CHA2DS2-VASc Score for Major Adverse Cardiovascular Events Stratification in Patients with Pneumonia with and without Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Study Participants
2.3. Statistical Analysis
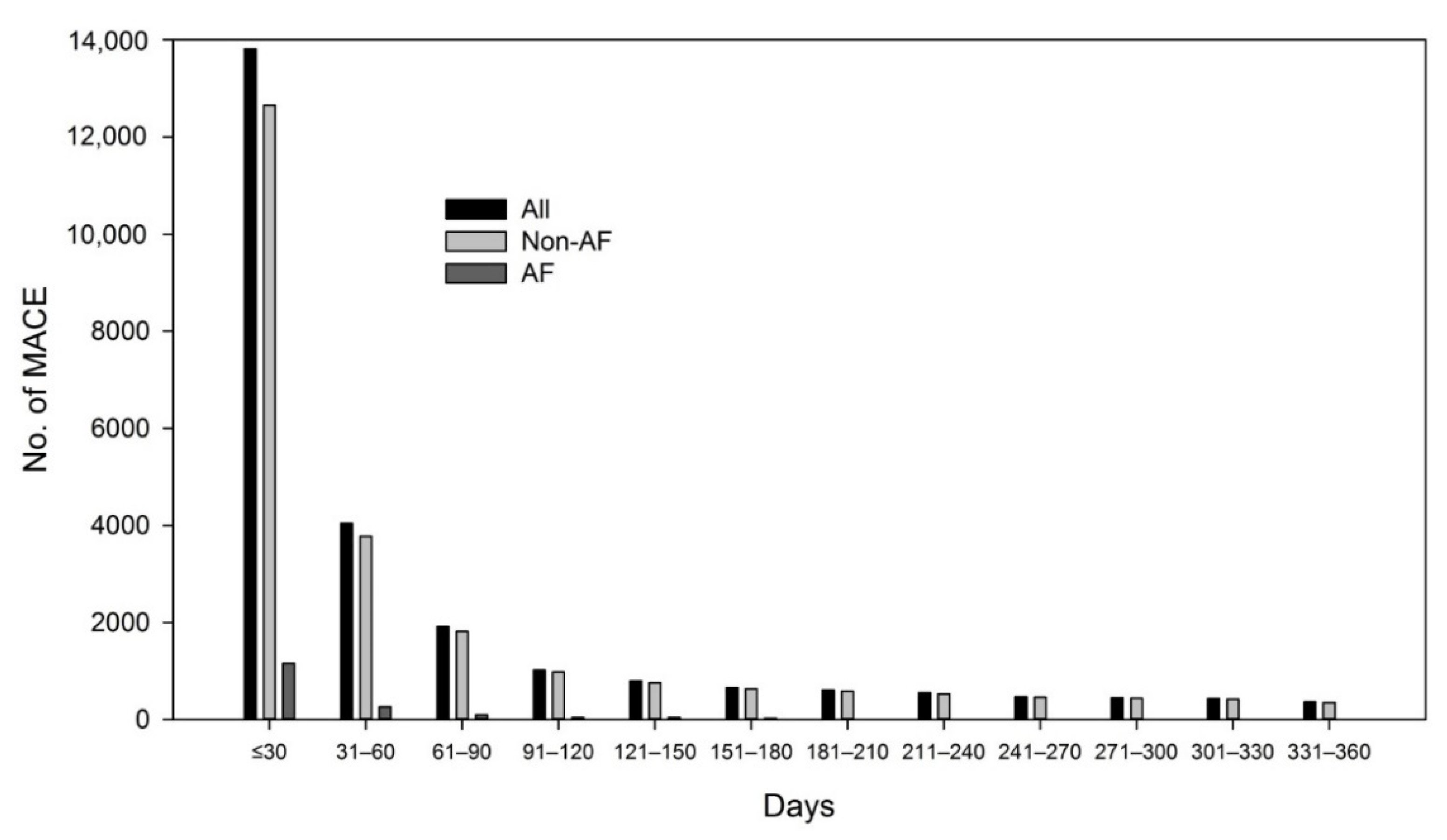
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Prina, E.; Ranzani, O.T.; Torres, A. Community-acquired pneumonia. Lancet 2015, 386, 1097–1108. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Musher, D.M.; Wells, G.A.; Chirinos, J.A.; Chen, L.; Fine, M.J. Cardiac complications in patients with community-acquired pneumonia: Incidence, timing, risk factors, and association with short-term mortality. Circulation 2012, 125, 773–781. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Suh, K.N.; Rose, G.; Chirinos, J.A.; Doucette, S.; Cameron, D.W.; Fergusson, D.A. Cardiac complications in patients with community-acquired pneumonia: A systematic review and meta-analysis of observational studies. PLoS Med. 2011, 8, e1001048. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.C.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association between hospitalization for pneumonia and subsequent risk of cardiovascular disease. JAMA 2015, 313, 264–274. [Google Scholar] [CrossRef]
- Perry, T.W.; Pugh, M.J.; Waterer, G.W.; Nakashima, B.; Orihuela, C.J.; Copeland, L.A.; Restrepo, M.I.; Anzueto, A.; Mortensen, E.M. Incidence of cardiovascular events after hospital admission for pneumonia. Am. J. Med. 2011, 124, 244–251. [Google Scholar] [CrossRef]
- Violi, F.; Cangemi, R.; Falcone, M.; Taliani, G.; Pieralli, F.; Vannucchi, V.; Nozzoli, C.; Venditti, M.; Chirinos, J.A.; SIXTUS (Thrombosis-Related Extrapulmonary Outcomes in Pneumonia) Study Group. Cardiovascular Complications and Short-term Mortality Risk in Community-Acquired Pneumonia. Clin. Infect. Dis. 2017, 64, 1486–1493. [Google Scholar] [CrossRef]
