Investigating Evolution and Balance of Grape Sugars and Organic Acids in Some New Pathogen-Resistant White Grapevine Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Treatment Layout
2.2. Phenological Stages, Vegetative Growth, and Yield Components
2.3. Grape Composition
2.4. HPLC Analysis
2.5. Statistical Analysis
3. Results
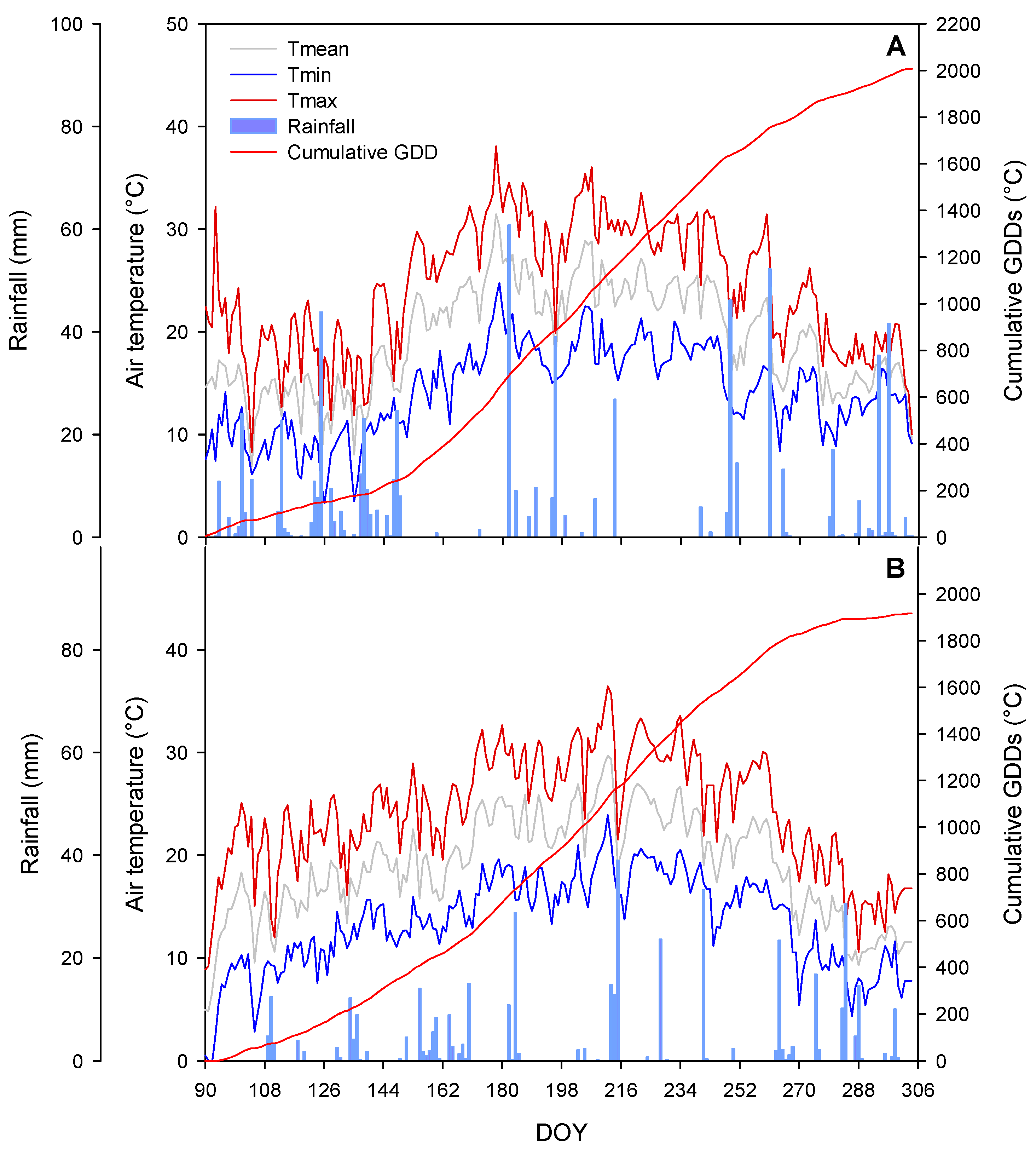
3.1. Weather Conditions and Phenology
3.2. Vegetative Growth and Shoot Fruitfulness
3.3. Yield, Bunch Morphology, and Vine Balance
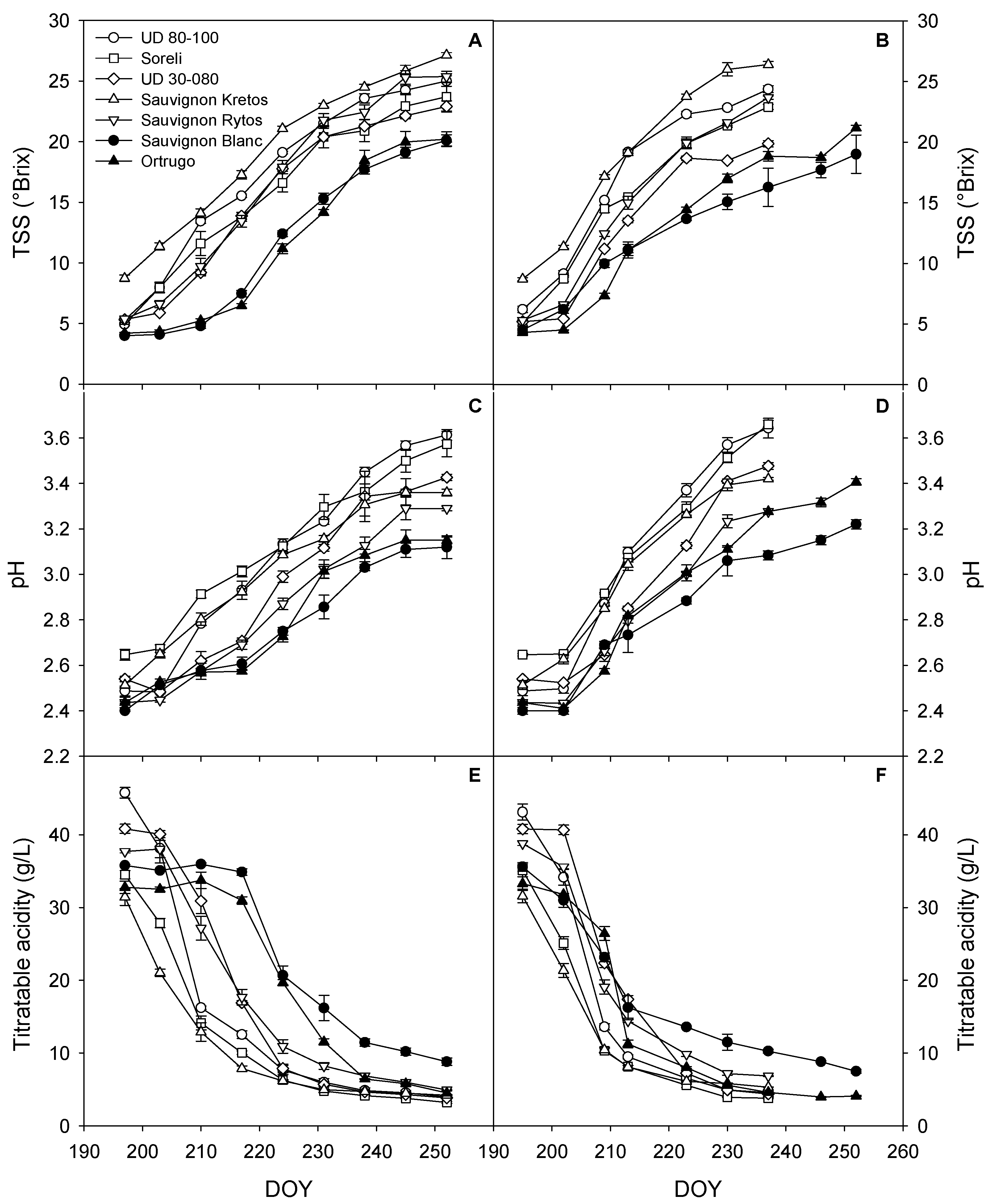
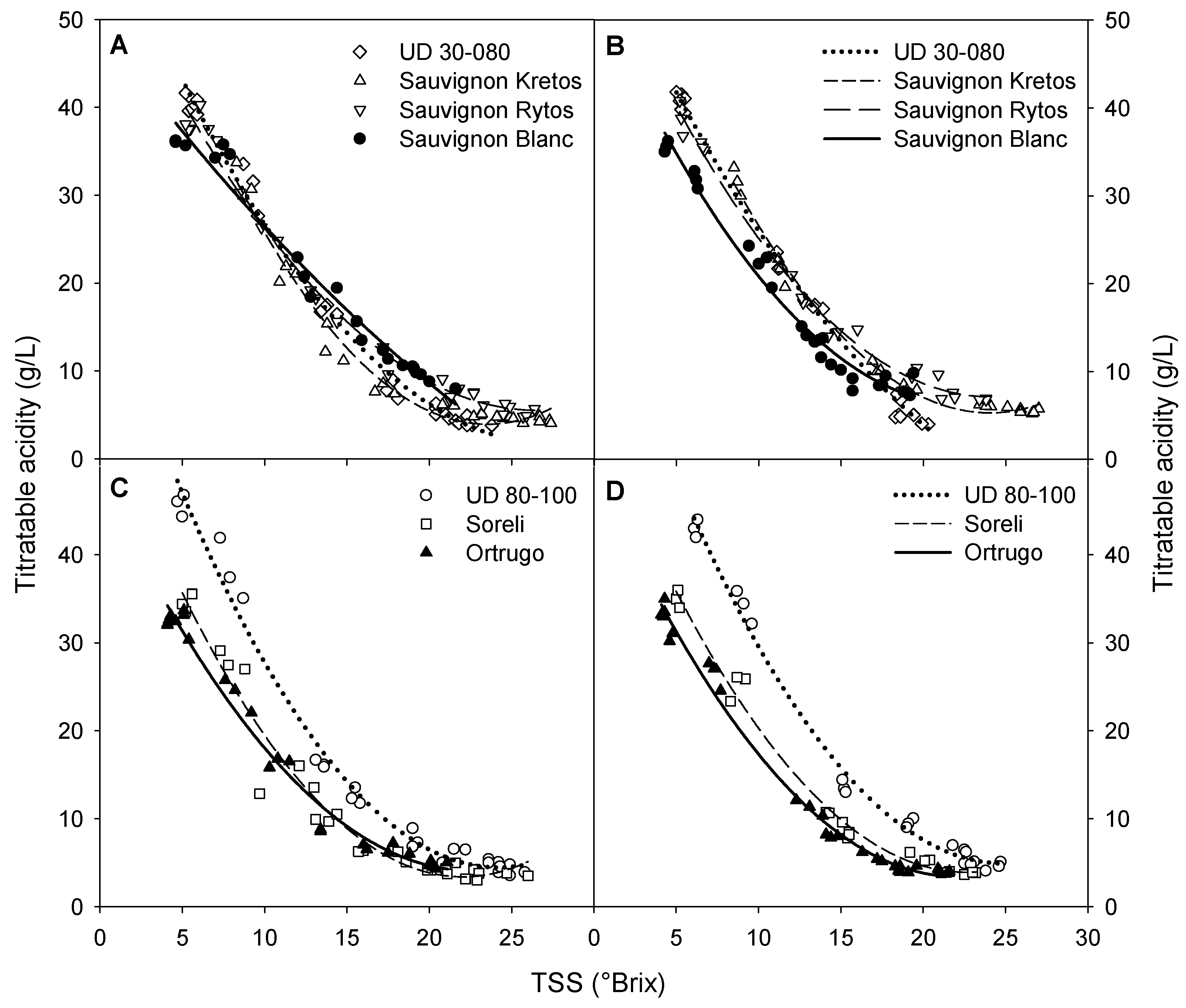
3.4. Grapes’ TSS, pH, and TA during Ripening
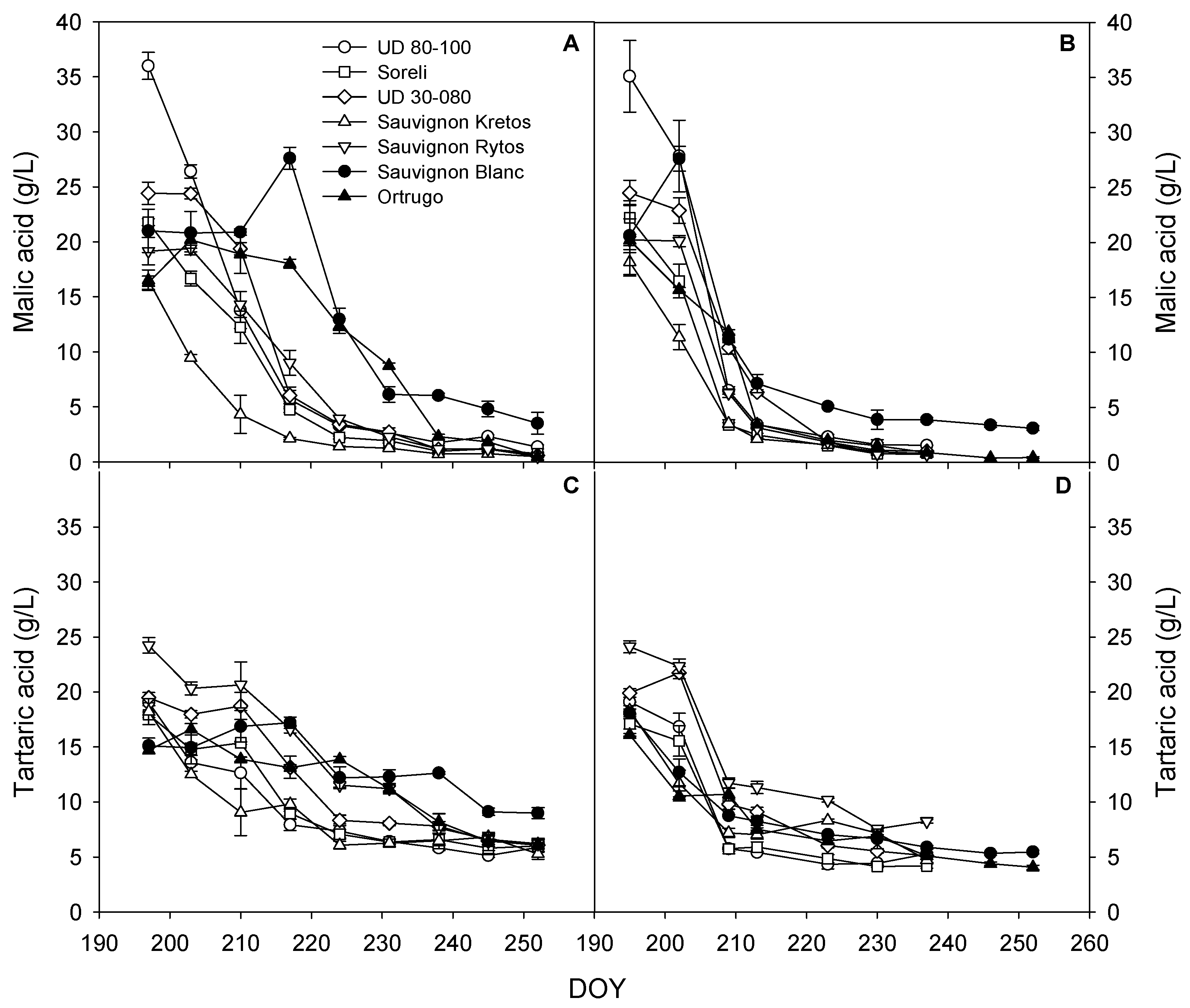
3.5. Trends for Grapes Organic Acids Concentration
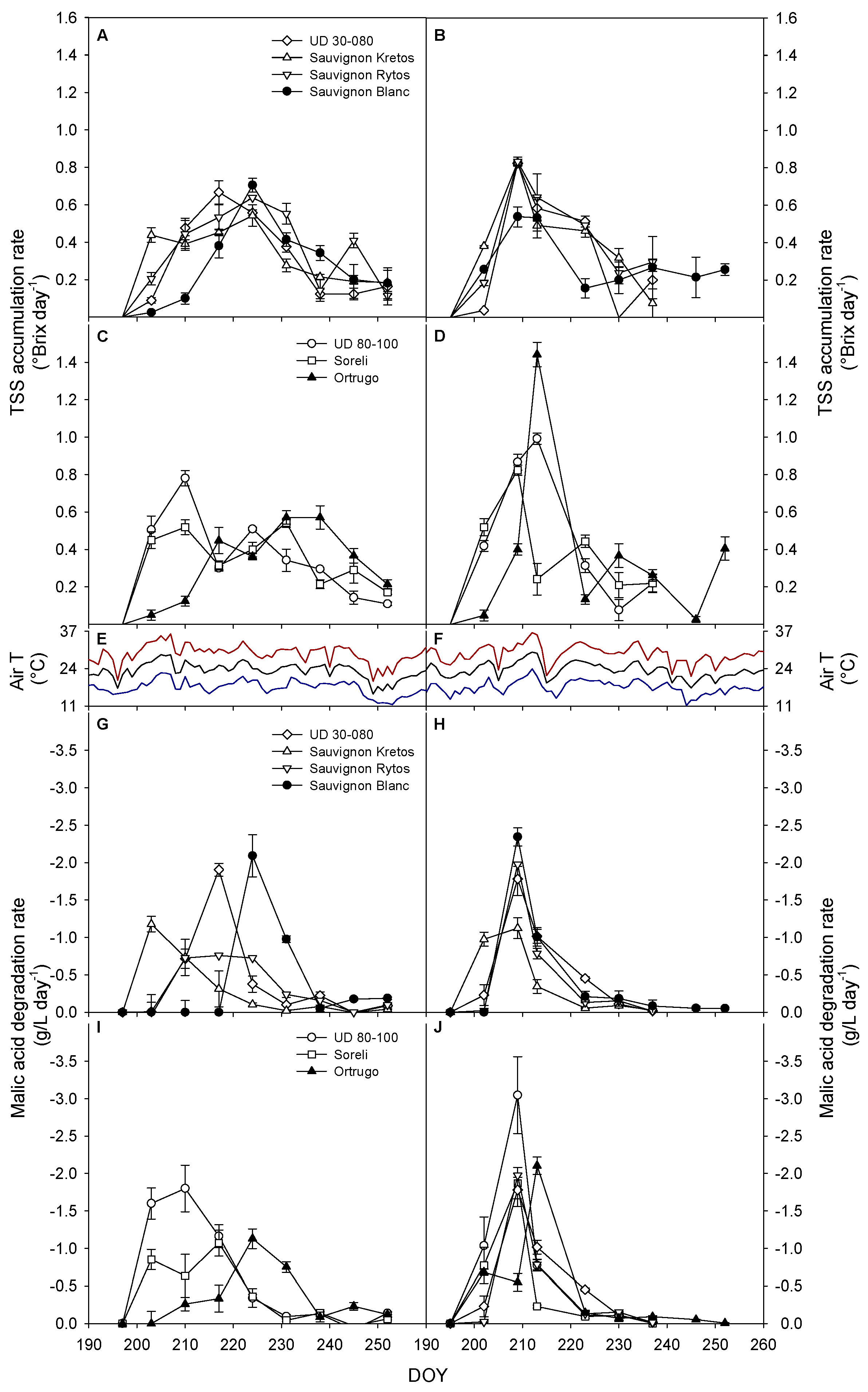
3.6. Sugar Accumulation and Malic Acid Degradation Rates
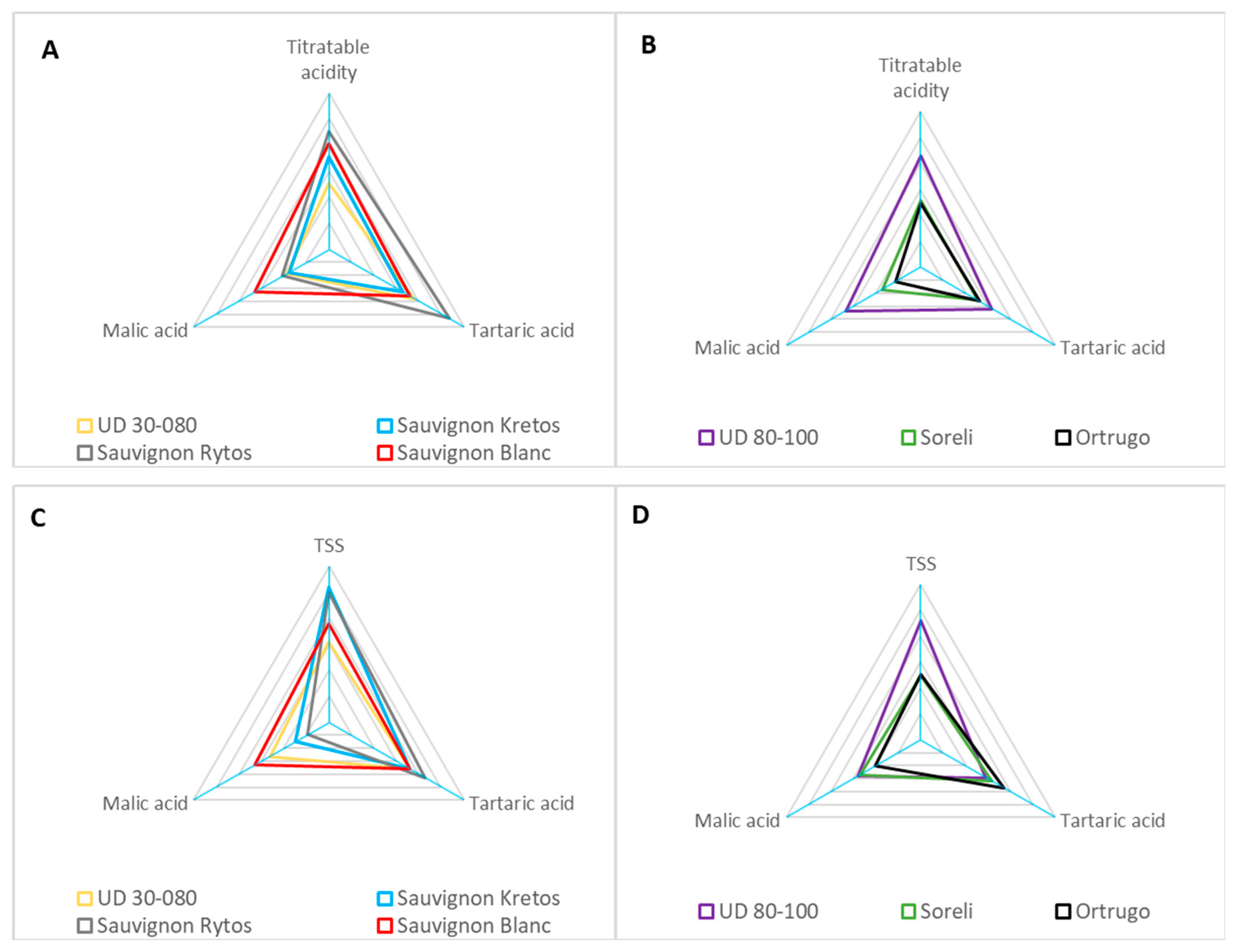
3.7. Fruit Composition at Harvest
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A.; et al. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38, 66. [Google Scholar] [CrossRef]
- Duchêne, E.; Huard, F.; Dumas, V.; Schneider, C.; Merdinoglu, D. The challenge of adapting grapevine varieties to climate change. Clim. Res. 2010, 41, 193–204. [Google Scholar] [CrossRef]
- Moran, M.A.; Sadras, V.O.; Petrie, P.R. Late pruning and carry-over effects on phenology, yield components and berry traits in Shiraz. Aust. J. Grape Wine Res. 2017, 23, 390–398. [Google Scholar] [CrossRef]
- Frioni, T.; Bertoloni, G.; Squeri, C.; Garavani, A.; Ronney, L.; Poni, S.; Gatti, M. Biodiversity of Local Vitis vinifera L. Germplasm: A Powerful Tool Toward Adaptation to Global Warming and Desired Grape Composition. Front. Plant Sci. 2020, 11, 608. [Google Scholar] [CrossRef] [PubMed]
- Garrett, K.A.; Nita, M.; De Wolf, E.D.; Esker, P.D.; Gomez-Montano, L.; Sparks, A.H. Plant pathogens as indicators of climate change. In Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 499–513. [Google Scholar]
- Zambito Marsala, R.; Capri, E.; Russo, E.; Bisagni, M.; Colla, R.; Lucini, L.; Gallo, A.; Suciu, N.A. First evaluation of pesticides occurrence in groundwater of Tidone Valley, an area with intensive viticulture. Sci. Total Environ. 2020, 736, 139730. [Google Scholar] [CrossRef] [PubMed]
