Polyaniline/Biopolymer Composite Systems for Humidity Sensor Applications: A Review
Abstract
1. Introduction
- To provide a literature overview of PANI/biopolymer sensor materials;
- To provide insight on the moisture-sensing mechanism of PANI and its hybrid composites that contain biopolymer systems;
- To develop a rational approach for the design of PANI-based materials that are suitable for humidity-sensing applications.
2. Key Features of Ceramic Humidity Sensors
2.1. Water Vapor Adsorption vs. Electrical Properties
2.2. Pore Structure, Grains and Grain Boundaries
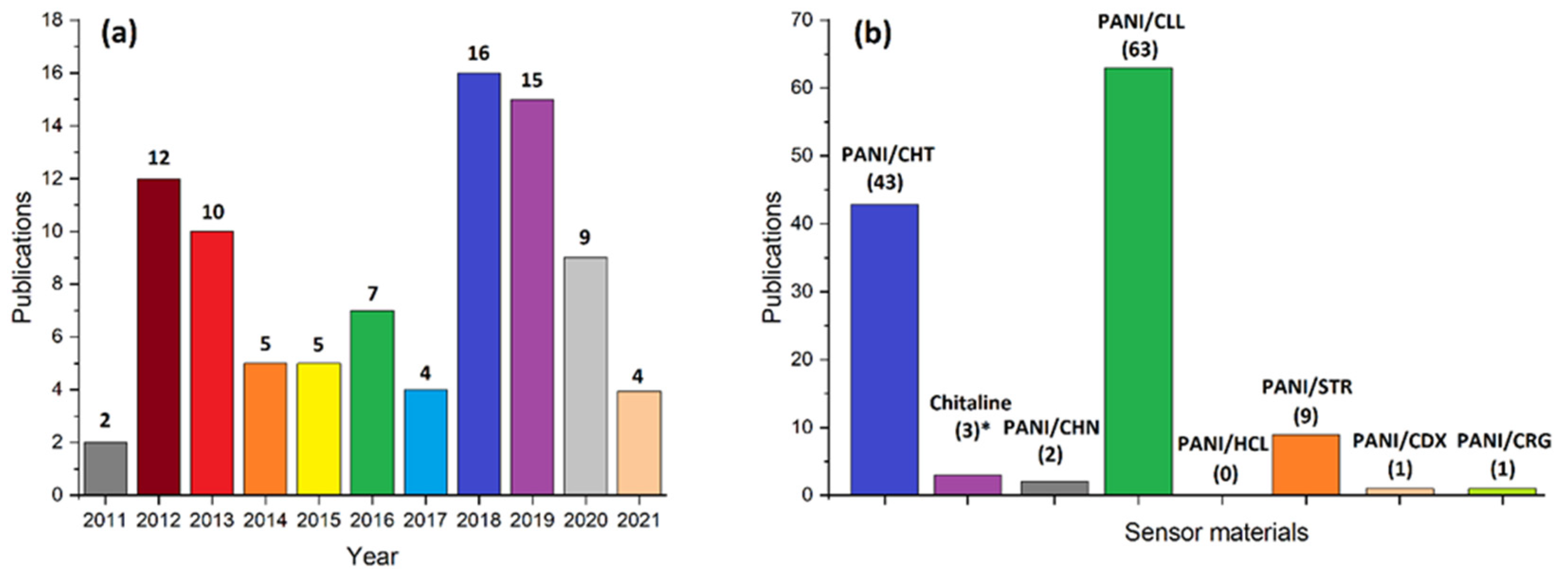
3. Structure and Physicochemical Properties of PANI/Biopolymer Composites
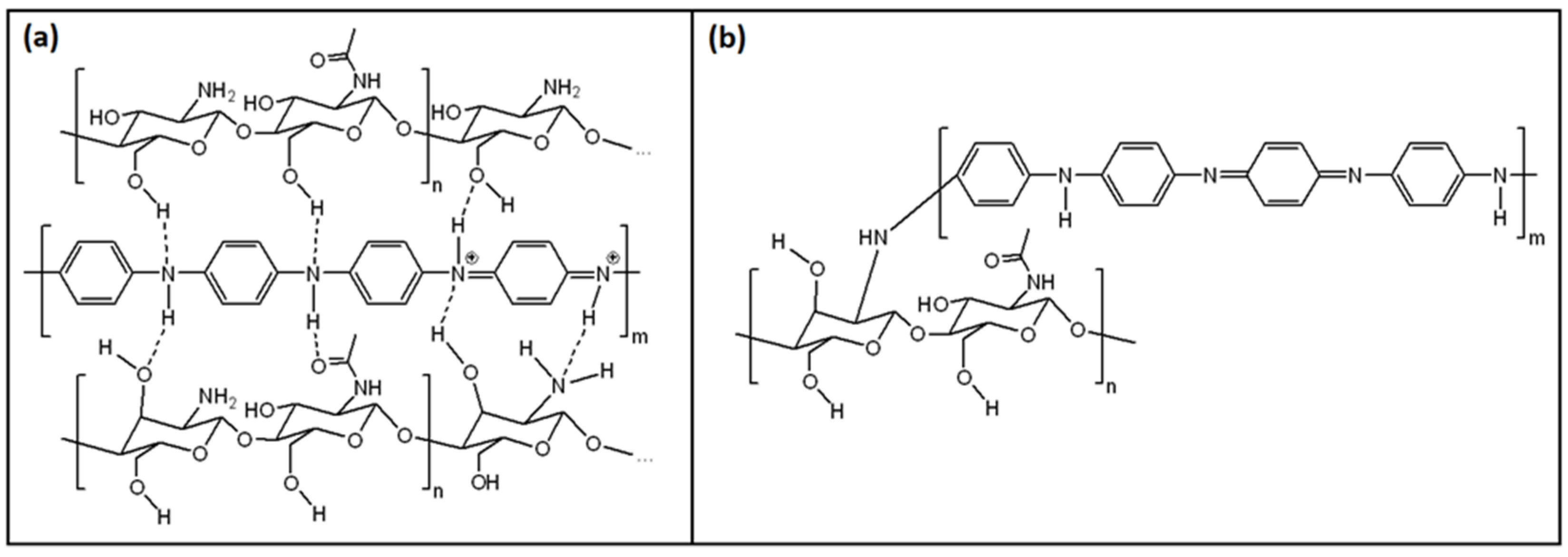
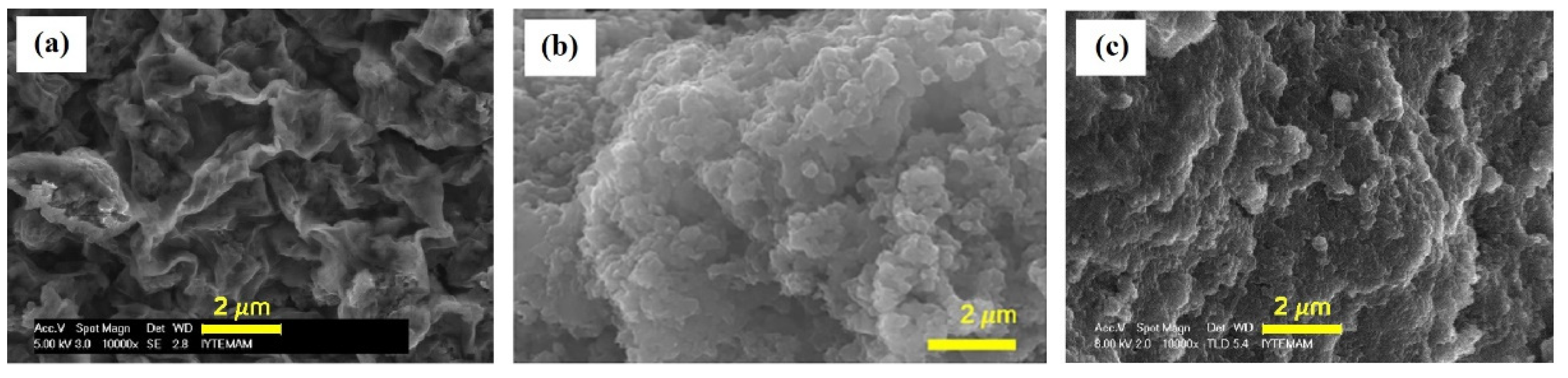
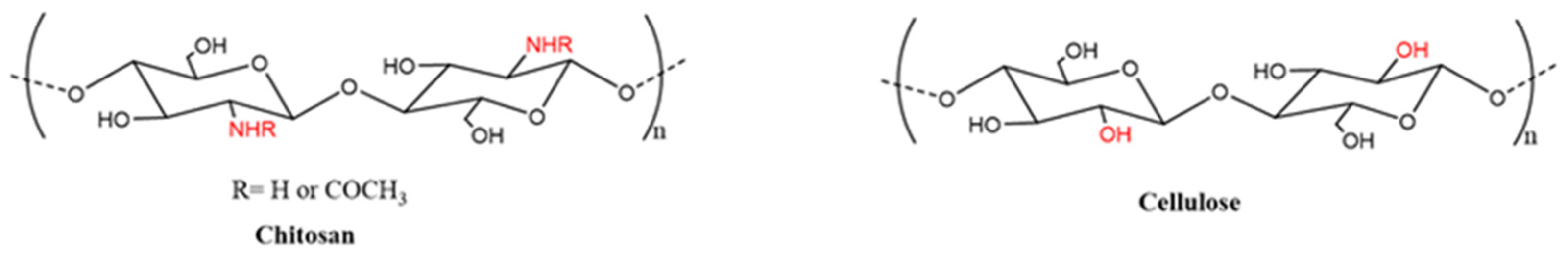