- Ramirez, J.; Aliberti, S.; Mirsaeidi, M.; Peyrani, P.; Filardo, G.; Amir, A.; Moffett, B.; Gordon, J.; Blasi, F.; Bordon, J. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin. Infect. Dis. 2008, 47, 182–187. [Google Scholar] [CrossRef]
- Clayton, T.C.; Thompson, M.; Meade, T.W. Recent respiratory infection and risk of cardiovascular disease: Case-control study through a general practice database. Eur. Heart J. 2008, 29, 96–103. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Musher, D.M.; Shachkina, S.; Chirinos, J.A. Acute pneumonia and the cardiovascular system. Lancet 2013, 381, 496–505. [Google Scholar] [CrossRef]
- Restrepo, M.I.; Reyes, L.F. Pneumonia as a cardiovascular disease. Respirology 2018, 23, 250–259. [Google Scholar] [CrossRef]
- Viasus, D.; Garcia-Vidal, C.; Manresa, F.; Dorca, J.; Gudiol, F.; Carratalà, J. Risk stratification and prognosis of acute cardiac events in hospitalized adults with community-acquired pneumonia. J. Infect. 2013, 66, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Chang, C.-J.; Chung, W.-J.; Lin, C.-J.; Hsueh, S.-K.; Lee, C.-H.; Wu, C.-J.; Tsai, T.-H.; Cheng, C.-I. Assessment of CHA2DS2-VASc score for predicting cardiovascular and cerebrovascular outcomes in acute myocardial infarction patients. Medicine 2018, 97, e11230. [Google Scholar] [CrossRef]
