Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland
Abstract
1. Introduction
1.1. Prostate Gland: Anatomy and Embryology
1.2. Localization and Expression of Estrogen and Androgen Receptors inside the Prostate Gland
1.3. The Role of Estrogens in the Prostate Gland
1.4. Prostate Diseases
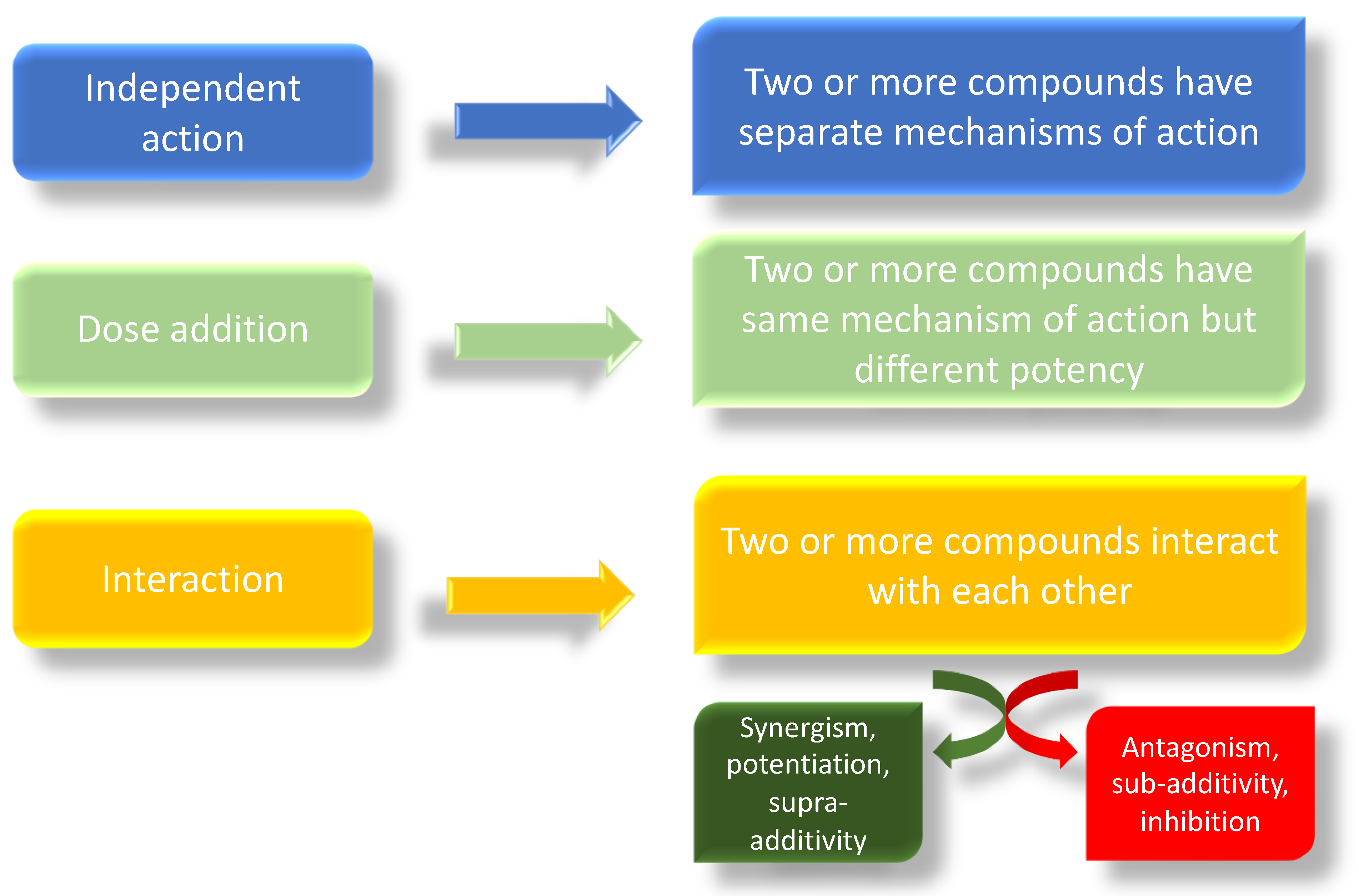
1.5. EDCs and Prostate Disease
2. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ARs | Androgen Receptors |
| BOO | Bladder Outlet Obstruction |
| BP | ButylParaben |
| BPA | BisPhenol A |
| BPH | Benign Prostatic Hyperplasia |
| DBP | DiButylPhtalate |
| DHT | 5α-DiHydroTestosterone |
| E | Estrogen |
| E2 | 17β-Estradiol |
| EDCs | Endocrine-Disrupting Chemicals |
| EFSA | European Food Safety Authority |
| ERs | Estrogen Receptors |
| GD | Gestation Day |
| LUTS | Lower Urinary Tract Symptoms |
| NOAELS | No Observed Adverse Effect Levels |
| NP | NonylPhenol |
| NPEO | NonylPhenol EthOxylate |
| OCPs | OrganoChlorine Pesticides |
| OP | OctylPhenol |
| PC | Prostate Cancer |
| PCBs | PolyChlorinated Biphenyls |
| PD | Pup Day |
| PND | Post Natal Day |
| POPs | Persistent Organic Pollutants |
| PRLR | Prolactin Receptor |
| PSA | Prostate-Specific Antigen |
| T | Testosterone |
| TSC | Triclosan |
| UGS | UroGenital Sinus |
| UNEP | UN Environment Programme |
| WHO | World Health Organization |
References
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Bleak, T.C.; Calaf, G.M. Breast and prostate glands affected by environmental substances. Oncol. Rep. 2021, 45, 20–37. [Google Scholar] [CrossRef]
- Quagliariello, V.; Rossetti, S.; Cavaliere, C.; Di Palo, R.; Lamantia, E.; Castaldo, L.; Nocerino, F.; Ametrano, G.; Cappuccio, F.; Malzone, G.; et al. Metabolic syndrome, endocrine disruptors and prostate cancer associations: Biochemical and pathophysiological evidences. Oncotarget 2017, 8, 30606–30616. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.; Zona, A.; Beccaloni, E.; Carere, M.; Comba, P. Incidence of breast, prostate, testicular, and thyroid cancer in Italian contaminated sites with presence of substances with endocrine disrupting properties. Int. J. Environ. Res. Public Health 2017, 14, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- WHO/UNEP. State of the Science of Endocrine Disrupting Chemicals-2012: An Assessment of the State of the Science of Endocrine Disruptors Prepared by a Group of Experts for the United Nations Environment Programme (UNEP) and WHO. 2012. Available online: https://www.who.int/ceh/risks/cehemerging2/en/ (accessed on 15 August 2021).
