Abstract
Coffee production is one of the main agricultural activities in Brazil, and several coffee cultivars with disease resistance have already been developed. The secondary metabolites produced by plants are closely associated with defense strategies, and the resistance of coffee cultivars to bacterial halo blight (BHB) can be related to these compounds. Therefore, this study aims to compare a partially resistant coffee cultivar (Iapar-59) and a susceptible cultivar (Mundo Novo 376/4) to BHB (Pseudomonas syringae pv. garcae) in relation to the chemical composition and antioxidant activity of the leaf extracts. In addition, this study determined the total phenolic and flavonoid contents and phenolic profiles of the Iapar-59 leaf extracts of plants inoculated with P. syringae pv. garcae. The Iapar-59 extract showed a higher content of phenolic compounds and flavonoids than the Mundo Novo 376/4 extract. Both cultivars contained gallic, chlorogenic and caffeic acids; however, the highest contents were quantified in the Iapar-59 cultivar. The leaf extracts from the Iapar-59 cultivar exhibited higher antioxidant activity. Higher concentrations of gallic, caffeic and chlorogenic acids and the presence of vanillin were detected in the extract of cultivar Iapar-59 inoculated with P. syringae pv. garcae.
1. Introduction
Plants produce large amounts of organic compounds without a direct function in their growth and development. These substances are secondary metabolites and are strongly involved in the interaction between plants and pathogens [1]. Phenolic compounds including phenylpropanoids and flavonoids are important secondary metabolites synthesized by plants. Phenolic compounds are toxic to pathogens and are rapidly produced and accumulate after infection, particularly in resistant varieties. Chlorogenic, caffeic and gallic acids are examples of some of these compounds. [1,2,3]. Flavonoids are involved in plant protection against pathogens, and defense-related flavonoids can be divided into preformed (basal resistance) or induced by stress, biotic/abiotic [4]. The phytobacterium Pseudomonas syringae trigger the synthesis of flavonoids in plants [5].
Studies demonstrating the induction of defense genes by sugars in the absence of pathogens elucidated the mechanism linking the carbohydrate metabolism with defense responses. Sugars provide energy and structural material for defense responses in plants. Moreover, they may also act as signal molecules that interact with the hormonal signaling network regulating the plant immune system [6,7,8].
Plants have many bioactive compounds with high antioxidant activity, which indicates a plant’s ability to scavenge free radicals [9,10], and this activity is related to the severity of diseases [11]. The antioxidant activity, flavonoids, phenolic compounds and sugars in plants can vary according to species, cultivar, resistance level to diseases, and are also dependent on the plant pathogen/pest interaction as Oidium xanthami in Xanthium strumarium [11], Leucoptera coffeella and Hemileia vastatrix in coffee [12,13], and Pseudomonas syringae in Arabidopsis thaliana, kiwi, and tomato [5,14,15,16].
Coffee is a crop of significant economic importance in many countries including Brazil, and several factors limit coffee production, including fungal and bacterial diseases [17]. The bacterial halo blight of coffee (BHB) caused by Pseudomonas syringae pv. garcae [18,19], is a disease that has caused losses in nurseries and fields established in regions subjected to strong winds, mild temperatures, and frequent and well-distributed rains [17,20]. Among the coffee (Coffea arabica L.) cultivars planted in Brazil, the Mundo Novo 376/4 cultivar corresponds to almost half of the planted area. This cultivar is susceptible to main coffee diseases, including BHB [21,22]. The Iapar-59 cultivar has an intermediate level of resistance to BHB [23,24] and is resistant to coffee leaf rust (Hemileia vastatrix) [23,25]. Coffee leaves have constitutive defense substances such as phenolic compounds [12,13], and information on the chemical composition of coffee cultivars under basal defense induction conditions against Pseudomonas syringae pv. garcae can help understand the mechanisms that act during pathogenesis and may assist resistance breeding programs to coffee diseases.
Therefore, this study aims to determine the difference in the content of secondary metabolites and antioxidant activities of coffee leaf extracts from Mundo Novo 376/4 and Iapar-59 cultivars. Additionally, this study determined the total phenolic and flavonoid contents and phenolic profiles of Iapar-59 leaf extracts of plants inoculated and non-inoculated with P. syringae pv. garcae.
