J. Alternative activation-skewed microglia/macrophages promote hematoma resolution in experimental intracerebral hemorrhage. Neurobiol. Dis. 2017, 103, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, G.; Ting, S.M.; Song, S.; Zhang, J.; Edwards, N.J.; Aronowski, J. Cleaning up after ICH: The role of Nrf2 in modulating microglia function and hematoma clearance. J. Neurochem. 2015, 133, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yihao, T.; Zhou, F.; Yin, N.; Qiang, T.; Haowen, Z.; Qianwei, C.; Jun, T.; Yuan, Z.; Gang, Z.; et al. Inflammatory Regulation by Driving Microglial M2 Polarization: Neuroprotective Effects of Cannabinoid Receptor-2 Activation in Intracerebral Hemorrhage. Front. Immunol. 2017, 8, 112. [Google Scholar] [CrossRef] [Green Version]
- Min, H.; Jang, Y.H.; Cho, I.H.; Yu, S.W.; Lee, S.J. Alternatively activated brain-infiltrating macrophages facilitate recovery from collagenase-induced intracerebral hemorrhage. Mol. Brain 2016, 9, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhu, Z.; Gao, S.; Zhang, L.; Cheng, X.; Li, S.; Li, M. Inhibition of fibrin formation reduces neuroinflammation and improves long-term outcome after intracerebral hemorrhage. Int. Immunopharmacol. 2019, 72, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Jing, C.; Bian, L.; Wang, M.; Keep, R.F.; Xi, G.; Hua, Y. Enhancement of Hematoma Clearance With CD47 Blocking Antibody in Experimental Intracerebral Hemorrhage. Stroke 2019, 50, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Z.; Lu, H.; Yang, Q.; Wu, H.; Wang, J. Microglial Polarization and Inflammatory Mediators After Intracerebral Hemorrhage. Mol. Neurobiol. 2017, 54, 1874–1886. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Han, R.; Chen, X.; Liu, X.; Wan, J.; Wang, L.; Yang, X.; Wang, J. Potential therapeutic targets for intracerebral hemorrhage-associated inflammation: An update. J. Cereb. Blood Flow Metab. 2020, 40, 1752–1768. [Google Scholar] [CrossRef]
- Neves, J.D.; Mestriner, R.G.; Netto, C.A. Astrocytes in the cerebral cortex play a role in the spontaneous motor recovery following experimental striatal hemorrhage. Neural. Regen. Res. 2018, 13, 67–68. [Google Scholar] [CrossRef]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Hermanns, S.; Klapka, N.; Gasis, M.; Muller, H.W. The collagenous wound healing scar in the injured central nervous system inhibits axonal regeneration. Adv. Exp. Med. Biol. 2006, 557, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Cui, Y.; Roberts, C.; Lu, M.; Wilhelmsson, U.; Pekny, M.; Chopp, M. Beneficial effects of gfap/vimentin reactive astrocytes for axonal remodeling and motor behavioral recovery in mice after stroke. Glia 2014, 62, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Nakano, T.; Irie, K.; Higuchi, S.; Fujioka, M.; Orito, K.; Iwasaki, K.; Jin, G.; Lo, E.H.; Mishima, K.; et al. Inhibition of reactive astrocytes with fluorocitrate retards neurovascular remodeling and recovery after focal cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 2010, 30, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, B.; Ioannidou, K.; Barnett, S.C. Astrocyte phenotypes and their relationship to myelination. J. Anat. 2011, 219, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Cottarelli, A.; Agalliu, D. Neuronal and glial regulation of CNS angiogenesis and barriergenesis. Development 2020, 147, dev182279. [Google Scholar] [CrossRef] [PubMed]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mackenzie, J.M.; Clayton, J.A. Early cellular events in the penumbra of human spontaneous intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 1999, 8, 1–8. [Google Scholar] [CrossRef]
- Gusdon, A.M.; Gialdini, G.; Kone, G.; Baradaran, H.; Merkler, A.E.; Mangat, H.S.; Navi, B.B.; Iadecola, C.; Gupta, A.; Kamel, H.; et al. Neutrophil-Lymphocyte Ratio and Perihematomal Edema Growth in Intracerebral Hemorrhage. Stroke 2017, 48, 2589–2592. [Google Scholar] [CrossRef]
- Lattanzi, S.; Cagnetti, C.; Provinciali, L.; Silvestrini, M. Neutrophil-to-Lymphocyte Ratio Predicts the Outcome of Acute Intracerebral Hemorrhage. Stroke 2016, 47, 1654–1657. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ting, S.M.; Liu, C.H.; Sun, G.; Kruzel, M.; Roy-O’Reilly, M.; Aronowski, J. Neutrophil polarization by IL-27 as a therapeutic target for intracerebral hemorrhage. Nat. Commun. 2017, 8, 602. [Google Scholar] [CrossRef] [Green Version]
- Wimmer, I.; Zrzavy, T.; Lassmann, H. Neuroinflammatory responses in experimental and human stroke lesions. J. Neuroimmunol. 2018, 323, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Liu, X.; Chen, S.; Xiao, Y.; Zhuang, W. Neutrophil-lymphocyte ratio predicts the outcome of intracerebral hemorrhage: A meta-analysis. Medicine (Baltimore) 2019, 98, e16211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Zhong, Q.; Wang, Y.C.; Xiong, X.Y.; Meng, Z.Y.; Zhao, T.; Zhu, W.Y.; Liao, M.F.; Wu, L.R.; Yang, Y.R.; et al. Regulatory T cells ameliorate intracerebral hemorrhage-induced inflammatory injury by modulating microglia/macrophage polarization through the IL-10/GSK3beta/PTEN axis. J. Cereb. Blood Flow Metab. 2017, 37, 967–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Yu, A.; Liu, Y.; Shen, H.; Lin, C.; Lin, L.; Wang, S.; Yuan, B. Regulatory T cells inhibit microglia activation and protect against inflammatory injury in intracerebral hemorrhage. Int. Immunopharmacol. 2014, 22, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Keep, R.F.; Hoff, J.T. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006, 5, 53–63. [Google Scholar] [CrossRef]
