Influence of Phosphite Supply in the MS Medium on Root Morphological Characteristics, Fresh Biomass and Enzymatic Behavior in Five Genotypes of Potato (Solanum tuberosum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Place of the Experiment and Materials
2.2. Source of Genotypes and Preparation of In Vitro Explants
2.3. Experimental Design and Treatments
2.4. Measurements of Data
2.4.1. Physio-Morphological Parameters
2.4.2. Tolerance Indexes Determination
2.4.3. Determination of Hydrogen Peroxide and Malonaldehyde Contents in Samples
2.4.4. Determination of Antioxidant Enzymes Activities and Proline Contents in Shoots
2.5. Analyses of Data
3. Results
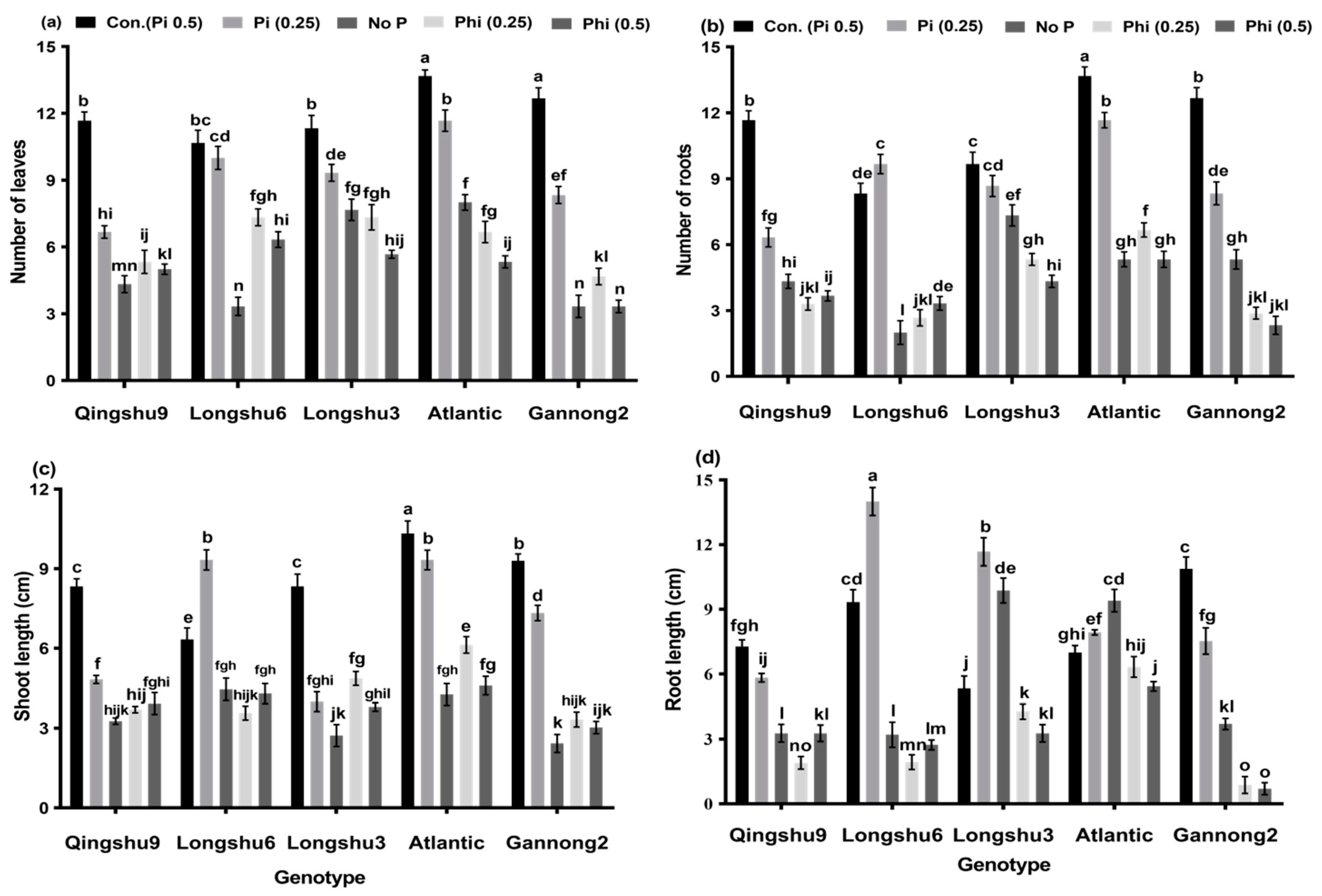
3.1. Influence of Phosphite and Phosphate on Physiological Parameters of Five Potato Genotypes after 20 Days’ Growth Period
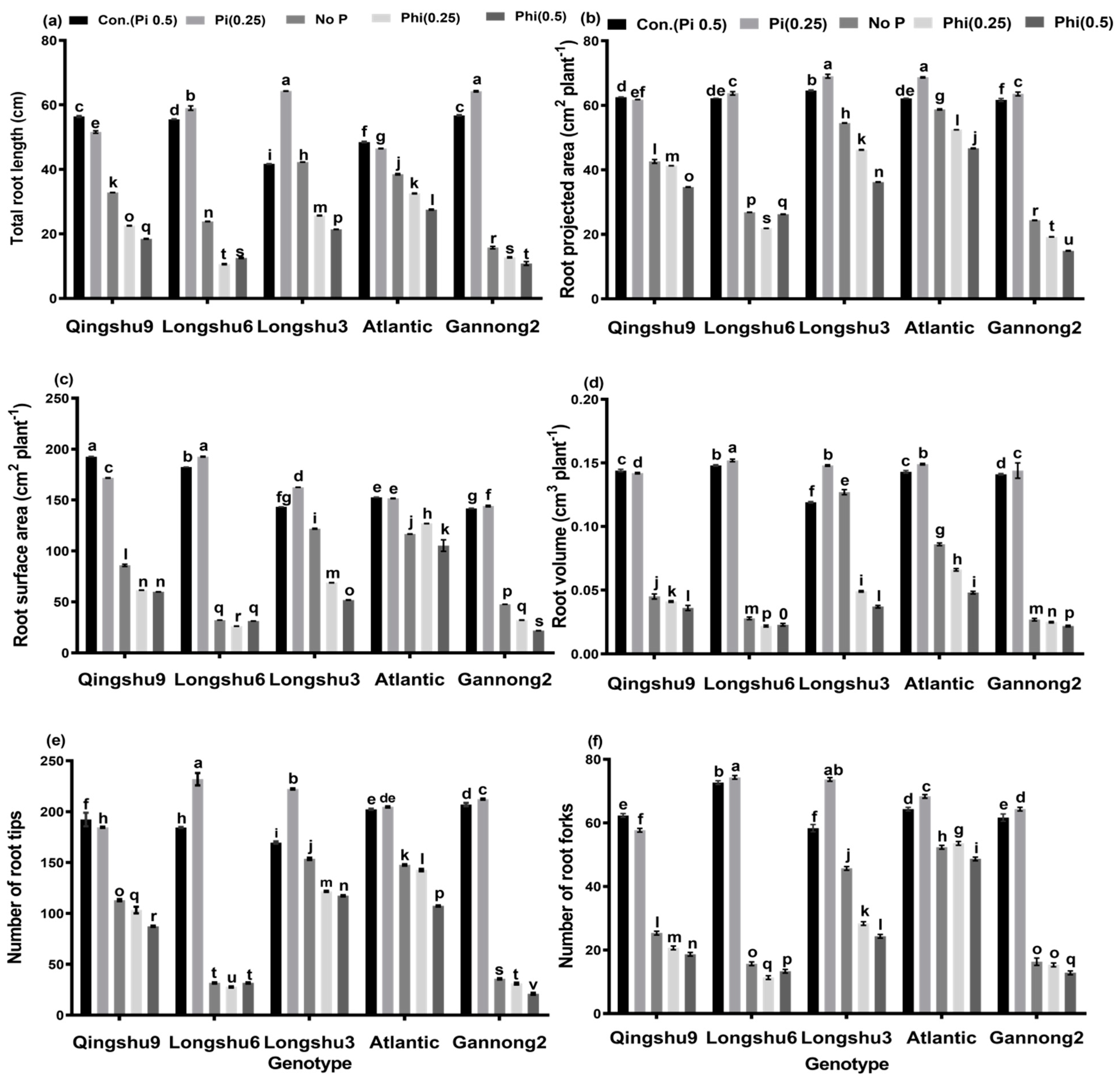
3.2. Effects of Phosphite, Phosphate and No-P Supply on Root Morphological Characteristics of Five Potato Genotypes for 20 Days Growth Period
3.3. Tolerance of the Five Potato Genotypes to Phosphite Stress
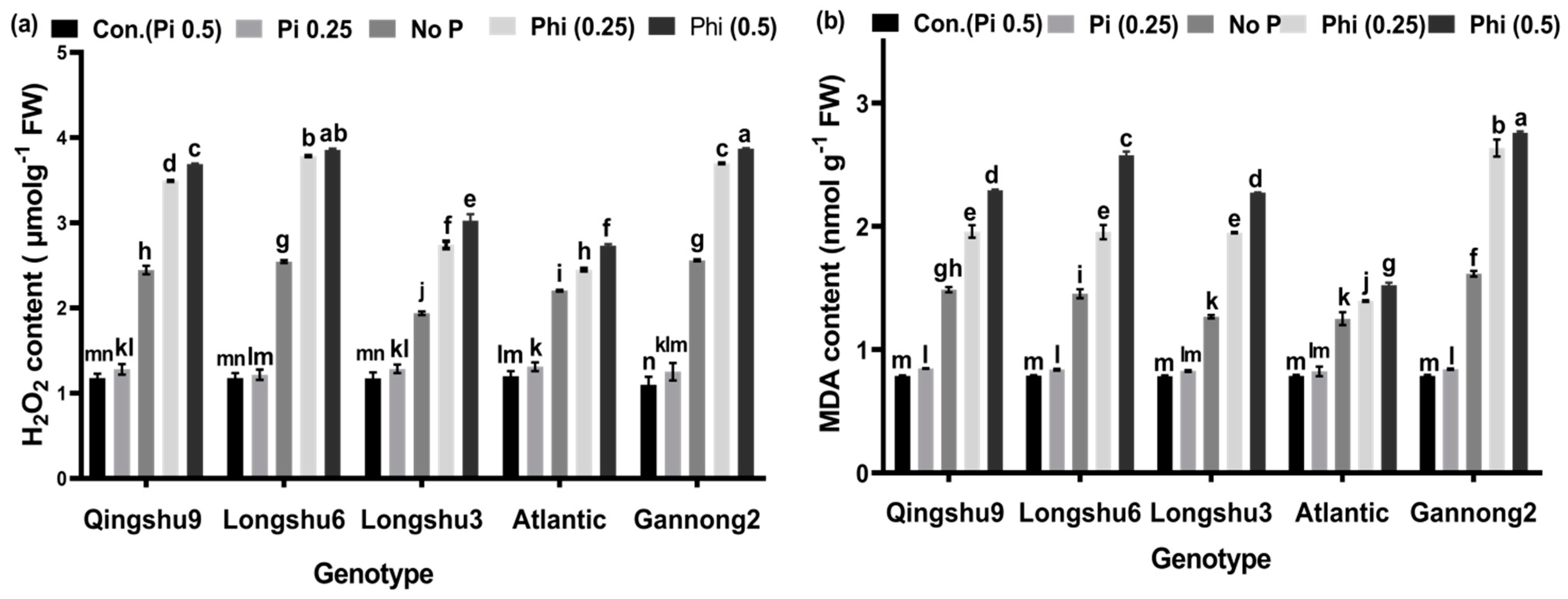
3.4. Content of H2O2 and MDA in the Shoots
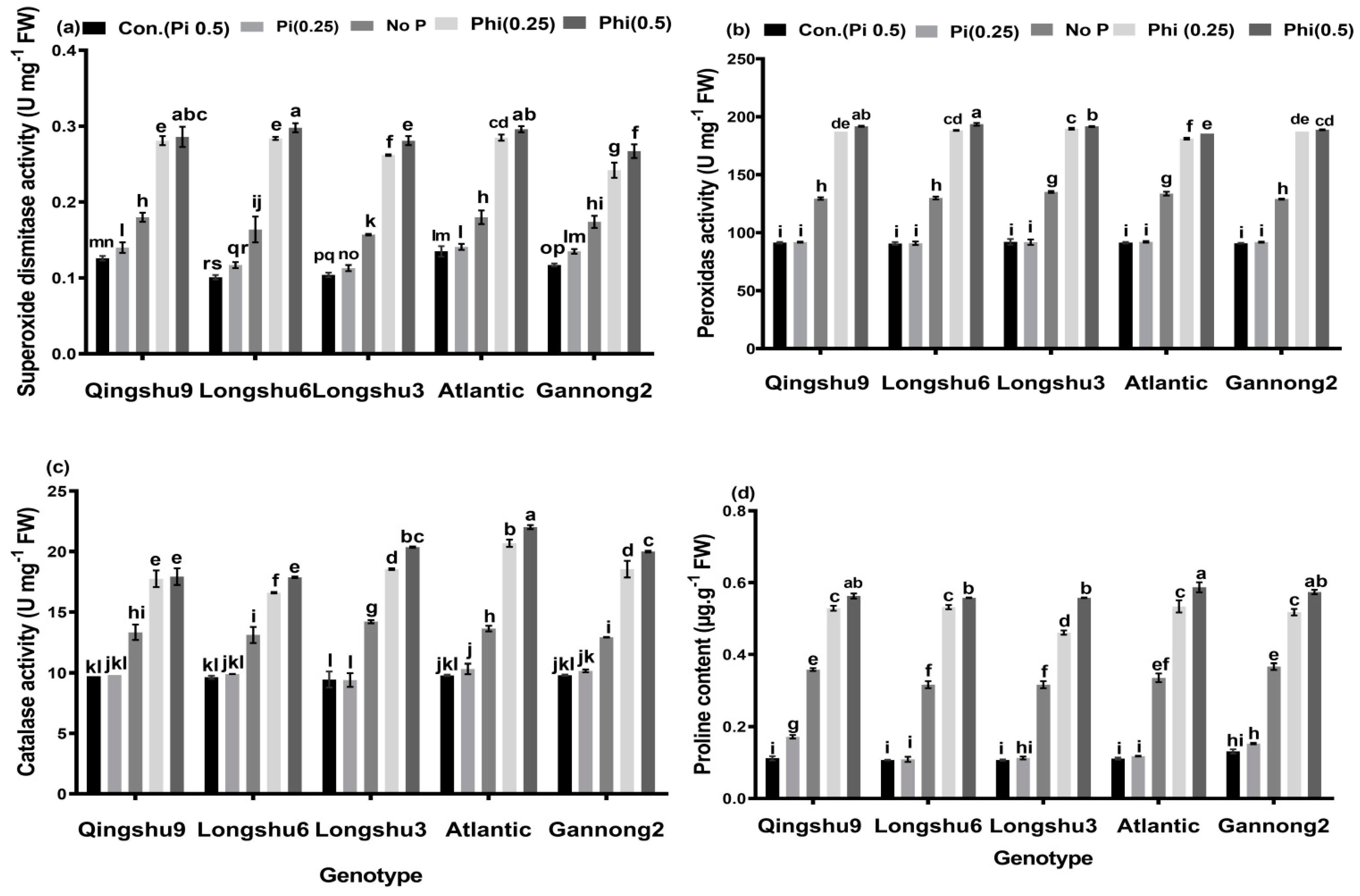
3.5. Antioxidant Enzymes Activities and Content of Proline
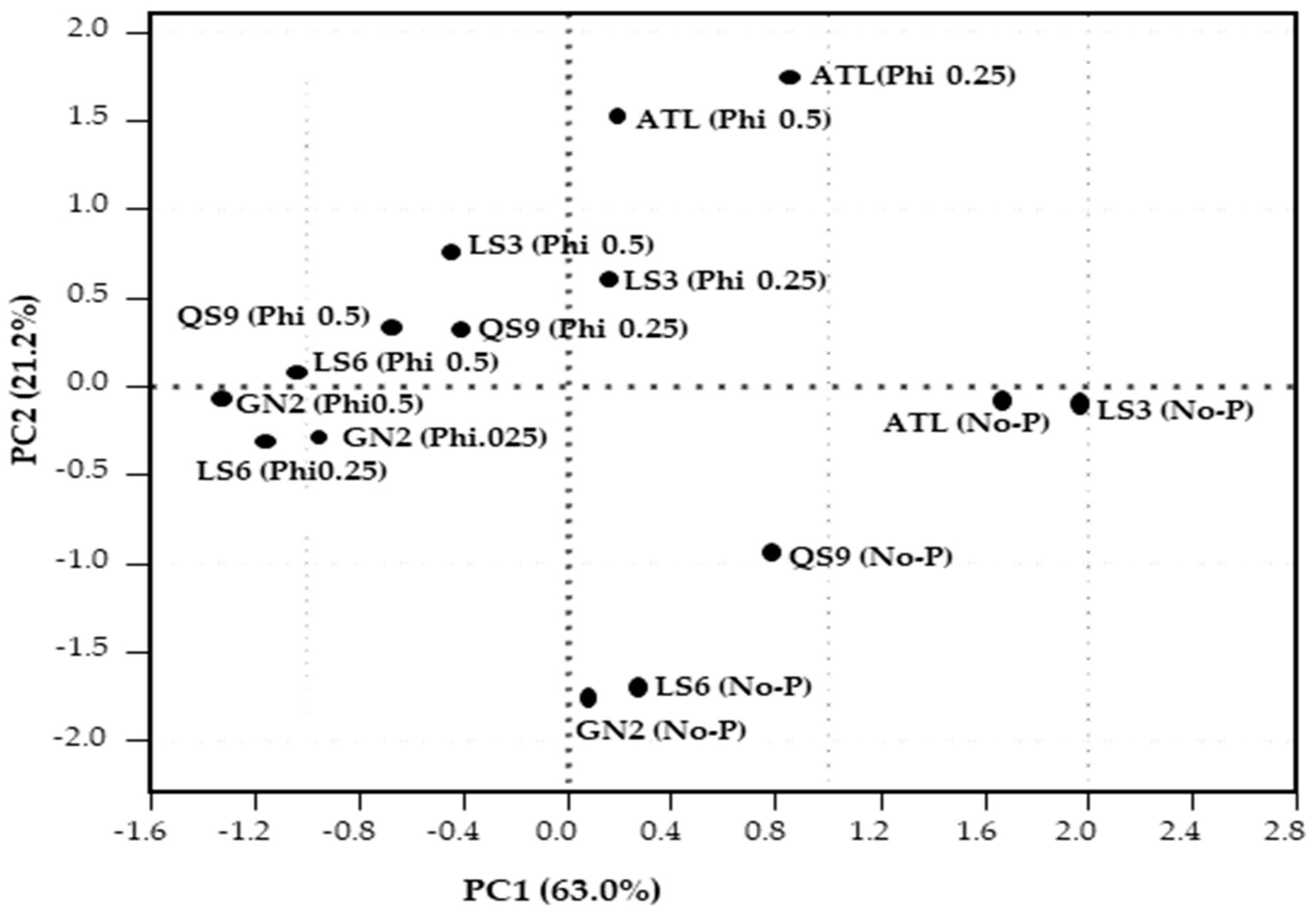
3.6. Relationships between Root Morphological Characteristics, Fresh Biomass and Biochemical Responses of the Five Potato Genotypes under Phi Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kalayu, G. Phosphate solubilizing microorganisms: Promising approach as biofertilizers. Int. J. Agron. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Achary, V.M.M.; Ram, B.; Manna, M.; Datta, D.; Bhatt, A.; Reddy, M.K.; Agrawal, P.K. Phosphite: A novel P fertilizer for weed management and pathogen control. Plant Biotech. J. 2017, 15, 1493–1508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manna, M.; Achary, V.M.M.; Islam, T.; Agrawal, P.K.; Reddy, M.K. The development of a phosphite-mediated fertilization and weed control system for rice. Sci. Rep. 2016, 6, 24941. [Google Scholar] [CrossRef] [PubMed]
- Ávila, F.W.; Faquin, V.; da Silva Lobato, A.K.; Ávila, P.A.; Marques, D.J.; Silva Guedes, E.M.; Tan, D.K.Y. Effect of phosphite supply in nutrient solution on yield, phosphorus nutrition and enzymatic behavior in common bean (‘Phaseolus vulgaris’ L.) Plants. Aust. J. Crop Sci. 2013, 7, 713. [Google Scholar]
- Heuer, S.; Gaxiola, R.; Schilling, R.; Herrera-Estrella, L.; López-Arredondo, D.; Wissuwa, M.; Delhaize, E.; Rouached, H. Improving phosphorus use efficiency: A complex trait with emerging opportunities. Plant J. 2017, 90, 868–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Yang, Z.; Zhong, X.; Xu, C.; Lin, Y.; Fan, Y.; Wang, M.; Chen, G.; Yang, Y. Decreases in soil P availability are associated with soil organic P declines following forest conversion in subtropical China. CATENA 2021, 205, 105459. [Google Scholar] [CrossRef]