- Saliba, W.; Gronich, N.; Barnett-Griness, O.; Rennert, G. The role of CHADS2 and CHA2 DS2-VASc scores in the prediction of stroke in individuals without atrial fibrillation: A population-based study. J. Thromb. Haemost. 2016, 14, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Topaz, G.; Haisraely, O.; Shacham, Y.; Beery, G.; Shilo, L.; Kassem, N.; Pereg, D.; Kitay-Cohen, Y. CHA2 DS2-VASc score and clinical outcomes of patients with chest pain discharged from internal medicine wards following acute coronary syndrome rule-out. Clin. Cardiol. 2018, 41, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, F.; Vannucchi, V.; Nozzoli, C.; Augello, G.; Dentali, F.; De Marzi, G.; Uomo, G.; Risaliti, F.; Morbidoni, L.; FADOI-ICECAP Study Group. Acute cardiovascular events in patients with community acquired pneumonia: Results from the observational prospective FADOI-ICECAP study. BMC Infect. Dis. 2021, 21, 116. [Google Scholar] [CrossRef]
- Yende, S.; D’Angelo, G.; Kellum, J.A.; Weissfeld, L.; Fine, J.; Welch, R.D.; Kong, L.; Carter, M.; Angus, D.C.; GenIMS Investigators. Inflammatory markers at hospital discharge predict subsequent mortality after pneumonia and sepsis. Am. J. Respir. Crit. Care Med. 2008, 177, 1242–1247. [Google Scholar] [CrossRef] [PubMed]
- Cangemi, R.; Pignatelli, P.; Carnevale, R.; Bartimoccia, S.; Nocella, C.; Falcone, M.; Taliani, G.; Violi, F.; Battaglia, S.; SIXTUS Study Group. Low-grade endotoxemia, gut permeability and platelet activation in community-acquired pneumonia. J. Infect. 2016, 73, 107–114. [Google Scholar] [CrossRef]
- O’Donnell, M.J.; Hankey, G.J.; Eikelboom, J.W. Antiplatelet therapy for secondary prevention of noncardioembolic ischemic stroke: A critical review. Stroke 2008, 39, 1638–1646. [Google Scholar] [CrossRef]