- Pedneault, K.; Provost, C. Fungus resistant grape varieties as a suitable alternative for organic wine production: Benefits, limits, and challenges. Sci. Hortic. 2016, 208, 57–77. [Google Scholar] [CrossRef]
- Töpfer, R.; Hausmann, L.; Harst, M.; Maul, E.; Zyprian, E.; Eibach, R. New horizons for grapevine breeding. Fruit Veg. Cereal Sci. Biotechnol. 2011, 5, 79–100. [Google Scholar]
- Montaigne, E.; Coelho, A.; Khefifi, L. Economic issues and perspectives on innovation in new resistant grapevine varieties in France. Wine Econ. Policy 2016, 5, 73–77. [Google Scholar] [CrossRef]
- Pertot, I.; Caffi, T.; Rossi, V.; Mugnai, L.; Hoffmann, C.; Grando, M.S.; Gary, C.; Lafond, D.; Duso, C.; Thiery, D.; et al. A critical review of plant protection tools for reducing pesticide use on grapevine and new perspectives for the implementation of IPM in viticulture. Crop Prot. 2017, 97, 70–84. [Google Scholar] [CrossRef]
- Bavaresco, L.; Gardiman, M.; Brancadoro, L.; Espen, L.; Failla, O.; Scienza, A.; Vezzulli, S.; Zulini, L.; Velasco, R.; Stefanini, M.; et al. Grapevine breeding programs in Italy. In Grapevine Breeding Programs for the Wine Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 135–157. ISBN 978-1-78242-080-4. [Google Scholar]
- Di Gaspero, G.; Foria, S. Molecular grapevine breeding techniques. In Grapevine Breeding Programs for the Wine Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 23–37. ISBN 978-1-78242-080-4. [Google Scholar]
- Vezzulli, S.; Dolzani, C.; Migliaro, D.; Banchi, E.; Stedile, T.; Zatelli, A.; Dallaserra, M.; Clementi, S.; Dorigatti, C.; Velasco, R.; et al. The Fondazione Edmund Mach grapevine breeding program for downy and powdery mildew resistances: Toward a green viticulture. Acta Hortic. 2019, 1248, 109–113. [Google Scholar] [CrossRef]
- Bove, F.; Bavaresco, L.; Caffi, T.; Rossi, V. Assessment of Resistance Components for Improved Phenotyping of Grapevine Varieties Resistant to Downy Mildew. Front. Plant Sci. 2019, 10, 1559. [Google Scholar] [CrossRef]
- Casagrande, K.; Falginella, L.; Castellarin, S.D.; Testolin, R.; Di Gaspero, G. Defence responses in Rpv3-dependent resistance to grapevine downy mildew. Planta 2011, 234, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Vezzulli, S.; Vecchione, A.; Stefanini, M.; Zulini, L. Downy mildew resistance evaluation in 28 grapevine hybrids promising for breeding programs in Trentino region (Italy). Eur. J. Plant Pathol. 2018, 150, 485–495. [Google Scholar] [CrossRef]
