Protein Kinase A Detection in Human Urine Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Biological Samples
2.2. Cell Culture
2.3. Cell Protein Extraction
2.4. Serum and Urine Protein Concentration
2.5. Samples Preparation
2.6. Western Blotting
2.7. PKA ELISA Assay
2.8. Statistical Analysis
3. Results
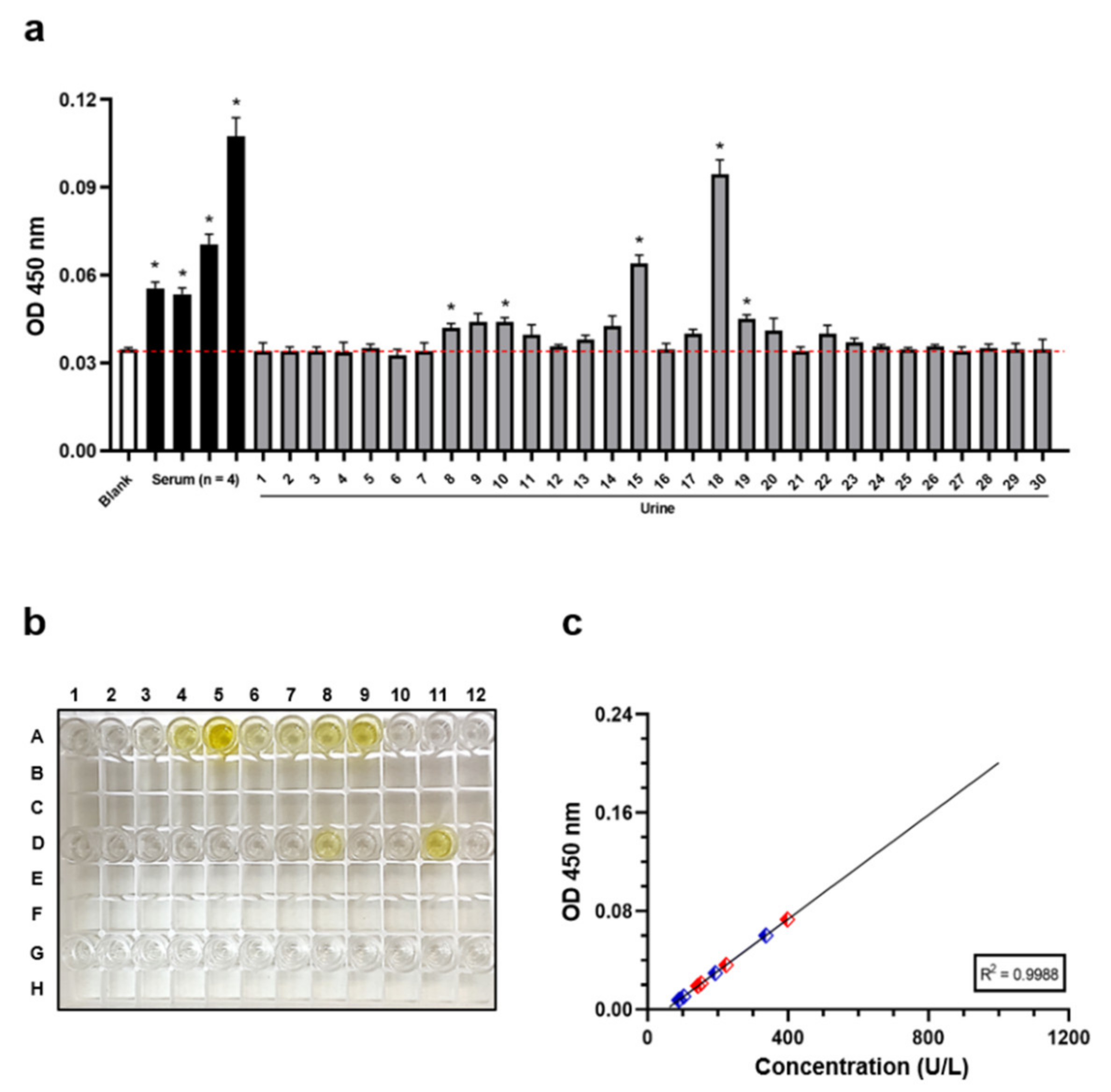
3.1. Human Urine Samples Can Sporadically Contain Detectable PKA Levels
3.2. Western Blotting Analysis Supports the Presence of PKA in Human Urine Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sassone-Corsi, P. The cyclic AMP pathway. Cold Spring Harb. Perspect Biol. 2012, 4, a011148. [Google Scholar] [CrossRef]
- London, E.; Bloyd, M.; Stratakis, C.A. PKA functions in metabolism and resistance to obesity: Lessons from mouse and human studies. J. Endocrinol. 2020, 246, R51–R64. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.S.; Zhang, P.; Steichen, J.M.; Keshwani, M.M.; Kornev, A.P. PKA: Lessons learned after twenty years. Biochim. Biophys. Acta. 2013, 1834, 1271–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgers, P.P.; Ma, Y.; Margarucci, L.; Mackey, M.; van der Heyden, M.A.; Ellisman, M.; Scholten, A.; Taylor, S.S.; Heck, A.J. A small novel A-kinase anchoring protein (AKAP) that localizes specifically protein kinase A-regulatory subunit I (PKA-RI) to the plasma membrane. J. Biol. Chem. 2012, 287, 43789–43797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizuno, R.; Kamioka, Y.; Kabashima, K.; Imajo, M.; Sumiyama, K.; Nakasho, E.; Ito, T.; Hamazaki, Y.; Okuchi, Y.; Sakai, Y.; et al. In vivo imaging reveals PKA regulation of ERK activity during neutrophil recruitment to inflamed intestines. J. Exp. Med. 2014, 211, 1123–1136. [Google Scholar]
- Zaccolo, M.; Zerio, A.; Lobo, M.J. Subcellular Organization of the cAMP Signaling Pathway. Pharmacol. Rev. 2021, 73, 278–309. [Google Scholar]
- Zhang, H.; Li, L.; Dong, Q.; Wang, Y.; Feng, Q.; Ou, X.; Zhou, P.; He, T.; Luo, J. Activation of PKA/CREB Signaling is Involved in BMP9-Induced Osteogenic Differentiation of Mesenchymal Stem Cells. Cell Physiol. Biochem. 2015, 37, 548–562. [Google Scholar] [CrossRef] [Green Version]
- Soberg, K.; Moen, L.V.; Skalhegg, B.S.; Laerdahl, J.K. Evolution of the cAMP-dependent protein kinase (PKA) catalytic subunit isoforms. PLoS one. 2017, 12, e0181091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bathon, K.; Weigand, I.; Vanselow, J.T.; Ronchi, C.L.; Sbiera, S.; Schlosser, A.; Fassnacht, M.; Calebiro, D. Alterations in Protein Kinase A Substrate Specificity as a Potential Cause of Cushing Syndrome. Endocrinology 2019, 160, 447–459. [Google Scholar] [CrossRef] [Green Version]
- Dagda, R.K.; Das Banerjee, T. Role of protein kinase A in regulating mitochondrial function and neuronal development: Implications to neurodegenerative diseases. Rev. Neurosci. 2015, 26, 359–370. [Google Scholar] [CrossRef] [Green Version]