- Frye, C.A.; Bo, E.; Calamandrei, G.; Calzà, L.; Dessi-Fulgheri, F.; Fernandez, M.; Fusani, L.; Kah, O.; Kajta, M.; Le Page, Y.; et al. Endocrine disrupters: A review of some sources, effects, and mechanisms of actions on behavior and neuroendocrine systems. J. Neuroendocrinol. 2012, 24, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.M.; Bornman, M.S.; Joubert, A.M.; Pitts, N.; Naidoo, V.; de Jager, C. Effects of environmental endocrine disruptors, including insecticides used for malaria vector control on reproductive parameters of male rats. Reprod. Toxicol. 2016, 61, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.; Zhou, C.; Chiang, C.; Mahalingam, S.; Brehm, E.; Flaws, J.A. Exposure to endocrine disruptors during adulthood: Consequences for female fertility. J. Endocrinol. 2017, 233, R109–R129. [Google Scholar] [CrossRef]
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Barra, T.; Rosati, L.; Valiante, S.; Capaldo, A.; De Falco, M.; Laforgia, V. Adrenal gland response to endocrine disrupting chemicals in fishes, amphibians and reptiles: A comparative overview. Gen. Comp. Endocrinol. 2020, 297, 113550. [Google Scholar] [CrossRef]
- EPRS. Endocrine Disruptors: An Overview of the Latest Developments at the European Level in the Context of Plant Protection Products; European Parliamentary Research Service: Brussels, Belguim, 2019; Available online: https://www.europarl.europa.eu/thinktank/en/document.html?reference=EPRS_STU(2019)631743 (accessed on 15 August 2021).
- De Falco, M.; Sellitti, A.; Sciarrillo, R.; Capaldo, A.; Valiante, S.; Iachetta, G.; Forte, M.; Laforgia, V. Nonylphenol effects on the HPA axis of the bioindicator vertebrate, Podarcis sicula lizard. Chemosphere 2014, 104, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef]
- Gore, A.C. Neuroendocrine targets of endocrine disruptors. Hormones 2010, 9, 16–27. [Google Scholar] [CrossRef]
- Giulivo, M.; Lopez de Alda, M.; Capri, E.; Barceló, D. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ. Res. 2016, 151, 251–264. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Ayers, S.; Gescher, A.J.; Glatt, H.R.; Meinl, W.; Jarrat, P.; Kirk, C.J.; Pettitt, T.; Rea, D.; Harris, R.M. Phytoestrogens and xenoestrogens: The contribution of diet and environment to endocrine disruption. J. Steroid Biochem. Mol. Biol. 2008, 108, 213–220. [Google Scholar] [CrossRef]
- Guo, Y.; Zang, Z.; Liu, L.; Li, Y.; Ren, N.; Kannan, K. Occurence and profiles of phthalates in foodstuffs from China and their implications for human exposure. J. Agric. Food Chem. 2012, 60, 6913–6919. [Google Scholar] [CrossRef] [PubMed]
- Kalfa, N.; Philibert, P.; Sultan, C. Is hypospadias a genetic, endocrine or environmental disease, or still an unexplained malformation? Int. J. Androl. 2009, 32, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Su, Z.J.; Ge, R.S. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules 2011, 16, 9983–10001. [Google Scholar] [CrossRef]
- Prusinski, L.; Al-Hendy, A.; Yang, Q. Developmental exposure to endocrine disrupting chemicals alters the epigenome: Identification of reprogrammed targets. Gynecol. Obstet. Res. 2016, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef]
- Ding, D.; Xu, L.; Fang, H.; Hong, H.; Perkins, R.; Harris, S.; Bearden, E.D.; Shi, L.; Tong, W. The EDKB: An established knowledge base for endocrine disrupting chemicals. BMC Bioinform. 2010, 11, 5. [Google Scholar] [CrossRef]
- European Commission. Endocrine Disruptors. 2015. Available online: https://ec.europa.eu/environment/chemicals/endocrine/documents/index_en.htm#SubThemes5 (accessed on 24 November 2015).
- International Programme on Chemical Safety. Global Assessment of State-of-the-Science for Endocrine Disruptors; World Health Organization: Geneva, Switzerland, 2012; Available online: https://www.who.int/ipcs/publications/new_issues/endocrine_disruptors/en (accessed on 6 October 2014).
- Trasande, L.; Shaffer, R.M.; Sathyanarayana, S. Food additives and child health. Pediatrics 2018, 142, e20181408. [Google Scholar] [CrossRef]
- Gray, L.E., Jr.; Ostby, J.; Furr, J.; Price, M.; Veeramachaneni, D.N.; Parks, L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol. Sci. 2000, 58, 350–365. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; Strüssmann, C.A.; Hashimoto, S. Assessment of short-term exposure to nonylphenol in Japanese medaka using sperm velocity and frequency of motile sperm. Arch. Environ. Contam. Toxicol. 2007, 53, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Tang, R.; Fu, G.; Xu, B.; Zhu, P.; Qiao, S.; Chen, X.; Xu, B.; Qin, Y.; Lu, C.; et al. Association of exposure to phenols and idiopathic male infertility. J. Hazard. Mater 2013, 250, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Namasivayam, V. Endocrine disrupting chemicals in the atmosphere: Their effects on humans and wildlife. Environ. Int. 2015, 76, 78–97. [Google Scholar] [CrossRef]
- Christiansen, S.; Boberg, J.; Axelstad, M.; Dalgaard, M.; Vingaard, A.M.; Metzdorff, S.B.; Hass, U. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces antiandrogenic effects in male rats. Reprod. Toxicol. 2010, 30, 313–321. [Google Scholar] [CrossRef]
- Forte, M.; Di Lorenzo, M.; Carrizzo, A.; Valiante, S.; Vecchione, C.; Laforgia, V.; De Falco, M. Nonylphenol effects on human prostate non tumorigenic cells. Toxicology 2016, 357-358, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.T.; Xu, R.; Cao, W.X.; Qian, L.L.; Wang, M.; Lu, L.; Xu, Q.; Yu, S.Q. Effects of six priority controlled phthalate esters with long-term low-dose integrated exposure on male reproductive toxicity in rats. Food Chem. Toxicol. 2017, 101, 94–104. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Forte, M.; Valiante, S.; Laforgia, V.; De Falco, M. Interference of dibutylphthahalte on human prostate cell viability. Ecotoxicol. Environ. Saf. 2018, 147, 565–573. [Google Scholar] [CrossRef]
- ECHA. European Chemicals Agency Substance Information for Bis(2-ethylhexyl)phthalate. 2019. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.003.829 (accessed on 15 August 2021).