2. Results
2.1. Chemical Composition and Antioxidant Activity of Coffee Leaf Extracts from Mundo Novo 376/4 and Iapar-59 Cultivars
The cultivar Iapar-59 showed the highest phenolic compounds and flavonoid contents, differing significantly from the Mundo Novo 376/4 cultivar (Table 1). The phenolic compounds and flavonoids contents were 66% and 53% higher, respectively, in leaf extracts from Iapar-59.

Table 1.
Contents of total phenolic compounds (mg GA g−1 extract), flavonoids (mg Q g−1 extract), gallic acid (mg 100 g−1), chlorogenic acid (mg 100 g−1), and caffeic acid (mg 100 g−1) of aqueous extracts of coffee leaves from the cultivars Iapar-59 and Mundo Novo 376/4.
The phenolic profile quantified by HPLC identified gallic, caffeic and chlorogenic acids in the extracts of both the Iapar-59 and Mundo Novo cultivars; however, the Iapar-59 cultivar showed a higher concentration of gallic and caffeic acid than the Mundo Novo cultivar (Table 1).
Coffee leaf extracts from Mundo Novo and Iapar-59 showed lower antioxidant activity than the standards used (Figure 1A,B). However, when the studied cultivars were compared, Iapar-59 showed higher antioxidant activity (quantified by the DPPH method) than the Mundo Novo 376/4 cultivar. The antioxidant activity (quantified by the FRAP method), was similar in both cultivars (Figure 1A,B).
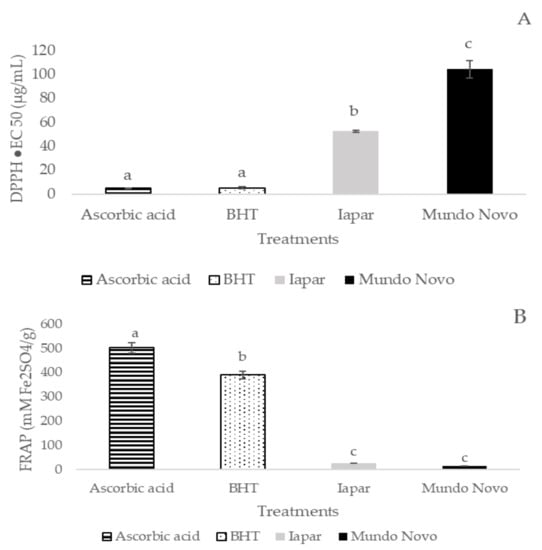
Figure 1.
Antioxidant activities of the Iapar-59 and Mundo Novo 376/4 cultivars and the standards (ascorbic acid and butylated hydroxytoluene: BHT) by the methods of free radical scavenging activity: DPPH (µg/mL) (A), and ferric reducing antioxidant power: FRAP (mM Fe2SO4/g) (B). Bars followed by the same lowercase letter do not differ statistically by Tukey test (p < 0.05) (n = 6 biological replicates).
2.2. Production of Secondary Metabolites in Iapar-59 Coffee Leaves Inoculated with P. syringae pv. garcae
Extracts from intact seedlings (non-inoculated with P. syringae pv. garcae) showed the highest content of total phenolic compounds, reducing sugars and flavonoids (Table 2). Higher concentrations of gallic, caffeic and chlorogenic acids and the presence of vanillin were detected in the extract of Iapar-59 inoculated with P. syringae pv. garcae than in the control samples (non-inoculated plants) (Table 2).

Table 2.
Contents of reducing sugars (mg glucose g−1 extract), flavonoids (mg Q g−1 extract), total phenolic compounds (mg GA g−1 extract), gallic acid (mg 100 g−1), chlorogenic acid and caffeic acid (mg 100 g−1) in extracts of Iapar-59 coffee leaves inoculated and non-inoculated with P. syringae pv. garcae.
The severity of BHB in Mundo Novo and Iapar-59 cultivars was 16.0% and 6% of the diseased leaf area, respectively.
3. Discussion
The Mundo Novo and Iapar-59 cultivars showed variation in disease severity, as reported by Andreazi et al. [22], who evaluated the resistance to BHB in trials performed under field conditions. According to the authors, the disease severity in Mundo Novo was five to ten times more than in Iapar-59.