- Zanghelini, J.A.; Bogo, A.; Dal Vesco, L.L.; Gomes, B.R.; Mecabô, C.V.; Herpich, C.H.; Welter, L.J. Response of PIWI grapevine cultivars to downy mildew in highland region of southern Brazil. Eur. J. Plant Pathol. 2019, 154, 1051–1058. [Google Scholar] [CrossRef]
- Poni, S.; Chiari, G.; Caffi, T.; Bove, F.; Tombesi, S.; Moncalvo, A.; Gatti, M. Canopy physiology, vine performance and host-pathogen interaction in a fungi resistant cv. Sangiovese x Bianca accession vs. a susceptible clone. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Casanova-Gascón, J.; Ferrer-Martín, C.; Bernad-Eustaquio, A.; Elbaile-Mur, A.; Ayuso-Rodríguez, J.M.; Torres-Sánchez, S.; Jarne-Casasús, A.; Martín-Ramos, P. Behavior of vine varieties resistant to fungal diseases in the Somontano region. Agronomy 2019, 9, 738. [Google Scholar] [CrossRef]
- Zambon, Y.; Khavizova, A.; Colautti, M.; Georgofili, E.S. Varietà di Vite Resistenti Alle Malattie e Rame: Opportunità e Limiti. In Riflessioni Sull’uso del Rame per la Protezione Delle Piante; Accademia dei Georgofili, Quaderni 2019-III, Società Editrice Fiorentina: Firenze, Italy, 2020. [Google Scholar]
- Gratl, V.; Sturm, S.; Zini, E.; Letschka, T.; Stefanini, M.; Vezzulli, S.; Stuppner, H. Comprehensive polyphenolic profiling in promising resistant grapevine hybrids including 17 novel breeds in northern Italy. J. Sci. Food Agric. 2021, 101, 2380–2388. [Google Scholar] [CrossRef]
- Gelmetti, A.; Roman, T.; Bottura, M.; Stefanini, M.; Pedò, S. Performance agronomiche di viti resistenti. Primi risultati di un progetto poliennale. Vite e Vino 2019, 2, 54–61. [Google Scholar]
- Nicolini, G.; Barp, L.; Roman, T.; Larcher, R.; Malacarne, M.; Bottura, M.; Tait, F.; Battisti, F.; Mereles, M.S.; Battistella, R. Resistenti bianchi e rossi: Primi dati da esperienze trentine sulla concentrazione nei vini di shikimico e flavonoidi. L’Enologo 2018, 3, 89–93. [Google Scholar]
- Ruehl, E.; Schmid, J.; Eibach, R.; Töpfer, R. Grapevine breeding programmes in Germany. In Grapevine Breeding Programs for the Wine Industry; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 77–101. ISBN 978-1-78242-080-4. [Google Scholar]