- Brödlin, D.; Kaiser, K.; Kessler, A.; Hagedorn, F. Drying and rewetting foster phosphorus depletion of forest soils. Soil Biol. Biochem. 2019, 128, 22–34. [Google Scholar] [CrossRef]
- Gianessi, L.P. The increasing importance of herbicides in worldwide crop production. Pest Manag. Sci. 2013, 69, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.A.; Hawkesford, M.J. Food security: Increasing yield and improving resource use efficiency. Proc. Nutr. Soc. 2010, 69, 592–600. [Google Scholar] [CrossRef] [Green Version]
- Haque, S.E. How Effective Are Existing Phosphorus Management Strategies in Mitigating Surface Water Quality Problems in the US? Sustainability 2021, 13, 6565. [Google Scholar] [CrossRef]
- Peng, L.; Dai, H.; Wu, Y.; Peng, Y.; Lu, X. A comprehensive review of the available media and approaches for phosphorus recovery from wastewater. Water Air Soil Pollut. 2018, 229, 1–28. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in action: Plants resistant to herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asaduzzaman, M.; Pratley, J.E.; Luckett, D.; Lemerle, D.; Wu, H. Weed management in canola (Brassica napus L): A review of current constraints and future strategies for Australia. Archives Agron. Soil Sci. 2020, 66, 427–444. [Google Scholar] [CrossRef]
- Guo, M.; Li, B.; Xiang, Q.; Wang, R.; Liu, P.; Chen, Q. Phosphite translocation in soybean and mechanisms of Phytophthora sojae inhibition. Pest. Biochem. Physiol. 2021, 172, 104757. [Google Scholar] [CrossRef] [PubMed]
- García-Gaytán, V.; Hernández-Mendoza, F.; Coria-Téllez, A.V.; García-Morales, S.; Sánchez-Rodríguez, E.; Rojas-Abarca, L.; Daneshvar, H. Fertigation: Nutrition, stimulation and bioprotection of the root in high performance. Plants 2018, 7, 88. [Google Scholar] [CrossRef] [Green Version]
- McDonald, A.E.; Grant, B.R.; Plaxton, W.C. Phosphite (phosphorous acid): Its relevance in the environment and agriculture and influence on plant phosphate starvation response. J. Plant Nutr. 2001, 24, 1505–1519. [Google Scholar] [CrossRef]
- Solis-Palacios, R.; Hernández-Ramírez, G.; Salinas-Ruiz, J.; Hidalgo-Contreras, J.V.; Gómez-Merino, F.C. Effect and Compatibility of Phosphite with Trichoderma sp. Isolates in the Control of the Fusarium Species Complex Causing Pokkah Boeng in Sugarcane. Agronomy 2021, 11, 1099. [Google Scholar] [CrossRef]
- Cerqueira, A.; Alves, A.; Berenguer, H.; Correia, B.; Gómez-Cadenas, A.; Diez, J.J.; Monteiro, P.; Pinto, G. Phosphite shifts physiological and hormonal profile of Monterey pine and delays Fusarium circinatum progression. Plant Physiol. Biochem. 2017, 114, 88–99. [Google Scholar] [CrossRef]