- Lee, K.R.; Colon, G.P.; Betz, A.L.; Keep, R.F.; Kim, S.; Hoff, J.T. Edema from intracerebral hemorrhage: The role of thrombin. J. Neurosurg. 1996, 84, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Song, S.; Hua, Y.; Nakamura, T.; Keep, R.F.; Xi, G. Effects of thrombin on neurogenesis after intracerebral hemorrhage. Stroke 2008, 39, 2079–2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.J.; Tang, T.; Cui, H.J.; Yang, A.L.; Luo, J.K.; Lin, Y.; Yang, Q.D.; Li, X.Q. Thrombin-triggered angiogenesis in rat brains following experimental intracerebral hemorrhage. J. Neurosurg. 2012, 117, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Keep, R.F.; Gu, Y.; Xi, G. Thrombin and brain recovery after intracerebral hemorrhage. Stroke 2009, 40, S88–S89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarzami, S.T.; Wang, G.; Li, W.; Green, L.; Singh, J.P. Thrombin and PAR-1 stimulate differentiation of bone marrow-derived endothelial progenitor cells. J. Thromb. Haemost. 2006, 4, 656–663. [Google Scholar] [CrossRef]
- Tsopanoglou, N.E.; Maragoudakis, M.E. Inhibition of angiogenesis by small-molecule antagonists of protease-activated receptor-1. Semin. Thromb. Hemost. 2007, 33, 680–687. [Google Scholar] [CrossRef]
- Hu, E.; Hu, W.; Yang, A.; Zhou, H.; Zhou, J.; Luo, J.; Wang, Y.; Tang, T.; Cui, H. Thrombin promotes pericyte coverage by Tie2 activation in a rat model of intracerebral hemorrhage. Brain Res. 2019, 1708, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Xi, G.; Reiser, G.; Keep, R.F. The role of thrombin and thrombin receptors in ischemic, hemorrhagic and traumatic brain injury: Deleterious or protective? J. Neurochem. 2003, 84, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Power, C.; Henry, S.; Del Bigio, M.R.; Larsen, P.H.; Corbett, D.; Imai, Y.; Yong, V.W.; Peeling, J. Intracerebral hemorrhage induces macrophage activation and matrix metalloproteinases. Ann. Neurol. 2003, 53, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Lischper, M.; Beuck, S.; Thanabalasundaram, G.; Pieper, C.; Galla, H.J. Metalloproteinase mediated occludin cleavage in the cerebral microcapillary endothelium under pathological conditions. Brain Res. 2010, 1326, 114–127. [Google Scholar] [CrossRef]
- Xue, M.; Yong, V.W. Matrix metalloproteinases in intracerebral hemorrhage. Neurol. Res. 2008, 30, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Aronowski, J.; Zhao, X. Molecular pathophysiology of cerebral hemorrhage: Secondary brain injury. Stroke 2011, 42, 1781–1786. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Hollenberg, M.D.; Yong, V.W. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J. Neurosci. 2006, 26, 10281–10291. [Google Scholar] [CrossRef] [Green Version]
- Florczak-Rzepka, M.; Grond-Ginsbach, C.; Montaner, J.; Steiner, T. Matrix metalloproteinases in human spontaneous intracerebral hemorrhage: An update. Cerebrovasc. Dis. 2012, 34, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Liu, Y.F.; Ma, L.; Worthmann, H.; Wang, Y.L.; Wang, Y.J.; Gao, Y.P.; Raab, P.; Dengler, R.; Weissenborn, K.; et al. Association of molecular markers with perihematomal edema and clinical outcome in intracerebral hemorrhage. Stroke 2013, 44, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tsirka, S.E. Neuroprotection by inhibition of matrix metalloproteinases in a mouse model of intracerebral haemorrhage. Brain 2005, 128, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Lin, S.; Zhang, C.; Tao, W.; Dong, W.; Hao, Z.; Liu, M.; Wu, B. Activation of cerebral recovery by matrix metalloproteinase-9 after intracerebral hemorrhage. Neuroscience 2013, 230, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.J.; Emanuel, B.A.; Mack, W.J.; Tsivgoulis, G.; Alexandrov, A.V. Matrix metalloproteinase-9: Dual role and temporal profile in intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2014, 23, 2498–2505. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Nozako, M.; Hazekawa, M.; Mishima, S.; Fujioka, M.; Orito, K.; Egashira, N.; Iwasaki, K.; Fujiwara, M. Delayed treatment with minocycline ameliorates neurologic impairment through activated microglia expressing a high-mobility group box1-inhibiting mechanism. Stroke 2008, 39, 951–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, M.; Katsuki, H.; Fukutomi, C.; Takahashi, M.; Motomura, M.; Fukunaga, M.; Matsuoka, Y.; Isohama, Y.; Izumi, Y.; Kume, T.; et al. HMGB1 inhibitor glycyrrhizin attenuates intracerebral hemorrhage-induced injury in rats. Neuropharmacology 2011, 61, 975–980. [Google Scholar] [CrossRef]
- Hoshino, K.; Takeuchi, O.; Kawai, T.; Sanjo, H.; Ogawa, T.; Takeda, Y.; Takeda, K.; Akira, S. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: Evidence for TLR4 as the Lps gene product. J. Immunol. 1999, 162, 3749–3752. [Google Scholar]
- Dobrovolskaia, M.A.; Medvedev, A.E.; Thomas, K.E.; Cuesta, N.; Toshchakov, V.; Ren, T.; Cody, M.J.; Michalek, S.M.; Rice, N.R.; Vogel, S.N. Induction of in vitro reprogramming by Toll-like receptor (TLR)2 and TLR4 agonists in murine macrophages: Effects of TLR “homotolerance” versus “heterotolerance” on NF-kappa B signaling pathway components. J. Immunol. 2003, 170, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Herold, K.; Moser, B.; Chen, Y.; Zeng, S.; Yan, S.F.; Ramasamy, R.; Emond, J.; Clynes, R.; Schmidt, A.M. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: Inflammatory signals gone awry in the primal response to stress. J. Leukoc. Biol. 2007, 82, 204–212. [Google Scholar] [CrossRef]
- Lei, C.; Lin, S.; Zhang, C.; Tao, W.; Dong, W.; Hao, Z.; Liu, M.; Wu, B. Effects of high-mobility group box1 on cerebral angiogenesis and neurogenesis after intracerebral hemorrhage. Neuroscience 2013, 229, 12–19. [Google Scholar] [CrossRef]
- Lei, C.; Wu, B.; Cao, T.; Zhang, S.; Liu, M. Activation of the high-mobility group box 1 protein-receptor for advanced glycation end-products signaling pathway in rats during neurogenesis after intracerebral hemorrhage. Stroke 2015, 46, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Lei, C.; Zhang, S.; Cao, T.; Tao, W.; Liu, M.; Wu, B. HMGB1 may act via RAGE to promote angiogenesis in the later phase after intracerebral hemorrhage. Neuroscience 2015, 295, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Leitner, G.R.; Wenzel, T.J.; Marshall, N.; Gates, E.J.; Klegeris, A. Targeting toll-like receptor 4 to modulate neuroinflammation in central nervous system disorders. Expert Opin. Ther. Targets 2019, 23, 865–882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Wang, P.F.; Fang, H.; Chen, J.; Xiong, X.Y.; Yang, Q.W. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke 2013, 44, 2545–2552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, C.; Wu, B.; Cao, T.; Liu, M.; Hao, Z. Brain recovery mediated by toll-like receptor 4 in rats after intracerebral hemorrhage. Brain Res. 2016, 1632, 1–8. [Google Scholar] [CrossRef]