- Forte, M.; Di Lorenzo, M.; Iachetta, G.; Mita, D.G.; Laforgia, V.; De Falco, M. Nonylphenol acts on prostate adenocarcinoma cells via estrogen molecular pathways. Ecotoxicol. Environ. Saf. 2019, 180, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Rosati, L.; Agnese, M.; Abagnale, L.; Andreuccetti, P.; Prisco, M. The mussel Mytilus galloprovincialis in the gulf of Naples: New insights on oogenic cycle and its hormonal control. Anat. Rec. 2019, 302, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Casals-Casas, C.; Descergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef]
- Gan, W.; Zhou, M.; Xiang, Z.; Han, X.; Li, D. Combined effects of nonylphenol and bisphenol A on the human prostate epithelial cell line RWPE-1. Int. J. Environ. Res. Public Health 2015, 12, 4141–4155. [Google Scholar] [CrossRef]
- Huggins, C.; Scott, W.W.; Heinen, J.H. Chemical composition of human semen and of the secretions of the prostate and seminal vesicles. Am. J. Physiol.-Leg. Content 1942, 136, 467–473. [Google Scholar] [CrossRef]
- Scarano, W.R.; Pinho, C.F.; Pissinatti, L.; Goncalves, B.F.; Mendes, L.O.; Campos, S.G.P. Cell junction in the prostate: An overview about the effects of endocrine disrupting chemicals (EDCs) in different experimental models. Reprod. Toxicol. 2018, 81, 147–154. [Google Scholar] [CrossRef]
- Klukovich, R.; Nilsson, E.; Sadler-Riggleman, I.; Beck, D.; Xie, Y.; Yan, W.; Skinner, M.K. Environmental toxicant induced epigenetic transgenerational inheritance of prostate pathology and stromal-epithelial cell epigenome and transcriptome alterations: Ancestral origins of prostate disease. Sci. Rep. 2019, 9, 2209. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S. Developmental estrogenization: Prostate gland reprogramming leads to increased disease risk with aging. Differentiation 2021, 118, 72–81. [Google Scholar] [CrossRef]
- Sugimura, Y.; Cunha, G.R.; Donjacour, A.A. Morphogenesis of ductal networks in the mouse prostate. Biol. Reprod. 1986, 34, 961–971. [Google Scholar] [CrossRef]
- Calderon-Gierszal, E.L.; Prins, G.L. Directed differentiation of human embryonic stem cells into prostate organoids in vitro and its perturbation by low-dose bisphenol A exposure. PLoS ONE 2015, 10, e0133238. [Google Scholar] [CrossRef]
- Leong, K.G.; Wang, B.E.; Johnson, L.; Gao, W.Q. Generation of a prostate from a single adult stem cell. Nature 2008, 456, 804–808. [Google Scholar] [CrossRef]
- Prins, G.S.; Putz, O. Molecular signaling pathways that regulate prostate gland development. Differentiation 2008, 76, 641–659. [Google Scholar] [CrossRef]
- Venditti, M.; Aniello, F.; Santillo, A.; Minucci, S. Study on PREP localization in mouse seminal vesicles and its possible involvement during regulated exocytosis. Zygote 2019, 27, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, C.J. The fine structure of the interstitial tissue of the rat prostate. Am. J. Anat. 1972, 134, 107–125. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.M.; Frydenberg, M.; Majewski, H. Testosterone- and phorbol ester-stimulated proliferation in human cultured prostatic stromal cells. Cell. Signal. 2001, 13, 703–709. [Google Scholar] [CrossRef]
- Prins, G.S.; Ye, S.H.; Birch, L.; Zhang, X.; Cheong, A.; Lin, H.; Calderon-Gierszal, E.; Groen, J.; Hu, W.-Y.; Ho, S.-M.; et al. Prostate cancer risk and DNA methylation signatures in aging rats following developmental BPA exposure: A dose-response analysis. Environ. Health Perspect. 2017, 125, 077007. [Google Scholar] [CrossRef] [PubMed]
- Nelles, J.L.; Hu, W.Y.; Prins, G.S. Estrogenaction and prostate cancer. Expert Rev. Endocrinol. Metab. 2011, 6, 437–451. [Google Scholar] [CrossRef]
- Prins, G.S.; Marmer, M.; Woodham, C.; Chang, W.; Kuiper, G.; Gustafsson, J.A.; Birch, L. Estrogen receptor-beta messenger ribonucleic acid ontogeny in the prostate of normal and neonatally estrogenized rats. Endocrinology 1998, 139, 874–883. [Google Scholar] [CrossRef]
- Omoto, Y.; Imamov, O.; Warner, M.; Gustafsson, J.A. Estrogen receptor alpha and imprinting of the neonatal mouse ventral prostate by estrogen. Proc. Natl. Acad. Sci. USA 2005, 102, 1484–1489. [Google Scholar] [CrossRef]
- Chen, M.; Hsu, I.; Wolfe, A.; Radovick, S.; Huang, K.; Yu, S.; Chang, C.; Messing, E.M.; Yeh, S. Defects of prostate development and reproductive system in the estrogen receptor-alpha null male mice. Endocrinology 2009, 150, 251–259. [Google Scholar] [CrossRef]
- Vitkus, S.; Yeh, C.R.; Lin, H.H.; Hsu, I.; Yu, J.; Chen, M.; Yeh, S. Distinct function of estrogen receptor alpha in smooth muscle and fibroblast cells in prostate development. Mol. Endocrinol. 2013, 27, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.Y.; Leav, I.; Lau, K.M.; Ho, S.M.; Pflueger, S.M. Expression of estrogen receptor beta in the fetal, neonatal, and prepubertal human prostate. Prostate 2002, 52, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, E.; Huang, H.; Masch, R.J.; McFadden, D.E.; Wilson, E.L.; Wu, X.R. Immunolocalization of estrogen receptor alpha and beta in human fetal prostate. J. Urol. 2005, 174, 2051–2053. [Google Scholar] [CrossRef]