- Rampersad, S.N. Pathogenomics and management of Fusarium diseases in plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Olivieri, F.P.; Feldman, M.L.; Machinandiarena, M.F.; Lobato, M.C.; Caldiz, D.O.; Daleo, G.R.; Andreu, A.B. Phosphite applications induce molecular modifications in potato tuber periderm and cortex that enhance resistance to pathogens. Crop Protect. 2012, 32, 1–6. [Google Scholar] [CrossRef]
- Silva, O.; Santos, H.; Dalla Pria, M.; May-De Mio, L. Potassium phosphite for control of downy mildew of soybean. Crop Protect. 2011, 30, 598–604. [Google Scholar] [CrossRef]
- Huang, Z.; Carter, N.; Lu, H.; Zhang, Z.; Wang-Pruski, G. Translocation of phosphite encourages the protection against Phytophthora infestans in potato: The efficiency and efficacy. Pest. Biochem. Physiol. 2018, 152, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Ghahremani, M.; Pérez-Fernández, M.; Tan, M.; Schläpfer, P.; Plaxton, W.C.; Uhrig, R.G. Phosphate and phosphite have a differential impact on the proteome and phosphoproteome of Arabidopsis suspension cell cultures. Plant J. 2021, 105, 924–941. [Google Scholar] [CrossRef] [PubMed]
- Thao, H.T.B.; Yamakawa, T. Phosphite (phosphorous acid): Fungicide, fertilizer or bio-stimulator? Soil Sci. Plant Nutr. 2009, 55, 228–234. [Google Scholar] [CrossRef]
- César Bachiega Zambrosi, F.; Mattos, D.J.; Syvertsen, J.P. Plant growth, leaf photosynthesis, and nutrient-use efficiency of citrus rootstocks decrease with phosphite supply. J. Plant Nutr. Soil Sci. 2011, 174, 487–495. [Google Scholar] [CrossRef]
- Xi, Y.; Han, X.; Zhang, Z.; Joshi, J.; Borza, T.; Aqa, M.M.; Zhang, B.; Yuan, H.; Wang-Pruski, G. Exogenous phosphite application alleviates the adverse effects of heat stress and improves thermotolerance of potato (Solanum tuberosum L.) seedlings. Ecotoxicol. Environ. Saf. 2020, 190, 110048. [Google Scholar] [CrossRef]
- Leong, S.J.; Lu, W.-C.; Chiou, T.-J. Phosphite-mediated suppression of anthocyanin accumulation regulated by mitochondrial ATP synthesis and sugars in Arabidopsis. Plant Cell Physiol. 2018, 59, 1158–1169. [Google Scholar] [CrossRef]
- Trejo-Téllez, L.I.; Estrada-Ortiz, E.; Gómez-Merino, F.C.; Becker, C.; Krumbein, A.; Schwarz, D. Flavonoid, nitrate and glucosinolate concentrations in Brassica species are differentially affected by photosynthetically active radiation, phosphate and phosphite. Front. Plant Sci. 2019, 10, 371. [Google Scholar] [CrossRef] [PubMed]
- Burra, D.D. Defence Related Molecular Signalling in Potato. Acta Univ. Agric. Sueciae 2016, 7, 1652–6880. [Google Scholar]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Biostimulant activity of phosphite in horticulture. Sci. Hort. 2015, 196, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Xi, Y.; Zhang, Z.; Mohammadi, M.A.; Joshi, J.; Borza, T.; Wang-Pruski, G. Effects of phosphite as a plant biostimulant on metabolism and stress response for better plant performance in Solanum tuberosum. Ecotoxicol. Environ. Saf. 2021, 210, 111873. [Google Scholar] [CrossRef]