- Wagner, K.R.; Xi, G.; Hua, Y.; Kleinholz, M.; de Courten-Myers, G.M.; Myers, R.E. Early metabolic alterations in edematous perihematomal brain regions following experimental intracerebral hemorrhage. J. Neurosurg. 1998, 88, 1058–1065. [Google Scholar] [CrossRef]
- Carhuapoma, J.R.; Wang, P.Y.; Beauchamp, N.J.; Keyl, P.M.; Hanley, D.F.; Barker, P.B. Diffusion-weighted MRI and proton MR spectroscopic imaging in the study of secondary neuronal injury after intracerebral hemorrhage. Stroke 2000, 31, 726–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schurr, A.; West, C.A.; Rigor, B.M. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science 1988, 240, 1326–1328. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A. Lactate: The ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006, 26, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Tarczyluk, M.A.; Nagel, D.A.; O’Neil, J.D.; Parri, H.R.; Tse, E.H.; Coleman, M.D.; Hill, E.J. Functional astrocyte-neuron lactate shuttle in a human stem cell-derived neuronal network. J. Cereb. Blood Flow Metab. 2013, 33, 1386–1393. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Liu, T.; Guo, H.; Cui, H.; Li, P.; Feng, D.; Hu, E.; Huang, Q.; Yang, A.; Zhou, J.; et al. Lactate potentiates angiogenesis and neurogenesis in experimental intracerebral hemorrhage. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef]
- Marin, M.C.; Jost, C.A.; Brooks, L.A.; Irwin, M.S.; O’Nions, J.; Tidy, J.A.; James, N.; McGregor, J.M.; Harwood, C.A.; Yulug, I.G.; et al. A common polymorphism acts as an intragenic modifier of mutant p53 behaviour. Nat. Genet. 2000, 25, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Dumont, P.; Leu, J.I.; Della Pietra, A.C., 3rd; George, D.L.; Murphy, M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat. Genet. 2003, 33, 357–365. [Google Scholar] [CrossRef]
- Bonafe, M.; Salvioli, S.; Barbi, C.; Trapassi, C.; Tocco, F.; Storci, G.; Invidia, L.; Vannini, I.; Rossi, M.; Marzi, E.; et al. The different apoptotic potential of the p53 codon 72 alleles increases with age and modulates in vivo ischaemia-induced cell death. Cell Death Differ. 2004, 11, 962–973. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, E.C.; Humbey, O.; Murphy, M.E. Polymorphisms in the p53 pathway. Oncogene 2006, 25, 1602–1611. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, C.; Sobrino, T.; Agulla, J.; Bobo-Jimenez, V.; Ramos-Araque, M.E.; Duarte, J.J.; Gomez-Sanchez, J.C.; Bolanos, J.P.; Castillo, J.; Almeida, A. Neovascularization and functional recovery after intracerebral hemorrhage is conditioned by the Tp53 Arg72Pro single-nucleotide polymorphism. Cell Death Differ. 2017, 24, 144–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pias-Peleteiro, J.; Campos, F.; Castillo, J.; Sobrino, T. Endothelial progenitor cells as a therapeutic option in intracerebral hemorrhage. Neural. Regen. Res. 2017, 12, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.Z.; Patil, P.; Gude, R.P. Role of STAT3 in cancer metastasis and translational advances. Biomed. Res. Int 2013, 2013, 421821. [Google Scholar] [CrossRef]
- Haitao, L.; Zhou, H. Activation of signal transducer and activator of transcription 3 signaling attenuates neurogenesis in a rat model of intracerebral hemorrhage. Turk. Neurosurg. 2018, 30, 793–798. [Google Scholar] [CrossRef]
- Akbik, F.; Cafferty, W.B.; Strittmatter, S.M. Myelin associated inhibitors: A link between injury-induced and experience-dependent plasticity. Exp. Neurol. 2012, 235, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Yiu, G.; He, Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006, 7, 617–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ma, C.; Li, H.; Shen, H.; Li, X.; Fu, X.; Wu, J.; Chen, G. Nogo-A/Pir-B/TrkB Signaling Pathway Activation Inhibits Neuronal Survival and Axonal Regeneration After Experimental Intracerebral Hemorrhage in Rats. J. Mol. Neurosci. 2019, 69, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Yuan, X.Y.; Zhang, X. Intracerebral hemorrhage influences hippocampal neurogenesis and neurological function recovery via Notch1 signaling. Neuroreport 2021, 32, 489–497. [Google Scholar] [CrossRef]
- Shao, Z.; Tu, S.; Shao, A. Pathophysiological Mechanisms and Potential Therapeutic Targets in Intracerebral Hemorrhage. Front. Pharmacol. 2019, 10, 1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.J.; Ding, D.; Ironside, N.; Buell, T.J.; Elder, L.J.; Warren, A.; Adams, A.P.; Ratcliffe, S.J.; James, R.F.; Naval, N.S.; et al. Statins for neuroprotection in spontaneous intracerebral hemorrhage. Neurology 2019, 93, 1056–1066. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Meschia, J.F.; Bushnell, C.; Boden-Albala, B.; Braun, L.T.; Bravata, D.M.; Chaturvedi, S.; Creager, M.A.; Eckel, R.H.; Elkind, M.S.; Fornage, M.; et al. Guidelines for the primary prevention of stroke: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2014, 45, 3754–3832. [Google Scholar] [CrossRef] [Green Version]
- Amarenco, P.; Labreuche, J. Lipid management in the prevention of stroke: Review and updated meta-analysis of statins for stroke prevention. Lancet Neurol. 2009, 8, 453–463. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A., 3rd; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E.; Sillesen, H.; Simunovic, L.; Szarek, M.; Welch, K.M.; et al. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar] [CrossRef]