- Hu, W.Y.; Shi, G.B.; Hu, D.P.; Nelles, J.L.; Prins, G.S. Actions of estrogens and endocrine disrupting chemicals on human prostate stem/progenitor cells and prostate cancer risk. Mol. Cell. Endocrinol. 2012, 354, 63–73. [Google Scholar] [CrossRef]
- Prins, G.S.; Hu, W.Y.; Shi, G.B.; Hu, D.P.; Majumdar, S.; Li, G.; Huang, K.; Nelles, J.L.; Ho, S.M.; Walker, C.L.; et al. Bisphenol A promotes human prostate stem-progenitor cell self-renewal and increases in vivo carcinogenesis in human prostate epithelium. Endocrinology 2014, 155, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.S.; Calderon-Gierszal, E.L.; Hu, W.Y. Stem cells as hormone targets that lead to increased cancer susceptibility. Endocrinology 2015, 156, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Imamov, O.; Morani, A.; Shim, G.J.; Omoto, Y.; Thulin-Andersson, C.; Warner, M.; Gustafsson, J.A. Estrogen receptor beta regulates epithelial cellular differentiation in the mouse ventral prostate. Proc. Natl. Acad. Sci. USA 2004, 101, 9375–9380. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Rinaldi, J.C.; Malhotra, N.R.; Xie, L.; Hu, D.P.; Gauntner, T.D.; Grewal, H.S.; Hu, W.Y.; Kim, S.H.; Katzenellenbogen, J.A.; et al. Differential actions of estrogen receptor α and β via nongenomic signaling in human prostate stem and progenitor cells. Endocrinology 2019, 160, 2692–2708. [Google Scholar] [CrossRef]
- Prins, G.S.; Ho, S.M. Early life estrogens and prostate cancer in an animal model. J. Dev. Orig. Health Dis. 2010, 1, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Ekbom, A. Growing evidence that several human cancers may originate in utero. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 1998; Volume 8, pp. 237–244. [Google Scholar]
- Rohrmann, S.; Nelson, W.G.; Rifai, N.; Brown, T.R.; Dobs, A.; Kanarek, N.; Yager, J.D.; Platz, E.A. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J. Clin. Endocrinol. Metab. 2007, 92, 2519–2525. [Google Scholar] [CrossRef]
- Prins, G.S.; Ye, S.H.; Birch, L.; Ho, S.M.; Kannan, K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod. Toxicol. 2011, 31, 1–9. [Google Scholar] [CrossRef]
- Wang, Q.; Trevino, L.S.; Wong, R.L.Y.; Medvedovic, M.; Chen, J.; Ho, S.M.; Shen, J.; Foulds, C.E.; Coarfa, C.; O’Malley, B.W.; et al. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol. Endocrinol. 2016, 30, 856–871. [Google Scholar] [CrossRef]
- Ho, S.M.; Tang, W.Y.; Belmonte de Frausto, J.; Prins, G.S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 2006, 66, 5624–5632. [Google Scholar] [CrossRef]
- Prins, G.S.; Huang, L.; Birch, L.; Pu, Y. The role of estrogens in normal and abnormal development of the prostate gland. Ann. N. Y. Acad. Sci. 2006, 1089, 1–13. [Google Scholar] [CrossRef]
- Nicholson, T.M.; Ricke, E.A.; Marker, P.C.; Miano, J.M.; Mayer, R.D.; Timms, B.G.; vom Saal, F.S.; Wood, R.W.; Ricke, W.A. Testosterone and 17β-Estradiol Induce Glandular Prostatic Growth, Bladder Outlet Obstruction, and Voiding Dysfunction in Male Mice. Endocrinology 2012, 153, 5556–5565. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Benign prostatic hyperplasia: An overview. Rev. Urol. 2005, 7, S3–S14. [Google Scholar]
- Oelke, M.; Kirschner-Hermanns, R.; Thiruchelvam, N.; Heesakkers, J. Can we identify men who will have complications from benign prostatic obstruction (BPO)?: ICI-RS 2011. Neurourol. Urodyn. 2012, 31, 322–326. [Google Scholar] [CrossRef]
- Irwin, D.E.; Milsom, I.; Kopp, Z.; Abrams, P.; Artibani, W.; Herschorn, S. Prevalence, severity, and symptom bother of lower urinary tract symptoms among men in the EPIC study: Impact of overactive bladder. Eur. Urol. 2009, 56, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Welch, G.; Weinger, K.; Barry, M.J. Quality-of-life impact of lower urinary tract symptom severity: Results from the Health Professionals Follow-up Study. Urology 2002, 59, 245–250. [Google Scholar] [CrossRef]
- Parsons, J.K. Benign prostatic hyperplasia and male lower urinary tract symptoms: Epidemiology and risk factors. Curr. Bladder Dysfunct. Rep. 2010, 5, 212–218. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Key Statistics for Prostate Cancer. 2016. Available online: http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-key-statistics (accessed on 15 July 2021).
- Lim, J.-E.; Nam, C.; Yang, J.; Rha, K.H.; Lim, K.-M.; Jee, S.H. Serum persistent organic pollutants (POPs) and prostate cancer risk: A case-cohort study. Int. J. Hyg. Environ. Health 2017, 220, 849–856. [Google Scholar] [CrossRef]
- Capocaccia, R.; Foschi, R.; Zucchetto, A.; Valdagni, R.; Nicolai, N.; Maffezzini, M.; Gatta, G. Estimates of prostate cancer burden in Italy. Cancer Epidemiol. 2016, 40, 166–172. [Google Scholar] [CrossRef]
- National Cancer Information Center, Korea. Available online: http://www.cancer.go.kr/mbs/cancer/subview.jsp?id=cancer040104000000 (accessed on 15 July 2021).