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. Conventional and novel uses of phosphite in horticulture: Potentialities and challenges. Italus Hortus 2016, 23, 1–13. [Google Scholar]
- Quandahor, P.; Lin, C.; Gou, Y.; Coulter, J.A.; Liu, C. Leaf morphological and biochemical responses of three potato (Solanum tuberosum L.) cultivars to drought stress and aphid (Myzus persicae Sulzer) infestation. Insects 2019, 10, 435. [Google Scholar] [CrossRef] [Green Version]
- Jia, H.; Wang, Q.; Tao, Q.; Xue, W. Analysis and circuit implementation for the fractional-order Chen system. In Proceedings of the 8th CHAOS Conference Proceedings, Paris, France, 26–29 May 2015. [Google Scholar]
- Cabello, R.; De Mendiburu, F.; Bonierbale, M.; Monneveux, P.; Roca, W.; Chujoy, E. Large-scale evaluation of potato improved varieties, genetic stocks and landraces for drought tolerance. Am. J. Potato Res. 2012, 89, 400–410. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Wilkins, D. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Dawuda, M.M.; Liao, W.; Hu, L.; Yu, J.; Xie, J.; Calderón-Urrea, A.; Jin, X.; Wu, Y. Root tolerance and biochemical response of Chinese lettuce (Lactuca sativa L.) genotypes to cadmium stress. PeerJ 2019, 7, e7530. [Google Scholar] [CrossRef] [Green Version]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Analyt. Chem. 2014, 5, 730. [Google Scholar] [CrossRef] [Green Version]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Pocketbook, F.S. World Food and Agriculture; Food and Agriculture Organization: Rome, Italy, 2015. [Google Scholar]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotech. 2005, 16, 123–132. [Google Scholar] [CrossRef]
- Zhu, J.-K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Ticconi, C.A.; Delatorre, C.A.; Abel, S. Attenuation of phosphate starvation responses by phosphite in Arabidopsis. Plant Physiol. 2001, 127, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Ram, B.; Fartyal, D.; Sheri, V.; Varakumar, P.; Borphukan, B.; James, D.; Yadav, R.; Bhatt, A.; Agrawal, P.K.; Achary, V.M.M. Characterization of phoA, a Bacterial Alkaline Phosphatase for Phi Use Efficiency in Rice Plant. Front. Plant Sci. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plaxton, W.C.; Carswell, M.C. Metabolic aspects of the phosphate starvation response in plants. In Plant Responses to Environmental Stresses; Routledge: Oxfordshire, UK, 2018; pp. 349–372. [Google Scholar]
- Carswell, M.C.; Grant, B.R.; Plaxton, W.C. Disruption of the phosphate-starvation response of oilseed rape suspension cells by the fungicide phosphonate. Planta 1997, 203, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Vinas, M.; Mendez, J.C.; Jiménez, V.M. Effect of foliar applications of phosphites on growth, nutritional status and defense responses in tomato plants. Sci. Hort. 2020, 265, 109200. [Google Scholar] [CrossRef]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the roots. Annu. Plant Rev. 2015, 48, 3–22. [Google Scholar]