Coffee leaf extracts from the Iapar-59 cultivar showed the highest phenolic compounds, flavonoids, caffeic acid and gallic acid. Flavonoids may be constitutively synthesized; however, their biosynthesis is often enhanced under the influence of stress including pathogens [4]. According to Treutter [4] phenolic compounds, including flavonoids, often accumulate in specialized cells and can be infused into attacked tissues. Such leaching is possibly involved in common mechanisms of pathogen defense such as programmed cell death and hypersensitive response. This action mode associated with the higher phenolic compound and flavonoids can explain the moderate resistance of Iapar-59 to BHB and its high level of resistance to coffee rust [23,24,25]. Caffeic acid is involved in the synthesis of lignin, and gallic acid has antioxidant and antimicrobial properties [3,26]. Phenolic compounds and lignin are produced and may accumulate as chemical and physical defenses against pathogens [2,3].
The aqueous extracts from both tested cultivars showed lower activity than the standard ascorbic acid and butylated hydroxytoluene (BHT). The highest antioxidant activity observed in the Iapar-59 cultivar correlated with the higher content of total phenolics. According to Ngamsuk et al. [27], the DPPH radical scavenging activity of coffee leaves is significantly correlated with total phenolic content. The cultivar Iapar-59 (cross between Villa Sarchi and Timor hybrid CIFC 832/2), has in its genealogy genes from Coffea canephora due to the Timor hybrid (natural interspecific cross between C. arabica and C. canephora) [28,29]. C. canephora has a higher soluble solid content, caffeine and chlorogenic acid [30]. Therefore, the results found higher phenolic compounds content and antioxidant activity in Iapar-59, which may be correlated with the genotypic origin of this cultivar.
The inoculation with P. syringae pv. garcae altered the contents of compounds evaluated. Studies have reported the increased accumulation of phenolic compounds in olive trees due to infection by Pseudomonas savastanoi pv. savastanoi [31,32]. Tomato plants respond to Pseudomonas syringae pv tomato DC3000 infection by producing flavonoids and other phenolic compounds [5]. However, in the present study, the total phenolic compounds, flavonoids and reducing sugars content decreased upon infection by P. syringae pv. garcae. The decrease in sugars was observed by Li et al. [15] in kiwi leaves infected with Pseudomonas syringae pv. actinidae (Psa). According to authors the reduced content of sugars indicated that Psa infection directly or indirectly inhibited carbon fixation and sugar metabolism.
Plant pathogens can actively suppress the expression of plant defense reactions during successful infection [33]. Resistant and susceptible olive cultivars inoculated with Verticillium dahliae showed a decrease in total phenolics. According to the authors, this decrease was associated with the fungal DNA level in the susceptible cultivar [34], indicating the influence of the infection process on the reduction in the synthesis of these compounds in the plant. Then, our results suggested that P. syringae pv. garcae may induce reduction in the compounds (sugars, phenolic compounds and flavonoids) in coffee seedlings during the infection process as observed in other pathosystems [15,34]. Higher concentrations of gallic, caffeic, and chlorogenic acids and the presence of vanillin were detected in the Iapar-59 cultivar inoculated with P. syringae pv. garcae than in the non-inoculated ones. Vanillin was detected only in the partially resistant Iapar-59 inoculated plants and may be considered a putative phytoalexin in coffee leaves, since it was not detected in non-inoculated plants. At present, no phytoalexin-like compound has been confirmed in coffee; however, the accumulation of caffeic, chlorogenic, and gallic acids has been reported as a defense response to phytopathogens such as Hemileia vastatrix in coffee [35], Xylella fastidiosa in grape plants [36], Pseudomonas syringae pv. tomato DC3000 in tomato [37], and Fusarium in cucumber [38].
In summary, this study provides information regarding the chemical composition of coffee cultivars under basal defense induction conditions against Pseudomonas syringae pv. garcae as well as the detection for the first time of vanillin in leaves of a partially resistant cultivar to BHB. These results can help to understand the mechanisms that act during pathogenesis and may assist resistance breeding programs regarding coffee diseases.