- McKinney, J.S.; Kostis, W.J. Statin therapy and the risk of intracerebral hemorrhage: A meta-analysis of 31 randomized controlled trials. Stroke 2012, 43, 2149–2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziff, O.J.; Banerjee, G.; Ambler, G.; Werring, D.J. Statins and the risk of intracerebral haemorrhage in patients with stroke: Systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 75–83. [Google Scholar] [CrossRef]
- Ribe, A.R.; Vestergaard, C.H.; Vestergaard, M.; Fenger-Gron, M.; Pedersen, H.S.; Lietzen, L.W.; Brynningsen, P.K. Statins and Risk of Intracerebral Haemorrhage in a Stroke-Free Population: A Nationwide Danish Propensity Score Matched Cohort Study. EClinicalMedicine 2019, 8, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Tapia-Perez, H.; Sanchez-Aguilar, M.; Torres-Corzo, J.G.; Rodriguez-Leyva, I.; Gonzalez-Aguirre, D.; Gordillo-Moscoso, A.; Chalita-Williams, C. Use of statins for the treatment of spontaneous intracerebral hemorrhage: Results of a pilot study. Cent. Eur. Neurosurg. 2009, 70, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Karki, K.; Knight, R.A.; Han, Y.; Yang, D.; Zhang, J.; Ledbetter, K.A.; Chopp, M.; Seyfried, D.M. Simvastatin and atorvastatin improve neurological outcome after experimental intracerebral hemorrhage. Stroke 2009, 40, 3384–3389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyfried, D.; Han, Y.; Lu, D.; Chen, J.; Bydon, A.; Chopp, M. Improvement in neurological outcome after administration of atorvastatin following experimental intracerebral hemorrhage in rats. J. Neurosurg. 2004, 101, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Han, Y.; Zhang, J.; Chopp, M.; Seyfried, D.M. Statins Enhance Expression of Growth Factors and Activate the PI3K/Akt-mediated Signaling Pathway after Experimental Intracerebral Hemorrhage. World J. Neurosci 2012, 2, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Knight, R.A.; Han, Y.; Karki, K.; Zhang, J.; Ding, C.; Chopp, M.; Seyfried, D.M. Vascular recovery promoted by atorvastatin and simvastatin after experimental intracerebral hemorrhage: Magnetic resonance imaging and histological study. J. Neurosurg. 2011, 114, 1135–1142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, Q.; Tan, Q.; Feng, Z.; He, Z.; Tang, J.; Feng, H.; Zhu, G.; Chen, Z. Simvastatin accelerates hematoma resolution after intracerebral hemorrhage in a PPARgamma-dependent manner. Neuropharmacology 2018, 128, 244–254. [Google Scholar] [CrossRef]
- Wu, J.; Yang, S.; Xi, G.; Fu, G.; Keep, R.F.; Hua, Y. Minocycline reduces intracerebral hemorrhage-induced brain injury. Neurol. Res. 2009, 31, 183–188. [Google Scholar] [CrossRef]
- Yang, H.; Gao, X.J.; Li, Y.J.; Su, J.B.; E, T.Z.; Zhang, X.; Ni, W.; Gu, Y.X. Minocycline reduces intracerebral hemorrhage-induced white matter injury in piglets. CNS Neurosci. Ther. 2019, 25, 1195–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tikka, T.; Fiebich, B.L.; Goldsteins, G.; Keinanen, R.; Koistinaho, J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J. Neurosci. 2001, 21, 2580–2588. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Imagama, S.; Ohgomori, T.; Hirano, K.; Uchimura, K.; Sakamoto, K.; Hirakawa, A.; Takeuchi, H.; Suzumura, A.; Ishiguro, N.; et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013, 4, e525. [Google Scholar] [CrossRef] [Green Version]
- Sun, N.; Shen, Y.; Han, W.; Shi, K.; Wood, K.; Fu, Y.; Hao, J.; Liu, Q.; Sheth, K.N.; Huang, D.; et al. Selective Sphingosine-1-Phosphate Receptor 1 Modulation Attenuates Experimental Intracerebral Hemorrhage. Stroke 2016, 47, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Shi, W.; Wang, Z.; Wang, R.; Guo, Z.; Liu, C.; Sun, J.; Gao, L.; Zhou, R. Effects of minocycline on the expression of NGF and HSP70 and its neuroprotection role following intracerebral hemorrhage in rats. J. Biomed. Res. 2011, 25, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Hao, D.; Shi, W.; Pu, J.; Wang, Z. Effects of minocycline on apoptosis and angiogenesis-related protein expression in a rat model of intracerebral hemorrhage. Neural. Regen. Res. 2012, 7, 595–600. [Google Scholar] [CrossRef]
- Fouda, A.Y.; Newsome, A.S.; Spellicy, S.; Waller, J.L.; Zhi, W.; Hess, D.C.; Ergul, A.; Edwards, D.J.; Fagan, S.C.; Switzer, J.A. Minocycline in Acute Cerebral Hemorrhage: An Early Phase Randomized Trial. Stroke 2017, 48, 2885–2887. [Google Scholar] [CrossRef]
- Chang, J.J.; Kim-Tenser, M.; Emanuel, B.A.; Jones, G.M.; Chapple, K.; Alikhani, A.; Sanossian, N.; Mack, W.J.; Tsivgoulis, G.; Alexandrov, A.V.; et al. Minocycline and matrix metalloproteinase inhibition in acute intracerebral hemorrhage: A pilot study. Eur. J. Neurol. 2017, 24, 1384–1391. [Google Scholar] [CrossRef]
- O’Sullivan, S.; Dev, K.K. Sphingosine-1-phosphate receptor therapies: Advances in clinical trials for CNS-related diseases. Neuropharmacology 2017, 113, 597–607. [Google Scholar] [CrossRef]
- Noda, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Fingolimod phosphate promotes the neuroprotective effects of microglia. J. Neuroimmunol. 2013, 256, 13–18. [Google Scholar] [CrossRef]
- Qin, C.; Fan, W.H.; Liu, Q.; Shang, K.; Murugan, M.; Wu, L.J.; Wang, W.; Tian, D.S. Fingolimod Protects Against Ischemic White Matter Damage by Modulating Microglia Toward M2 Polarization via STAT3 Pathway. Stroke 2017, 48, 3336–3346. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, S.; Zheng, Q.; Zhang, L.; Tan, X.; Zou, J.; Yan, B.; Chen, Y. FTY720 attenuates iron deposition and glial responses in improving delayed lesion and long-term outcomes of collagenase-induced intracerebral hemorrhage. Brain Res. 2019, 1718, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Chang, G.Q.; Liu, Y.; Gong, Y.; Yang, C.; Wood, K.; Shi, F.D.; Fu, Y.; Yan, Y. Fingolimod alters inflammatory mediators and vascular permeability in intracerebral hemorrhage. Neurosci. Bull. 2015, 31, 755–762. [Google Scholar] [CrossRef]
- Rolland, W.B.; Lekic, T.; Krafft, P.R.; Hasegawa, Y.; Altay, O.; Hartman, R.; Ostrowski, R.; Manaenko, A.; Tang, J.; Zhang, J.H. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Exp. Neurol. 2013, 241, 45–55. [Google Scholar] [CrossRef] [Green Version]