- Devaiah, B.N.; Karthikeyan, A.S.; Raghothama, K.G. WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis. Plant Physiol. 2007, 143, 1789–1801. [Google Scholar] [CrossRef] [Green Version]
- Correa, J.; Postma, J.A.; Watt, M.; Wojciechowski, T. Soil compaction and the architectural plasticity of root systems. J. Exp. Bot. 2019, 70, 6019–6034. [Google Scholar] [CrossRef]
- Vanhees, D.J.; Loades, K.W.; Bengough, A.G.; Mooney, S.J.; Lynch, J.P. The ability of maize roots to grow through compacted soil is not dependent on the amount of roots formed. Field Crop. Res. 2021, 264, 108013. [Google Scholar] [CrossRef]
- Polania, J.; Rao, I.M.; Cajiao, C.; Grajales, M.; Rivera, M.; Velasquez, F.; Raatz, B.; Beebe, S.E. Shoot and root traits contribute to drought resistance in recombinant inbred lines of MD 23–24× SEA 5 of common bean. Front. Plant Sci. 2017, 8, 296. [Google Scholar] [CrossRef]
- Varadarajan, D.K.; Karthikeyan, A.S.; Matilda, P.D.; Raghothama, K.G. Phosphite, an analog of phosphate, suppresses the coordinated expression of genes under phosphate starvation. Plant Physiol. 2002, 129, 1232–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, T.M.; Tsai, P.F.; Shyu, Y.T.; Sheu, F. The Effects of Phosphite on Phosphate Starvation Responses of Ulva Lactuca (Ulvales, Chlorophyta) 1. J. Phycol. 2005, 41, 975–982. [Google Scholar] [CrossRef]
- Schroetter, S.; Angeles-Wedler, D.; Kreuzig, R.; Schnug, E. Effects of phosphite on phosphorus supply and growth of corn (Zea mays). Landbauforsch. Volkenrode 2006, 56, 87. [Google Scholar]
- Thao, H.T.B.; Yamakawa, T.; Myint, A.K.; Sarr, P.S. Effects of phosphite, a reduced form of phosphate, on the growth and phosphorus nutrition of spinach (Spinacia oleracea L.). Soil Sci. Plant Nutrit. 2008, 54, 761–768. [Google Scholar] [CrossRef] [Green Version]
- Avila, F.W.; Faquin, V.; Araujo, J.L.; Marques, D.J.; Júnior, P.M.R.; da Silva Lobato, A.K.; Ramos, S.J.; Baliza, D.P. Phosphite supply affects phosphorus nutrition and biochemical responses in maize plants. Aust. J. Crop Sci. 2011, 5, 646–653. [Google Scholar]
- Zambrosi, F.C.B.; Mattos, D., Jr.; Quaggio, J.A.; Cantarella, H.; Boaretto, R.M. Phosphorus uptake by young citrus trees in low-P soil depends on rootstock varieties and nutrient management. Commun. Soil Sci. Plant Anal. 2013, 44, 2107–2117. [Google Scholar] [CrossRef]
- Hirosse, E.H.; Creste, J.E.; Custódio, C.C.; Machado-Neto, N.B. In vitro growth of sweet potato fed with potassium phosphite. Acta Scient. Agron. 2012, 34, 85–91. [Google Scholar] [CrossRef]