- Hedlund, J.; Hansson, L.O. Procalcitonin and C-reactive protein levels in community-acquired pneumonia: Correlation with etiology and prognosis. Infection 2000, 28, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003, 107, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Quisi, A.; Alıcı, G.; Harbalıoğlu, H.; Genç, Ö.; Er, F.; Allahverdiyev, S.; Yıldırım, A.; Kurt, I.H. The CHA2DS2-VASc score and in-hospital mortality in patients with COVID-19: A multicenter retrospective cohort study. Turk. Kardiyol. Dern. Ars. 2020, 48, 656–663. (In English) [Google Scholar] [CrossRef] [PubMed]
- Uribarri, A.; Núñez-Gil, I.J.; Aparisi, Á.; Arroyo-Espliguero, R.; Eid, C.M.; Romero, R.; Becerra-Muñoz, V.M.; Feltes, G.; Molina, M.; HOPE COVID-19 Investigators. Atrial fibrillation in patients with COVID-19. Usefulness of the CHA2DS2-VASc score: An analysis of the international HOPE COVID-19 registry. Rev. Esp. Cardiol. 2021, 74, 608–615. [Google Scholar] [CrossRef]
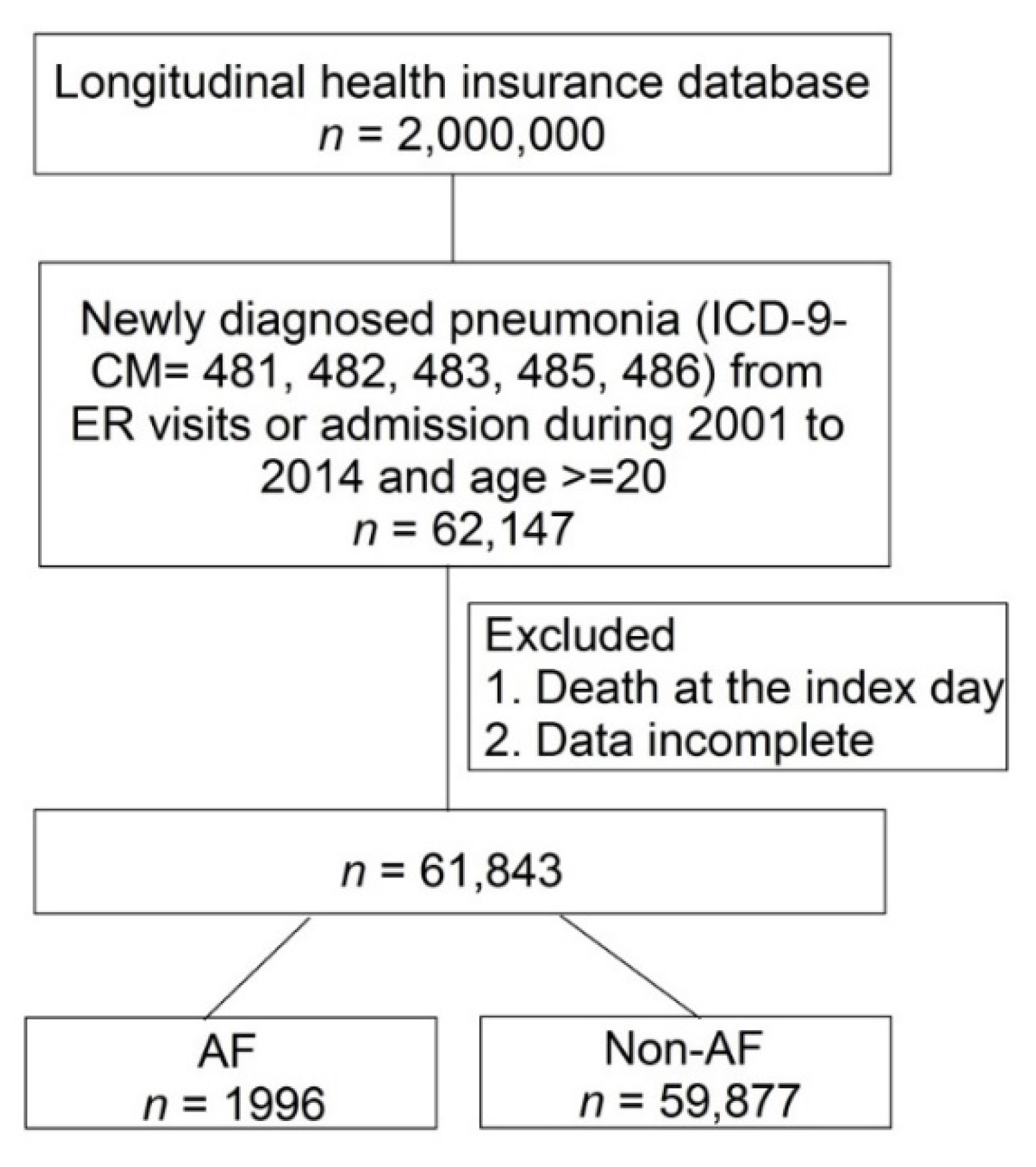
| All (n = 61873) | Non-Atrial Fibrillation (n = 59877) | Atrial Fibrillation (n = 1996) | p Value | |
|---|---|---|---|---|
| Age | <0.001 | |||
| 20–39 | 13,471 (21.8) | 13,460 (22.5) | 11 (0.6) | |
| 40–64 | 19,422 (31.4) | 19,216 (32.1) | 206 (10.5) | |
| ≥65 | 28,950 (46.8) | 27,201 (45.4) | 1749 (89.0) | |
| Mean ± SD | 59.9 ± 20.8 | 59.3 ± 20.8 | 78.1 ± 10.8 | <0.001 |
| Sex | 0.025 | |||
| Female | 26,882 (43.4) | 26,076 (43.5) | 806 (41.0) | |
| Male | 34,961 (56.5) | 33,801 (56.5) | 1160 (59.0) | |
| CHA2DS2-VASc | ||||
| Congestive heart failure | 3921 (6.3) | 3094 (5.2) | 827 (42.1) | <0.0001 |
| Hypertension | 23,116 (37.4) | 21,742 (36.3) | 1374 (69.9) | <0.0001 |
| Diabetes | 12,223 (19.8) | 11,627 (19.4) | 596 (30.3) | <0.0001 |
| Vascular disease | 1683 (2.7) | 1541 (2.6) | 142 (7.2) | <0.0001 |
| Prior ischemic stroke/transient ischemic attack | 8851 (14.3) | 8028 (13.4) | 823 (41.9) | <0.0001 |