3.1. PANI/Chitosan Binary Composites
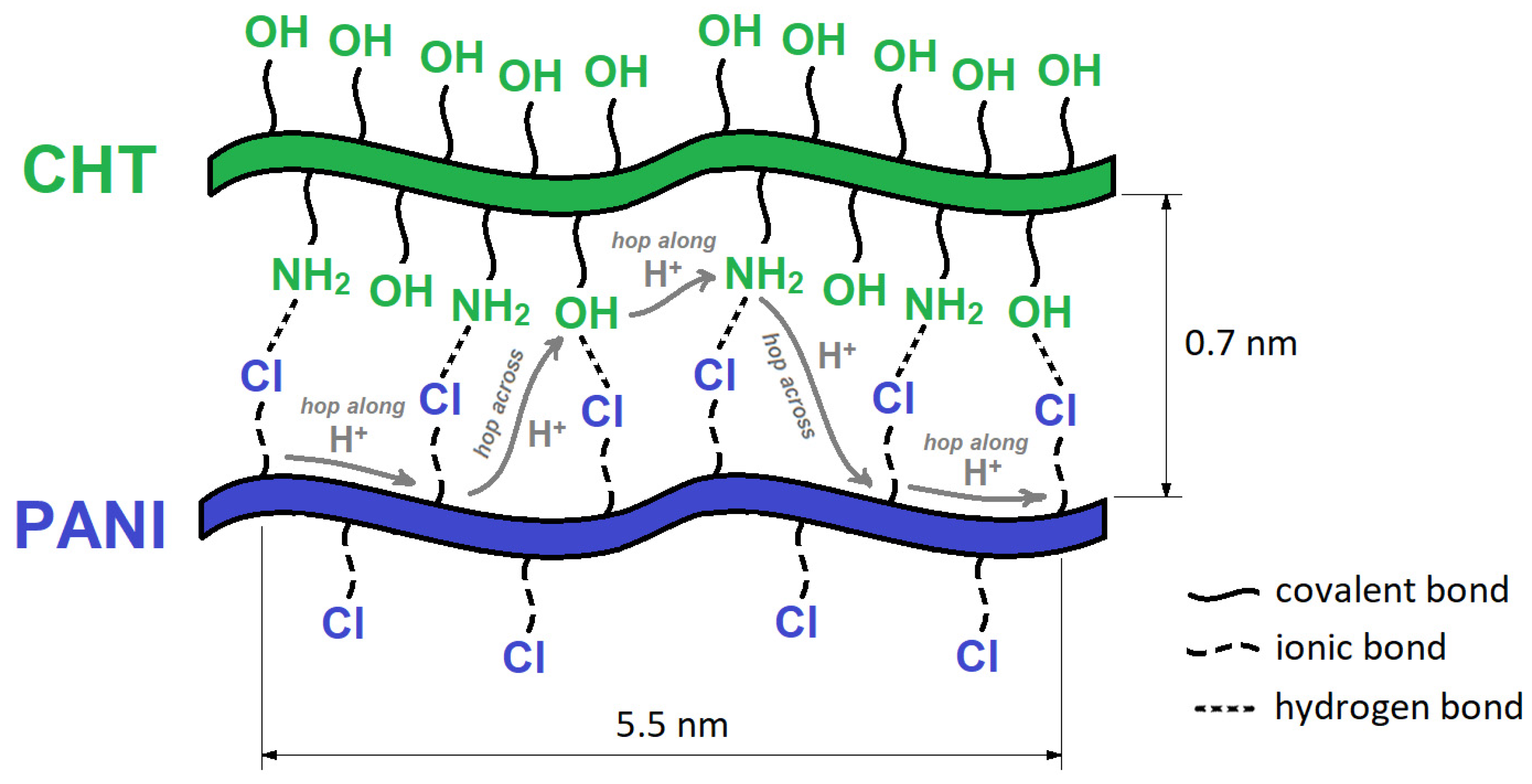
3.2. Humidity Sensing Mechanism of PANI/CHT Composites
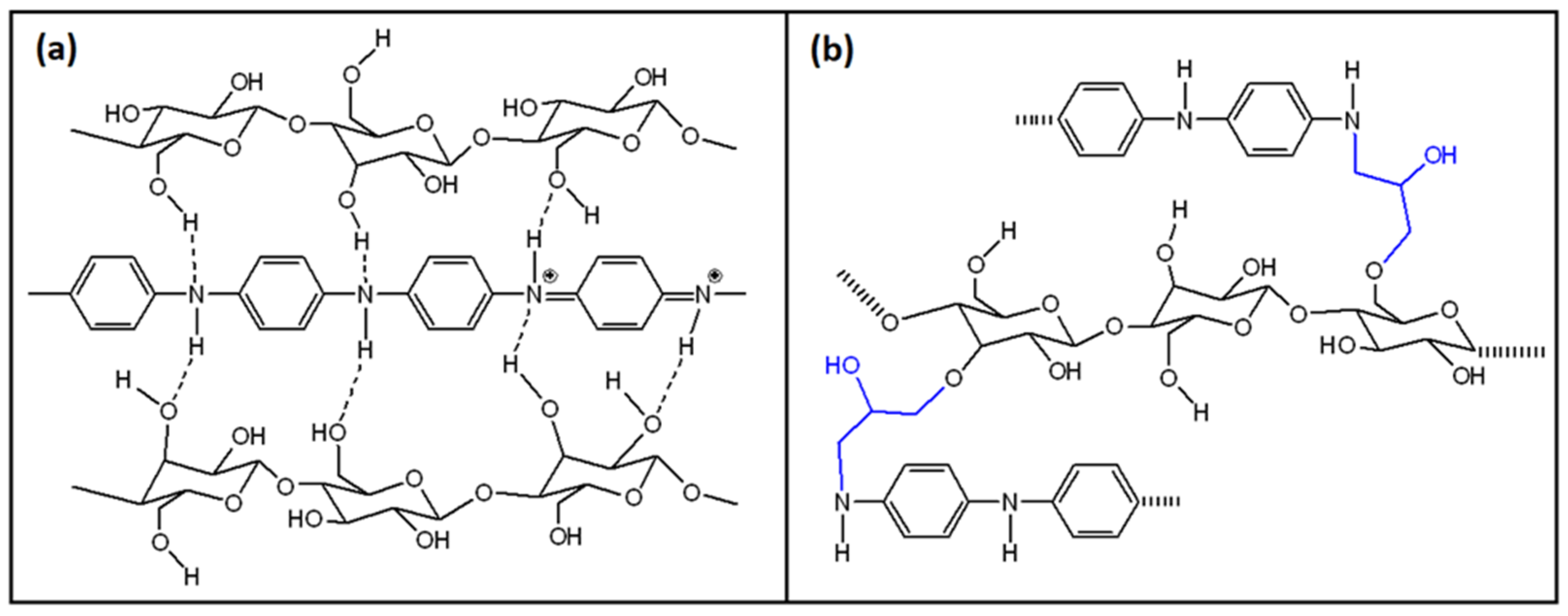
3.3. PANI/Cellulose Binary Composites
3.3.1. PANI/Bacterial Cellulose Composites
3.3.2. PANI/Carboxymethylcellulose (CMC) Composite
3.4. PANI/CLL Ternary Composites
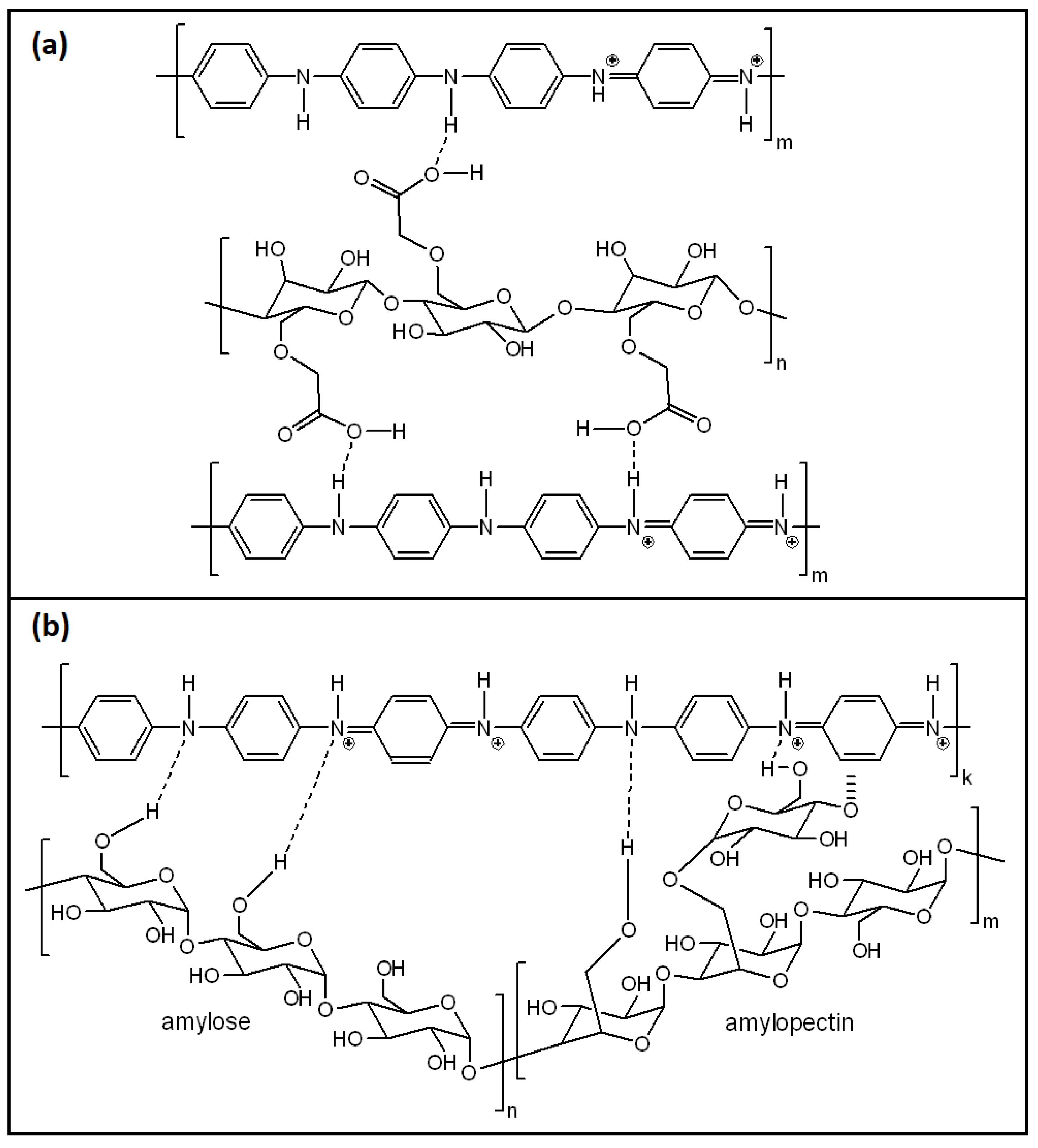
3.5. PANI/Starch Binary Composites
3.6. Concluding Remarks
4. Carbon-Based Humidity Sensors
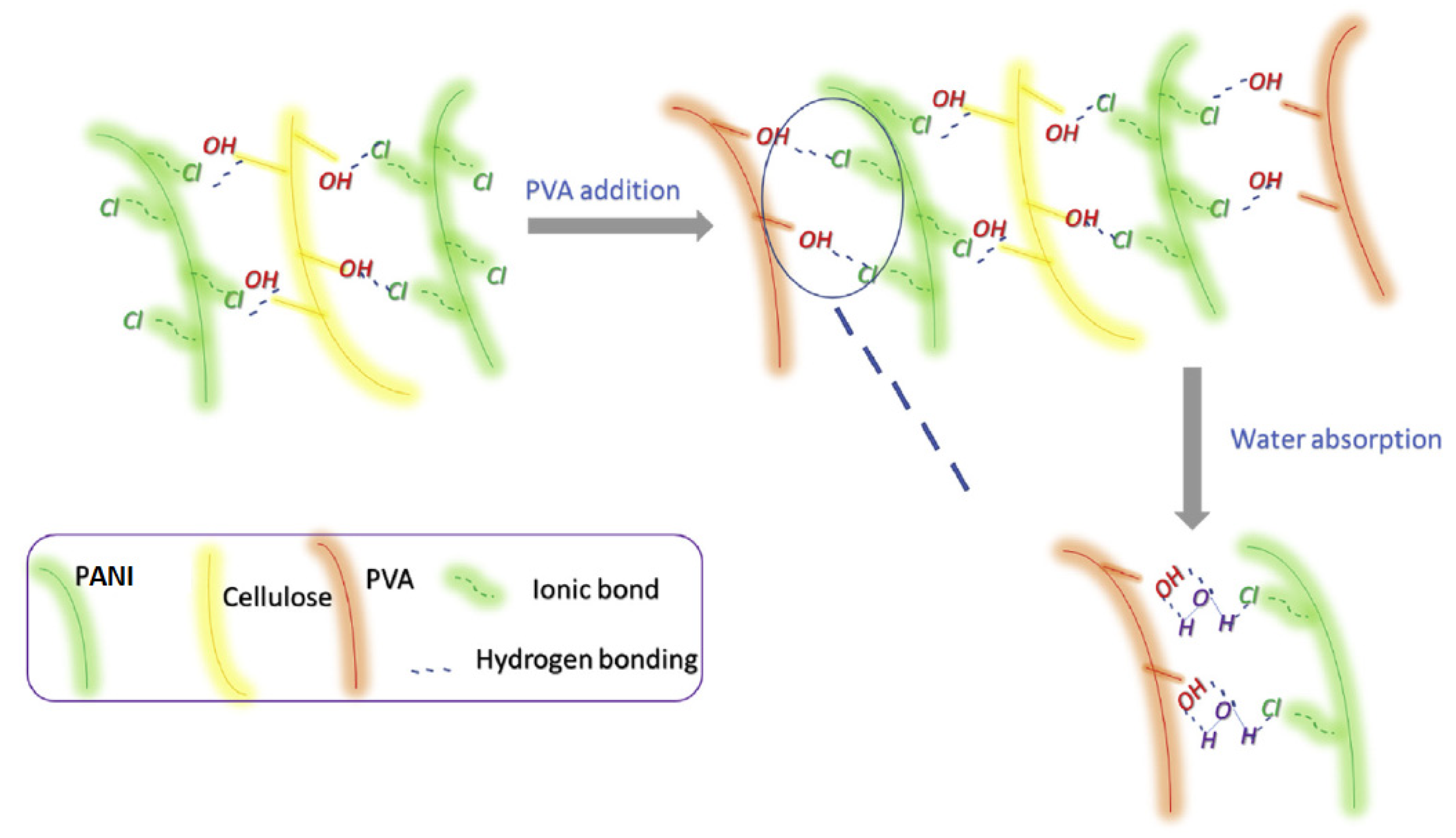
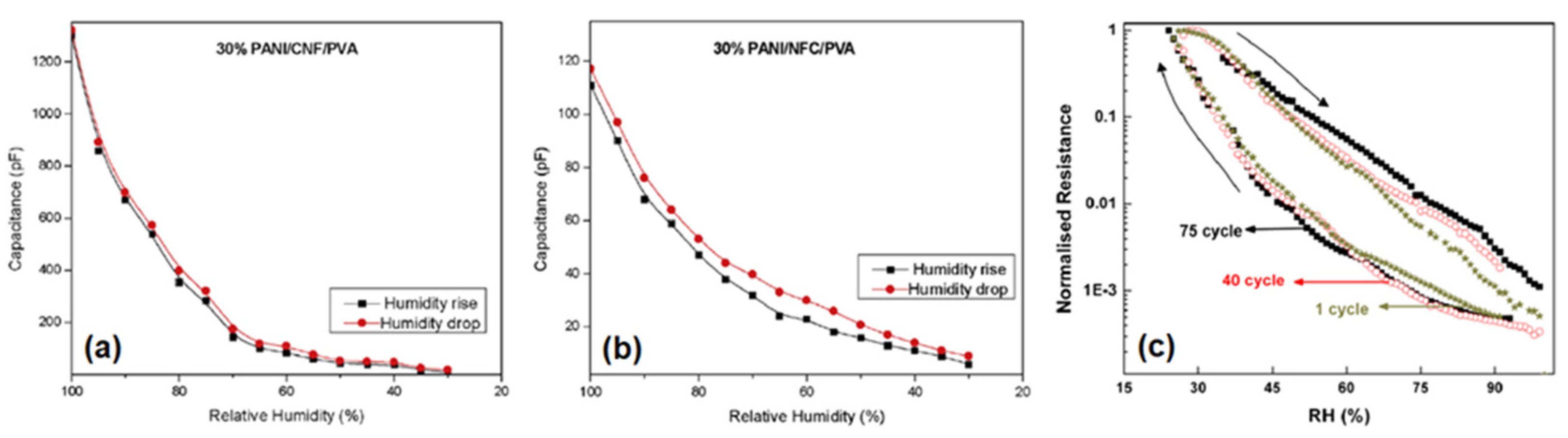
4.1. PANI/Carbon vs. PANI/Biopolymer Sensors
- Response time (how rapidly the sensor responds to humidity);
- Recovery time (how rapidly the sensor returns to its initial state after response);
- Hysteresis (how the sensor recovers fully after each measurement).