- Balemi, T.; Negisho, K. Management of soil phosphorus and plant adaptation mechanisms to phosphorus stress for sustainable crop production: A review. J. Soil Sci. Plant Nutrit. 2012, 12, 547–562. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Lambers, H.; Clode, P.L.; Hawkins, H.J.; Laliberté, E.; Oliveira, R.S.; Reddell, P.; Shane, M.W.; Stitt, M.; Weston, P. Metabolic adaptations of the non-mycotrophic Proteaceae to soils with low phosphorus availability. Annu. Plant Rev. Phosphorus Metab. Plants 2015, 48, 289. [Google Scholar]
- Wang, Y.; Yang, R.; Zheng, J.; Shen, Z.; Xu, X. Exogenous foliar application of fulvic acid alleviate cadmium toxicity in lettuce (Lactuca sativa L.). Ecotoxico. Environ. Saf. 2019, 167, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Meena, S.K.; Krishnapriya, V.; Ahmad, A.; Kishora, N. Root carboxylate exudation capacity under phosphorus stress does not improve grain yield in green gram. Plant Cell Rep. 2014, 33, 919–928. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 2004, 260, 47–57. [Google Scholar] [CrossRef]
- Wu, P.; Ma, X.; Tigabu, M.; Wang, C.; Liu, A.; Oden, P.C. Root morphological plasticity and biomass production of two Chinese fir clones with high phosphorus efficiency under low phosphorus stress. Can. J. For. Res. 2011, 41, 228–234. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, V.P.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol. Biochem. 2017, 110, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, H.; Wang, G.; Xu, L.; Shen, Z. Cadmium-induced accumulation of hydrogen peroxide in the leaf apoplast of Phaseolus aureus and Vicia sativa and the roles of different antioxidant enzymes. J. Haz. Mat. 2009, 168, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Oyarburo, N.S.; Machinandiarena, M.F.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B.; Olivieri, F.P. Potassium phosphite increases tolerance to UV-B in potato. Plant Physiol. Biochem. 2015, 88, 1–8. [Google Scholar] [CrossRef]
- Ramos, S.; Faquin, V.; Guilherme, L.; Castro, E.; Ávila, F.; Carvalho, G.; Bastos, C.; Oliveira, C. Selenium biofortification and antioxidant activity in lettuce plants fed with selenate and selenite. Plant Soil Environ. 2010, 56, 584–588. [Google Scholar] [CrossRef] [Green Version]

| Genotype | Biomass Accumulation (g) | Root/Shoot Ratio | * BTI (%) | |||
|---|---|---|---|---|---|---|
| Treatment | Fresh Root Weight | Fresh Shoot Weight | Fresh Plant Weight | |||
| Qingshu9 | Con.Pi 0.5 | 0.087 ± 0.002 a | 0.156 ± 0.001 a | 0.243 ± 0.003 ab | 0.560 ± 0.006 ef | 100 |
| Pi0.25 mM | 0.067 ± 0.004 b | 0.143 ± 0.0008 a | 0.210 ± 0.004 bc | 0.467 ± 0.031 fgh | 96.42 | |
| No-P | 0.033 ± 0.001 ef | 0. 054 ± 0.0008 c | 0. 087 ± 0.002 fg | 0.611 ± 0.019 de | 35.80 | |
| Phi0.25 mM | 0.019 ± 0.004 gh | 0.064 ± 0.001 bc | 0.083 ± 0.003 fg | 0.297 ± 0.072 jk | 30.04 | |
| Phi 0.5 mM | 0.018 ± 0.006 gh | 0.065 ± 0.001 bc | 0.083 ± 0.006 g | 0.277 ± 0.085 jk | 30.04 | |
| Longshu6 | Control | 0.087 ± 0.0006 a | 0.154 ± 0.002 a | 0.241 ± 0.002 ab | 0.565 ± 0.015 ef | 100 |