| Mean ± SD | 2.2 ± 1.8 | 2.1 ± 1.8 | 4.3 ± 1.7 | <0.0001 |
| Other comorbidities | ||||
| Hyperlipidemia | 7404 (12.0) | 7093 (11.8) | 311 (15.8) | <0.0001 |
| Chronic kidney disease | 3173 (5.1) | 2892 (4.8) | 191 (9.7) | <0.0001 |
| Chronic obstructive pulmonary disease | 5483 (8.9) | 5035 (8.4) | 448 (22.8) | <0.0001 |
| Antiplatelet agents | 13,716 (22.2) | 12,977 (21.7) | 739 (37.6) | <0.0001 |
| Aspirin | 21,414 (34.6) | 20,670 (34.5) | 744 (37.8) | 0.002 |
| Follow-up durations (years), Mean ± SD | 3.4 ± 4.0 | 3.5 ± 4.0 | 0.5 ± 1.3 | <0.0001 |
| Univariate | Multivariate † | |||||
|---|---|---|---|---|---|---|
| n | No. of MACE | HR (95% C.I.) | p Value | HR (95% C.I.) | p Value | |
| Non-AF | ||||||
| CHA2DS2-VASc | ||||||
| 0 | 13,167 | 3706 | Reference | Reference | ||
| 1 | 16,032 | 5357 | 1.25 (1.36–1.31) | <0.0001 | 1.60 (1.76–1.68) | <0.0001 |
| 2 | 8672 | 6517 | 4.58 (4.97–4.77) | <0.0001 | 2.96 (3.30–3.12) | <0.0001 |
| 3 | 8103 | 6865 | 6.69 (7.26–6.97) | <0.0001 | 4.17 (4.68–4.42) | <0.0001 |
| 4 | 6279 | 5697 | 9.27 (10.10–9.68) | <0.0001 | 5.90 (6.68–6.28) | <0.0001 |
| 5 | 4262 | 4102 | 13.74 (15.07–14.39) | <0.0001 | 8.64 (9.85–9.23) | <0.0001 |
| 6 | 2320 | 2281 | 18.00 (20.07–19.01) | <0.0001 | 11.14 (12.85–11.97) | <0.0001 |
| >6 | 1042 | 1032 | 19.91 (22.94–21.37) | <0.0001 | 14.62 (17.42–15.96) | <0.0001 |
| AF | ||||||
| CHA2DS2-VASc | ||||||
| 0 | 22 | 17 | Reference | Reference | ||
| 1 | 98 | 82 | 1.34 (0.79–2.25) | 0.277 | 1.72 (1.02–2.92) | 0.044 |
| 2 | 182 | 175 | 1.61 (0.98–2.65) | 0.061 | 2.27 (1.35–3.81) | 0.002 |
| 3 | 318 | 300 | 1.93 (1.18–3.15) | 0.009 | 2.95 (1.75–4.97) | <0.0001 |
| 4 | 396 | 391 | 2.85 (1.75–4.64) | <0.0001 | 4.30 (2.54–7.26) | <0.0001 |