5. Experimental Strategies for the Study of Hydration
6. Material Design Approach for Unique Hydration Properties
7. Discussion and Knowledge Gaps
8. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, R.; Jenjeti, R.N.; Sampath, S. Bulk and few-layer 2d, p-MnPS3 for sensitive and selective moisture sensing. Adv. Mater. Interfaces 2019, 6, 1900666. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Wang, R.; Zhang, T. An excellent humidity sensor with rapid response based on BaTiO3 nanofiber via electrospinning. Sens. Lett. 2011, 9, 262–265. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Urban, G.A. Perovskites as surface-assisted room temperature protonic conductor humidity sensor. In Proceedings of the 2019 IEEE International Conference on Sensors and Nanotechnology, Montreal, QC, Canada, 27–30 October 2019. [Google Scholar] [CrossRef]
- Almara, L.; Tarancóna, A.; Andreua, T.; Torrella, M.; Hub, Y.; Dezanneaub, G.; Morata, A. Mesoporous ceramic oxides as humidity sensors: A case study for gadolinium-doped ceria. Sens. Actuators B 2015, 216, 41–48. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, N.; Geng, W.; Chi, Y.; Tu, J.; Li, X.; Shao, C. Humidity sensing properties of mesoporous iron oxide/silica composite prepared via hydrothermal process. Sens. Actuators B 2011, 160, 334–340. [Google Scholar] [CrossRef]
- Wang, D.; Lou, Y.; Wang, R.; Wang, P.; Zheng, X.; Zhang, Y.; Jiang, N. Humidity sensor based on Ga2O3 nanorods doped with Na+ and K+ from GaN powder. Ceram. Int. 2015, 41, 14790–14797. [Google Scholar] [CrossRef]
- Prajapati, M.J.; Vardhan, R.V.; Mandal, S. Effect of lanthanum on the phase evolution of perovskite barium stannate synthesized through polymerized complex method. Ceram. Int. 2019, 45, 17420–17428. [Google Scholar] [CrossRef]
- Ga, A.; Zhao, Q.; He, D.; Zhao, Y.; Chang, A. Rapid response humidity sensor based on DC magnetron sputtered Mn1.2Co1.5Ni0.3O4 thin film. Mater. Lett. 2020, 271, 127685. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, M.; Wen, Z.; Ma, J.; Zhang, J.; Hou, Z. Progress on preparation and application of nanostructured WO3/TiO2 composite thin films. J. Artif. Cryst. 2020, 49, 144–151. [Google Scholar]
- Ramaprasad, A.T.; Rao, V. Chitin-polyaniline blend as humidity sensor. Sens. Actuators B 2010, 148, 117–125. [Google Scholar] [CrossRef]
- Putri, N.P.; Kusumawati, D.H.; Widiyanti, N.; Munasir. Synthesis of polyaniline/cellulose composite as humidity sensor. IOP Conf. Ser. J. Phys. Conf. Ser. 2018, 997, 012009. [Google Scholar] [CrossRef]
- Anju, V.P.; Jithesh, P.R.; Narayanankutty, S.K. A novel humidity and ammonia sensor based on nanofibers/polyaniline/polyvinyl alcohol. Sens. Actuators A 2019, 285, 35–44. [Google Scholar] [CrossRef]
- Kotresh, S.; Ravikiran, Y.T.; Raj Prakash, H.G.; Ramana, C.H.V.V.; Vijayakumari, S.C.; Thomas, S. Humidity sensing performance of spin coated polyaniline–carboxymethyl cellulose composite at room temperature. Cellulose 2016, 23, 3177–3186. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D.; Yang, A.; Wang, D.; Zong, X. Fabrication of polypyrrole/graphene oxide hybrid nanocomposite for ultrasensitive humidity sensing with unprecedented sensitivity. J. Mater. Sci. Mater. Electron. 2019, 30, 4967–4976. [Google Scholar] [CrossRef]
- Zhou, T.; Wu, S.; Cai, J.; Ruan, W. Rapid humidity sensors based on poly (o-phenylenediamine-co-aniline) spherical nanoparticles. Polym. Bull. 2020, 77, 1095–1105. [Google Scholar] [CrossRef]
- Jang, Y.J.; Jung, Y.E.; Kim, G.W.; Lee, C.Y.; Park, Y.D. Metal–organic frameworks in a blended polythiophene hybrid film with surface-mediated vertical phase separation for the fabrication of a humidity sensor. RSC Adv. 2019, 9, 529–535. [Google Scholar] [CrossRef]
- Cherrington, R.; Liang, J. Chapter 2–Materials and deposition processes for multifunctionality. In Design and Manufacture of Plastic Components for Multifunctionality; Elsevier: Walthem, MA, USA, 2016; pp. 19–52. [Google Scholar]
- Ragab, E.; Shaban, M.; Abdel Khalek, A.; Mohamed, F. Design and characterization of PANI/starch/Fe2O3 biocomposite for wastewater remediation. Int. J. Biol. Macromol. 2021, 181, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Yeamin, M.B.; Islam, M.M.; Chowdhury, A.-N.; Awual, M.R. Efficient encapsulation of toxic dyes from wastewater using several biodegradable natural polymers and their composites. J. Clean. Prod. 2021, 291, 125920. [Google Scholar] [CrossRef]
- Arrieta-Almario, A.; Mendoza-Fandiño, J.; Palencia-Luna, M. Composite material elaborated from conducting biopolymer cassava starch and polyaniline. Rev. Mex. Ing. Química 2019, 19, 707–715. [Google Scholar] [CrossRef]
- Hadi Salehi, M.; Golbaten-Mofrad, H.; Jafari, S.H.; Goodarzi, V.; Entezari, M.; Hashemi, M.; Zamanlui, S. Electrically conductive biocompatible composite aerogel based on nanofibrillated template of bacterial cellulose/polyaniline/nano-clay. Int. J. Biol. Macromol. 2021, 173, 467–480. [Google Scholar] [CrossRef]
- Ragazzini, I.; Gualandi, I.; Selli, S.; Polizzi, C.; Cassani, M.C.; Nanni, D.; Gambassi, F.; Tarterini, F.; Tonelli, D.; Scavetta, E.; et al. A simple and industrially scalable method for making a PANI-modified cellulose touch sensor. Carbohydr. Polym. 2021, 254, 117304. [Google Scholar] [CrossRef] [PubMed]
- Dubey, N.; Arora, S. Surfactant assisted synthesis of pH responsive polyaniline-cellulose biocomposite for sensor applications. Polym. Plast. Tech. Mat. 2021, 60, 1135–1147. [Google Scholar]
- Rathore, B.R.S.; Chauhan, N.P.S.; Rawal, M.K.; Ameta, S.C.; Ameta, R. Synthesis and characterization of chitosan-polyaniline-manganese dioxide nanocomposite for removal of methyl orange dye. Asian J. Chem. 2021, 33, 671–676. [Google Scholar] [CrossRef]
- Jasenská, D.; Kašpárková, V.; Radaszkiewicz, K.A.; Capáková, Z.; Pacherník, J.; Trchová, M.; Minařík, A.; Vajďák, J.; Bárta, T.; Stejskal, J.; et al. Conducting composite films based on chitosan or sodium hyaluronate. Properties and cytocompatibility with human induced pluripotent stem cells. Carbohydr. Polym. 2021, 253, 117244. [Google Scholar] [CrossRef] [PubMed]
- Rozova, E.Y.; Zoolshoev, Z.F.; Kuryndin, I.S.; Saprykina, N.N.; Elyashevich, G.K. Physicochemical properties and morphological features of modified chitosan/polyaniline composite films. Russ. J. Phys. Chem. 2021, 95, 193–198. [Google Scholar] [CrossRef]
- Draper, J.W. A Textbook on Chemistry; Harper & Bros: New York, NY, USA, 1861; p. 55. [Google Scholar]
- Wyszyński, P.; Przybylak, R. Variability of humidity conditions in the Arctic during the first International Polar Year, 1882–1883. Polar Res. 2014, 33, 23896. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lee, G.B. Humidity Sensors: A Review. Sens. Lett. 2005, 3, 1–15. [Google Scholar] [CrossRef]
- Harito, C.; Utari, L.; Putra, B.R.; Yuliarto, B.; Purwanto, S.; Zaidi, S.Z.J.; Bavykin, D.V.; Marken, F.; Walsh, F.C. Review—The development of wearable polymer-based sensors: Perspectives. J. Electrochem. Soc. 2020, 167, 037566. [Google Scholar] [CrossRef]
- Farahani, H.; Wagiran, R.; Hamidon, M.N. Humidity sensors principle, mechanism, and fabrication technologies: A comprehensive review. Sensors 2014, 14, 7881–7939. [Google Scholar] [CrossRef]
- Miyake, T.; Rolandi, M. Grotthuss mechanisms: From proton transport in proton wires to bioprotonic devices. J. Phys. Condens. Matter 2016, 28, 023001. [Google Scholar] [CrossRef]
- Conway, B.E.; Bockris, J.O.; Linton, H. Proton conductance and the existence of the H3O ion. J. Chem. Phys. 1956, 24, 834. [Google Scholar] [CrossRef]
- Rafique, M.S.; Tahir, M.B.; Rafique, M.; Shakil, M. Chapter 12—Photocatalytic nanomaterials for air purification and self-cleaning. In Nanotechnology and Photocatalysis for Environmental Applications; Elsevier: Cambridge, MA, USA, 2020; pp. 203–219. [Google Scholar]
- Gong, M.-S.; Kimb, J.-U.; Kim, J.-G. Preparation of water-durable humidity sensor by attachment of polyelectrolyte membrane to electrode substrate by photochemical crosslinking reaction. Sens. Actuators B 2010, 147, 539–547. [Google Scholar] [CrossRef]
- Romero, F.J.; Rivadeneyra, A.; Becherer, M.; Morales, D.P.; Rodríguez, N. Fabrication and characterization of humidity sensors based on graphene oxide–PEDOT:PSS composites on a flexible substrate. Micromachines 2020, 11, 148. [Google Scholar] [CrossRef]
- Gong, M.-S.; Lee, C.-W.; Joo, S.-W.; Cho, B.-K. Humidity-sensitive properties of phosphonium salt-containing polyelectrolytes. J. Mater. Sci. 2002, 37, 4615–4620. [Google Scholar] [CrossRef]