- Keil, M.F.; Briassoulis, G.; Stratakis, C.A.; Wu, T.J. Protein Kinase A and Anxiety-Related Behaviors: A Mini-Review. Front. Endocrinol. (Lausanne) 2016, 7, 83. [Google Scholar] [CrossRef] [Green Version]
- Sapio, L.; Di Maiolo, F.; Illiano, M.; Esposito, A.; Chiosi, E.; Spina, A.; Naviglio, S. Targeting protein kinase A in cancer therapy: An update. EXCLI. J. 2014, 13, 843–855. [Google Scholar]
- Sapio, L.; Gallo, M.; Illiano, M.; Chiosi, E.; Naviglio, D.; Spina, A.; Naviglio, S. The Natural cAMP Elevating Compound Forskolin in Cancer Therapy: Is It Time? J. Cell Physiol. 2017, 232, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Kong, Q.; Wang, J.; Jiang, Y.; Hua, H. Complex roles of cAMP-PKA-CREB signaling in cancer. Exp. Hematol. Oncol. 2020, 9, 32. [Google Scholar]
- Sapio, L.; Salzillo, A.; Ragone, A.; Illiano, M.; Spina, A.; Naviglio, S. Targeting CREB in Cancer Therapy: A Key Candidate or One of Many? An Update. Cancers 2020, 12, 3166. [Google Scholar] [CrossRef]
- Kondrashin, A.; Nesterova, M.; Cho-Chung, Y.S. Cyclic adenosine 3′: 5′-monophosphate-dependent protein kinase on the external surface of LS-174T human colon carcinoma cells. Biochemistry 1999, 38, 172–179. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Lin, W.; Wang, W.; Zhang, Z.; Rayburn, E.R.; Lu, J.; Chen, D.; Yue, X.; Shen, F.; et al. Extracellular activity of cyclic AMP-dependent protein kinase as a biomarker for human cancer detection: Distribution characteristics in a normal population and cancer patients. Cancer Epidemiol. Biomarkers Prev. 2007, 16, 789–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.S.; Lee, Y.N.; Cho-Chung, Y.S. Biochemical characterization of extracellular cAMP-dependent protein kinase as a tumor marker. Biochem. Biophys. Res. Commun. 2000, 278, 679–684. [Google Scholar] [CrossRef]
- Cho, Y.S.; Park, Y.G.; Lee, Y.N.; Kim, M.K.; Bates, S.; Tan, L.; Cho-Chung, Y.S. Extracellular protein kinase A as a cancer biomarker: Its expression by tumor cells and reversal by a myristate-lacking Calpha and RIIbeta subunit overexpression. Proc. Natl. Acad. Sci. 2000, 97, 835–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szkudlarek, M.; Bosio, R.M.; Wu, Q.; Chin, K.V. Inhibition of angiogenesis by extracellular protein kinase A. Cancer Lett. 2009, 283, 68–73. [Google Scholar] [CrossRef] [Green Version]
- Nesterova, M.V.; Johnson, N.; Cheadle, C.; Bates, S.E.; Mani, S.; Stratakis, C.A.; Khan, I.U.; Gupta, R.K.; Cho-Chung, Y.S. Autoantibody cancer biomarker: Extracellular protein kinase A. Cancer Res. 2006, 66, 8971–8974. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, Y.F.; Liu, J.Q.; Chen, M.J.; Li, W.; Chen, Y.C.; Xu, M. The diagnostic value of extracellular protein kinase A (ECPKA) in serum for gastric and colorectal cancer. Transl. Cancer Res. 2020, 9, 3870–3878. [Google Scholar] [CrossRef]