| 5 | 443 | 439 | 3.24 (1.99–5.28) | <0.0001 | 5.08 (3.00–8.61) | <0.0001 |
| 6 | 286 | 286 | 3.72 (2.27–6.09) | <0.0001 | 5.91 (3.46–10.10) | <0.0001 |
| >6 | 221 | 221 | 3.91 (2.38–6.43) | <0.0001 | 6.59 (3.82–11.35) | <0.0001 |
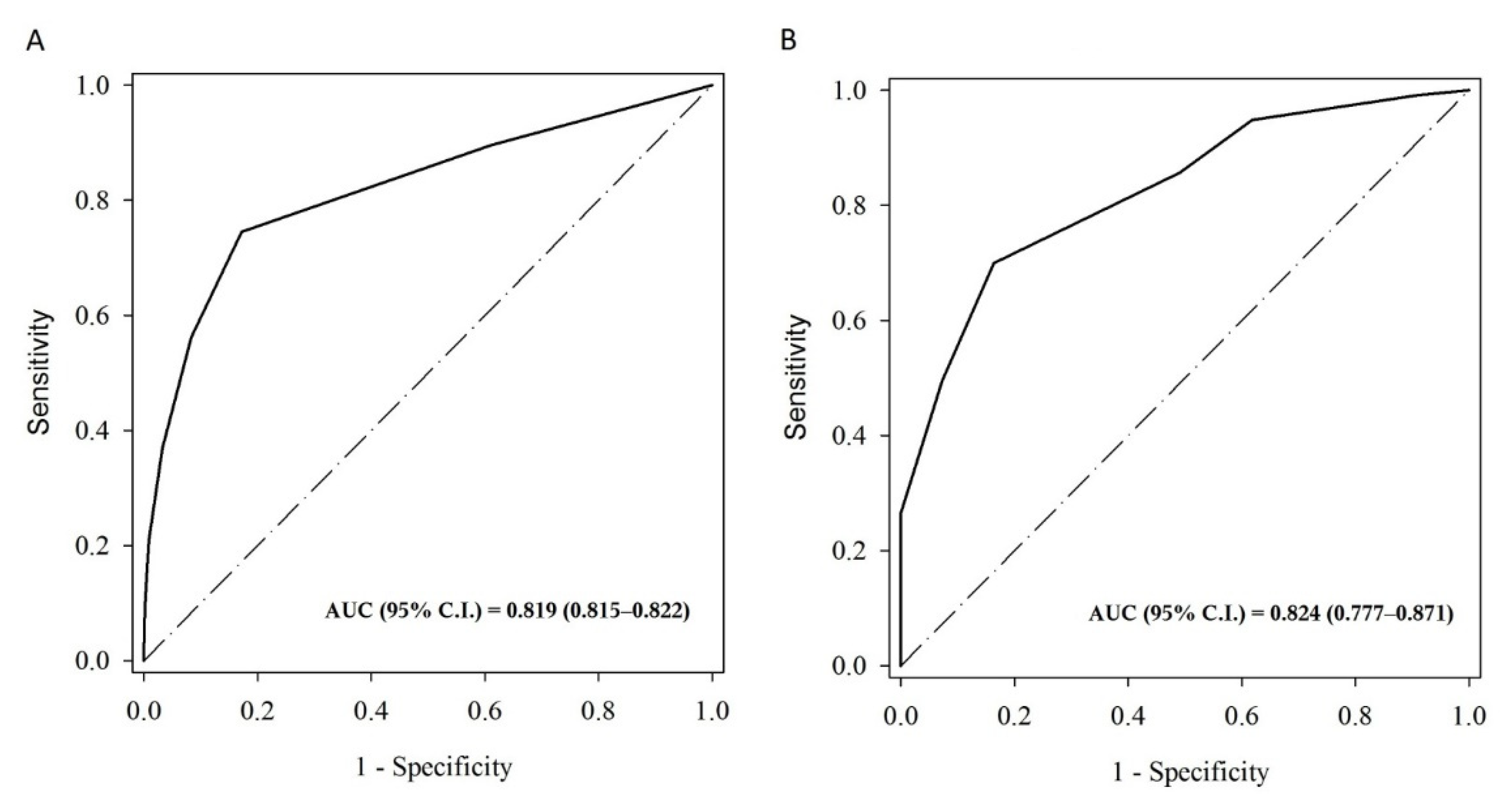
| n | No. of Event | AUC (95% C.I.) | Cut-Off | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|
| All event | ||||||||
| All | 61,843 | 37,468 | 0.8247 (0.8215–0.8278) | 2.0 | 0.7555 | 0.8270 | 0.8703 | 0.68751 |
| NonAF | 59,877 | 35,557 | 0.8185 (0.8152–0.8217) | 2.0 | 0.7451 | 0.8280 | 0.8636 | 0.68961 |
| AF | 1966 | 1911 | 0.8240 (0.7773–0.8708) | 4.0 | 0.6996 | 0.8364 | 0.9933 | 0.07419 |
| Ischemic heart disease | ||||||||
| All | 61,843 | 16,658 | 0.6588 (0.6542–0.6634) | 2.0 | 0.7391 | 0.5527 | 0.3786 | 0.85177 |
| NonAF | 59,877 | 15,810 | 0.6602 (0.6555–0.6649) | 2.0 | 0.7283 | 0.5651 | 0.3753 | 0.85287 |
| AF | 1966 | 848 | 0.5399 (0.5146–0.5653) | 4.0 | 0.5554 | 0.5125 | 0.4071 | 0.51613 |
| Heart failure | ||||||||
| All | 61,843 | 12,180 | 0.7424 (0.7379–0.7470) | 2.0 | 0.8489 | 0.5533 | 0.3179 | 0.93724 |
| NonAF | 59,877 | 11,064 | 0.7379 (0.7332–0.7427) | 2.0 | 0.8382 | 0.5615 | 0.3023 | 0.93870 |
| AF | 1966 | 1116 | 0.5480 (0.5227–0.5733) | 5.0 | 0.5117 | 0.5541 | 0.6011 | 0.46358 |
| Acute ischemic stroke | ||||||||
| All | 61,843 | 15,463 | 0.7720 (0.7678–0.7763) | 3.0 | 0.6957 | 0.7216 | 0.4545 | 0.87672 |
| NonAF | 59,877 | 14,543 | 0.7710 (0.7667–0.7754) | 2.0 | 0.8296 | 0.5894 | 0.3933 | 0.91513 |
| AF | 1966 | 920 | 0.6731 (0.6498–0.6964) | 5.0 | 0.6261 | 0.6425 | 0.6063 | 0.66142 |
| Intracranial hemorrhage | ||||||||
| All | 61,843 | 2678 | 0.6488 (0.6387–0.6589) | 2.0 | 0.7737 | 0.4853 | 0.0637 | 0.97933 |