4. Material and Methods
4.1. Chemical Composition and Antioxidant Activity of Coffee Leaf Extracts from Mundo Novo 376/4 and Iapar-59 Cultivars
4.1.1. Preparation of Coffee Seedlings
Coffee seedlings from the cultivars Iapar-59 and Mundo Novo 376/4 with four pairs of fully expanded leaves were grown in black polyethylene bags (0.11 × 0.20 m) containing a substrate composed of soil: sand: substrate for vegetables (Plantmax) at a 2:1:1 ratio. The seedlings were maintained in a growth chamber at 23 ± 2 °C and at 70% relative humidity.
4.1.2. Preparation of Aqueous Extracts from Coffee Leaves
The second pair of coffee leaves (from the apex to the base) was used to prepare the aqueous extracts. The leaves were oven dried at 50 °C for 48 h and then ground. The extraction was performed as described by Moreira et al. [39] with some modifications. A total of 100 mL of water was added to 10 g of leaves, and the extraction was performed by heating to 100 °C. Thereafter, the extracts were filtered on filter paper and freeze-dried.
4.1.3. Determination of the Contents of Total Phenolics and Flavonoids
The Folin–Ciocalteu reagent was used to determine the total phenolic compound content. An aliquot of each extract (125 μL) was mixed with 625 μL of Folin–Ciocalteu reagent (diluted 1:10 in distilled water) and 500 μL of 4% (w/v) sodium carbonate in distilled water. After 2 h of incubation in the dark, the absorbance was measured in a spectrophotometer (750 nm). The total phenolic compound content was expressed as gallic acid equivalents (mg GA g−1), calculated using a curve constructed with concentrations ranging from 5 to 100 μg mL−1 [40]. The limits of detection and quantification used for this method were 3.45 mg g−1 and 10.45 mg g−1, respectively.
The determination of flavonoid content was performed using a 125 μL aliquot of extract, which was mixed with 375 μL of ethanol, 25 μL of 10% (w/v) aluminum chloride, 25 μL of 1 M potassium acetate and 700 μL of distilled water, totaling 1250 μL of reaction. After 30 min, the absorbance was measured in a spectrophotometer (425 nm). The analytical curve for total flavonoids was constructed using the quercetin solution as standard. The total flavonoid content was expressed as quercetin (Q) equivalents (mg Q g−1 extract) [41]. The limits of detection and quantification used for this method were 3.59 mg g−1 and 10.89 mg g−1, respectively.
4.1.4. Determination of the Phenolic Compound Profile by High Performance Liquid Chromatography
The determination of the phenolic compound profile by high performance liquid chromatography was conducted using the following standard compounds for analysis: gallic acid, catechin, chlorogenic acid (5-caffeoylquinic acid), caffeic acid, vanillin, p-coumaric acid, ferulic acid, m-coumaric acid, coumarin, o-coumaric acid, rosmarinic acid, quercetin and trans-cinnamic acid. The samples were quantified using external standardization [42]. Analytical curves were constructed by triplicate injection of the standard solutions obtained by dilutions of the stock solution containing each standard at a concentration of 4 × 10−5 mol L−1. The analytical curves were obtained by linear regression considering the minimum correlation coefficient at 0.995. The identification of the analytes contained in the extracts was confirmed by the retention time and sample peaks in relation to the standards. The elution solvents used in the mobile phase were 2% acetic acid solution in water (phase A) and 70% methanol in 2% acetic acid solution in water (phase B). The detection wavelength was set at 271 nm. The identity of the analytes contained in the extracts was confirmed by the retention time and by the sample peaks in relation to the standards.
4.1.5. Antioxidant Activity: Free Radical Scavenging Activity (DPPH) and Ferric Reducing Antioxidant Power (FRAP)
The free radical scavenging activity was evaluated by the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging method using the stable radical DPPH [43]. The extracts were prepared in microtubes with four different dilutions in triplicate to determine EC50. A 25 μL aliquot of each extract dilution was transferred to microtubes with 975 μL of a 0.06 mM solution of DPPH. The reduction in the DPPH radical was determined at 515 nm. The results were expressed in EC50, characterized as the value in which it expresses the amount of antioxidant needed to decrease its radical concentration by 50% [43,44]. The EC50 value is negatively related to the antioxidant activity, the lower the EC50 value, the higher the antioxidant activity of the tested sample.