- Frioni, T.; Green, A.; Emling, J.E.; Zhuang, S.; Palliotti, A.; Sivilotti, P.; Falchi, R.; Sabbatini, P. Impact of spring freeze on yield, vine performance and fruit quality of Vitis interspecific hybrid Marquette. Sci. Hortic. 2017, 219, 302–309. [Google Scholar] [CrossRef]
- Teissedre, P.L. Composition of grape and wine from resistant vine varieties. OENO One 2018, 52, 197–203. [Google Scholar] [CrossRef]
- Pedò, S.; Bottura, M.; Porro, D. Development, yield potential and nutritional aspects of resistant grapevine varieties in Trentino Alto Adige. BIO Web Conf. 2019, 13, 02004. [Google Scholar] [CrossRef][Green Version]
- Smith, M.S.; Centinari, M. Young grapevines exhibit interspecific differences in hydraulic response to freeze stress but not in recovery. Planta 2019, 250, 495–505. [Google Scholar] [CrossRef]
- Hed, B.; Centinari, M. Gibberellin application improved bunch rot control of vignoles grape, but response to mechanical defoliation varied between training systems. Plant Dis. 2021, 105, 339–345. [Google Scholar] [CrossRef]
- Tóth-Lencsés, A.K.; Kozma, P.; Szőke, A.; Kerekes, A.; Veres, A.; Kiss, E. Parentage analysis in Hungarian grapevine cultivars of “Seibel’-’Seyve-Villard” origin. Vitis 2015, 54, 27–29. [Google Scholar]
- Venuti, S.; Copetti, D.; Foria, S.; Falginella, L.; Hoffmann, S.; Bellin, D.; Cindrić, P.; Kozma, P.; Scalabrin, S.; Morgante, M.; et al. Historical Introgression of the Downy Mildew Resistance Gene Rpv12 from the Asian Species Vitis amurensis into Grapevine Varieties. PLoS ONE 2013, 8, e61228. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A. General Viticulture; University of California Press: Berkeley, CA, USA, 1965. [Google Scholar]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Gatti, M.; Garavani, A.; Krajecz, K.; Ughini, V.; Parisi, M.G.; Frioni, T.; Poni, S. Mechanical mid-shoot leaf removal on ortrugo (Vitis vinifera L.) at pre-or mid-veraison alters fruit growth and maturation. Am. J. Enol. Vitic. 2019, 70, 88–97. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf Area/Crop Weight Ratios of Grapevines: Influence on Fruit Composition and Wine Quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Poni, S.; Tombesi, S.; Palliotti, A.; Ughini, V.; Gatti, M. Mechanical winter pruning of grapevine: Physiological bases and applications. Sci. Hortic. 2016, 204, 88–98. [Google Scholar] [CrossRef]