| NonAF | 59,877 | 2559 | 0.6514 (0.6410–0.6617) | 2.0 | 0.7644 | 0.4989 | 0.0638 | 0.97935 |
| AF | 1966 | 119 | 0.5343 (0.4821–0.5866) | 5.0 | 0.5378 | 0.5203 | 0.0674 | 0.94587 |
| Death | ||||||||
| All | 61,843 | 24,182 | 0.7938 (0.7902–0.7974) | 2.0 | 0.8358 | 0.6731 | 0.6214 | 0.86459 |
| NonAF | 59,877 | 22,684 | 0.7899 (0.7862–0.7936) | 2.0 | 0.8271 | 0.6796 | 0.6115 | 0.86565 |
| AF | 1966 | 1498 | 0.6854 (0.6576–0.7131) | 4.0 | 0.7517 | 0.5299 | 0.8366 | 0.40000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-Y.; Lin, F.-Y.; Ku, M.-S.; Wang, Y.-H.; Lee, K.-Y.; Ho, S.-W. CHA2DS2-VASc Score for Major Adverse Cardiovascular Events Stratification in Patients with Pneumonia with and without Atrial Fibrillation. J. Clin. Med. 2021, 10, 4093. https://doi.org/10.3390/jcm10184093
Wang B-Y, Lin F-Y, Ku M-S, Wang Y-H, Lee K-Y, Ho S-W. CHA2DS2-VASc Score for Major Adverse Cardiovascular Events Stratification in Patients with Pneumonia with and without Atrial Fibrillation. Journal of Clinical Medicine. 2021; 10(18):4093. https://doi.org/10.3390/jcm10184093
Chicago/Turabian StyleWang, Bo-Yuan, Fei-Yi Lin, Min-Sho Ku, Yu-Hsun Wang, Kun-Yu Lee, and Sai-Wai Ho. 2021. "CHA2DS2-VASc Score for Major Adverse Cardiovascular Events Stratification in Patients with Pneumonia with and without Atrial Fibrillation" Journal of Clinical Medicine 10, no. 18: 4093. https://doi.org/10.3390/jcm10184093
APA StyleWang, B.-Y., Lin, F.-Y., Ku, M.-S., Wang, Y.-H., Lee, K.-Y., & Ho, S.-W. (2021). CHA2DS2-VASc Score for Major Adverse Cardiovascular Events Stratification in Patients with Pneumonia with and without Atrial Fibrillation. Journal of Clinical Medicine, 10(18), 4093. https://doi.org/10.3390/jcm10184093