- Di Donato, M.; Cernera, G.; Giovannelli, P.; Galasso, G.; Bilancio, A.; Migliaccio, A.; Castoria, G. Recent advances on bisphenol-A and endocrine disruptors effects on huma prostate cancer. Mol. Cell. Endocrinol. 2017, 457, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Mizokami, A.; Lin, W.J.; Lai, K.-P.; Chang, C. Androgen receptor roles in the development of benign prostate hyperplasia. Am. J. Pathol. 2013, 182, 1942–1949. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Huang, D.; Su, X.; Yan, H.; Wu, J.; Sun, Z. Oral exposure to low-dose bisphenol A induces hyperplasia of dorsolateral prostate and upregulates EGFR expression in adult Sprague-Dawley rats. Toxicol. Ind. Health 2019, 35, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.G.; Gan, L.P.; Yu, G.Q.; Ye, Z.Q.; Mi, Z.G.; Wang, Q.H.; Han, C.Z.; Ren, L.S.; Wang, H.Z. Testosterone induces different-featured prostate hyperplasia in castrated and uncastrated mice. Zhonghua Nan Ke Xue 2009, 15, 153–157. [Google Scholar]
- Heinlein, C.A.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef]
- Härkönen, P.L.; Mäkelä, S.I. Role of estrogens in development of prostate cancer. J. Steroid Biochem. Mol. Biol. 2004, 92, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Prezioso, D.; Denis Louis, J.; Klocker, H.; Sciarra, A.; Reis, M.; Naber, K.; Lobe, B.L.; Pacik, D.; Griffiths, K. Estrogens and aspects of prostate disease. Int. J. Urol. 2007, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, X.; Yang, R.; Liu, J.; Xu, D.; Shi, J.; Chen, L.; Shao, R.; Fan, G.; Gao, X.; et al. The prevention and treatment effects of tanshinone IIA on oestrogen/androgen-induced benign prostatic hyperplasia in rats. J. Steroid Biochem. Mol. Biol. 2015, 145, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Kanno, J. Increase in prostate stem cell antigen expression in prostatic hyperplasia induced by testosterone and 17β-estradiol in C57BL mice. J. Steroid Biochem. Mol. Biol. 2016, 158, 56–62. [Google Scholar] [CrossRef]
- Suzuki, K.; Ito, K.; Suzuki, T.; Honma, S.; Yamanaka, H. Synergistic effects of estrogen and androgen on the prostate: Effects of estrogen on androgen- and estrogen-receptors, BrdU uptake, immunohistochemical study of AR, and responses to antiandrogens. Prostate 1995, 26, 151–163. [Google Scholar] [CrossRef]
- Drago, J.R. The induction of NB rat prostatic carcinomas. Anticancer Res. 1984, 4, 255–256. [Google Scholar] [PubMed]
- Noble, R.L. The development of prostatic adenocarcinoma in Nb rats following prolonged sex hormone administration. Cancer Res. 1977, 37, 1929–1933. [Google Scholar] [PubMed]
- Nicholson, T.M.; Ricke, W.A. Androgens and estrogens in benign prostatic hyperplasia: Past, present and future. Differentiation 2011, 82, 184–199. [Google Scholar] [CrossRef]
- Kaufman, J.M.; Vermeulen, A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr. Rev. 2005, 26, 833–876. [Google Scholar] [CrossRef] [PubMed]
- King, K.J.; Nicholson, H.D.; Assinder, S.J. Effect of increasing ratio of estrogen: Androgen on proliferation of normal human prostate stromal and epithelial cells, and the malignant cell line LNCaP. Prostate 2006, 66, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Risbridger, G.; Wang, H.; Young, P.; Kurita, T.; Wang, Y.Z.; Lubahn, D.; Gustafsson, J.A.; Cunha, G. Evidence that epithelial and mesenchymal estrogen receptor-alpha mediates effects of estrogen on prostatic epithelium. Dev. Biol. 2001, 229, 432–442. [Google Scholar] [CrossRef][Green Version]
- Eskenazi, B.; Bradman, A.; Castorina, R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ. Health Perspect. 1999, 107, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, P.J.; Claudio, L.; Markowitz, S.B.; Berkowitz, G.S.; Brenner, B.L.; Romero, H.; Wetmur, J.G.; Matte, T.D.; Gore, A.C.; Godbold, J.H.; et al. Pesticides and inner-city children: Exposures, risks, and prevention. Environ. Health Perspect. 1999, 107, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.; Rajapakse, N.; Kortenkamp, A. Something from “nothing”—Eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ. Sci. Technol. 2002, 36, 1751–1756. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef]
- Wolff, M.S.; Teitelbaum, S.L.; Windham, G.; Pinney, S.M.; Britton, J.A.; Chelimo, C.; Godbold, J.; Biro, F.; Kushi, L.H.; Pfeiffer, C.M.; et al. Pilot study of urinary biomarkers of phytoestrogens, phthalates, and phenols in girls. Environ. Health Perspect. 2007, 115, 116–121. [Google Scholar] [CrossRef]
- Wolff, M.S.; Engel, S.M.; Berkowitz, G.S.; Ye, X.; Silva, M.J.; Zhu, C.; Wetmur, J.; Calafat, A.M. Prenatal phenol and phthalate exposures and birth outcomes. Environ. Health Perspect. 2008, 116, 1092–1097. [Google Scholar] [CrossRef]
- Rider, C.V.; Furr, J.R.; Wilson, V.S.; Gray, L.E., Jr. Cumulative effects of in utero administration of mixtures of reproductive toxicants that disrupt common target tissues via diverse mechanisms of toxicity. Int. J. Androl. 2010, 33, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Nolan, M.; Tyler, C.R.; Brighty, G.; Sumpter, J.P. Widespread sexual disruption in wild fish. Environ. Sci. Technol. 1998, 32, 2498–2506. [Google Scholar] [CrossRef]
- Ankley, G.T.; Brooks, B.W.; Huggett, D.B.; Sumpter, J.P. Repeating history: Pharmaceuticals in the environment. Environ. Sci. Technol. 2007, 41, 8211–8217. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Tyler, C.R. Introduction: The ecological relevance of chemically induced endocrine disruption in wildlife. Environ. Health Perspect. 2006, 114, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Jobling, S.; Williams, R.; Johnson, A.; Taylor, A.; Gross-Sorokin, M.; Nolan, M.; Tyler, C.R.; van Aerle, R.; Santos, E.; Brighty, G. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environ. Health Perspect. 2006, 114, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Akhbarizadeh, R.; Russo, G.; Rossi, S.; Golianova, K.; Moore, F.; Guida, M.; De Falco, M.; Grumetto, L. Emerging endocrine disruptors in two edible fish from the Persian Gulf: Occurrence, congener profile, and human health risk assessment. Mar. Pollut. Bull. 2021, 166, 112241. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.J.; Thomas, G.O. Polychlorinated biphenyls, DDT, polybrominated diphenyl ethers, and organic pesticides in United Kingdom harbor seals (Phoca vitulina)—Mixed exposures and thyroid homeostasis. Environ. Toxicol. Chem. 2007, 26, 851–861. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Sciarrillo, R.; Rosati, L.; Sellitti, A.; Barra, T.; De Luca, A.; Laforgia, V.; De Falco, M. Effects of alkylphenols mixture on the adrenal gland of the lizard Podarcis sicula. Chemosphere 2020, 258, 127239. [Google Scholar] [CrossRef]