- Jarvis, C.; Darbyshire, R.; Goodwin, I.; Barlow, E.W.R.; Eckard, R. Advancement of winegrape maturity continuing for winegrowing regions in Australia with variable evidence of compression of the harvest period. Aust. J. Grape Wine Res. 2018, 25, 101–108. [Google Scholar] [CrossRef]
- Gladstones, J. Viticulture and Environment; Winetitles: Adelaide, Australia, 1992. [Google Scholar]
- Kemp, B.; Pedneault, K.; Pickering, G.; Usher, K.; Willwerth, J. Red Winemaking in Cool Climates. In Red Wine Technology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 341–356. ISBN 978-0-12814-400-8. [Google Scholar]
- Frioni, T.; Bronzoni, V.; Moncalvo, A.; Poni, S.; Gatti, M. Evaluation of local minor cultivars and marginal areas to improve wines and increase the sustainability of the district ‘Colli Piacentini’. Acta Hortic. 2020, 1276, 111–118. [Google Scholar] [CrossRef]
- Nistor, E.; Dobrei, A.G.; Dobrei, A.; Camen, D. Growing Season Climate Variability and its Influence on Sauvignon Blanc and Pinot Gris Berries and Wine Quality: Study Case in Romania (2005–2015). S. Afr. J. Enol. Vitic. 2018, 39, 196–207. [Google Scholar] [CrossRef]
- Gatti, M.; Garavani, A.; Cantatore, A.; Parisi, M.G.; Bobeica, N.; Merli, M.C.; Vercesi, A.; Poni, S. Interactions of summer pruning techniques and vine performance in the white Vitis vinifera cv. Ortrugo. Aust. J. Grape Wine Res. 2015, 21, 80–89. [Google Scholar] [CrossRef]
- Hale, C.R. Synthesis of Organic Acids in the Fruit of the Grape. Nature 1962, 195, 917–918. [Google Scholar] [CrossRef]
- Sweetman, C.; Deluc, L.G.; Cramer, G.R.; Ford, C.M.; Soole, K.L. Regulation of malate metabolism in grape berry and other developing fruits. Phytochemistry 2009, 70, 1329–1344. [Google Scholar] [CrossRef]
- Ford, C.M. The Biochemistry of organic acids in the Grape. In The Biochemistry of the Grape Berry; Geròs, H., Chaves, M.M., Delrot, S., Eds.; Bentham Books: Dubai, United Arab Emirates, 2012; pp. 67–88. [Google Scholar]
- Famiani, F.; Farinelli, D.; Frioni, T.; Palliotti, A.; Battistelli, A.; Moscatello, S.; Walker, R.P. Malate as substrate for catabolism and gluconeogenesis during ripening in the pericarp of different grape cultivars. Biol. Plant. 2016, 60, 155–162. [Google Scholar] [CrossRef]
- Famiani, F.; Battistelli, A.; Moscatello, S.; Cruz-Castillo, J.G.; Walker, R.P. The organic acids that are accumulated in the flesh of fruits: Occurrence, metabolism and factors affecting their contents—A review. Rev. Chapingo. Ser. Hortic. 2015, 21, 97–128. [Google Scholar] [CrossRef]