- Mahapure, P.D.; Gosavi, S.W.; Aiyer, R.C. Studies on PVP, PVA and their nAg composites based humidity sensors. In Proceedings of the 2015 2nd International Symposium on Physics and Technology of Sensors, Pune, India, 8–10 March 2015. [Google Scholar]
- Kanitkar, P.; Adhyapak, P.; Aiyer, R.; Mulik, U.; Amalnerkar, D. Synthesis and electrical characterization of Ag, Au-PVP nanocomposites for humidity sensing. Sens. Lett. 2012, 10, 932–940. [Google Scholar] [CrossRef]
- Jeeva, A.; Vijayanand, P.S.; Ashokan, S.; Kojima, T.; Kato, S.; Deepalekshmi, P. A facile synthesis of poly(aniline-co-3-trifluoromethyl aniline) doped silver nanoparticles in micellar solution: Its humidity sensor application. Polym. Sci. Ser. B 2018, 60, 505–515. [Google Scholar] [CrossRef]
- Hatamie, S.; Dhas, V.; Kale, B.B.; Mulla, I.S.; Kale, S.N. Polymer-embedded stannic oxide nanoparticles as humidity sensors. Mater. Sci. Eng. C 2009, 29, 847–850. [Google Scholar] [CrossRef]
- Park, T.; Kim, N.; Kim, D.; Kim, S.-W.; Oh, Y.; Yoo, J.-K.; You, J.; Um, M.-K. An organic/inorganic nanocomposite of cellulose nanofibers and ZnO nanorods for highly sensitive, reliable, wireless, and wearable multifunctional sensor applications. ACS Appl. Mater. Interfaces 2019, 11, 48239–48248. [Google Scholar] [CrossRef] [PubMed]
- Ashokan, S.; Jayamurugan, P.; Ponnuswamy, V. Effects of CuO and oxidant on the morphology and conducting properties of PANI:CuO hybrid nanocomposites for humidity sensor application. Polym. Sci. Ser. B 2019, 61, 86–97. [Google Scholar] [CrossRef]
- Chani, M.T.S.; Karimov, K.S.; Khan, S.B.; Fatima, N.; Asir, A.M. Impedimetric humidity and temperature sensing properties of chitosan-CuMn2O4 spinel nanocomposite. Ceram. Int. 2019, 45, 10565–10571. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Manna, A.; Shah, N.F.A.; Shasmin, H.N.; Radzi, Z.; Osman, N.A.A. Synthesis and characterizations of novel Ca-Mg-Ti-Fe-oxides based ceramic nanocrystals and flexible film of polydimethylsiloxane composite with improved mechanical and dielectric properties for sensors. Sensors 2016, 16, 292. [Google Scholar] [CrossRef] [PubMed]
- Machappa, T.; Ambika Prasad, M.V.N. Humidity sensing behaviour of polyaniline/magnesium chromate (MgCrO4) composite. Bull. Mater. Sci. 2012, 35, 75–81. [Google Scholar] [CrossRef]
- Ganiger, S.K.; Murugendrappa, M.V. Lab scale study on humidity sensing and DC conductivity of polypyrrole/strontium arsenate (Sr3(AsO4)2) ceramic composites. Polym. Sci. Ser. B 2018, 60, 395–404. [Google Scholar] [CrossRef]
- Zhuang, Z.; Qi, D.; Ru, C.; Pan, J.; Zhao, C.; Na, H. Fast response and highly sensitive humidity sensors based on CaCl2-doped sulfonated poly (ether ether ketone)s. Sens. Actuators B 2017, 253, 666–676. [Google Scholar] [CrossRef]
- Hashim, A.; Hamad, Z.H. Fabrication and characterization of polymer blend doped with metal carbide nanoparticles for humidity sensors. J. Nanostruct. 2019, 9, 340–348. [Google Scholar]
- Edwin Suresh Raj, A.M.; Mallika, C.; Swaminathan, K.; Sreedharan, O.M.; Nagaraja, K.S. Zinc(II) Oxide-zinc(II) molybdate composite humidity sensor. Sens. Actuators B 2002, 81, 229–236. [Google Scholar] [CrossRef]
- Meng, X.; Yang, J.; Liu, Z.; Lu, W.; Sun, Y.; Dai, Y. Non-contact, fibrous cellulose acetate/aluminum flexible electronic-sensor for humidity detecting. Compos. Commun. 2020, 20, 100347. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Sun, H.; Yang, Y.; Ye, Y.; Cui, J.; He, W.; Yong, X.; Xie, Y. An optical fiber sensor based on carboxymethyl cellulose/carbon nanotubes composite film for simultaneous measurement of relative humidity and temperature. Opt. Commun. 2020, 467, 125740. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, K.; Zhang, Q. Giant humidity response using a chitosan-based protonic conductive sensor. IEEE Sens. J. 2016, 16, 8884–8889. [Google Scholar] [CrossRef]
- Yadav, C.G.; Sharma, G.; Singh, V.; Kumar, M.; Srivastava, N.; Kumar, S.; Gupta, V. Coupled mode surface plasmon resonance sensor: In situ detection of humidity with starch biofilm. Opt. Quantum Electron. 2018, 50, 11. [Google Scholar] [CrossRef]
- Hashim, A.; Jassim, A. Novel of biodegradable polymers-inorganic nanoparticles: Structural, optical and electrical properties as humidity sensors and gamma radiation shielding for biological applications. J. Bionanosci. 2018, 12, 170–176. [Google Scholar] [CrossRef]
- Hashim, A.; Habeeb, M.A.; Hadi, A. Synthesis of novel polyvinyl alcohol–starch-copper oxide nanocomposites for humidity sensors applications with different temperatures. Sens. Lett. 2017, 15, 758–761. [Google Scholar] [CrossRef]
- Baatout, Z.; Teka, S.; Jaballah, N.; Sakly, N.; Sun, X.; Maurel, F.; Majdoub, M. Water-insoluble cyclodextrin membranes for humidity detection: Green synthesis, characterization and sensing performances. J. Mater. Sci. 2018, 53, 1455–1469. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Xu, H.; Wu, Q.; Liu, C.; Yang, B.-R.; Gui, X.; Xie, X.; Tao, K.; Shen, Y.; et al. An intrinsically stretchable humidity sensor based on anti-drying, self-healing and transparent organohydrogels. Mater. Horiz. 2019, 6, 595–603. [Google Scholar] [CrossRef]
- Singh, P.; Shukla, S.K. Advances in polyaniline-based nanocomposites. J. Mater. Sci. 2020, 55, 1331–1365. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Gholaminejad, A.; Omidfar, K.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: A review on recent advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef]
- Vijayan, A.; Fuke, M.; Hawaldar, R.; Kulkarni, M.; Amalnerkar, D.; Aiyer, R.C. Optical fibre based humidity sensor using Co-polyaniline clad. Sens. Actuators B 2008, 129, 106–112. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Blank, T.A.; Eksperiandova, L.P.; Belikov, K.N. Recent trends of ceramic humidity sensors development: A review. Sens. Actuators B 2016, 228, 416–442. [Google Scholar] [CrossRef]
- Sen, T.; Mishra, S.; Shimp, N.G. Synthesis and sensing applications of polyaniline nanocomposites: A review. RSC Adv. 2016, 6, 42196–42222. [Google Scholar] [CrossRef]
- Do Nascimento, G.M. Spectroscopy of polyaniline nanofibers. In Nanofibers; Kumar, A., Ed.; InTech: Rijeka, Croatia, 2010; ISBN 978-953-7619-86-2. [Google Scholar]
- Stejskal, J.; Kratochvíl, P.; Jenkins, A.D. Polyaniline: Forms and formation. Collect. Czech. Chem. Commun. 1995, 60, 1747–1755. [Google Scholar] [CrossRef]
- Yang, Q.-H.; Hao, Q.; Lei, J.-P.; Ju, H.-X. Photoelectron-regulated redox reaction of polyaniline for visual detection of trace copper. Chin. J. Anal. Chem. 2017, 45, 1895–1902. [Google Scholar] [CrossRef]
- Liu, J.; Agarwal, M.; Varahramyan, K.; Berney, E.S.; Hodo, W.D. Polymer-based microsensor for soil moisture measurement. Sens. Actuators B 2008, 129, 599–604. [Google Scholar] [CrossRef]
- Song, X.; Qi, Q.; Zhang, T.; Wang, C. A humidity sensor based on KCl-doped SnO2 nanofibers. Sens. Actuators B 2009, 138, 368–373. [Google Scholar] [CrossRef]
- Pang, S.; Li, G.; Zhang, Z. Synthesis of polyaniline–vanadium oxide nanocomposite nanosheets. Macromol. Rapid Commun. 2005, 26, 1262–1265. [Google Scholar] [CrossRef]
- Spain, E.; Kojima, R.; Kaner, R.B.; Wallace, G.G.; O’Grady, J.; Lacey, K.; Barry, T.; Keyes, T.E.; Forster, R.J. High sensitivity DNA detection using gold nanoparticle functionalised polyaniline nanofibres. Biosens. Bioelectron. 2011, 26, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Tamer, U.; Seçkin, A.İ.; Temur, E.; Torul, H. Fabrication of biosensor based on polyaniline/gold nanorod composite. Int. J. Electrochem. 2011, 2011, 869742. [Google Scholar] [CrossRef]
- Rakhi, R.B.; Chena, W.; Alshareef, H.N. Conducting polymer/carbon nanocoil composite electrodes for efficient supercapacitors. J. Mater. Chem. 2012, 22, 5177–5183. [Google Scholar] [CrossRef]
- Nobya, H.; El-Shazly, A.H.; Elkady, M.F.; Ohshima, M. Strong acid doping for the preparation of conductive polyaniline nanoflowers, nanotubes, and nanofibers. Polymer 2019, 182, 121848. [Google Scholar] [CrossRef]
- Freitas, T.V.; Sousa, E.A.; Fuzari, G.C., Jr.; Arlindo, E.P.S. Different morphologies of polyaniline nanostructures synthesized by interfacial polymerization. Mater. Lett. 2018, 224, 42–45. [Google Scholar] [CrossRef]
- Bae, J.; Shin, K.; Kwon, O.S.; Hwang, Y.H.; An, J.; Jang, A.; Kim, H.J.; Lee, C.-S. A succinct review of refined chemical sensor systems based on conducting polymer-cyclodextrin hybrids. J. Ind. Eng. Chem. 2019, 79, 19–28. [Google Scholar] [CrossRef]
- Anisimov, Y.A.; Cree, D.E.; Wilson, L.D. Preparation of multi-component biocomposites and characterization of their physico-chemical and mechanical properties. J. Compos. Sci. 2020, 4, 18. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.-H.; Wang, X.-D. Study on dielectric properties of humidity sensing nanometer materials. Sens. Actuators B 2005, 108, 445–449. [Google Scholar] [CrossRef]