- Di Lorenzo, M.; Mileo, A.; Laforgia, V.; De Falco, M.; Rosati, L. Alkyphenol Exposure Alters Steroidogenesis in Male Lizard Podarcis siculus. Animals 2021, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Sciarrillo, R.; Di Lorenzo, M.; Valiante, S.; Rosati, L.; De Falco, M. OctylPhenol (OP) Alone and in Combination with NonylPhenol (NP) Alters the Structure and the Function of Thyroid Gland of the Lizard Podarcis siculus. Arch. Environ. Contam. Toxicol. 2021, 80, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Fussell, K.C.; Melching-Kollmuss, S.; Buesen, R.; Gröters, S.; Strauss, V.; Jiang, X.; van Ravenzwaay, B. Investigations on the dose-response relationship of combined exposure to low doses of three anti-androgens in Wistar rats. Arch. Toxicol. 2017, 91, 3961–3989. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Bae, S.G.; Lee, S.H.; Jacobs, D.R., Jr.; Lee, D.H. Persistent organic pollutants and hyperuricemia in the U.S. general population. Atherosclerosis 2013, 230, 1–5. [Google Scholar] [CrossRef]
- Lim, J.E.; Park, S.H.; Jee, S.H.; Park, H. Body concentrations of persistent organic pollutants and prostate cancer: A meta-analysis. Environ. Sci. Pollut. Res. Int. 2015, 22, 11275–11284. [Google Scholar] [CrossRef]
- Scott, H.M.; Mason, J.I.; Sharpe, R.M. Steroidogenesis in the fetal testis and its susceptibility to disruption by exogenous compounds. Endocr. Rev. 2009, 30, 883–925. [Google Scholar] [CrossRef] [PubMed]
- Habauzit, D.; Flouriot, G.; Pakdel, F.; Saligaut, C. Effects of estrogens and endocrine-disrupting chemicals on cell differentiation-survival-proliferation in brain: Contributions of neuronal cell lines. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 300–327. [Google Scholar] [CrossRef]
- Jones, L.A.; Hajek, R.A. Effects of estrogenic chemicals on development. Environ. Health Perspect. 1995, 103, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kortenkamp, A. Ten years of mixing cocktails: A review of combination effects of endocrine-disrupting chemicals. Environ. Health Perspect. 2007, 115, 98–105. [Google Scholar] [CrossRef]
- Riad, M.A.; Abd-Rabo, M.M.; Abd El Aziz, S.A.; El Behairy, A.M.; Badawy, M.M. Reproductive toxic impact of subchronic treatment with combined butylparaben and triclosan in weanling male rats. J. Biochem. Mol. Toxicol. 2018, 32, e22037. [Google Scholar] [CrossRef]
- Hass, U.; Boberg, J.; Christiansen, S.; Jacobsen, P.R.; Vinggaard, A.M.; Taxvig, C.; Poulsen, M.E.; Herrmann, S.S.; Jensen, B.H.; Petersen, A.; et al. Adverse effects on sexual development in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod. Toxicol. 2012, 34, 261–274. [Google Scholar] [CrossRef]
- Wilkinson, C.F.; Christoph, G.R.; Julien, E.; Kelley, J.M.; Kronenberg, J.; McCarthy, J.; Reiss, R. Assessing the risks of exposures to multiple chemicals with a common mechanism of toxicity: How to cumulate? Regul. Toxicol. Pharmacol. 2000, 31, 30–43. [Google Scholar] [CrossRef]
- Feron, V.J.; Groten, J.P. Toxicological evaluation of chemical mixtures. Food Chem. Toxicol. 2002, 40, 825–839. [Google Scholar] [CrossRef]
- EFSA. Scientific Opinion on the identification of pesticides to be included in cumulative assessment groups on the basis of their toxicological profile. In EFSA J.; 2013; 11, p. 3293. [Google Scholar]
- EFSA. Scientific Opinion on the relevance of dissimilar mode of action and its appropriate application for cumulative risk assessment of pesticides residues in food. In EFSA J.; 2013; 11, p. 3472. [Google Scholar]
- Blystone, C.R.; Lambright, C.S.; Cardon, M.C.; Furr, J.; Rider, C.V.; Hartig, P.C.; Wilson, V.S.; Gray, L.E., Jr. Cumulative and antagonistic effects of a mixture of the antiandrogens vinclozolin and iprodione in the pubertal male rat. Toxicol. Sci. 2009, 111, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hass, U.; Scholze, M.; Christiansen, S.; Dalgaard, M.; Vinggaard, A.M.; Axelstad, M.; Metzdorff, S.B.; Kortenkamp, A. Combined exposure to anti-androgens exacerbates disruption of sexual differentiation in the rat. Environ. Health Perspect. 2007, 115, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Metzdorff, S.B.; Dalgaard, M.; Christiansen, S.; Axelstad, M.; Hass, U.; Kiersgaard, M.K.; Scholze, M.; Kortenkamp, A.; Vinggaard, A.M. Dysgenesis and histological changes of genitals and perturbations of gene expression in male rats after in utero exposure to antiandrogen mixtures. Toxicol. Sci. 2007, 98, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Howdeshell, K.L.; Wilson, V.S.; Furr, J.; Lambright, C.R.; Rider, C.V.; Blystone, C.R.; Hotchkiss, A.K.; Gray, L.E., Jr. A mixture of five phthalate esters inhibits fetal testicular testosterone production in the sprague-dawley rat in a cumulative, dose-additive manner. Toxicol. Sci. 2008, 105, 153–165. [Google Scholar] [CrossRef]
- Rider, C.V.; Furr, J.; Wilson, V.S.; Gray, L.E., Jr. A mixture of seven antiandrogens induces reproductive malformations in rats. Int. J. Androl. 2008, 31, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Scholze, M.; Dalgaard, M.; Vinggaard, A.M.; Axelstad, M.; Kortenkamp, A.; Hass, U. Synergistic disruption of external male sex organ development by a mixture of four antiandrogens. Environ. Health Perspect. 2009, 117, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.; Kortenkamp, A.; Axelstad, M.; Boberg, J.; Scholze, M.; Jacobsen, P.R.; Faust, M.; Lichtensteiger, W.; Schlumpf, M.; Burdorf, A.; et al. Mixtures of endocrine disrupting contaminants modelled on human high end exposures: An exploratory study in rats. Int. J. Androl. 2012, 35, 303–316. [Google Scholar] [CrossRef]
- Hellwig, J.; van Ravenzwaay, B.; Mayer, M.; Gembardt, C. Pre- and postnatal oral toxicity of vinclozolin in Wistar and Long-Evans rats. Regul. Toxicol. Pharmacol. 2000, 32, 42–50. [Google Scholar] [CrossRef]