- Ewart, A.J.W.; Gawel, R.; Thistlewood, S.P.; McCarthy, M.G. Evaluation of must composition and wine quality of six clones of Vitis vinifera cv. Sauvignon Blanc. J. Exp. Agric. 1993, 33, 945–951. [Google Scholar] [CrossRef]
- Costantini, L.; Battilana, J.; Lamaj, F.; Fanizza, G.; Grando, M.S. Berry and phenology-related traits in grapevine (Vitis vinifera L.): From Quantitative Trait Loci to underlying genes. BMC Plant Biol. 2008, 8, 1–17. [Google Scholar] [CrossRef]
- Duchêne, É. How can grapevine genetics contribute to the adaptation to climate change? OENO One 2016, 50, 113–124. [Google Scholar] [CrossRef]
- Bayo-Canha, A.; Costantini, L.; Fernández-Fernández, J.I.; Martínez-Cutillas, A.; Ruiz-García, L. QTLs Related to Berry Acidity Identified in a Wine Grapevine Population Grown in Warm Weather. Plant Mol. Biol. Report. 2019, 37, 157–169. [Google Scholar] [CrossRef]
- Bigard, A.; Berhe, D.T.; Maoddi, E.; Sire, Y.; Boursiquot, J.M.; Ojeda, H.; Péros, J.P.; Doligez, A.; Romieu, C.; Torregrosa, L. Vitis vinifera L. fruit diversity to breed varieties anticipating climate changes. Front. Plant Sci. 2018, 9, 455. [Google Scholar] [CrossRef]
- Bigard, A.; Romieu, C.; Sire, Y.; Torregrosa, L. Vitis vinifera L. Diversity for Cations and Acidity Is Suitable for Breeding Fruits Coping With Climate Warming. Front. Plant Sci. 2020, 11, 1175. [Google Scholar] [CrossRef] [PubMed]
- Bernizzoni, F.; Gatti, M.; Civardi, S.; Poni, S. Long-term Performance of Barbera Grown under Different Training Systems and Within-Row Vine Spacings. Am. J. Enol. Vitic. 2009, 60, 339–348. [Google Scholar]
- Reynolds, A.G.; Vanden Heuvel, J.E. Influence of Grapevine Training Systems on Vine Growth and Fruit Composition: A Review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar]
- Dinis, L.T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Gonçalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of grapevine physiology and yield under summer stress by kaolin-foliar application: Water relations, photosynthesis and oxidative damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
| Cultivar | |||||||
|---|---|---|---|---|---|---|---|