The Fe-ion reducing power assay was performed with the leaf extracts using quercetin as the standard compound [10,45]. The FRAP reagent was prepared by mixing 300 mM sodium acetate buffer (pH 3.6); 10 mM 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ) solution in 40 mM HCl; and 20 mM ferric chloride solution. Absorbance was carried at 593 nm, and ferrous sulfate heptahydrate was used as the standard.
The experimental design was completely randomized with two treatments (Mundo Novo and Iapar-59 cultivars), with six replicates and the experimental plot consisted of three coffee seedlings.
4.2. Production of Secondary Metabolites in Iapar-59 Coffee Leaves Inoculated with P. syringae pv. garcae
4.2.1. Inoculation with P. syringae pv. garcae
Coffee seedlings of the cultivar Iapar-59 with four pairs of fully expanded leaves were used in this trial. A virulent isolate of P. syringae pv. garcae (obtained from the collection of microorganisms, Laboratory of Plant Bacteriology at the Federal University of Lavras) was used to inoculate the coffee seedlings. The bacterial suspension used as inoculum was prepared from cultures grown for 48 h on nutrient agar medium (0.5% peptone, 0.3% meat extract, 0.1% NaCl and 18 g agar in 1 L of distilled water), suspended in a saline solution (0.85% NaCl), and standardized in a spectrophotometer to contain approximately 5.1 × 109 CFU.mL−1 (A600 = 0.8). The bacterial suspension was sprayed on the abaxial surface of the second pair of leaves. Control plants were inoculated only with saline solution. Plants were kept in a humid chamber for 24 h after inoculation. The collection of leaf samples was performed five days after inoculation. The samples were stored individually in aluminum foil, immediately immersed in liquid nitrogen (−196 °C) and then stored in an ultra-freezer at −80 °C until the preparation of the extracts and the analysis of the metabolites (total phenolic compounds, flavonoids, and reducing sugars).
4.2.2. Quantification of Total Phenolic and Flavonoid Compounds, Reducing Sugars and Determination of Phenolic Profile in Leaves
Samples were obtained as described in Section 4.1.2. After extraction, the extracts were utilized to determine the total phenolic compound [40], flavonoid [41] and reducing sugar contents [46]. The phenolic profile was determined by high-performance liquid chromatography (HPLC) as reported in the Section 4.1.4.
The experimental design of experiment production of secondary metabolites in Iapar-59 coffee leaves inoculated with P. syringae pv. garcae was completely randomized with two treatments (inoculated and non-inoculated plants), six replicates and the experimental plot consisted of three coffee seedlings.
4.2.3. Severity Disease in Mundo Novo and Iapar-59 Cultivars
Ten coffee seedlings from the cultivars Iapar-59 and Mundo Novo 376/4 were used in the pathogenicity test. The severity of BHB was determined using the diagrammatic scale proposed by Belan et al. [47], 20 days after inoculation with P. syringae pv. garcae.
4.3. Statistical Analysis
The original data of the total phenolic compounds, flavonoid and reducing sugar contents, free radical scavenging activity (DPPH), and ferric reducing antioxidant power (FRAP) were subjected to analysis of variance, and their means were compared by Tukey’s test at 5% probability.
Author Contributions
Conceptualization, M.L.V.d.R.; Methodology, J.A.G.d.S., I.S.R., L.R.M.A., A.R.L.; Validation, J.A.G.d.S., M.H.B.P. and A.C.A.M.; Formal Analysis, J.A.G.d.S., I.S.R., A.R.L., L.R.M.A. and M.H.B.P.; Data Curation, D.M.d.S.B. and M.L.V.d.R.; Writing—Original Draft Preparation, J.A.G.d.S., D.M.d.S.B., I.S.R.; A.R.L. and A.C.A.M.; Writing—Review and Editing, J.A.G.d.S., D.M.d.S.B., I.S.R. and M.L.V.d.R.; Project Administration, M.L.V.d.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available from the corresponding author upon reasonable request.