- Gray, L.E., Jr.; Wilson, V.S.; Stoker, T.; Lambright, C.; Furr, J.; Noriega, N.; Howdeshell, K.; Ankley, G.T.; Guillette, L. Adverse effects of environmental antiandrogens and androgens on reproductive development in mammals. Int. J. Androl. 2006, 29, 96–104. [Google Scholar] [CrossRef]
- Kang, K.S.; Che, J.H.; Ryu, D.Y.; Kim, T.W.; Li, G.X.; Lee, Y.S. Decreased sperm number and motile activity on the F1 offspring maternally exposed to butyl p-hydroxybenzoic acid (butyl paraben). J. Vet. Med. Sci. 2002, 64, 227–235. [Google Scholar] [CrossRef]
- Timms, B.G.; Howdeshell, K.L.; Barton, L.; Bradley, S.; Richter, C.A.; vom Saal, F.S. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc. Natl. Acad. Sci. USA 2005, 102, 7014–7019. [Google Scholar] [CrossRef] [PubMed]
- Durrer, S.; Ehnes, C.; Fuetsch, M.; Maerkel, K.; Schlumpf, M.; Lichtensteiger, W. Estrogen sensitivity of target genes and expression of nuclear receptor co-regulators in rat prostate after pre- and postnatal exposure to the ultraviolet filter 4-methylbenzylidene camphor. Environ. Health Perspect. 2007, 115, 42–50. [Google Scholar] [CrossRef]
- Prins, G.S.; Tang, W.Y.; Belmonte, J.; Ho, S.M. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin. Pharmacol. Toxicol. 2008, 102, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Salian, S.; Doshi, T.; Vanage, G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009, 85, 742–752. [Google Scholar] [CrossRef]
- Axelstad, M.; Boberg, J.; Hougaard, K.S.; Christiansen, S.; Jacobsen, P.R.; Mandrup, K.R.; Nellemann, C.; Lund, S.P.; Hass, U. Effects of pre- and postnatal exposure to the UV-filter octyl methoxycinnamate (OMC) on the reproductive, auditory and neurological development of rat offspring. Toxicol. Appl. Pharmacol. 2011, 250, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Willems, J.L. Prostate cancer among pesticide applicators: A meta-analysis. Int. Arch. Occup. Environ. Health 2004, 77, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Van Maele-Fabry, G.; Libotte, V.; Willems, J.; Lison, D. Review and meta-analysis of risk estimates for prostate cancer in pesticide manufacturing workers. Cancer Causes Control 2006, 17, 353–373. [Google Scholar] [CrossRef]
- Ritchie, J.M.; Vial, S.L.; Fuortes, L.J.; Guo, H.; Reedy, V.E.; Smith, E.M. Organochlorines and risk of prostate cancer. J. Occup. Environ. Med. 2003, 45, 692–702. [Google Scholar] [CrossRef]
- Ritchie, J.M.; Vial, S.L.; Fuortes, L.J.; Robertson, L.W.; Guo, H.; Reedy, V.E.; Smith, E.M. Comparison of proposed frameworks for grouping polychlorinated biphenyl congener data applied to a case-control pilot study of prostate cancer. Environ. Res. 2005, 98, 104–113. [Google Scholar] [CrossRef]
- Koutros, S.; Langseth, H.; Grimsrud, T.K.; Barr, D.B.; Vermeulen, R.; Portengen, L.; Wacholder, S.; Freeman, L.E.; Blair, A.; Hayes, R.B.; et al. Prediagnostic Serum Organochlorine Concentrations and Metastatic Prostate Cancer: A Nested Case-Control Study in the Norwegian Janus Serum Bank Cohort. Environ. Health Perspect. 2015, 123, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Mikhael, A.M.; Olmedo-Requena, R.; Martínez-Ruiz, V.; Bueno-Cavanillas, A.; Jiménez-Moleón, J.J. Organochlorine pesticides and prostate cancer, Is there an association? A meta-analysis of epidemiological evidence. Cancer Causes Control 2015, 26, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Vakonaki, E.; Androutsopoulos, V.P.; Liesivuori, J.; Tsatsakis, A.M.; Spandidos, D.A. Pesticides and oncogenic modulation. Toxicology 2013, 307, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Hess-Wilson, J.K.; Webb, S.; Daly, H.; Godoy-Tundidor, S.; Kim, J.; Boldison, J.; Daaka, Y.; Knudsen, K.E. 2,2-bis(4-chlorophenyl)-1,1-dichloroethylene stimulates androgen independence in prostate cancer cells through combinatorial activation of mutant androgen receptor and mitogen-activated protein kinase pathways. Mol. Cancer Res. 2008, 6, 1507–1520. [Google Scholar] [CrossRef][Green Version]
- Pflieger-Bruss, S.; Hagemann, S.; Körner, W.; Hanf, V.; Köhn, F.M.; Müller, C.; Schill, W.B. Effects of single non-ortho, mono-ortho, and di-ortho chlorinated biphenyls on human sperm functions in vitro. Reprod. Toxicol. 2006, 21, 280–284. [Google Scholar] [CrossRef]
- Rusiecki, J.A.; Baccarelli, A.; Bollati, V.; Tarantini, L.; Moore, L.E.; Bonefeld-Jorgensen, E.C. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ. Health Perspect. 2008, 116, 1547–1552. [Google Scholar] [CrossRef]
- Kim, K.Y.; Kim, D.S.; Lee, S.K.; Lee, I.K.; Kang, J.H.; Chang, Y.S.; Jacobs, D.R.; Steffes, M.; Lee, D.H. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ. Health Perspect. 2010, 118, 370–374. [Google Scholar] [CrossRef]
- Isling, L.K.; Boberg, J.; Jacobsen, P.R.; Mandrup, K.R.; Axelstad, M.; Christiansen, S.; Vinggaard, A.M.; Taxvig, C.; Kortenkamp, A.; Hass, U. Late-life effects on rat reproductive system after developmental exposure to mixtures of endocrine disrupters. Reproduction 2014, 147, 465–476. [Google Scholar] [CrossRef]
- Jacobsen, P.R.; Axelstad, M.; Boberg, J.; Isling, L.K.; Christiansen, S.; Mandrup, K.R.; Berthelsen, L.O.; Vinggaard, A.M.; Hass, U. Persistent developmental toxicity in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod. Toxicol. 2012, 34, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Boberg, J.; Johansson, H.K.; Hadrup, N.; Dreisig, K.; Berthelsen, L.; Almstrup, K.; Vinggaard, A.M.; Hass, U. Perinatal exposure to mixtures of anti-androgenic chemicals causes proliferative lesions in rat prostate. Prostate 2015, 75, 126–140. [Google Scholar] [CrossRef]
- Prins, G.S.; Birch, L.; Tang, W.Y.; Ho, S.M. Developmental estrogen exposures predispose to prostate carcinogenesis with aging. Reprod. Toxicol. 2007, 23, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Vinyals, G.; Carrasco, E.; Lorente, J.A.; Sabaté, Y.; Cirac-Claveras, J.; Pollán, M.; Kogevinas, M. Anogenital distance and the risk of prostate cancer. BJU Int. 2012, 110, E707–E710. [Google Scholar] [CrossRef]
- Weihua, Z.; Makela, S.; Andersson, L.C.; Salmi, S.; Saji, S.; Webster, J.I.; Jensen, E.V.; Nilsson, S.; Warner, M.; Gustafsson, J.A. A role for estrogen receptor beta in the regulation of growth of the ventral prostate. Proc. Natl. Acad. Sci. USA 2001, 98, 6330–6335. [Google Scholar] [CrossRef] [PubMed]