- Napier, J.; Rose, L.; Adeoye, O.; Hooker, E.; Walsh, K.B. Modulating acute neuroinflammation in intracerebral hemorrhage: The potential promise of currently approved medications for multiple sclerosis. Immunopharmacol. Immunotoxicol. 2019, 41, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Hao, J.; Zhang, N.; Ren, L.; Sun, N.; Li, Y.J.; Yan, Y.; Huang, D.; Yu, C.; Shi, F.D. Fingolimod for the treatment of intracerebral hemorrhage: A 2-arm proof-of-concept study. JAMA Neurol. 2014, 71, 1092–1101. [Google Scholar] [CrossRef] [Green Version]
- Bobinger, T.; Manaenko, A.; Burkardt, P.; Beuscher, V.; Sprugel, M.I.; Roeder, S.S.; Bauerle, T.; Seyler, L.; Nagel, A.M.; Linker, R.A.; et al. Siponimod (BAF-312) Attenuates Perihemorrhagic Edema And Improves Survival in Experimental Intracerebral Hemorrhage. Stroke 2019, 50, 3246–3254. [Google Scholar] [CrossRef] [PubMed]
- Bobinger, T.; Bauerle, T.; Seyler, L.; v Horsten, S.; Schwab, S.; Huttner, H.B.; Manaenko, A. A Sphingosine-1-Phosphate Receptor Modulator Attenuated Secondary Brain Injury and Improved Neurological Functions of Mice after ICH. Oxid Med. Cell Longev. 2020, 2020, 3214350. [Google Scholar] [CrossRef]
- Baldessarini, R.J.; Tondo, L.; Davis, P.; Pompili, M.; Goodwin, F.K.; Hennen, J. Decreased risk of suicides and attempts during long-term lithium treatment: A meta-analytic review. Bipolar. Disord. 2006, 8, 625–639. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Wu, X.; Yu, Z.; Zhao, S.; Tang, X. Lithium chloride promoted hematoma resolution after intracerebral hemorrhage through GSK-3beta-mediated pathways-dependent microglia phagocytosis and M2-phenotype differentiation, angiogenesis and neurogenesis in a rat model. Brain Res. Bull. 2019, 152, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xia, M.; Chen, W.; Wang, J.; Yin, Y.; Guo, C.; Li, C.; Tang, X.; Zhao, H.; Tan, Q.; et al. Lithium treatment mitigates white matter injury after intracerebral hemorrhage through brain-derived neurotrophic factor signaling in mice. Transl. Res. 2020, 217, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Mao, S.; Xi, G.; Keep, R.F.; Hua, Y. Role of Erythrocyte CD47 in Intracerebral Hematoma Clearance. Stroke 2016, 47, 505–511. [Google Scholar] [CrossRef] [Green Version]
- Tao, C.; Keep, R.F.; Xi, G.; Hua, Y. CD47 Blocking Antibody Accelerates Hematoma Clearance After Intracerebral Hemorrhage in Aged Rats. Transl. Stroke Res. 2020, 11, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, B.; Zhang, J.; Ding, W.; Xiao, Z.; Zhu, Z.; Han, Q.; Wu, C.; Sun, Y.; Tong, W.; et al. Nerve regeneration and functional recovery by collagen-binding brain-derived neurotrophic factor in an intracerebral hemorrhage model. Tissue Eng. Part A 2015, 21, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.Q.; Jin, W.; Xiao, Z.F.; Huang, J.C.; Ni, H.B.; Kong, J.; Wu, J.; Chen, B.; Liang, W.B.; Dai, J.W. The promotion of neurological recovery in an intracerebral hemorrhage model using fibrin-binding brain derived neurotrophic factor. Biomaterials 2011, 32, 3244–3252. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Jia, Y.; Tian, Y.; Sun, J.; Wei, Y.; Yue, S.; Lin, L.; Wei, Y.; Li, Y.; Lei, P.; et al. Mouse nerve growth factor promotes neurological recovery in patients with acute intracerebral hemorrhage: A proof-of-concept study. J. Neurol. Sci. 2020, 418, 117069. [Google Scholar] [CrossRef]
- Chen, L.; Xi, H.; Huang, H.; Zhang, F.; Liu, Y.; Chen, D.; Xiao, J. Multiple cell transplantation based on an intraparenchymal approach for patients with chronic phase stroke. Cell Transplant. 2013, 22 (Suppl. 1), S83–S91. [Google Scholar] [CrossRef]
- Li, Z.M.; Zhang, Z.T.; Guo, C.J.; Geng, F.Y.; Qiang, F.; Wang, L.X. Autologous bone marrow mononuclear cell implantation for intracerebral hemorrhage-a prospective clinical observation. Clin. Neurol. Neurosurg. 2013, 115, 72–76. [Google Scholar] [CrossRef]
- Chang, Z.; Mao, G.; Sun, L.; Ao, Q.; Gu, Y.; Liu, Y. Cell therapy for cerebral hemorrhage: Five year follow-up report. Exp. Ther. Med. 2016, 12, 3535–3540. [Google Scholar] [CrossRef] [Green Version]
- Tsang, K.S.; Ng, C.P.S.; Zhu, X.L.; Wong, G.K.C.; Lu, G.; Ahuja, A.T.; Wong, K.S.L.; Ng, H.K.; Poon, W.S. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J. Stem. Cells 2017, 9, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, M.F.; Horn, A.P. Stem cell therapy in intracerebral hemorrhage rat model. World J. Stem. Cells 2015, 7, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Marsh, S.E.; Blurton-Jones, M. Neural stem cell therapy for neurodegenerative disorders: The role of neurotrophic support. Neurochem. Int. 2017, 106, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, W.; Li, T.; Chen, J.; Shao, A.; Yan, F.; Chen, G. Stem Cell Therapy: A Promising Therapeutic Method for Intracerebral Hemorrhage. Cell Transplant. 2018, 27, 1809–1824. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Chen, S.L.; Wang, D.Y.; Chiu, I.M. Stem cell-based therapy in neural repair. Biomed. J. 2013, 36, 98–105. [Google Scholar] [CrossRef]
- Ryu, S.; Lee, S.H.; Kim, S.U.; Yoon, B.W. Human neural stem cells promote proliferation of endogenous neural stem cells and enhance angiogenesis in ischemic rat brain. Neural. Regen. Res. 2016, 11, 298–304. [Google Scholar] [CrossRef]
- Giusto, E.; Donega, M.; Cossetti, C.; Pluchino, S. Neuro-immune interactions of neural stem cell transplants: From animal disease models to human trials. Exp. Neurol. 2014, 260, 19–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Zhang, R.; Lu, Y.; Wen, L.; Li, Y.; Duan, R.; Yao, Y.; Jia, Y. Lin28B regulates the fate of grafted mesenchymal stem cells and enhances their protective effects against Alzheimer’s disease by upregulating IGF-2. J. Cell Physiol. 2019, 234, 21860–21876. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.P.; Hsu, Y.H.; Wu, M.S.; Tsai, H.H.; Su, C.Y.; Ling, T.Y.; Hsu, S.H.; Lai, D.M. Potential of stem cell therapy in intracerebral hemorrhage. Mol. Biol. Rep. 2020, 47, 4671–4680. [Google Scholar] [CrossRef] [PubMed]