- Biswas, P.; Kundu, S.; Banerji, P.; Bhunia, S. Super rapid response of humidity sensor based on MOCVD grown ZnO nanotips array. Sens. Actuators B 2013, 178, 331–338. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, H.Y.; Wang, W.C.; Li, K.; Wang, X.C.; Li, X.J. Capacitive humidity sensing properties of ZnO cauliflowers grown on silicon nanoporous pillar array. Sens. Actuators B 2013, 177, 740–744. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Li, X.; Lu, G.; You, L.; Liang, X.; Liu, F.; Zhang, T.; Du, Y. Highly sensitive humidity sensor based on high surface area mesoporous LaFeO3 prepared by a nanocasting route. Sens. Actuators B 2013, 181, 802–809. [Google Scholar] [CrossRef]
- Bayhan, B.; Kavasoğlu, N. A study on the humidity sensing properties of ZnCr2O4–K2CrO4 ionic conductive ceramic sensor. Sens. Actuators B 2006, 117, 261–265. [Google Scholar] [CrossRef]
- Nitta, T.; Terada, Z.; Hayakawa, S. Humidity-sensitive electrical conduction of MgCr2O4-TiO2 porous ceramics. J. Am. Ceram. Soc. 1980, 63, 295–300. [Google Scholar] [CrossRef]
- Yavuz, A.G.; Uygun, A.; Can, H.K. The effect of synthesis media on the properties of substituted polyaniline/chitosan composites. Carbohydr. Res. 2011, 346, 2063–2069. [Google Scholar] [CrossRef]
- Lee, S.; Choi, D.; Son, Y. Hazardous acid detection based on chitosan-grafted-polyaniline copolymer. Polym. Eng. Sci. 2019, 59, E105–E110. [Google Scholar] [CrossRef]
- Rajeev, K.K.; Kim, E.; Nam, J.; Lee, S.; Mun, J.; Kim, T.-H. Chitosan-grafted-polyaniline copolymer as an electrically conductive and mechanically stable binder for high-performance Si anodes in Li-ion batteries. Electrochim. Acta 2020, 333, 135532. [Google Scholar] [CrossRef]
- Ratuchne, F.; Danczuk, M.; de Castro, E.G. Enhanced stability and conductivity of (polyaniline-chitosan) composites. Orbital Electron. J. Chem. 2018, 10, 239–246. [Google Scholar] [CrossRef]
- Minisy, I.M.; Salahuddin, N.A.; Ayad, M.A. Adsorption of methylene blue onto chitosan–montmorillonite/polyaniline nanocomposite. Appl. Clay Sci. 2021, 203, 105993. [Google Scholar] [CrossRef]
- Mohammadi, B.; Pirsa, S.; Alizadeh, M. Preparing chitosan-polyaniline nanocomposite film and examining its mechanical, electrical, and antimicrobial properties. Polym. Polym. Compos. 2019, 27, 507–517. [Google Scholar] [CrossRef]
- Karunanithy, P.; Prasad, R.G.S.V.; Jakka, V.S.; Aparna, R.S.L.; Phani, A.R.; Prabhakara, G.S.; Ahmed, S.A. Enhanced antimicrobial activity of polyaniline grafted chitosan. Adv. Sci. Eng. Med. 2013, 5, 420–426. [Google Scholar] [CrossRef]
- Yavuz, A.G.; Uygun, A.; Bhethanabotla, V.R. Substituted polyaniline/chitosan composites: Synthesis and characterization. Carbohydr. Polym. 2009, 75, 448–453. [Google Scholar] [CrossRef]
- Abdi, Z.; Sedaghat, S. Synthesis and characterization of functionalized single-walled carbon nanotube/chitosan/polyaniline nanocomposite. Int. J. Nano Dimens. 2016, 7, 25–32. [Google Scholar]
- Li, W.; Jang, D.M.; An, S.Y.; Kim, D.; Hong, S.-K.; Kim, H. Polyaniline-chitosan nanocomposite: High performance hydrogen sensor from new principle. Sens. Actuators B 2011, 160, 1020–1025. [Google Scholar] [CrossRef]
- Yang, S.; Tirmizi, S.A.; Burns, A.; Barney, A.A.; Risen, W.M. Chitaline materials: Soluble chitosan-polyaniline copolymers and their conductive doped forms. Synth. Met. 1989, 32, 191–200. [Google Scholar] [CrossRef]
- Ramaprasad, A.T.; Rao, V.; Sanjeev, G.; Ramanani, S.P.; Sabharwal, S. Grafting of polyaniline onto the radiation crosslinked chitosan. Synth. Met. 2009, 159, 1983–1990. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Simiari, J.; Farhadpour, B. Chemical and electrochemical grafting of polyaniline onto chitosan. Iran. Polym. J. 2009, 18, 3–13. [Google Scholar]
- Zhao, S.; Xu, H.; Wang, L.; Zhu, P.; Risen, W.M.; Suggs, J.W. Synthesis of novel chitaline–silica aerogels with spontaneous Au and Ag nanoparticles formation in aerogels matrix. Micropor. Mesopor. Mat. 2013, 171, 147–155. [Google Scholar] [CrossRef]
- Lee, G.-W.; Park, M.; Kim, J.; Lee, J.I.; Yoon, H.G. Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos. Appl. Sci. Manuf. 2006, 37, 727–734. [Google Scholar] [CrossRef]
- Xue, C.; Wilson, L.D. An overview of the design of chitosan-based fiber composite materials. J. Compos. Sci. 2021, 5, 160. [Google Scholar] [CrossRef]
- Mohamed, M.H.; Dolatkhah, A.; Aboumourad, T.; Dehabadi, L.; Wilson, L.D. Investigation of templated and supported polyaniline adsorbent materials. RSC Adv. 2015, 5, 6976–6984. [Google Scholar] [CrossRef]
- Udoetok, I.A.; Wilson, L.D.; Headley, J.V. Self-assembled and cross-linked animal and plant-based polysaccharides: Chitosan–cellulose composites and their anion uptake properties. ACS Appl. Mater. Interfaces 2016, 8, 33197–33209. [Google Scholar] [CrossRef]
- Cabuk, M.; Yavuz, M.; Unal, H.I. Electrokinetic properties of biodegradable conducting polyaniline-graft-chitosan copolymer in aqueous and non-aqueous media. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 494–501. [Google Scholar] [CrossRef]
- Casey, L.S.; Wilson, L.D. Investigation of chitosan-PVA composite films and their adsorption properties. J. Geosci. Environ. Prot. 2015, 3, 78–84. [Google Scholar] [CrossRef]
- He, Y.; Zhu, B.; Inoue, Y. Hydrogen bonds in polymer blends. Prog. Polym. Sci. 2004, 29, 1021–1051. [Google Scholar] [CrossRef]
- Wang, T.; Gunasekaran, S. State of water in chitosan–PVA hydrogel. J. Appl. Polym. Sci. 2006, 101, 3227–3232. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K. Spectroscopic, thermal and electrical properties of sulphonic acids doped poly (o-anisidine) and their application as humidity sensor. Sens. Actuators B 2005, 107, 791–797. [Google Scholar] [CrossRef]
- McGovern, S.T.; Spinks, G.M.; Wallace, G.G. Micro-humidity sensors based on a processable polyaniline blend. Sens. Actuators B 2005, 107, 657–665. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. The Polyanilines: A Novel Class of Conducting Polymers; Technical Report No. 1992-35; University of Pennsylvania: Philadelphia, PA, USA, 1992; pp. 1–28. [Google Scholar]
- Feng, C.D.; Sun, S.L.; Wang, H.; Segre, C.U.; Stetter, J.R. Humidity sensing properties of nation and sol–gel derived SiO2/Nafion composite thin films. Sens. Actuators B 1997, 40, 217–222. [Google Scholar] [CrossRef]
- Nechtschein, M.; Santier, C.; Travers, J.P.; Chroboczek, J.; Alix, A.; Ripert, M. Water effects in polyaniline: NMR and transport properties. Synth. Met. 1987, 18, 311–316. [Google Scholar] [CrossRef]
- Chiang, J.C.; MacDiarmid, A.G. Polyaniline: Protonic acid doping of the emeraldine form to the metallic regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Angelopoulos, M.; Ray, A.; McDiarmid, A.G. Polyaniline: Processability from aqueous solutions and effect of water vapor on conductivity. Synth. Met. 1987, 21, 21–30. [Google Scholar] [CrossRef]
- Travers, J.P.; Nechtschein, M. Water effects in polyaniline: A new conduction process. Synth. Met. 1987, 21, 135–141. [Google Scholar] [CrossRef]
- Abdi, M.M.; Liyana, R.; Md Tahir, P.; Heng, L.Y.; Sulaiman, Y.; Waheeda, N.F.; Abu Hassan, N. Physical and structural properties of polyaniline/microcrystalline cellulose nanocomposite. AIP Conf. Proceed. 2017, 1901, 100018. [Google Scholar]
- Abdi, M.M.; Md Tahir, P.; Liyana, R.; Javahershenas, R. A surfactant directed microcrystalline cellulose/polyaniline composite with enhanced electrochemical properties. Molecules 2018, 23, 2470. [Google Scholar] [CrossRef]
- Zhang, Z.; Chu, F. Study on conductivity of the composites of polyaniline/nano-cellulose. In Applied Sciences in Graphic Communication and Packaging, Lecture Notes in Electrical Engineering 477; Springer Nature: Singapore, 2018; pp. 877–883. [Google Scholar]
- Razalli, R.L.; Abdi, M.M.; Tahir, P.M.; Moradbak, A.; Sulaiman, Y.; Heng, L.Y. Polyaniline-modified nanocellulose prepared from Semantan bamboo by chemical polymerization: Preparation and characterization. RSC Adv. 2017, 7, 25191–25198. [Google Scholar] [CrossRef]
- Fei, G.; Wang, Y.; Wang, H.; Ma, Y.; Guo, Q.; Huang, W.; Yang, D.; Shao, Y.; Ni, Y. Fabrication of bacterial cellulose/polyaniline nanocomposite paper with excellent conductivity, strength, and flexibility. ACS Sustain. Chem. Eng. 2019, 7, 8215–8225. [Google Scholar] [CrossRef]
- Pleumphon, C.; Thiangtham, S.; Pechyen, C.; Manuspiya, H.; Ummartyotin, S. Development of conductive bacterial cellulose composites: An approach to bio-based substrates for solar cells. J. Biobased Mater. Bioenergy 2017, 11, 321–329. [Google Scholar] [CrossRef]