| Pi0.25 mM | 0.097 ± 0.0006 a | 0.157 ± 0.001 a | 0.254 ± 0.001 a | 0.618 ± 0.007 de | 105.39 | |
| No-P | 0.025 ± 0.001 fg | 0.062 ± 0.003 c | 0.087 ± 0.003 fg | 0.403 ± 0.033 ghi | 36.51 | |
| Phi0.25 mM | 0.014 ± 0.0006 gh | 0.054 ± 0.0006 c | 0.068 ± 0.0006 g | 0.259 ± 0.015 k | 27.80 | |
| Phi 0.5 mM | 0.026 ± 0.001 fg | 0.057 ± 0.001 c | 0.083 ± 0.001 g | 0.456 ± 0.018 fghi | 34.44 | |
| Longshu3 | Control | 0.048 ± 0.001 cd | 0.147 ± 0.001 c | 0.195 ± 0.002 c | 0.327 ± 0.001 jk | 100 |
| Pi0.25 mM | 0.085 ± 0.0006 a | 0.089 ± 0.0006 b | 0.174 ± 0.000 cd | 0.955 ± 0.013 b | 89.23 | |
| No-P | 0.067 ± 0.002 b | 0.055 ± 0.001 c | 0.122 ± 0.003 ef | 1.218 ± 0.007 a | 63.08 | |
| Phi0.25 mM | 0.037 ± 0.0006 def | 0.064 ± 0.0006 bc | 0.101 ± 0.001 fg | 0.578 ± 0.002 ef | 57.95 | |
| Phi 0.5 mM | 0.024 ± 0.001 fg | 0.048 ± 0.002 c | 0.072 ± 0.001 g | 0.500 ± 0.037 efg | 36.92 | |
| Atlantic | Control | 0.054 ± 0.0006 bc | 0.148 ± 0.0006 a | 0.202 ± 0.000 c | 0.365 ± 0.005 hij | 100 |
| Pi 0.25 mM | 0.067 ± 0.001 b | 0.135 ± 0.001 a | 0.202 ± 0.0006 c | 0.496 ± 0.012 fg | 100 | |
| No-P | 0.057 ± 0.001 bc | 0.058 ± 0.0006 c | 0.115 ± 0.002 ef | 0.983 ± 0.019 b | 56.93 | |
| Phi0.25 mM | 0.058 ± 0.0006 bc | 0.084 ± 0.0006 b | 0.142 ± 0.0006 de | 0.690 ± 0.010 d | 69.80 | |
| Phi 0.5 mM | 0.046 ± 0.001 cde | 0.058 ± 0.001 c | 0.104 ± 0.002 efg | 0.793 ± 0.005 d | 51.48 | |
| Gannong2 | Control | 0.063 ± 0.001 b | 0.137 ± 0.001 a | 0.200 ± 0.001 c | 0.460 ± 0.006 fghi | 100 |
| Pi0.25 mM | 0.067 ± 0.001 b | 0.135 ± 0.001 a | 0.202 ± 0.0006 c | 0.496 ± 0.012 fg | 101.00 | |
| No-P | 0.024 ± 0.001 fg | 0.044 ± 0.001 cd | 0.068 ± 0.001 g | 0.545 ± 0.031 ef | 34.00 | |
| Phi0.25 mM | 0.016 ± 0.0006 fg | 0.052 ± 0.001 c | 0.078 ± 0.001 g | 0.500 ± 0.032 efg | 18.00 | |
| Phi0.5 mM | 0.008 ± 0.001 h | 0.022 ± 0.003 d | 0.039 ± 0.003 h | 0.350 ± 0.052 ijk | 19.50 | |
| Index | TRL | RPA | RSA | RV | NRT | NRF | FRW | FSW | TPW |
|---|---|---|---|---|---|---|---|---|---|
| MDA | −0.87 ** | −0.77 ** | −0.81 ** | −0.74 ** | −0.78 ** | −0.78 ** | −0.67 * | −0.75 ** | −0.84 ** |
| H2O2 | −0.94 ** | −0.90 ** | −0.92 ** | −0.86 ** | −0.90 ** | −0.91 ** | −0.75 ** | −0.79 ** | −0.92 ** |
| CAT | −0.80 ** | −0.71 ** | −0.74 ** | −0.68 ** | −0.70 ** | −0.70 ** | −0.62 * | −0.74 ** | −0.81 ** |
| SOD | −0.83 ** | −0.71 ** | −0.75 ** | −0.71 ** | −0.71 ** | −0.73 ** | −0.69 * | −0.72 ** | −0.78 ** |
| POD | −0.87 ** | −0.77 ** | −0.82 ** | −0.75 ** | −0.77 ** | −0.80 ** | −0.69 * | −0.76 ** | −0.85 ** |
| Pro | −0.89 ** | −0.80 ** | −0.84 ** | −0.77 ** | −0.80 ** | −0.82 ** | −0.72 ** | −0.81 ** | −0.89 ** |
| Principal Component | PC 1 | PC 2 |
|---|---|---|
| Total root length | 0.323 | 0.098 |
| Root projected area | 0.284 | 0.234 |
| Root surface area | 0.290 | 0.235 |
| Root volume | 0.260 | 0.224 |
| Number of root tips | 0.259 | 0.266 |