- Bedini, G.; Bersano, A.; Zanier, E.R.; Pischiutta, F.; Parati, E.A. Mesenchymal Stem Cell Therapy in Intracerebral Haemorrhagic Stroke. Curr. Med. Chem. 2018, 25, 2176–2197. [Google Scholar] [CrossRef]
- Wang, S.P.; Wang, Z.H.; Peng, D.Y.; Li, S.M.; Wang, H.; Wang, X.H. Therapeutic effect of mesenchymal stem cells in rats with intracerebral hemorrhage: Reduced apoptosis and enhanced neuroprotection. Mol. Med. Rep. 2012, 6, 848–854. [Google Scholar] [CrossRef] [Green Version]
- Liang, H.; Yin, Y.; Lin, T.; Guan, D.; Ma, B.; Li, C.; Wang, Y.; Zhang, X. Transplantation of bone marrow stromal cells enhances nerve regeneration of the corticospinal tract and improves recovery of neurological functions in a collagenase-induced rat model of intracerebral hemorrhage. Mol. Cells 2013, 36, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Ding, R.; Lin, C.; Wei, S.; Zhang, N.; Tang, L.; Lin, Y.; Chen, Z.; Xie, T.; Chen, X.; Feng, Y.; et al. Therapeutic Benefits of Mesenchymal Stromal Cells in a Rat Model of Hemoglobin-Induced Hypertensive Intracerebral Hemorrhage. Mol. Cells 2017, 40, 133–142. [Google Scholar] [CrossRef]
- Cui, J.; Cui, C.; Cui, Y.; Li, R.; Sheng, H.; Jiang, X.; Tian, Y.; Wang, K.; Gao, J. Bone Marrow Mesenchymal Stem Cell Transplantation Increases GAP-43 Expression via ERK1/2 and PI3K/Akt Pathways in Intracerebral Hemorrhage. Cell Physiol. Biochem. 2017, 42, 137–144. [Google Scholar] [CrossRef]
- Kobayashi, T.; Ahlenius, H.; Thored, P.; Kobayashi, R.; Kokaia, Z.; Lindvall, O. Intracerebral infusion of glial cell line-derived neurotrophic factor promotes striatal neurogenesis after stroke in adult rats. Stroke 2006, 37, 2361–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.S.; Patel, N.K.; Hotton, G.R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D.J.; Svendsen, C.N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Xin, T.; Zhao, L.; Hunter, R.L.; Chen, Y.; Bing, G. Glial cell line-derived neurotrophic factor protects midbrain dopaminergic neurons against lipopolysaccharide neurotoxicity. J. Neuroimmunol. 2010, 225, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L.; Gao, X.; Fan, G.; Yang, C. Effects of GDNF-Transfected Marrow Stromal Cells on Rats with Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2019, 28, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cao, J.; Duan, S.; Xu, R.; Yu, H.; Huo, X.; Qian, Y. Effect of MicroRNA-126a-3p on Bone Marrow Mesenchymal Stem Cells Repairing Blood-brain Barrier and Nerve Injury after Intracerebral Hemorrhage. J. Stroke Cerebrovasc. Dis. 2020, 29, 104748. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kitada, M.; Wakao, S.; Nishikawa, K.; Tanimura, Y.; Makinoshima, H.; Goda, M.; Akashi, H.; Inutsuka, A.; Niwa, A.; et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc. Natl. Acad. Sci. USA 2010, 107, 8639–8643. [Google Scholar] [CrossRef] [Green Version]
- Wakao, S.; Kuroda, Y.; Ogura, F.; Shigemoto, T.; Dezawa, M. Regenerative Effects of Mesenchymal Stem Cells: Contribution of Muse Cells, a Novel Pluripotent Stem Cell Type that Resides in Mesenchymal Cells. Cells 2012, 1, 1045–1060. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Yang, Z.; Xiao, R.; Pan, B. Regenerative potential of pluripotent nontumorgenetic stem cells: Multilineage differentiating stress enduring cells (Muse cells). Regen. Ther. 2020, 15, 92–96. [Google Scholar] [CrossRef]
- Shimamura, N.; Kakuta, K.; Wang, L.; Naraoka, M.; Uchida, H.; Wakao, S.; Dezawa, M.; Ohkuma, H. Neuro-regeneration therapy using human Muse cells is highly effective in a mouse intracerebral hemorrhage model. Exp. Brain Res. 2017, 235, 565–572. [Google Scholar] [CrossRef]
- Kuramoto, Y.; Takagi, T.; Tatebayashi, K.; Beppu, M.; Doe, N.; Fujita, M.; Yoshimura, S. Intravenous administration of human adipose-derived stem cells ameliorates motor and cognitive function for intracerebral hemorrhage mouse model. Brain Res. 2019, 1711, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tang, Y.X.; Liu, Y.M.; Chen, J.; Hu, X.Q.; Liu, N.; Wang, S.X.; Zhang, Y.; Zeng, W.G.; Ni, H.J.; et al. Transplantation of adipose-derived stem cells is associated with neural differentiation and functional improvement in a rat model of intracerebral hemorrhage. CNS Neurosci. Ther. 2012, 18, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Deng, H.; Hu, Y.; Pan, C.; Wu, G.; Li, Q.; Tang, Z. Adipose-derived mesenchymal stem cells stereotactic transplantation alleviate brain edema from intracerebral hemorrhage. J. Cell Biochem. 2019, 120, 14372–14382. [Google Scholar] [CrossRef]
- Re, D.B.; Przedborski, S. Fractalkine: Moving from chemotaxis to neuroprotection. Nat. Neurosci. 2006, 9, 859–861. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Rostene, W.; Apartis, E.; Banisadr, G.; Biber, K.; Milligan, E.D.; White, F.A.; Zhang, J. Chemokine action in the nervous system. J. Neurosci. 2008, 28, 11792–11795. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.K.; Jiang, Y.; Chen, S.; Xia, Y.; Maciejewski, D.; McNamara, R.K.; Streit, W.J.; Salafranca, M.N.; Adhikari, S.; Thompson, D.A.; et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 1998, 95, 10896–10901. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Yu, H.; Liu, N.; Zhang, P.; Tang, Y.; Hu, Y.; Zhang, Y.; Pan, C.; Deng, H.; Wang, J.; et al. Overexpression of CX3CR1 in Adipose-Derived Stem Cells Promotes Cell Migration and Functional Recovery After Experimental Intracerebral Hemorrhage. Front. Neurosci. 2019, 13, 462. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Han, Y.; Zhang, J.; Seyda, A.; Chopp, M.; Seyfried, D.M. Therapeutic effect of human umbilical tissue-derived cell treatment in rats with experimental intracerebral hemorrhage. Brain Res. 2012, 1444, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.M.; Lu, G.; Tsang, K.S.; Li, G.; Wu, Y.; Huang, Z.S.; Ng, H.K.; Kung, H.F.; Poon, W.S. Umbilical cord-derived mesenchymal stem cells with forced expression of hepatocyte growth factor enhance remyelination and functional recovery in a rat intracerebral hemorrhage model. Neurosurgery 2010, 67, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Chu, K.; Jung, K.H.; Kim, S.U.; Kim, M.; Roh, J.K. Human neural stem cell transplantation promotes functional recovery in rats with experimental intracerebral hemorrhage. Stroke 2003, 34, 2258–2263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Kim, K.S.; Kim, E.J.; Choi, H.B.; Lee, K.H.; Park, I.H.; Ko, Y.; Jeong, S.W.; Kim, S.U. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem. Cells 2007, 25, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, C.; Li, Q.; Zhou, S.; Fu, J.; Wang, X.; Zhuge, Q. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J. Cell Mol. Med. 2011, 15, 2624–2633. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Ma, X.; Qi, H.; Song, B.; Wang, Y.; Wen, X.; Wang, Q.M.; Sun, S.; Li, Y.; Zhang, R.; et al. Transplantation of Induced Pluripotent Stem Cells Alleviates Cerebral Inflammation and Neural Damage in Hemorrhagic Stroke. PLoS ONE 2015, 10, e0129881. [Google Scholar] [CrossRef]
- Qin, J.; Song, B.; Zhang, H.; Wang, Y.; Wang, N.; Ji, Y.; Qi, J.; Chandra, A.; Yang, B.; Zhang, Y.; et al. Transplantation of human neuro-epithelial-like stem cells derived from induced pluripotent stem cells improves neurological function in rats with experimental intracerebral hemorrhage. Neurosci. Lett. 2013, 548, 95–100. [Google Scholar] [CrossRef]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem. Cell 2020, 27, 523–531. [Google Scholar] [CrossRef]
- Branscome, H.; Paul, S.; Yin, D.; El-Hage, N.; Agbottah, E.T.; Zadeh, M.A.; Liotta, L.A.; Kashanchi, F. Use of Stem Cell Extracellular Vesicles as a “Holistic” Approach to CNS Repair. Front. Cell Dev. Biol. 2020, 8, 455. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Barreto, G.E.; Read, M.I.; Tafti, H.A.; Sahebkar, A. The Therapeutic Potential of Mesenchymal Stem Cell-Derived Exosomes in Treatment of Neurodegenerative Diseases. Mol. Neurobiol. 2019, 56, 8157–8167. [Google Scholar] [CrossRef]
- Otero-Ortega, L.; Gomez de Frutos, M.C.; Laso-Garcia, F.; Rodriguez-Frutos, B.; Medina-Gutierrez, E.; Lopez, J.A.; Vazquez, J.; Diez-Tejedor, E.; Gutierrez-Fernandez, M. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2018, 38, 767–779. [Google Scholar] [CrossRef]
- Han, Y.; Seyfried, D.; Meng, Y.; Yang, D.; Schultz, L.; Chopp, M.; Seyfried, D. Multipotent mesenchymal stromal cell-derived exosomes improve functional recovery after experimental intracerebral hemorrhage in the rat. J. Neurosurg. 2018, 131, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wang, F.; Cao, J.; Wang, C. Exosomes Derived from MicroRNA-146a-5p-Enriched Bone Marrow Mesenchymal Stem Cells Alleviate Intracerebral Hemorrhage by Inhibiting Neuronal Apoptosis and Microglial M1 Polarization. Drug Des. Devel. Ther. 2020, 14, 3143–3158. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yao, X.; Li, H.; Li, X.; Zhang, T.; Sun, Q.; Ji, C.; Chen, G. Role of Exosomes Derived from miR-133b Modified MSCs in an Experimental Rat Model of Intracerebral Hemorrhage. J. Mol. Neurosci. 2018, 64, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Nih, L.R.; Carmichael, S.T.; Segura, T. Hydrogels for brain repair after stroke: An emerging treatment option. Curr. Opin. Biotechnol. 2016, 40, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Nih, L.R.; Gojgini, S.; Carmichael, S.T.; Segura, T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 2018, 17, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Duan, Z.; Qi, X.; Ou, Y.; Guo, X.; Zi, L.; Wei, Y.; Liu, H.; Ma, L.; Li, H.; et al. Injectable Gelatin Hydrogel Suppresses Inflammation and Enhances Functional Recovery in a Mouse Model of Intracerebral Hemorrhage. Front. Bioeng. Biotechnol. 2020, 8, 785. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, N.; Zhang, P.; Pan, C.; Zhang, Y.; Tang, Y.; Deng, H.; Aimaiti, M.; Zhang, Y.; Zhou, H.; et al. Preclinical Studies of Stem Cell Transplantation in Intracerebral Hemorrhage: A Systemic Review and Meta-Analysis. Mol. Neurobiol. 2016, 53, 5269–5277. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.C.; Mandeville, E.; Weng, D.; Wang, L.S.; Kurisawa, M.; Leite-Morris, K.; Selim, M.H.; Lo, E.H.; Spector, M. Hydrogel-Based Therapy for Brain Repair After Intracerebral Hemorrhage. Transl. Stroke Res. 2020, 11, 412–417. [Google Scholar] [CrossRef]
- Schneider, A.; Garlick, J.A.; Egles, C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS ONE 2008, 3, e1410. [Google Scholar] [CrossRef] [Green Version]
- Sang, L.Y.; Liang, Y.X.; Li, Y.; Wong, W.M.; Tay, D.K.; So, K.F.; Ellis-Behnke, R.G.; Wu, W.; Cheung, R.T. A self-assembling nanomaterial reduces acute brain injury and enhances functional recovery in a rat model of intracerebral hemorrhage. Nanomedicine 2015, 11, 611–620. [Google Scholar] [CrossRef]
- Zhang, N.; Luo, Y.; He, L.; Zhou, L.; Wu, W. A self-assembly peptide nanofibrous scaffold reduces inflammatory response and promotes functional recovery in a mouse model of intracerebral hemorrhage. Nanomedicine 2016, 12, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lu, J.; Mei, S.; Wu, H.; Sun, Z.; Fang, Y.; Xu, S.; Wang, X.; Shi, L.; Xu, W.; et al. Ceria nanoparticles ameliorate white matter injury after intracerebral hemorrhage: Microglia-astrocyte involvement in remyelination. J. Neuroinflammation 2021, 18, 43. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [Green Version]