Acknowledgments
The Foundation for Research Support of the State of Minas Gerais (FAPEMIG), the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), and the National Institute for Coffee Science and Technology (INCT-Café).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Taiz, L. Fisiologia Vegetal; Artmed: Porto Alegre, Brazil, 2010; ISBN 9788536316147. [Google Scholar]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic Compounds and Their Role in Disease Resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Riaz, U.; Kharal, M.A.; Murtaza, G.; Zaman, Q.; Javaid, S.; Malik, H.A.; Aziz, H.; Abbas, Z. Prospective Roles and Mechanisms of Caffeic Acid in Counter Plant Stress: A Mini Review. PJAR 2018, 32, 8–19. [Google Scholar] [CrossRef]
- Treutter, D. Significance of Flavonoids in Plant Resistance and Enhancement of Their Biosynthesis. Plant. Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef]
- Vargas, P.; Farias, G.A.; Nogales, J.; Prada, H.; Carvajal, V.; Barón, M.; Rivilla, R.; Martín, M.; Olmedilla, A.; Gallegos, M.-T. Plant Flavonoids Target Pseudomonas Syringae Pv. Tomato DC3000 Flagella and Type III Secretion System: Flavonoids Affect Pto DC3000 Virulence. Environ. Microbiol. Rep. 2013, 5, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Morkunas, I.; Ratajczak, L. The Role of Sugar Signaling in Plant Defense Responses against Fungal Pathogens. Acta Physiol. Plant 2014, 36, 1607–1619. [Google Scholar] [CrossRef]
- Moghaddam, M.R.B.; Van den Ende, W. Sugars and Plant Innate Immunity. J. Exp. Bot. 2012, 63, 3989–3998. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of Primary Plant Metabolism during Plant-Pathogen Interactions and Its Contribution to Plant Defense. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Kang, H.-M.; Saltveit, M.E. Reduced Chilling Tolerance in Elongating Cucumber Seedling Radicles Is Related to Their Reduced Antioxidant Enzyme and DPPH-Radical Scavenging Activity. Physiol. Plant. 2002, 115, 244–250. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Devaki, M. The ferric reducing/antioxidant power (FRAP) assay for non-enzymatic antioxidant capacity: Concepts, procedures, limitations and applications. In Measurement of Antioxidant Activity & Capacity; Apak, R., Capanoglu, E., Shahidi, F., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 77–106. ISBN 9781119135388. [Google Scholar]
- Thite, S.V.; Aparadht, V.T.; Kore, S.A. Effect of powdery mildew infection on DPPH radical scavenging activity and ferric-re ducing antioxidant power of plants. World J. Pharm. Res. 2013, 2, 1–6. [Google Scholar]
- Ramiro, D.A.; Guerreiro-Filho, O.; Mazzafera, P. Phenol Contents, Oxidase Activities, and the Resistance of Coffee to the Leaf Miner Leucoptera Coffeella. J. Chem. Ecol. 2006, 32, 1977–1988. [Google Scholar] [CrossRef]
- Melo, G.A.; Shimizu, M.M.; Mazzafera, P. Polyphenoloxidase Activity in Coffee Leaves and Its Role in Resistance against the Coffee Leaf Miner and Coffee Leaf Rust. Phytochemistry 2006, 67, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Kempthorne, C.J.; Nielsen, A.J.; Wilson, D.C.; McNulty, J.; Cameron, R.K.; Liscombe, D.K. Metabolite Profiling Reveals a Role for Intercellular Dihydrocamalexic Acid in the Response of Mature Arabidopsis thaliana to Pseudomonas syringae. Phytochemistry 2021, 187, 112747. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zeng, Y.; Liu, P. Metabolic Profiling Reveals Local and Systemic Responses of Kiwifruit to Pseudomonas Syringae Pv. Actinidiae. Plant Direct 2020, 4, e00297. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Langlois-Meurinne, M.; Bellvert, F.; Garmier, M.; Didierlaurent, L.; Massoud, K.; Saindrenan, P. The differential spatial distribution of secondary metabolites in Arabidopsis leaves reacting hypersensitively to Pseudomonas syringae pv. tomato is dependent on the oxidative burst. J. Exp. Bot. 2010, 61, 3355–3370. [Google Scholar] [CrossRef] [PubMed]
- Badel, J.L.; Zambolim, L. Coffee Bacterial Diseases: A Plethora of Scientific Opportunities. Plant Pathol. 2019, 68, 411–425. [Google Scholar] [CrossRef]
- Amaral, J.F.; Teixeira, C.G.; Pinheiro, E.D. Obacterio causador da Mancha Aureolada Do Cafeeiro. Arq. Inst. Biológico 1956, 23, 151–155. [Google Scholar]
- Young, J.M.; Dye, D.W.; Bradbury, J.F.; Panagopoulos, C.G.; Robbs, C.F. A Proposed Nomenclature and Classification for Plant Pathogenic Bacteria. N. Z. J. Agric. Res. 1978, 21, 153–177. [Google Scholar] [CrossRef]
- Zoccoli, D.M.; Takatsu, A.; Uesugi, C.H. Ocorrência de Mancha Aureolada Em Cafeeiros Na Região Do Triângulo Mineiro e Alto Paranaíba. Bragantia 2011, 70, 843–849. [Google Scholar] [CrossRef]
- Sera, G.H.; Sera, T.; Fazuoli, L.C. IPR 102—Dwarf Arabica Coffee Cultivar with Resistance to Bacterial Halo Blight. Crop. Breed. Appl. Biotechnol. 2017, 17, 403–407. [Google Scholar] [CrossRef]
- Andreazi, E.; Sera, G.H.; Sera, T.; Fonseca, I.C.d.B.; Carducci, F.C.; Shigueoka, L.H.; dos Santos, W.G.; Pereira, C.T.M. Resistance to Bacterial Halo Blight in Arabica Coffee Lines Derivative from the Genotype C1195-5-6-2 under Natural Infection Conditions. Crop. Breed. Appl. Biotechnol. 2018, 18, 110–115. [Google Scholar] [CrossRef]
- Petek, M.R.; Sera, T.; Sera, G.H.; Fonseca, I.C.d.B.; Ito, D.S. Seleção de Progênies de Coffea Arabica Com Resistência Simultânea à Mancha Aureolada e à Ferrugem Alaranjada. Bragantia 2006, 65, 65–73. [Google Scholar] [CrossRef]
- Ito, D.S.; Sera, T.; Sera, G.H.; Grossi, L.D.; Kanayama, F.S. Resistance to Bacterial Blight in Arabica Coffee Cultivars. CBAB 2008, 8, 99–103. [Google Scholar] [CrossRef]
- Sera, G.H.; Sera, T.; Ito, D.S.; Fonseca, I.C.d.B.; Kanayama, F.S.; Grossi, L.D.; Shigueoka, L.H. Seleção para a resistência à ferrugem em progênies das cultivares de café IPR 99 e IPR 107. Bragantia 2010, 69, 547–554. [Google Scholar] [CrossRef][Green Version]
- Karamać, M.; Kosińska, A.; Pegg, R.B. Content of gallic acid in selected plant extracts. Pol. J. Food Nutr. Sci. 2006, 15, 55–58. [Google Scholar]
- Ngamsuk, S.; Huang, T.-C.; Hsu, J.-L. Determination of Phenolic Compounds, Procyanidins, and Antioxidant Activity in Processed Coffea Arabica L. Leaves. Foods 2019, 8, 389. [Google Scholar] [CrossRef] [PubMed]
- Lashermes, P.; Andrzejewski, S.; Bertrand, B.; Combes, M.C.; Dussert, S.; Graziosi, G.; Trouslot, P.; Anthony, F. Molecular Analysis of Introgressive Breeding in Coffee (Coffea Arabica L.). Theor. Appl. Genet. 2000, 100, 139–146. [Google Scholar] [CrossRef]
- Carvalho, F.G.; Sera, G.H.; Andreazi, E.; Sera, T.; Fonseca, I.C.D.B.; Carducci, F.C.; Shigueoka, L.H.; Holderbaum, M.M.; Costa, K.C. Tolerância Ao Déficit Hídrico Em Mudas de Genótipos de Café Portadores de Genes de Diferentes Espécies. C. Sci. 2017, 12, 156. [Google Scholar] [CrossRef]