| Number of root forks | 0.270 | 0.279 |
| Fresh root weight | 0.298 | 0.044 |
| Fresh root/shoot ratio | 0.295 | 0.095 |
| Malonaldehyde | −0.232 | 0.353 |
| Hydrogen peroxide | −0.319 | 0.044 |
| Catalase | −0.162 | 0.409 |
| Superoxide dismutase | −0.229 | 0.374 |
| Peroxidases | −0.238 | 0.348 |
| Proline | −0.245 | 0.339 |
| Eigenvalue | 8.818 | 3.805 |
| Contribution rate % | 62.98 | 27.18 |
| Cumulative contribution rate % | 62.98 | 90.16 |
| Genotype | Treatment | CI(1) | CI(2) | µ(X1) | µ(X2) | Comprehensive Assessment Value (D) | Ranking |
|---|---|---|---|---|---|---|---|
| Qingshu9 | No-P | 2.394 | −1.940 | 0.639 | 0.235 | 0.517 | |
| Phi 0.25 | −1.252 | 0.648 | 0.277 | 0.597 | 0.373 | ||
| Phi 0.5 | −2.079 | 0.689 | 0.195 | 0.603 | 0.318 | 3 | |
| Longshu6 | No-P | 0.832 | −3.531 | 0.484 | 0.013 | 0.342 | |
| Phi 0.25 | −3.567 | −0.622 | 0.047 | 0.419 | 0.159 | ||
| Phi 0.5 | −3.172 | 0.169 | 0.086 | 0.539 | 0.220 | 4 | |
| Longshu3 | No-P | 6.034 | −0.266 | 1.000 | 0.469 | 0.849 | |
| Phi 0.25 | 0.474 | 1.228 | 0.448 | 0.678 | 0.517 | ||
| Phi 0.5 | −1.384 | 1.539 | 0.264 | 0.721 | 0.402 | 2 | |
| Atlantic | No-P | 5.160 | −0.213 | 0.913 | 0.477 | 0.782 | |
| Phi 0.25 | 2.649 | 3.536 | 0.663 | 1.000 | 0.765 | ||
| Phi 0.5 | 0.589 | 3.088 | 0.469 | 0.937 | 0.604 | 1 | |
| Gannong2 | No-P | 0.249 | −3.627 | 0.426 | 0.000 | 0.297 | |
| Phi 0.25 | −2.876 | −0.582 | 0.116 | 0.425 | 0.209 | ||
| Phi 0.5 | −4.043 | −0.117 | 0.000 | 0.499 | 0.148 | 5 | |
| Weight | 0.699 | 0.301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dormatey, R.; Sun, C.; Ali, K.; Qin, T.; Xu, D.; Bi, Z.; Bai, J. Influence of Phosphite Supply in the MS Medium on Root Morphological Characteristics, Fresh Biomass and Enzymatic Behavior in Five Genotypes of Potato (Solanum tuberosum L.). Horticulturae 2021, 7, 265. https://doi.org/10.3390/horticulturae7090265
Dormatey R, Sun C, Ali K, Qin T, Xu D, Bi Z, Bai J. Influence of Phosphite Supply in the MS Medium on Root Morphological Characteristics, Fresh Biomass and Enzymatic Behavior in Five Genotypes of Potato (Solanum tuberosum L.). Horticulturae. 2021; 7(9):265. https://doi.org/10.3390/horticulturae7090265
Chicago/Turabian StyleDormatey, Richard, Chao Sun, Kazim Ali, Tianyuan Qin, Derong Xu, Zhenzhen Bi, and Jiangping Bai. 2021. "Influence of Phosphite Supply in the MS Medium on Root Morphological Characteristics, Fresh Biomass and Enzymatic Behavior in Five Genotypes of Potato (Solanum tuberosum L.)" Horticulturae 7, no. 9: 265. https://doi.org/10.3390/horticulturae7090265
APA StyleDormatey, R., Sun, C., Ali, K., Qin, T., Xu, D., Bi, Z., & Bai, J. (2021). Influence of Phosphite Supply in the MS Medium on Root Morphological Characteristics, Fresh Biomass and Enzymatic Behavior in Five Genotypes of Potato (Solanum tuberosum L.). Horticulturae, 7(9), 265. https://doi.org/10.3390/horticulturae7090265