- Tissera, N.D.; Wijesena, R.N.; Rathnayake, S.; de Silva, R.M.; de Silva, K.M.N. Heterogeneous in situ polymerization of polyaniline (PANI) nanofibers on cotton textiles: Improved electrical conductivity, electrical switching, and tuning properties. Carbohydr. Polym. 2018, 186, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Sanches, A.O.; Medeiros, E.S.; Mattoso, L.H.C.; McMahan, C.M.; Malmonge, J.A. Nanocomposites of natural rubber and polyaniline-modified cellulose nanofibrils. J. Therm. Anal. Calorim. 2014, 117, 387–392. [Google Scholar] [CrossRef]
- Takano, T.; Tagaya, M.; Kobayashi, T. Dual-layer hollow fiber of polyaniline–cellulose acetate prepared with simple wet technique of chemical polymerization of aniline. Polym. Bull. 2013, 70, 3019–3030. [Google Scholar] [CrossRef]
- De Paoli, M.A.; Duek, E.R.; Rodrigues, M.A. Poly(aniline)/cellulose acetate composites: Conductivity and electrochromic properties. Synth. Met. 1991, 41–43, 973–978. [Google Scholar] [CrossRef]
- Megha, R.; Ravikiran, Y.T.; Kotresh, S.; Vijaya Kumari, S.C.; Raj Prakash, H.G.; Thomas, S. Carboxymethyl cellulose: An efficient material in enhancing alternating current conductivity of HCl doped polyaniline. Cellulose 2018, 25, 1147–1158. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Abd El-Aziz, M.E.; Kamel, S.; Turky, G. Synthesis and characterization of polyaniline/tosylcellulose stearate composites as promising semiconducting materials. Synth. Met. 2018, 236, 44–53. [Google Scholar] [CrossRef]
- Janaki, V.; Vijayaraghavan, K.; Oh, B.-T.; Ramasamy, A.K.; Kamala-Kannan, S. Synthesis, characterization and application of cellulose/polyaniline nanocomposite for the treatment of simulated textile effluent. Cellulose 2013, 20, 1153–1166. [Google Scholar] [CrossRef]
- Saikia, A.; Karak, N. Cellulose nanofiber-polyaniline nanofiber-carbon dot nanohybrid and its nanocomposite with sorbitol based hyperbranched epoxy: Physical, thermal, biological and sensing properties. Colloids Surf. A Physicochem. Eng. Asp. 2020, 584, 124049. [Google Scholar] [CrossRef]
- Kim, H.; Song, J.E.; Silva, C.; Kim, H.R. Production of conductive bacterial cellulose-polyaniline membranes in the presence of metal salts. Text. Res. J. 2020, 90, 1517–1526. [Google Scholar] [CrossRef]
- Alonso, E.; Faria, M.; Mohammadkazemi, F.; Resnik, M.; Ferreira, A.; Cordeiro, N. Conductive bacterial cellulose-polyaniline blends: Influence of the matrix and synthesis conditions. Carbohydr. Polym. 2018, 183, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Jasim, A.; Ullah, M.W.; Shi, Z.; Lin, X.; Yang, G. Fabrication of bacterial cellulose/polyaniline/single-walled carbon nanotubes membrane for potential application as biosensor. Carbohydr. Polym. 2017, 163, 62–69. [Google Scholar] [CrossRef]
- Alonso, E.; Faria, M.; Ferreira, A.; Cordeiro, N. Influence of the matrix and polymerization methods on the synthesis of BC/PANi nanocomposites: An IGC study. Cellulose 2018, 25, 2343–2354. [Google Scholar] [CrossRef]
- Wan, Y.; Li, J.; Yang, Z.; Ao, H.; Xiong, L.; Luo, H. Simultaneously depositing polyaniline onto bacterial cellulose nanofibers and graphene nanosheets toward electrically conductive nanocomposites. Curr. Appl. Phys. 2018, 18, 933–940. [Google Scholar] [CrossRef]
- Yue, L.-N.; Zheng, Y.-D.; Luan, J.; Yu, Y. Effects of water content on preparation and properties of bacterial cellulose/polyaniline composite gel-membranes. Acta Polym. Sin. 2014, 9, 1228–1237. [Google Scholar]
- Zhou, Z.; Yang, Y.; Han, Y.; Guo, Q.; Zhang, X.; Lu, C. In situ doping enables the multifunctionalization of templately synthesized polyaniline@cellulose nanocomposites. Carbohydr. Polym. 2017, 177, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Marins, J.A.; Soares, B.G.; Dahmouche, K.; Ribeiro, S.J.L.; Barud, H.; Bonemer, D. Structure and properties of conducting bacterial cellulose-polyaniline nanocomposites. Cellulose 2011, 18, 1285–1294. [Google Scholar] [CrossRef]
- Lee, H.-J.; Chung, T.-J.; Kwon, H.-J.; Kim, H.-J.; Yin Tze, W.T. Fabrication and evaluation of bacterial cellulose-polyaniline composites by interfacial polymerization. Cellulose 2012, 19, 1251–1258. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of bacterial cellulose based biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef]
- Müller, D.; Mandelli, J.S.; Marins, J.A.; Soares, B.G.; Porto, L.M.; Rambo, C.R.; Barra, G.M.O. Electrically conducting nanocomposites: Preparation and properties of polyaniline (PANI)-coated bacterial cellulose nanofibers (BC). Cellulose 2012, 19, 1645–1654. [Google Scholar] [CrossRef]
- Gautam, V.; Srivastava, A.; Singh, K.P.; Yadav, V.L. Vibrational and gravimetric analysis of polyaniline/polysaccharide composite materials. Polym. Sci. A 2016, 58, 206–219. [Google Scholar] [CrossRef]
- Pandi, N.; Sonawane, S.H.; Gumfekar, S.P.; Kola, A.K.; Borse, P.H.; Ambade, S.B.; Guptha, S.; Ashokkumar, M. Electrochemical performance of starch-polyaniline nanocomposites synthesized by sonochemical process intensification. J. Renew. Mater. 2019, 7, 1279–1293. [Google Scholar] [CrossRef]
- Alkhursani, S.A.; Ghobashy, M.M.; Madani, M. Radiation synthesis of organostarch as fluorescence label. Asian J. Chem. 2020, 32, 1799–1805. [Google Scholar] [CrossRef]
- Sarma, T.K.; Chattopadhyay, A. Reversible encapsulation of nanometer-size polyaniline and polyaniline−Au-nanoparticle composite in starch. Langmuir 2004, 20, 4733–4737. [Google Scholar] [CrossRef] [PubMed]
- Sammanan, B.; Sekar, J.R.; Thavasikan, J. Synthesis and characterization of polyacid doped conducting and non-conducting polymers: A comparative study. Asian J. Chem. 2018, 30, 799–803. [Google Scholar] [CrossRef]
- Noreen, S.; Bhatti, H.N.; Iqbal, M.; Hussain, F.; Sarim, F.M. Chitosan, starch, polyaniline and polypyrrole biocomposite with sugarcane bagasse for the efficient removal of Acid Black dye. Int. J. Biol. Macromol. 2020, 147, 439–452. [Google Scholar] [CrossRef]
- Sapurina, I.; Stejskal, J. The mechanism of the oxidative polymerization of aniline and the formation of supramolecular polyaniline structures. Polym. Int. 2008, 57, 1295–1325. [Google Scholar] [CrossRef]
- Jian, M.; Wang, C.; Wang, Q.; Wang, H.; Xia, K.; Yin, Z.; Zhang, M.; Liang, X.; Zhang, Y. Advanced carbon materials for flexible and wearable sensors. Sci. China Mater. 2017, 60, 1026–1062. [Google Scholar] [CrossRef]
- He, S.; Chen, P.; Qiu, L.; Wang, B.; Sun, X.; Xu, Y.; Peng, H. A mechanically actuating carbon-nanotube fiber in response to water and moisture. Angew. Chem. Int. Ed. 2015, 54, 14880–14884. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Zhao, Y.; Hu, C.; Zhang, Z.; Chen, N.; Jiang, L.; Qu, L. Graphene fibers with predetermined deformation as moisture-triggered actuators and robots. Angew. Chem. Int. Ed. 2013, 52, 10482–10486. [Google Scholar] [CrossRef]
- Kundu, S.; Majumder, R.; Ghosh, R.; Pradhan, M.; Roy, S.; Singha, P.; Ghosh, D.; Banerjee, A.; Banerjee, D.; Chowdhury, M.P. Relative humidity sensing properties of doped polyaniline-encased multiwall carbon nanotubes: Wearable and flexible human respiration monitoring application. J. Mater. Sci. 2020, 55, 3884–3901. [Google Scholar] [CrossRef]
- Bibi, A.; Rubio, Y.R.M.; Santiago, K.S.; Jia, H.-W.; Ahmed, M.M.M.; Lin, Y.-F.; Yeh, J.-M. H2S-sensing studies using interdigitated electrode with spin-coated carbon aerogel-polyaniline composites. Polymers 2021, 13, 1457. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Karouei, S.; Milani Moghaddam, H.; Saadat Niavol, S. Characterization and gas sensing properties of graphene/polyaniline nanocomposite with long-term stability under high humidity. J. Mater. Sci. 2021, 56, 4239–4253. [Google Scholar] [CrossRef]
- Li, C.; Yang, S.; Guo, Y.; Huang, H.; Chen, H.; Zuo, X.; Fan, Z.; Liang, H.; Pan, L. Flexible, multi-functional sensor based on all-carbon sensing medium with low coupling for ultrahigh-performance strain, temperature and humidity sensing. Chem. Eng. J. 2021, 426, 130364. [Google Scholar] [CrossRef]
- Liu, K.; Yang, P.; Li, S.; Li, J.; Ding, T.; Xue, G.; Chen, Q.; Feng, G.; Zhou, J. Induced potential in porous carbon films through water vapor absorption. Angew. Chem. Int. Ed. 2016, 55, 8003–8007. [Google Scholar] [CrossRef]
- Dehabadi, L.; Karoyo, A.H.; Wilson, L.D. Spectroscopic and thermodynamic study of biopolymer adsorption phenomena in heterogeneous solid−liquid systems. ACS Omega 2018, 3, 15370–15379. [Google Scholar] [CrossRef] [PubMed]
- Miege, A.; Steffen, F.; Luschtinetz, T.; Jakubith, S.; Freitag, M. Real time water detection for adaptive control strategy in pemfc-systems. Energy Procedia 2012, 29, 431–437. [Google Scholar] [CrossRef][Green Version]