- Lee, J.E.; Song, W.J.; Lee, H.; Kim, B.G.; Kim, T.; Lee, C.; Jang, B.; Youn, H.Y.; Choi, U.S.; Bhang, D.H. AniScan Using Extracellular Cyclic AMP-Dependent Protein Kinase A as a Serum Biomarker Assay for the Diagnosis of Malignant Tumors in Dogs. Sensors 2020, 20, 4075. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.O.; Kim, B.G.; Choi, U.S.; Baek, K.H.; Song, Y.K.; Li, Q.; Seo, K.W.; Ryeom, S.; Youn, H.Y.; Bhang, D.H. Extracellular cyclic adenosine monophosphate-dependent protein kinase A autoantibody and C-reactive protein as serum biomarkers for diagnosis of cancer in dogs. Vet. Comp. Oncol. 2019, 17, 99–106. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Kyaw, Y.M.E.; Tan, E.K.M.; Bekale, L.; Kang, M.W.C.; Kim, S.S.; Tan, I.; Lam, K.P.; Kah, J.C.Y. Quantitative and Label-Free Detection of Protein Kinase A Activity Based on Surface-Enhanced Raman Spectroscopy with Gold Nanostars. Anal. Chem. 2018, 90, 6071–6080. [Google Scholar] [CrossRef] [PubMed]
- Kasari, M.; Padrik, P.; Vaasa, A.; Saar, K.; Leppik, K.; Soplepmann, J.; Uri, A. Time-gated luminescence assay using nonmetal probes for determination of protein kinase activity-based disease markers. Anal. Biochem. 2012, 422, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.; Goel, S.; Nesterova, M.; Martin, R.M.; Grindel, J.M.; Rothenberg, M.L.; Zhang, R.; Tortora, G.; Cho-Chung, Y.S. Clinical studies in patients with solid tumors using a second-generation antisense oligonucleotide (GEM 231) targeted against protein kinase A type I. Ann. N. Y. Acad. Sci. 2003, 1002, 252–262. [Google Scholar] [CrossRef]
- Zhang, K.; Zeng, K.; Shen, C.; Tian, S.; Yang, M. Determination of protein kinase A activity and inhibition by using hydroxyapatite nanoparticles as a fluorescent probe. Mikrochim. Acta. 2018, 185, 225. [Google Scholar] [CrossRef]
- Yang, L.S.; Kadam, A.L.; Koide, S.S. Identification of a cAMP-dependent protein kinase in bovine and human follicular fluids. Biochem. Mol. Biol. Int. 1993, 31, 521–525. [Google Scholar]
- Bhang, D.H.; Choi, U.S.; Kim, B.G.; Lee, S.N.; Lee, S.; Roh, H.S.; Chung, W.J.; Jeon, K.O.; Song, W.J.; Youn, H.Y.; et al. Characteristics of extracellular cyclic AMP-dependent protein kinase as a biomarker of cancer in dogs. Vet. Comp. Oncol. 2017, 15, 1585–1589. [Google Scholar] [CrossRef]
- Hawkes, N. Cancer survival data emphasise importance of early diagnosis. Bmj. Brit. Med. J. 2019, 364. [Google Scholar] [CrossRef]
- Holdenrieder, S.; Pagliaro, L.; Morgenstern, D.; Dayyani, F. Clinically Meaningful Use of Blood Tumor Markers in Oncology. Biomed. Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martellucci, S.; Orefice, N.S.; Angelucci, A.; Luce, A.; Caraglia, M.; Zappavigna, S. Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy? Int. J. Mol. Sci. 2020, 21, 6486. [Google Scholar] [CrossRef] [PubMed]