- Bostwick, D.G.; Burke, H.B.; Djakiew, D.; Euling, S.; Ho, S.M.; Landolph, J.; Morrison, H.; Sonawane, B.; Shifflett, T.; Waters, D.J.; et al. Human prostate cancer risk factors. Cancer 2004, 101, 2371–2490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dong, L.; Ding, S.; Qiao, P.; Wang, C.; Zhang, M.; Zhang, L.; Du, Q.; Li, Y.; Tang, N.; et al. Effects of n-butylparaben on steroidogenesis and spermatogenesis through changed E₂ levels in male rat offspring. Environ. Toxicol. Pharmacol. 2014, 37, 705–717. [Google Scholar] [CrossRef]
- Ishibashi, H.; Matsumura, N.; Hirano, M.; Matsuoka, M.; Shiratsuchi, H.; Ishibashi, Y.; Takao, Y.; Arizono, K. Effects of triclosan on the early life stages and reproduction of medaka Oryzias latipes and induction of hepatic vitellogenin. Aquat. Toxicol. 2004, 67, 167–179. [Google Scholar] [CrossRef]
- Cariati, F.; Carbone, L.; Conforti, A.; Bagnulo, F.; Peluso, S.R.; Carotenuto, C.; Buonfantino, C.; Alviggi, E.; Alviggi, C.; Strina, I. Bisphenol A-Induced Epigenetic Changes and Its Effects on the Male Reproductive System. Front. Endocrinol. 2020, 11, 453. [Google Scholar] [CrossRef]
- Welshons, W.V.; Thayer, K.A.; Judy, B.M.; Taylor, J.A.; Curran, E.M.; vom Saal, F.S. Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity. Environ. Health Perspect. 2003, 111, 994–1006. [Google Scholar] [CrossRef]
- Derouiche, S.; Warnier, M.; Mariot, P.; Gosset, P.; Mauroy, B.; Bonnal, J.L.; Slomianny, C.; Delcourt, P.; Prevarskaya, N.; Roudbaraki, M. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling. Springerplus 2013, 2, 54. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.; Ying, J.; Ouyang, B.; Burke, B.; Bracken, B.; Ho, S.M. Exposure to bisphenol A correlates with early-onset prostate cancer and promotes centrosome amplification and anchorage-independent growth in vitro. PLoS ONE 2014, 9, e90332. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005, 113, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Nam, K.H.; Hwang, K.A.; Choi, K.C. Influence of hexabromocyclododecane and 4-nonylphenol on the regulation of cell growth, apoptosis and migration in prostatic cancer cells. Toxicol. In Vitro 2016, 32, 240–247. [Google Scholar] [CrossRef]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in male physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.H.; Myers, J.P.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod. Toxicol. 2013, 38, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N. Non-monotonic dose responses in studies of endocrine disrupting chemicals: Bisphenol a as a case study. Dose Response 2013, 12, 259–276. [Google Scholar] [CrossRef] [PubMed]
- Mileo, A.; Chianese, T.; Riccio, L.; Rosati, L.; Laforgia, V.; De Falco, M. Effect of EDC mixture on human prostate cells. Eur. J. Histochem. 2021, 65, 14. [Google Scholar]
- Shirai, M.; Wakui, S.; Wempe, M.F.; Mutou, T.; Oyama, N.; Motohashi, M.; Takahashi, H.; Kansaku, N.; Asari, M.; Hano, H.; et al. Male Sprague-Dawley rats exposed to in utero di(n-butyl) phthalate: Dose dependent and age-related morphological changes in Leydig cell smooth endoplasmic reticulum. Toxicol. Pathol. 2013, 41, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Wakui, S.; Takahashi, H.; Mutou, T.; Shirai, M.; Jutabha, P.; Anzai, N.; Wempe, M.F.; Kansaku, N.; Hano, H.; Inomata, T.; et al. Atypical Leydig cell hyperplasia in adult rats with low T and high LH induced by prenatal Di(n-butyl) phthalate exposure. Toxicol. Pathol. 2013, 41, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Wakui, S.; Shirai, M.; Motohashi, M.; Mutou, T.; Oyama, N.; Wempe, M.F.; Takahashi, H.; Inomata, T.; Ikegami, M.; Endou, H.; et al. Effects of in utero exposure to di(n-butyl) phthalate for estrogen receptors α, β, and androgen receptor of Leydig cell on rats. Toxicol. Pathol. 2014, 42, 877–887. [Google Scholar] [CrossRef]
- Guo, Y.; Kannan, K. A survey of phthalates and parabens in personal care products from the United States and its implications for human exposure. Environ. Sci. Technol. 2013, 47, 14442–14449. [Google Scholar] [CrossRef]
- Hubinger, J.C. A survey of phthalate esters in consumer cosmetic products. J. Cosmet. Sci. 2010, 61, 457–465. [Google Scholar]
- Schettler, T. Human exposure to phthalates via consumer products. Int. J. Androl. 2006, 29, 134–139. [Google Scholar] [CrossRef]
- Xu, Y.; Cohen Hubal, E.A.; Little, J.C. Predicting residential exposure to phthalate plasticizer emitted from vinyl flooring: Sensitivity, uncertainty, and implications for biomonitoring. Environ. Health Perspect. 2010, 118, 253–258. [Google Scholar] [CrossRef]
- Barlow, N.J.; Foster, P.M. Pathogenesis of male reproductive tract lesions from gestation through adulthood following in utero exposure to Di(n-butyl) phthalate. Toxicol. Pathol. 2003, 31, 397–410. [Google Scholar]
- Blount, B.C.; Silva, M.J.; Caudill, S.P.; Needham, L.L.; Pirkle, J.L.; Sampson, E.J.; Lucier, G.W.; Jackson, R.J.; Brock, J.W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 2000, 108, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Kay, V.R.; Chambers, C.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol. 2013, 43, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Lioy, P.J.; Hauser, R.; Gennings, C.; Koch, H.M.; Mirkes, P.E.; Schwetz, B.A.; Kortenkamp, A. Assessment of phthalates/phthalate alternatives in children’s toys and childcare articles: Review of the report including conclusions and recommendation of the Chronic Hazard Advisory Panel of the Consumer Product Safety Commission. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 343–353. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Falco, M.; Laforgia, V. Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland. Int. J. Environ. Res. Public Health 2021, 18, 9772. https://doi.org/10.3390/ijerph18189772
De Falco M, Laforgia V. Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland. International Journal of Environmental Research and Public Health. 2021; 18(18):9772. https://doi.org/10.3390/ijerph18189772
Chicago/Turabian StyleDe Falco, Maria, and Vincenza Laforgia. 2021. "Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland" International Journal of Environmental Research and Public Health 18, no. 18: 9772. https://doi.org/10.3390/ijerph18189772
APA StyleDe Falco, M., & Laforgia, V. (2021). Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland. International Journal of Environmental Research and Public Health, 18(18), 9772. https://doi.org/10.3390/ijerph18189772