- Blokzijl, W.; Engberts, J.B.F.N. Hydrophobic effects. Opinions and facts. Angew. Chem. Int. Ed. Engl. 1993, 32, 1545–1579. [Google Scholar] [CrossRef]
- Biedermann, F.; Nau, W.M.; Schneider, H.-J. The hydrophobic effect revisited—studies with supramolecular complexes imply high-energy water as a noncovalent driving force. Angew. Chem. Int. Ed. 2014, 53, 2–16. [Google Scholar] [CrossRef]
- Smithrud, D.B.; Wyman, T.B.; Diederich, F. Enthalpically driven cyclophane–arene inclusion complexation: Solvent-dependent calorimetric studies. J. Am. Chem. Soc. 1991, 113, 5420–5426. [Google Scholar] [CrossRef]
- Merzel, F.; Avbelj, F. Why do water molecules around small hydrophobic solutes form stronger hydrogen bonds than in the bulk? BBA-Gen. Subj. 2020, 1864, 129537. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yukiyama, T.; Fujisawa, M. Thermodynamic properties of inclusion complexes of α-cyclodextrin + aliphatic nitriles (H(CH2)nCN: n = 1–8) in aqueous solution. J. Therm. Anal. Calorim. 2012, 108, 695–704. [Google Scholar] [CrossRef]
- Betzel, C.; Saenger, W.; Hingerty, B.E.; Brown, G.M. Topography of cyclodextrin inclusion complexes, part 20. Circular and flip-flop hydrogen bonding in beta-cyclodextrin undecahydrate: A neutron diffraction study. J. Am. Chem. Soc. 1984, 106, 7545–7557. [Google Scholar] [CrossRef]
- Koehler, J.E.H.; Saenger, W.; van Gunsteren, W.F. The flip-flop hydrogen bonding phenomenon. Molecular dynamics simulation of crystalline β-cyclodextrin. Eur. Biophys. J. 1988, 16, 153–168. [Google Scholar] [CrossRef]
- Vaitheeswaran, S.; Yin, H.; Rasaiah, J.C.; Hummer, G. Water clusters in nonpolar cavities. Proc. Natl. Acad. Sci. USA 2004, 101, 17002–17005. [Google Scholar] [CrossRef]
- Thiessen, A.N.; Verbeek, W.; Gritter, K.; Ooms, K.J. Assessment of the sensitivity of DQF/ZQF 2H NMR of D2O for studying modified Nafion membranes at 20 °C and 80 °C. Solid State Nucl. Magn. Reson. 2018, 93, 1–6. [Google Scholar] [CrossRef]
- Alix, A.; Lemoine, V.; Nechtschein, M.; Travers, J.P.; Menardo, C. Water absorption study in polyaniline. Synth. Met. 1989, 29, 457–462. [Google Scholar] [CrossRef]
- Gocho, H.; Shimizu, H.; Tanioka, A.; Chou, T.-J.; Nakajima, T. Effect of polymer chain end on sorption isotherm of water by chitosan. Carbohydr. Polym. 2000, 41, 87–90. [Google Scholar] [CrossRef]
- Aizamddin, M.F.; Roslan, N.C.; Kamarudin, M.A.; Omar, S.N.I.; Safian, M.F.; Halim, M.I.A.; Mahat, M.M. Study of conductivity and thermal properties of polyaniline doped with p-toluene sulfonic acid. Malays. J. Anal. Sci. 2020, 24, 413–421. [Google Scholar]
- Ming Li, M.; Gu, S.; Jin, J.; Zheng, Y.; Xie, D. Research on the influence of polyanionic cellulose on the microstructure and properties of oil well cement. Constr. Build. Mater. 2020, 259, 119841. [Google Scholar]
- Qiao, D.; Zhang, B.; Huang, J.; Xie, F.; Wang, D.K.; Jiang, F.; Zhao, S.; Zhu, J. Hydration-induced crystalline transformation of starch polymer under ambient conditions. Int. J. Biol. Macromol. 2017, 103, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, A.; Htoon, A.; Gilbert, E.P. Influence of extrusion and digestion on the nanostructure of high-amylose maize starch. Biomacromolecules 2007, 8, 1564–1572. [Google Scholar] [CrossRef]
- Chanajaree, R.; Sriuttha, M.; Lee, V.S.; Wittayanarakul, K. Thermodynamics and kinetics of cationic/anionic dyes adsorption on cross-linked chitosan. J. Mol. Liq. 2021, 322, 114507. [Google Scholar] [CrossRef]
- Alghunaim, N.S. Effect of hydration on the electronic properties of Si/PANi/3ZnO nanocomposite. J. Inorg. Organomet. Polym. Mater. 2020, 30, 451–456. [Google Scholar] [CrossRef]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular view into the cyclodextrin cavity: Structure and hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef]
- Grosu, I.A.; Filip, X.; Miclăuș, M.O.; Filip, C. Hydrogen-mediated noncovalent interactions in solids: What can nmr crystallography tell about? Molecules 2020, 25, 3757. [Google Scholar] [CrossRef]
- Eggers, D.K.; Fu, S.; Ngo, D.V.; Vuong, E.H.; Brotin, T. Thermodynamic contribution of water in cryptophane host−guest binding reaction. J. Phys. Chem. B 2020, 124, 6585–6591. [Google Scholar] [CrossRef]
- Hähnel, T.; Möllmer, J.; Klauck, M.; Kalies, G. Vapor adsorption and liquid immersion experiments on carbon molecular sieves. Adsorption 2020, 26, 361–373. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D.; Cree, D.E. Gas and solution uptake properties of graphene oxide-based composite materials: Organic vs. inorganic cross-linkers. J. Compos. Sci. 2019, 3, 80. [Google Scholar] [CrossRef]
- Kraml, J.; Kamenik, A.S.; Waibl, F.; Schauperl, M.; Liedl, K.R. Solvation free energy as a measure of hydrophobicity: Application to serine protease binding interfaces. J. Chem. Theory Comput. 2019, 15, 5872–5882. [Google Scholar] [CrossRef]
- Tang, D.; Dwyer, T.; Bukannan, H.; Blackmon, O.; Delpo, C.; Barnett, J.W.; Gibb, B.C.; Ashbaugh, H.S. Pressure induced wetting and dewetting of the nonpolar pocket of deep-cavity cavitands in water. J. Phys. Chem. B 2020, 124, 4781–4792. [Google Scholar] [CrossRef] [PubMed]
- Lyuleeva, A.; Helbich, T.; Bobinger, M.; Rieger, B.; Becherer, M.; Lugli, P.; Rivadeneyra, A. Functionalized and oxidized silicon nanosheets: Customized design for enhanced sensitivity towards relative humidity. Sens. Actuators B 2019, 283, 451–457. [Google Scholar] [CrossRef]
- Wang, X.-H.; Wang, L.-X.; Jing, X.-B.; Wang, F.-S. Synthesis and characterization of chlorinated polyaniline. Synth. Met. 1995, 69, 149–150. [Google Scholar] [CrossRef]
- Saidani, N.; Morallo, E.; Huerta, F.; Besbes-Hentati, S.; Montilla, F. Electrochemical synthesis of fluorinated polyanilines. Electrochim. Acta 2020, 348, 136329. [Google Scholar] [CrossRef]
- Skopalová, K.; Capáková, Z.; Bober, P.; Pelková, J.; Stejskal, J.; Kašpárková, V.; Lehocký, M.; Junkar, I.; Mozetič, M.; Humpolíček, P. In-vitro hemocompatibility of polyaniline functionalized by bioactive molecules. Polymers 2019, 11, 1861. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Hung, H.-L.; Han, C.-C.; Chiu, H.-C. Correlation between nanoscale elasticity, semiconductivity, and structural order in functionalized polyaniline thin films. Langmuir 2020, 36, 4153–4164. [Google Scholar] [CrossRef]
- Chen, X.P.; Jiang, J.K.; Liang, Q.H.; Yang, N.; Ye, H.Y.; Cai, M.; Shen, L.; Yang, D.G.; Ren, T.L. First-principles study of the effect of functional groups on polyaniline backbone. Sci. Rep. 2015, 5, 16907. [Google Scholar] [CrossRef]
- Noboru Yamazoe, N.; Yasuhiro Shimizu, Y. Humidity sensors: Principles and applications. Sens. Actuators 1986, 10, 379–398. [Google Scholar] [CrossRef]
- Packirisamy, M.; Stiharu, I.; Li, X.; Rinaldi, G. A polyimide based resistive humidity sensor. Sens. Rev. 2005, 25, 271–276. [Google Scholar] [CrossRef]
- Harris, F.K. (Ed.) Electrical Measurements; Wiley: New York, NY, USA, 1952. [Google Scholar]
- Singh, Y. Electrical resistivity measurements: A review. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 745–756. [Google Scholar] [CrossRef]
- Wenner, F. The four-terminal conductor and the Thomson bridge. Bull. Bur. Stand. 1912, 8, 559–610. [Google Scholar] [CrossRef]
- Chandra, H.; Allen, S.W.; Oberloier, S.W.; Bihari, N.; Gwamuri, J.; Pearce, J.M. Open-source automated mapping four-point probe. Materials 2017, 10, 110. [Google Scholar] [CrossRef]
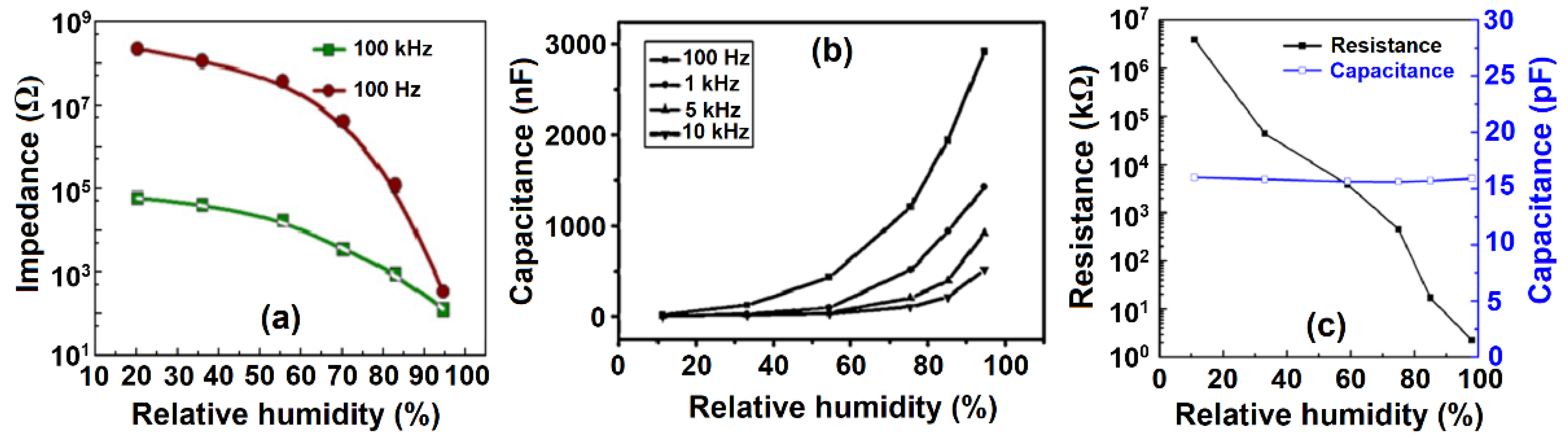
- Chethan, B.; Raj Prakash, H.G.; Ravikiran, Y.T.; Vijayakumari, S.C.; Ramanad, C.H.V.V.; Thomas, S.; Kim, D. Enhancing humidity sensing performance of polyaniline/water soluble graphene oxide composite. Talanta 2019, 196, 337–344. [Google Scholar] [CrossRef]
- Nagaraju, S.C.; Roy, A.S.; Kumar, J.B.P.; Anilkumar, K.R.; Ramagopal, G. Humidity sensing properties of surface modified polyaniline metal oxide composites. J. Eng. 2014, 2014, 925020. [Google Scholar] [CrossRef]
- Lavrič, P.K.; Warmoeskerken, M.M.C.G.; Jocic, D. Functionalization of cotton with poly-NiPAAm/chitosan microgel. Part I. Stimuli-responsive moisture management properties. Cellulose 2012, 19, 257–271. [Google Scholar] [CrossRef]
- Box, G.E.P.; Behnken, D.W. Some new three level designs for the study of quantitative variables. Technometrics 1960, 2, 455–475. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Salehi, R.; Irani, M.; Rashidi, M.-R.; Aroujalian, A.; Raisi, A.; Eskandani, M.; Haririand, I.; Davaran, S. Stimuli-responsive nanofibers prepared from poly(N-isopropylacrylamide-acrylamide-vinylpyrrolidone) by electrospinning as an anticancer drug delivery. Des. Monomers Polym. 2013, 16, 515–527. [Google Scholar] [CrossRef]