- Shoamanesh Co-Chair, A.; Patrice Lindsay, M.; Castellucci, L.A.; Cayley, A.; Crowther, M.; de Wit, K.; English, S.W.; Hoosein, S.; Huynh, T.; Kelly, M.; et al. Canadian stroke best practice recommendations: Management of Spontaneous Intracerebral Hemorrhage, 7th Edition Update 2020. Int. J. Stroke 2021, 16, 321–341. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.C.; Jeng, J.S.; Chen, W.S.; Pan, G.S.; Chuang Pt Bs, W.Y.; Lee, Y.Y.; Teng, T. Early Mobilization of Mild-Moderate Intracerebral Hemorrhage Patients in a Stroke Center: A Randomized Controlled Trial. Neurorehabil. Neural. Repair. 2020, 34, 72–81. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, Y.; Wu, Y.; Zhu, Y.; He, Q.; Jiang, C.; Sun, L.; Fan, W. A prospective, randomized, single-blinded trial on the effect of early rehabilitation on daily activities and motor function of patients with hemorrhagic stroke. J. Clin. Neurosci. 2012, 19, 1376–1379. [Google Scholar] [CrossRef]
- Liu, N.; Cadilhac, D.A.; Andrew, N.E.; Zeng, L.; Li, Z.; Li, J.; Li, Y.; Yu, X.; Mi, B.; Li, Z.; et al. Randomized controlled trial of early rehabilitation after intracerebral hemorrhage stroke: Difference in outcomes within 6 months of stroke. Stroke 2014, 45, 3502–3507. [Google Scholar] [CrossRef] [Green Version]
- Sreekrishnan, A.; Leasure, A.C.; Shi, F.D.; Hwang, D.Y.; Schindler, J.L.; Petersen, N.H.; Gilmore, E.J.; Kamel, H.; Sansing, L.H.; Greer, D.M.; et al. Functional Improvement Among Intracerebral Hemorrhage (ICH) Survivors up to 12 Months Post-injury. Neurocrit. Care 2017, 27, 326–333. [Google Scholar] [CrossRef]
- Mestriner, R.G.; Pagnussat, A.S.; Boisserand, L.S.; Valentim, L.; Netto, C.A. Skilled reaching training promotes astroglial changes and facilitated sensorimotor recovery after collagenase-induced intracerebral hemorrhage. Exp. Neurol. 2011, 227, 53–61. [Google Scholar] [CrossRef]
- Santos, M.V.; Pagnussat, A.S.; Mestriner, R.G.; Netto, C.A. Motor Skill Training Promotes Sensorimotor Recovery and Increases Microtubule-Associated Protein-2 (MAP-2) Immunoreactivity in the Motor Cortex after Intracerebral Hemorrhage in the Rat. ISRN Neurol. 2013, 2013, 159184. [Google Scholar] [CrossRef]
- MacLellan, C.L.; Plummer, N.; Silasi, G.; Auriat, A.M.; Colbourne, F. Rehabilitation promotes recovery after whole blood-induced intracerebral hemorrhage in rats. Neurorehabil. Neural. Repair. 2011, 25, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Auriat, A.M.; Colbourne, F. Delayed rehabilitation lessens brain injury and improves recovery after intracerebral hemorrhage in rats. Brain Res. 2009, 1251, 262–268. [Google Scholar] [CrossRef]
- Auriat, A.M.; Wowk, S.; Colbourne, F. Rehabilitation after intracerebral hemorrhage in rats improves recovery with enhanced dendritic complexity but no effect on cell proliferation. Behav. Brain Res. 2010, 214, 42–47. [Google Scholar] [CrossRef]
- Caliaperumal, J.; Colbourne, F. Rehabilitation improves behavioral recovery and lessens cell death without affecting iron, ferritin, transferrin, or inflammation after intracerebral hemorrhage in rats. Neurorehabil. Neural. Repair. 2014, 28, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Tamakoshi, K.; Ishida, A.; Takamatsu, Y.; Hamakawa, M.; Nakashima, H.; Shimada, H.; Ishida, K. Motor skills training promotes motor functional recovery and induces synaptogenesis in the motor cortex and striatum after intracerebral hemorrhage in rats. Behav. Brain Res. 2014, 260, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Ishida, A.; Hamakawa, M.; Tamakoshi, K.; Jung, C.G.; Ishida, K. Treadmill running improves motor function and alters dendritic morphology in the striatum after collagenase-induced intracerebral hemorrhage in rats. Brain Res. 2010, 1355, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.A.; Khodaparast, N.; Hulsey, D.R.; Ruiz, A.; Sloan, A.M.; Rennaker, R.L., 2nd; Kilgard, M.P. Vagus nerve stimulation during rehabilitative training improves functional recovery after intracerebral hemorrhage. Stroke 2014, 45, 3097–3100. [Google Scholar] [CrossRef] [Green Version]
- Lo, A.C.; Guarino, P.D.; Richards, L.G.; Haselkorn, J.K.; Wittenberg, G.F.; Federman, D.G.; Ringer, R.J.; Wagner, T.H.; Krebs, H.I.; Volpe, B.T.; et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N. Engl. J. Med. 2010, 362, 1772–1783. [Google Scholar] [CrossRef] [Green Version]
- Gross, B.A.; Jankowitz, B.T.; Friedlander, R.M. Cerebral Intraparenchymal Hemorrhage: A Review. JAMA 2019, 321, 1295–1303. [Google Scholar] [CrossRef]
- Tatlisumak, T.; Cucchiara, B.; Kuroda, S.; Kasner, S.E.; Putaala, J. Nontraumatic intracerebral haemorrhage in young adults. Nat. Rev. Neurol. 2018, 14, 237–250. [Google Scholar] [CrossRef]
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Approaches | Possible Benefits | |
|---|---|---|
| Medications | Statins | Neurogenesis, angiogenesis, phagocytosis |
| Minocycline | “M2” polarization, neurogenesis | |
| Fingolimod/Siponimod | Neurogenesis, remyelination | |
| Lithium | Trophic factors, white matter repair | |
| CD47 antibody | Hematoma clearance | |
| Stem cells | BM-MSCs | Axonal regeneration, BBB reconstruction |
| Muse cells | Cell replacement of neurons | |
| ADSCs | Neuron-like differentiation, VEGF | |
| UCSCs | Neurogenesis, angiogenesis, remyelination | |
| Exosomes | Remyelination, axonal sprouting, neurogenesis | |
| Biomaterials | Hydrogel | Regulatory polarization, neurogenesis |
| SAPNS | Oligodendrogenesis, remyelination | |
| Rehabilitation | Skilled reach training | Dendritic reorganization, BDNF |
| Enriched environment | Dendritic length | |
| Acrobatic training | Neuronal activity, synaptic remodeling | |
| Aerobic training | Dendritic length and complexity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Xue, M.; Yong, V.W. Central Nervous System Tissue Regeneration after Intracerebral Hemorrhage: The Next Frontier. Cells 2021, 10, 2513. https://doi.org/10.3390/cells10102513
Zhang R, Xue M, Yong VW. Central Nervous System Tissue Regeneration after Intracerebral Hemorrhage: The Next Frontier. Cells. 2021; 10(10):2513. https://doi.org/10.3390/cells10102513
Chicago/Turabian StyleZhang, Ruiyi, Mengzhou Xue, and Voon Wee Yong. 2021. "Central Nervous System Tissue Regeneration after Intracerebral Hemorrhage: The Next Frontier" Cells 10, no. 10: 2513. https://doi.org/10.3390/cells10102513