| UD 80–100 | Soreli | UD 30–080 | Sauvignon Kretos | Sauvignon Rytos | Sauvignon Blanc | Ortrugo | |
| 2019 | (DOY) 1 | ||||||
| BBCH09 2 | 92 | 90 | 92 | 93 | 92 | 92 | 94 |
| BBCH81 2 | 203 | 203 | 210 | 197 | 210 | 217 | 217 |
| TSStr 3 | 224 | 231 | 231 | 224 | 231 | 252 | 245 |
| Matr 3 | 224 | 224 | 231 | 217 | 231 | 252 | 238 |
| 2020 | |||||||
| BBCH09 2 | 90 | 90 | 92 | 92 | 92 | 92 | 95 |
| BBCH81 2 | 202 | 202 | 209 | 195 | 202 | 209 | 209 |
| TSStr 3 | 213 | 223 | 237 | 213 | 223 | 252 | 252 |
| Matr 3 | 223 | 213 | 223 | 213 | 213 | 252 | 223 |
| Cultivar | Main Shoots Leaf Area | Lateral Shoots Leaf Area | Vine Total Leaf Area | Shoot Fruitfulness | Shoot Fruitfulness Nodes 0–2 |
|---|---|---|---|---|---|
| (m2/Vine) | (m2/Vine) | (m2/Vine) | (N. Inflorescences/Shoot) | (N. Inflorescences/Shoot) | |
| UD 80–100 | 2.38 b 2 | 0.35 | 2.73 ab | 1.36 b | 1.16 ab |
| Soreli | 2.96 ab | 0.32 | 3.27 ab | 1.76 a | 1.51 a |
| UD 30–080 | 3.17 a | 0.24 | 3.41 a | 1.47 b | 1.20 ab |
| Sauvignon Kretos | 3.01 ab | 0.23 | 3.24 ab | 1.58 ab | 1.19 ab |
| Sauvignon Rytos | 2.27 b | 0.12 | 2.39 b | 1.73 a | 1.44 a |
| Sauvignon Blanc | 3.12 ab | 0.45 | 3.55 a | 1.23 bc | 0.75 b |
| Ortrugo | 2.95 ab | 0.39 | 3.34 a | 0.93 c | 0.33 c |
| 2019 | 3.12 | 0.38 a | 3.50 a | 1.62 a | 1.26 b |
| 2020 | 2.58 | 0.24 b | 2.82 b | 1.17 b | 1.02 a |
| V 1 | ** 3 | ns | * | *** | *** |
| Y | ns | * | ** | *** | *** |
| VxY | ns | ns | ** | ** | ns |
| Cultivar | Yield | Bunches Per vine | Leaf Area to Yield Ratio | Bunch Weight | Berry Mass | Berries Per Bunch | Bunch Compactness |
|---|---|---|---|---|---|---|---|
| (kg/vine) | (n.) | (m2/kg) | (g) | (g) | (n.) | (g/cm) | |
| UD 80–100 | 2.31 b 2 | 22 b | 1.18 b | 110 ab | 1.17 ab | 106 b | 12.4 b |
| Soreli | 3.53 a | 30 a | 0.92 b | 120 ab | 1.15 ab | 104 b | 11.1 b |
| UD 30–080 | 2.08 b | 22 b | 1.64 a | 103 bc | 1.36 a | 76 c | 10.4 b |
| Sauvignon Kretos | 2.43 b | 25 ab | 1.33 b | 107 ab | 1.33 ab | 80 c | 10.1 b |
| Sauvignon Rytos | 2.41 b | 29 a | 0.99 b | 85 c | 1.02 b | 83 c | 9.3 b |
| Sauvignon Blanc | 2.86 ab | 20 b | 1.24 b | 143 ab | 1.45 a | 99 bc | 13.5 ab |
| Ortrugo | 2.35 b | 13 c | 1.42 ab | 181 a | 1.06 b | 170 a | 17.1 a |
| 2019 | 2.28 b | 25 a | 1.61 | 92 b | 1.11 a | 83 b | 10.1 b |
| 2020 | 2.82 a | 20 b | 1.40 | 141 a | 1.32 b | 126 a | 13.3 a |
| V 1 | *** 3 | *** | ** | *** | *** | *** | *** |