- Hierlemann, A.; Weimar, U.; Kraus, G.; Schweizer-Berberich, M.; Göpel, W. Polymer-based sensor arrays and multicomponent analysis for the detection of hazardous organic vapours in the environment. Sens. Actuators B 1995, 26–27, 126–134. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Ravi, R.; Tharanathan, R.N. Effect of storage conditions on the tensile properties of eco-friendly chitosan films by response surface methodology. J. Food Eng. 2007, 80, 184–189. [Google Scholar] [CrossRef]
- Ruotolo, L.A.M.; Gubulin, J.C. A factorial-design study of the variables affecting the electrochemical reduction of Cr(VI) at polyaniline-modified electrodes. Chem. Eng. J. 2005, 110, 113–121. [Google Scholar] [CrossRef]
- Czitrom, V. One-Factor-at-a-Time versus Designed Experiments. Am. Stat. 1999, 53, 126–131. [Google Scholar]
- Li, S.; He, Y.; Guan, Y.; Liu, X.; Liu, H.; Xie, M.; Zhou, L.; Wei, C.; Yu, C.; Chen, Y. Cellulose nanofibril-stabilized Pickering emulsion and in situ polymerization lead to hybrid aerogel for high-efficiency solar steam generation. ACS Appl. Polym. Mater. 2020, 2, 4581–4591. [Google Scholar] [CrossRef]
- Shukla, S.K. Synthesis of polyaniline grafted cellulose suitable for humidity sensing. Indian J. Eng. Mater. Sci. 2012, 19, 417–420. [Google Scholar]



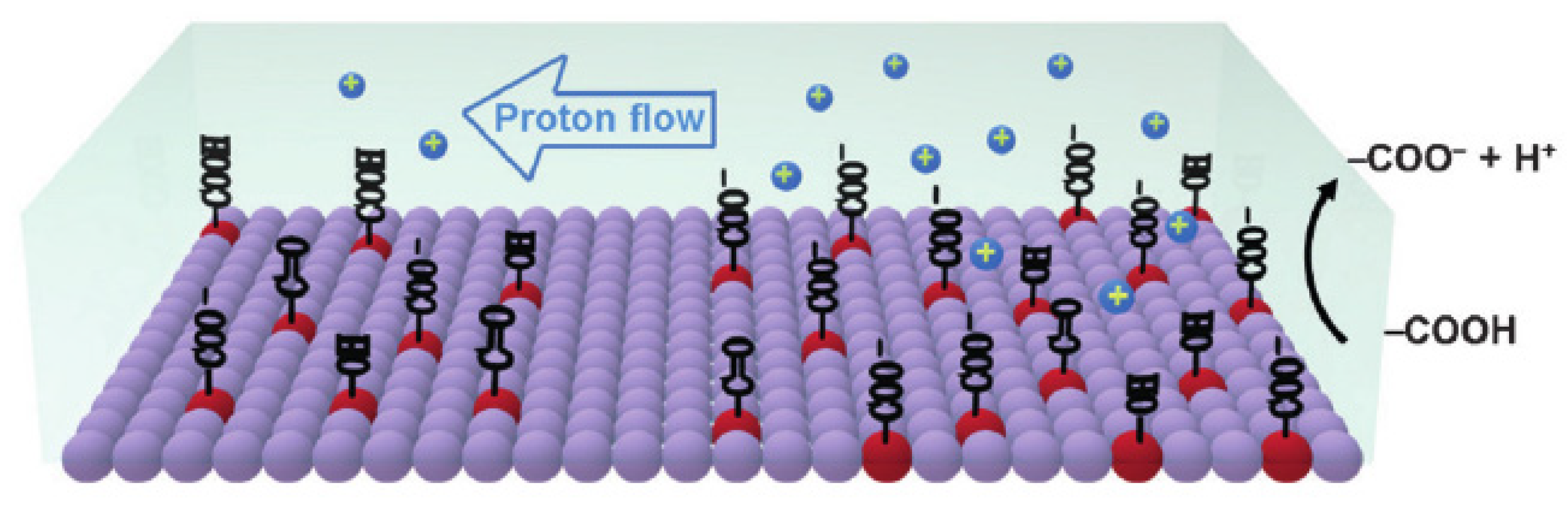
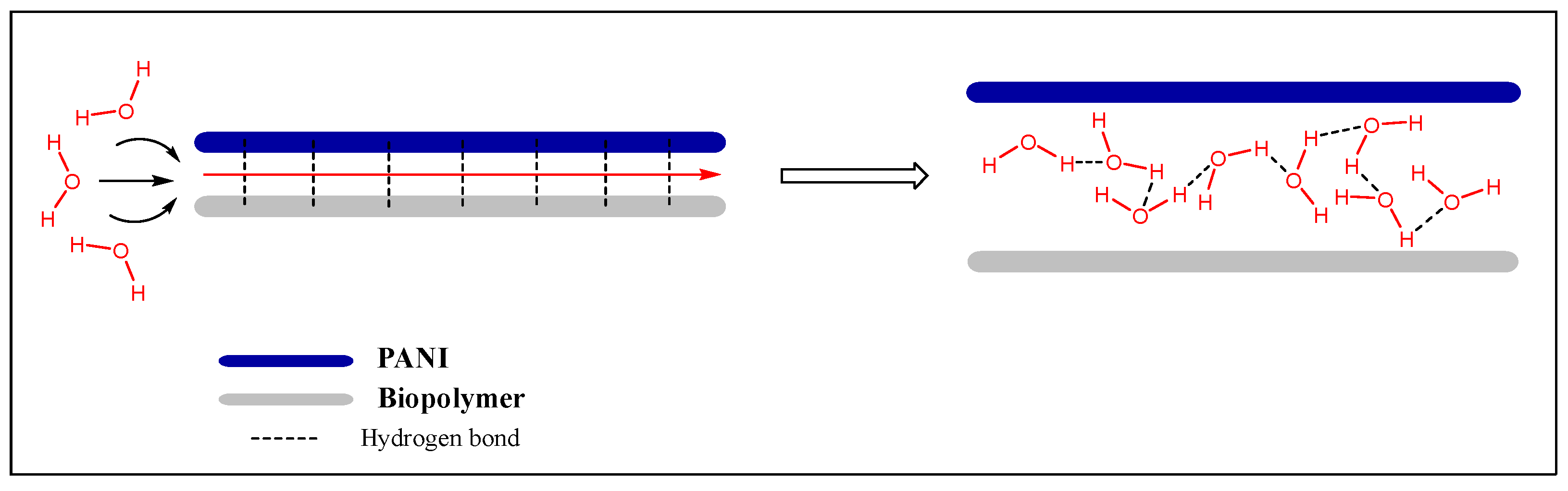

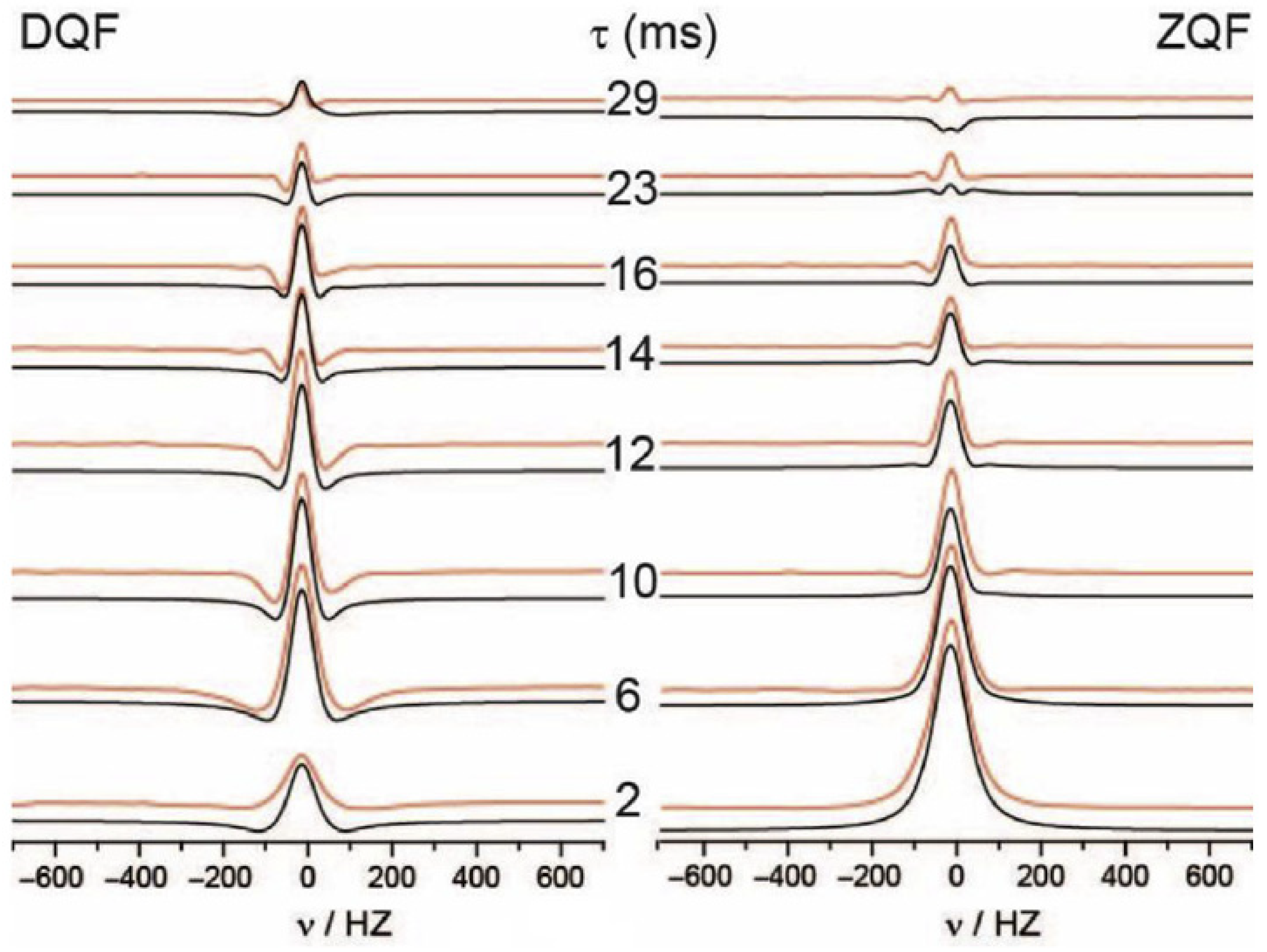

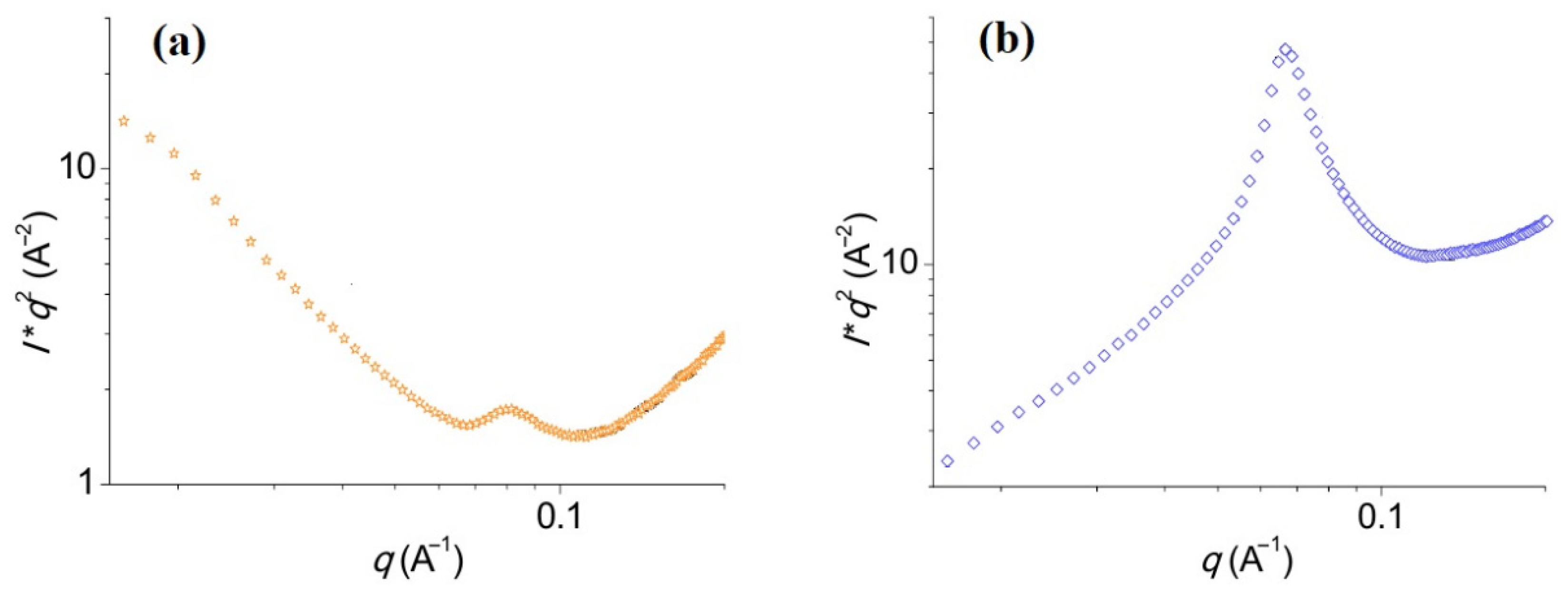
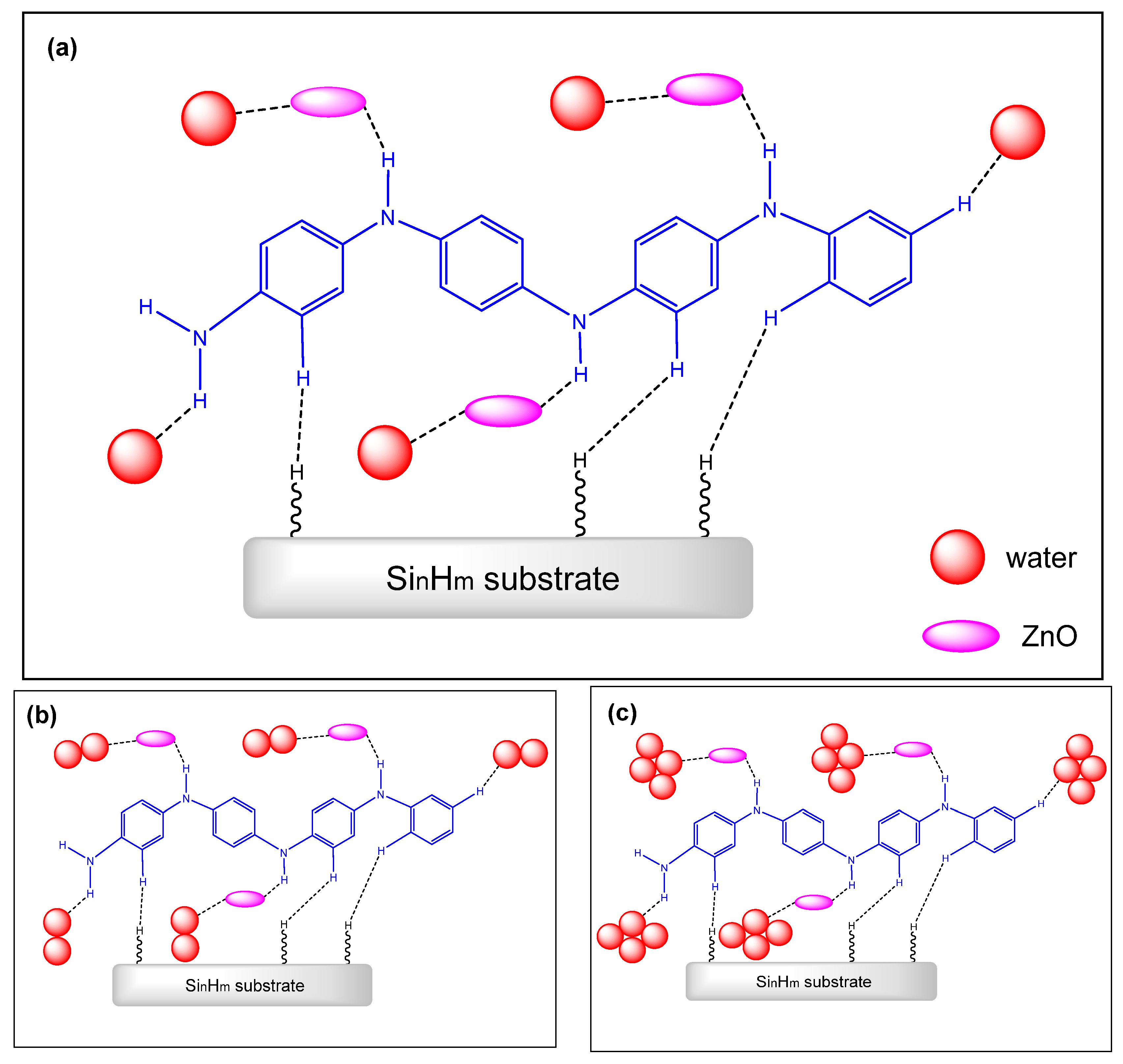
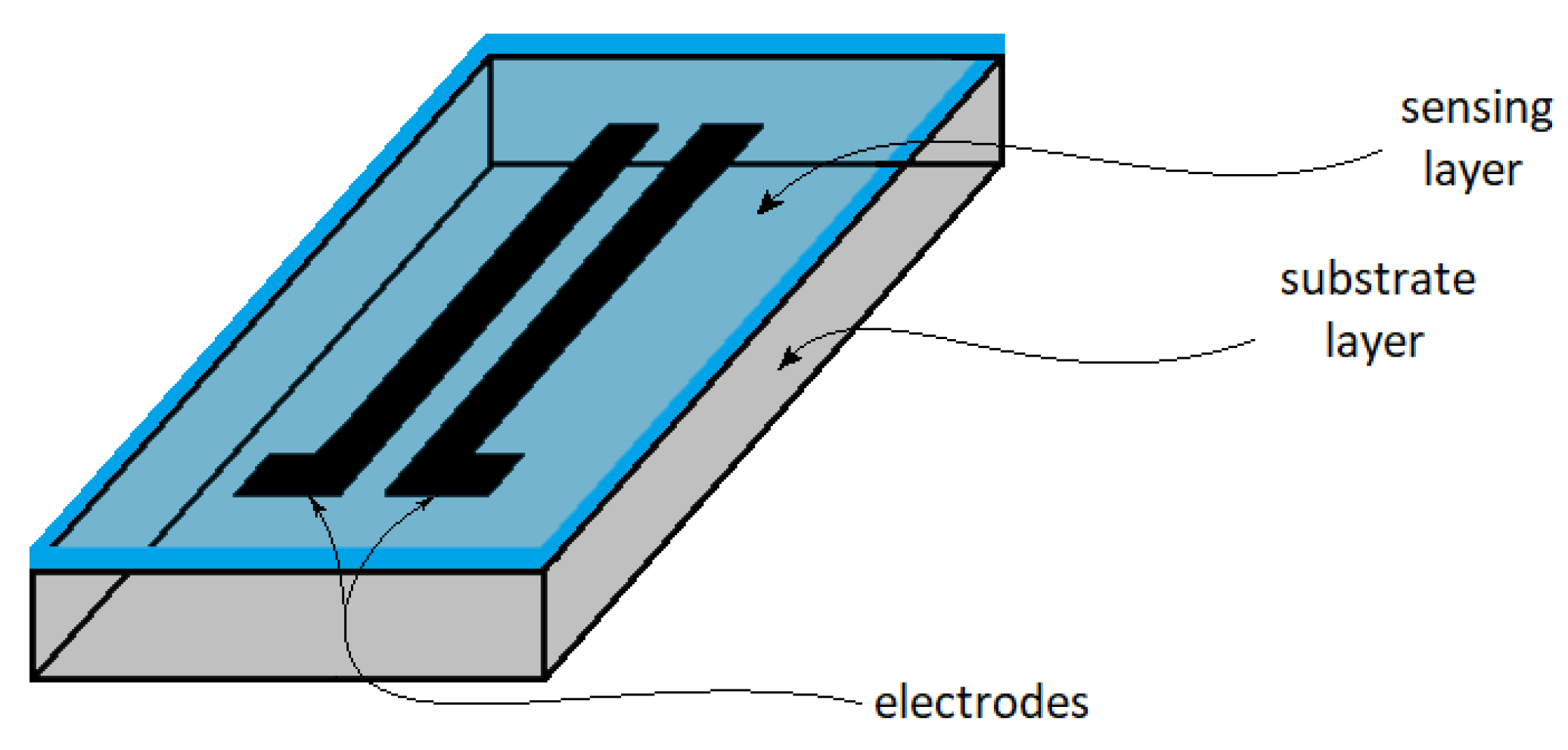
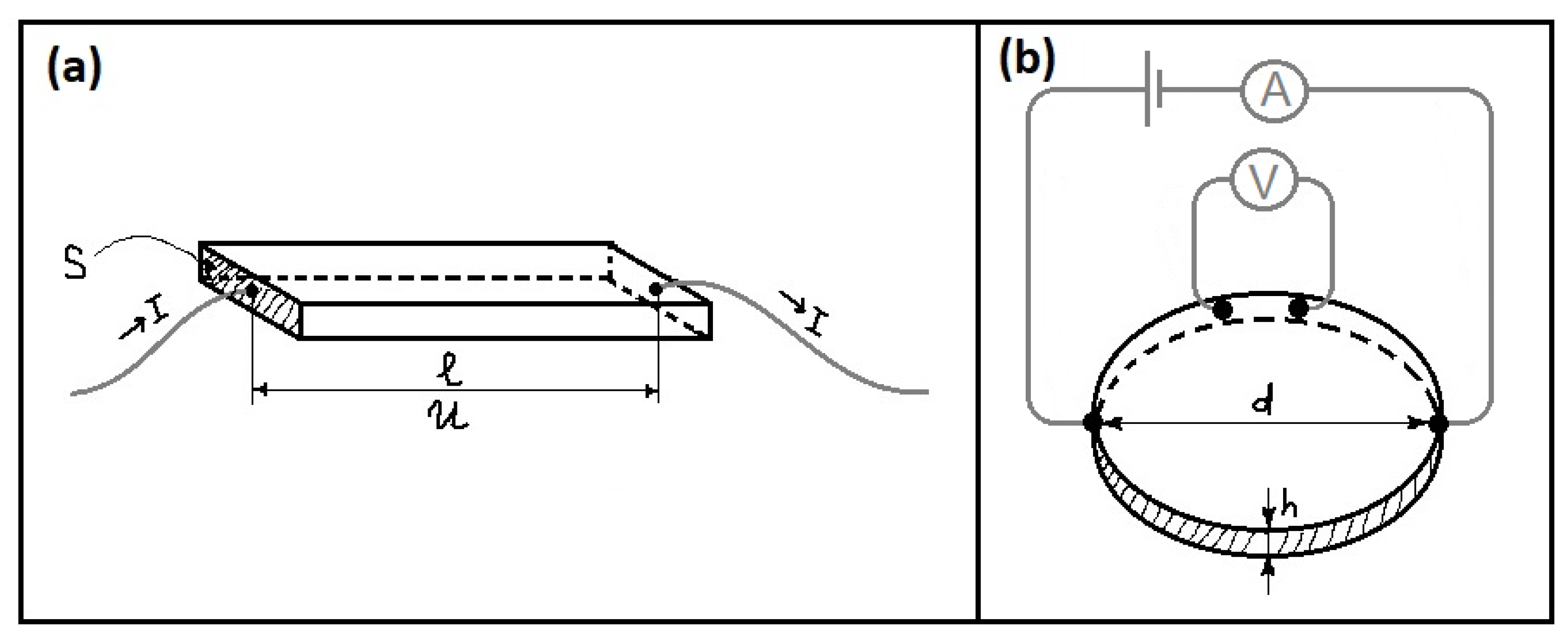
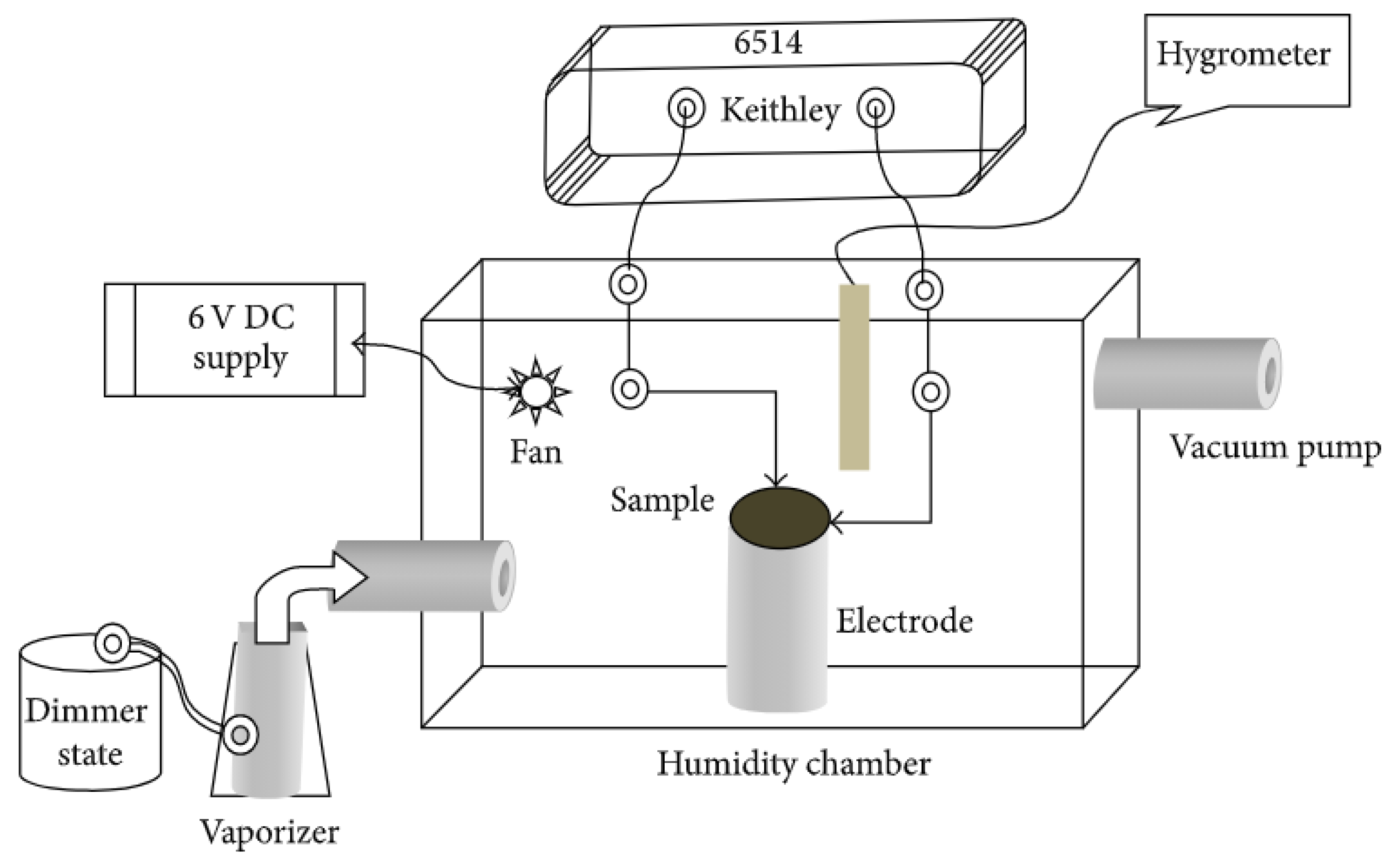
| Type | Resistive | Capacitive | Thermal | |
|---|---|---|---|---|
| Properties | ||||
| Basis | Conducting moisture-absorbing film | Two conducting plates with a hygroscopic dielectric located in between | Two thermal sensors: one encased in dry air (or N2), where another is exposed to humid air | |
| Measurand | Resistance | Capacitance | Temperature | |
| RH range (%) | 5–90 | Full range (0–100) | Measures the absolute humidity in g/m3 | |
| Temperature range (°C) | −40–100 | −50–150 | 90–300 | |
| Accuracy | Moderate | High | Depends on the temperature, where a value above 90 °C is optimal | |
| Price | Low to moderate | High | Varies | |
| Potential applications | Smart food packaging, automotive devices, residential environments | Medicine, scientific research | Industry, machinery; clothes, textile and wood drying | |
| Material Type | Classification | Sub-Classification | Examples |
|---|---|---|---|
| Polymer | Polymer-electrolyte | Quaternary ammonium, sulfonate and phosphonium salts | AEPAB/styrene [35] PEDOT:PSS/GO [36] VBTPC/n-butylacrylate [37] |
| Conducting polymers | Polyaniline, polypyrrole, poly(ortho-phenylenediamine), polythiophene | PANI/CHN [10] PPy/graphene oxide [14] PoPD/PANI [15] P3HT/Cu3(BTC)2 [16] | |
| Ceramic | Electronic/ionic conduction mechanism | Perovskites | MnPS3 [1], BaTiO3 [2], Ba0.5Sr0.5TiO3 [3] |
| Thick film ceramics | Gd-doped CeO2 [4], Fe2O3/SiO2 [5] | ||
| Cation-doped ceramics | Na+/K+-doped Ga2O3 [6], La3+-doped BaSnO3 [7] | ||
| Thin-film ceramics | Mn1.2Co1.5Ni0.3O4 thin film [8], WO3/TiO2 thin films [9] | ||
| Polymer/Ceramic | Electronic/ionic conduction mechanism | Polymer/metal | PVP/Ag [38], PVA/Ag [38], PVP/Au [39], PANI/PTFMA/Ag [40] |
| Polymer/metal oxide | PVA/SnO2 [41], CLNF/ZnO [42], PANI/CuO [43], CHT/CuMn2O4 [44], PDMS/Armalcolite [45] | ||
| Polymer/inorganic salt | PANI/MgCrO4 [46] PPy/Sr3(AsO4)2 [47] SPEEK/CaCl2 [48] PVA-PAA/NbC [49] |
| x Value | Oxidation State | Name | Base Form | Color | Salt Form | Color |
|---|---|---|---|---|---|---|
| 1 | fully reduced | leucoemeraldine | PANI-LB | colorless | PANI-LS | light yellow |
| 0.5 | half-oxidized | emeraldine ** | PANI-EB | blue | PANI-ES | green |
| 0 | fully oxidized | pernigraniline | PANI-PB | violet | PANI-PS | dark blue |
| Specimen | Grain Size (µm) | Pore Size (nm) | Surface Area (m2/g) | Resistivity (Ω·cm) | Conductivity (S/cm) |
|---|---|---|---|---|---|
| MgCr2O4 | 0.2 | 100 | 1.6 | 1.3 × 1010 | 7.7 × 10−11 |
| 7 MgCr2O4·3TiO2 | 1 | 270 | 0.3 | 2.6 × 1012 | 3.8 × 10−12 |
| 3 MgCr2O4·7TiO2 | 4 | 350 | 0.1 | 1.0 × 109 | 1.0 × 10−9 |
| Feature | PANI/Biopolymer | PANI/Carbon |
|---|---|---|
| Conductivity of components | One is non-conductive | Both are conductive |
| Hydrophilicity | Biopolymer is hydrophilic | Carbon is hydrophobic |
| Type of interactions | H- or covalent bonding | van der Waals interactions |
| Size of a fiber | Biopolymer is thin | Carbon is thick |
| Material | Type of Sensor | Response Time (s) | Recovery Time (s) | Hysteresis Error (%) | Ref. |
|---|---|---|---|---|---|
| PANI/carbon sensors | |||||
| PANI/MWCNT | Resistive | 60 | 140 | 0.5 | [149] |
| PANI/CNF/PVA | Capacitive | 41 | 50 | 1 | [12] |
| PANI/CA (H2S) | Resistive | 1 | 960 | — | [150] |
| PANI/G (CO2) | Resistive | 81 | 20 | — | [151] |
| PANI/biopolymer sensors | |||||
| PANI/NFC/PVA | Capacitive | 47 | 58 | 5 | [12] |
| PANI/CHN | Resistive | 30 | 180 | 18–20 * | [10] |
| PANI/CMC | Resistive | 10 | 90 | 2 | [13] |
| Category | Experimental Method | Application | Ref. |
|---|---|---|---|
| Spectroscopy | 2H NMR diffusion | Detection of hydrophilic pockets | [165] |
| 1H NMR | Intramolecular H-bonding by calculation of J-coupling constants | [174] | |
| SEM | Morphology as a function of variable hydration | [169] | |
| SAXS/WAXS | Crystallinity as a function of variable hydration | [170] | |
| NMR crystallography | Noncovalent interactions, detection of labile H atoms | [175] | |
| Raman spectroscopy: D2O spectral probe | Bound-water fraction by detection of HOD uncoupled oscillators via isotopic dilution | [154] | |
| Calorimetry | DSC | Enthalpies of hydration and dehydration (solid-vapor or solid-liquid systems) | [77,168] |
| ITC | Solvent binding enthalpy (solid-liquid system) | [176] | |
| Immersion calorimetry | Immersion enthalpies in water (liquid or vapor phase) | [177] | |
| Thermodynamic | Water vapor adsorption isotherms | Surface area and pore volume at variable temperature for solid-vapor systems | [177,178] |
| Equilibrium dye adsorption | Use of dye probes to estimate the hydrophile-lipophile character of adsorbent and water adsorption capacity | [77] | |
| GIST | Enthalpy and entropy contributions to the free energy of solvation | [179] | |
| Hydration distribution model | Thermodynamics of pocket hydration | [180] | |
| Computational | Quantum-Mechanical DFT | Accumulation of water molecules at hydrophilic sites | [173] |
| Correlation of dye-based adsorption with hydration | [172] | ||
| Physical models | Derivation of the equations for water binding (free energies versus chemical potentials and activities) | [176] |
| −1 | 0 | 1 | |
|---|---|---|---|
| PANI (% w/w) | 25 | 50 | 75 |
| RH, % | 35 | 65 | 95 |
| T, °C | 15 | 25 | 35 |
| Material | Porosity | Electrical Conductivity (S/cm) | Response Time (s) | Recovery Time (s) | Ref. | |
|---|---|---|---|---|---|---|
| BET Surface Area (m2/g) | Pore Diameter (nm) | |||||
| PANI/NFC/PVA | 17 | 17.1 | — | 47 | 58 | [12] |
| PANI/CNF/PVA | 34 | 21.2 | — | 41 | 50 | [12] |
| PANI/CHT/PVA | — | — | 3.9·10−6 | — | — | [77] |
| PANI/NFC/PLA | — | — | — | — | — | [203] |
| PANI/CHN | — | — | 2.2·10−5 | 120 | — | [10] |
| PANI/CMC | — | — | 7.6·10−4 | 10 | 90 | [13,124] |
| PANI/MCC | — | — | 1.25·10−2 | 40 | 60 | [204] |
| PANI/NC | — | — | 0.65 | — | — | [116] |
| PANI/BC | — | — | 0.12 | — | — | [118] |
| PANI/EBC | — | — | 1.1 | — | — | [118] |
| PANI/EBC/PAM | — | — | 1.4 | — | — | [118] |
| PANI/CA | — | — | 4.0·10−3 | — | — | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anisimov, Y.A.; Evitts, R.W.; Cree, D.E.; Wilson, L.D. Polyaniline/Biopolymer Composite Systems for Humidity Sensor Applications: A Review. Polymers 2021, 13, 2722. https://doi.org/10.3390/polym13162722
Anisimov YA, Evitts RW, Cree DE, Wilson LD. Polyaniline/Biopolymer Composite Systems for Humidity Sensor Applications: A Review. Polymers. 2021; 13(16):2722. https://doi.org/10.3390/polym13162722
Chicago/Turabian StyleAnisimov, Yuriy A., Richard W. Evitts, Duncan E. Cree, and Lee D. Wilson. 2021. "Polyaniline/Biopolymer Composite Systems for Humidity Sensor Applications: A Review" Polymers 13, no. 16: 2722. https://doi.org/10.3390/polym13162722
APA StyleAnisimov, Y. A., Evitts, R. W., Cree, D. E., & Wilson, L. D. (2021). Polyaniline/Biopolymer Composite Systems for Humidity Sensor Applications: A Review. Polymers, 13(16), 2722. https://doi.org/10.3390/polym13162722