- Herrera, J.C.; Lambot, C. The Coffee Tree—Genetic Diversity and Origin. In The Craft and Science of Coffee; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–16. ISBN 9780128035207. [Google Scholar]
- Roussos, P.A.; Pontikis, C.A.; Tsantili, E. Root Promoting Compounds Detected in Olive Knot Extract in High Quantities as a Response to Infection by the Bacterium Pseudomonas Savastanoi Pv. Savastanoi. Plant. Sci. 2002, 163, 533–541. [Google Scholar] [CrossRef]
- Cayuela, J.A.; Rada, M.; Rios, J.J.; Albi, T.; Guinda, A. Changes in Phenolic Composition Induced by Pseudomonas Savastanoi Pv. Savastanoi Infection in Olive Tree: Presence of Large Amounts of Verbascoside in Nodules of Tuberculosis Disease. J. Agric. Food Chem. 2006, 54, 5363–5368. [Google Scholar] [CrossRef] [PubMed]
- Heath, M.C. The Absence of Active Defense Mechanisms in Compatible Hostpathogen Interactions. In Active Defense Mechanisms in Plants; Wood, R.K.S., Ed.; Plenum Press: New York, NY, USA, 1982; pp. 143–156. [Google Scholar]
- Markakis, E.A.; Tjamos, S.E.; Antoniou, P.P.; Roussos, P.A.; Paplomatas, E.J.; Tjamos, E.C. Phenolic responses of resistant and susceptible olive cultivars induced by defoliating and nondefoliating Verticillium dahliae pathotypes. Plant Dis. 2010, 94, 1156–1162. [Google Scholar] [CrossRef]
- Rodrigues, F.Á.; Carré-Missio, V.; Jham, G.N.; Berhow, M.; Schurt, D.A. Chlorogenic acid levels in leaves of coffee plants supplied with silicon and infected by Hemileia vastatrix. Trop. Plant. Pathol. 2011, 36. [Google Scholar] [CrossRef]
- Maddox, C.E.; Laur, L.M.; Tian, L. Antibacterial Activity of Phenolic Compounds against the Phytopathogen Xylella Fastidiosa. Curr. Microbiol. 2010, 60, 53–58. [Google Scholar] [CrossRef]
- Dadáková, K.; Heinrichová, T.; Lochman, J.; Kašparovský, T. Production of Defense Phenolics in Tomato Leaves of Different Age. Molecules 2020, 25, 4952. [Google Scholar] [CrossRef]
- Zhou, X.; Jia, H.; Ge, X.; Wu, F. Effects of Vanillin on the Community Structures and Abundances of Fusarium and Trichoderma Spp. in Cucumber Seedling Rhizosphere. J. Plant Interact. 2018, 13, 45–50. [Google Scholar] [CrossRef]
- Moreira, M.E.d.C.; Pereira, R.G.F.A.; Dias, D.F.; Gontijo, V.S.; Vilela, F.C.; de Moraes, G.d.O.I.; Giusti-Paiva, A.; dos Santos, M.H. Anti-Inflammatory Effect of Aqueous Extracts of Roasted and Green Coffea Arabica L. J. Funct. Foods 2013, 5, 466–474. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 299, pp. 152–178. ISBN 9780121822002. [Google Scholar]
- Kalia, K.; Sharma, K.; Singh, H.P.; Singh, B. Effects of Extraction Methods on Phenolic Contents and Antioxidant Activity in Aerial Parts of Potentilla Atrosanguinea Lodd. and Quantification of Its Phenolic Constituents by RP-HPLC. J. Agric. Food Chem. 2008, 56, 10129–10134. [Google Scholar] [CrossRef]
- Aquino, F.W.B.; Rodrigues, S.; do Nascimento, R.F.; Casimiro, A.R.S. Simultaneous Determination of Aging Markers in Sugar Cane Spirits. Food Chem. 2006, 98, 569–574. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH. Free Radical Method. LWT—Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Belan, L.L.; Pozza, E.A.; Freitas, M.L.d.O.; de Souza, R.M.; Junior, W.C.d.J.; Oliveira, J.M. Diagrammatic Scale for Assessment of Bacterial Blight in Coffee Leaves. J. Phytopathol. 2014, 162, 801–810. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).