- Piskor, B.M.; Przylipiak, A.; Dabrowska, E.; Sidorkiewicz, I.; Niczyporuk, M.; Szmitkowski, M.; Lawicki, S. Plasma Concentrations of Matrilysins MMP-7 and MMP-26 as Diagnostic Biomarkers in Breast Cancer. J. Clin. Med. 2021, 10, 1436. [Google Scholar] [CrossRef] [PubMed]
- Formica, V.; Lucchetti, J.; Doldo, E.; Riondino, S.; Morelli, C.; Argiro, R.; Renzi, N.; Nitti, D.; Nardecchia, A.; Dell’Aquila, E.; et al. Clinical Utility of Plasma KRAS, NRAS and BRAF Mutational Analysis with Real Time PCR in Metastatic Colorectal Cancer Patients-The Importance of Tissue/Plasma Discordant Cases. J. Clin. Med. 2020, 10, 87. [Google Scholar] [CrossRef]
- Elalamy, I.; Said, F.A.; Singer, M.; Couetil, J.P.; Hatmi, M. Inhibition by extracellular cAMP of phorbol 12-myristate 13-acetate-induced prostaglandin H synthase-2 expression in human pulmonary microvascular endothelial cells. Involvement of an ecto-protein kinase A activity. J. Biol. Chem. 2000, 275, 13662–13667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgenstern, E.; Gnad, U.; Preissner, K.T.; Dierichs, R.; Belleli, A.; Chestukhin, A.; Schvartz, I.; Shaltiel, S. Localization of protein kinase A and vitronectin in resting platelets and their translocation onto fibrin fibers during clot formation. Eur. J. Cell Biol. 2001, 80, 87–98. [Google Scholar] [CrossRef]
- Shaltiel, S.; Schvartz, I.; Korc-Grodzicki, B.; Kreizman, T. Evidence for an extra-cellular function for protein kinase A. Mol. Cell Biochem. 1993, 127, 283–291. [Google Scholar] [CrossRef]
- Feng, X.; Wang, J.; Gao, Z.; Tian, Y.; Zhang, L.; Chen, H.; Zhang, T.; Xiao, L.; Yao, J.; Xing, W.; et al. An alternative strategy to western blot as a confirmatory diagnostic test for HIV infection. J. Clin. Virol. 2017, 88, 8–11. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragone, A.; Salzillo, A.; Spina, A.; Zappavigna, S.; Caraglia, M.; Sapio, L.; Naviglio, S. Protein Kinase A Detection in Human Urine Samples. J. Clin. Med. 2021, 10, 4096. https://doi.org/10.3390/jcm10184096
Ragone A, Salzillo A, Spina A, Zappavigna S, Caraglia M, Sapio L, Naviglio S. Protein Kinase A Detection in Human Urine Samples. Journal of Clinical Medicine. 2021; 10(18):4096. https://doi.org/10.3390/jcm10184096
Chicago/Turabian StyleRagone, Angela, Alessia Salzillo, Annamaria Spina, Silvia Zappavigna, Michele Caraglia, Luigi Sapio, and Silvio Naviglio. 2021. "Protein Kinase A Detection in Human Urine Samples" Journal of Clinical Medicine 10, no. 18: 4096. https://doi.org/10.3390/jcm10184096
APA StyleRagone, A., Salzillo, A., Spina, A., Zappavigna, S., Caraglia, M., Sapio, L., & Naviglio, S. (2021). Protein Kinase A Detection in Human Urine Samples. Journal of Clinical Medicine, 10(18), 4096. https://doi.org/10.3390/jcm10184096