| Y | *** | *** | ns | *** | * | *** | * |
| VxY | ns | ns | ns | ** | ** | * | ns |
| Cultivar | TSS | pH | TA | TSS/TA | Malic Acid | Tartaric Acid | HT/HM |
|---|---|---|---|---|---|---|---|
| (°Brix) | (g/L) | (g/L) | (g/L) | ||||
| UD 80–100 | 22.6 ab 2 | 3.43 a | 5.07 c | 4.46 a | 1.38 b | 5.34 bc | 3.87 d |
| Soreli | 22.0 b | 3.42 a | 4.08 d | 5.39 a | 0.90 c | 4.67 c | 5.19 c |
| UD 30–080 | 20.1 bc | 3.32 ab | 4.66 cd | 4.29 ab | 1.04 bc | 5.35 bc | 5.14 c |
| Sauvignon Kretos | 24.5 a | 3.33 ab | 5.07 c | 4.83 a | 0.75 c | 6.36 b | 8.48 a |
| Sauvignon Rytos | 23.4 ab | 3.23 b | 6.50 b | 3.60 b | 0.89 c | 7.44 a | 8.36 a |
| Sauvignon Blanc | 19.0 c | 3.06 c | 8.81 a | 2.16 c | 3.45 a | 7.61 a | 2.21 e |
| Ortrugo | 19.5 c | 3.15 bc | 4.95 cd | 3.94 b | 0.81 c | 5.61 bc | 6.93 b |
| 2019 | 21.6 | 3.26 | 4.56 | 4.74 | 1.24 | 6.15 | 4.96 |
| 2020 | 21.4 | 3.35 | 5.59 | 3.83 | 1.39 | 5.92 | 4.26 |
| V 1 | *** 3 | *** | *** | *** | *** | *** | *** |
| Y | ns | ns | ns | ns | ns | ns | ns |
| VxY | ns | ns | ns | ns | ns | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frioni, T.; Squeri, C.; Del Zozzo, F.; Guadagna, P.; Gatti, M.; Vercesi, A.; Poni, S. Investigating Evolution and Balance of Grape Sugars and Organic Acids in Some New Pathogen-Resistant White Grapevine Varieties. Horticulturae 2021, 7, 229. https://doi.org/10.3390/horticulturae7080229
Frioni T, Squeri C, Del Zozzo F, Guadagna P, Gatti M, Vercesi A, Poni S. Investigating Evolution and Balance of Grape Sugars and Organic Acids in Some New Pathogen-Resistant White Grapevine Varieties. Horticulturae. 2021; 7(8):229. https://doi.org/10.3390/horticulturae7080229
Chicago/Turabian StyleFrioni, Tommaso, Cecilia Squeri, Filippo Del Zozzo, Paolo Guadagna, Matteo Gatti, Alberto Vercesi, and Stefano Poni. 2021. "Investigating Evolution and Balance of Grape Sugars and Organic Acids in Some New Pathogen-Resistant White Grapevine Varieties" Horticulturae 7, no. 8: 229. https://doi.org/10.3390/horticulturae7080229
APA StyleFrioni, T., Squeri, C., Del Zozzo, F., Guadagna, P., Gatti, M., Vercesi, A., & Poni, S. (2021). Investigating Evolution and Balance of Grape Sugars and Organic Acids in Some New Pathogen-Resistant White Grapevine Varieties. Horticulturae, 7(8), 229. https://doi.org/10.3390/horticulturae7080229









