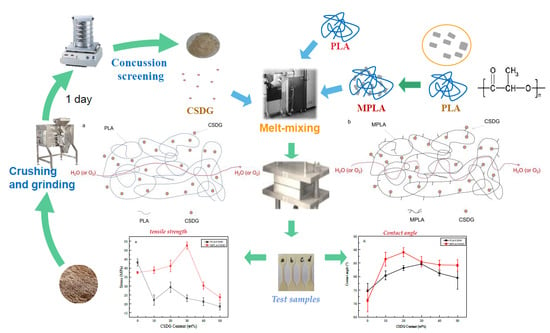
Barrier Properties and Hydrophobicity of Biodegradable Poly(lactic acid) Composites Reinforced with Recycled Chinese Spirits Distiller’s Grains
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Preparation of Composites
- (1)
- Drying of materials: Before the sample preparation, PLA and CSDG were placed in a vacuum oven at a drying temperature of 85 °C for a drying time of 8 h.
- (2)
- Preparation of PLA/CSDG or MPLA/CSDG composites: A torque rheometer (HAAKE PolyLab OS) was used. The conditions at which it was operated were at a temperature of 180 °C and a speed of 100 rpm. First, PLA or MPLA were added to melt them for 1 min. Then, CSDG powder was added, and its content was varied (10, 20, 30, 40, and 50 wt %). The two materials were blended for 10 min to prepare PLA/CSDG or MPLA/CSDG composites with different concentrations of CSDG.
2.3. Fourier Transform Infrared Spectroscopy
2.4. Mechanical Properties
2.5. X-ray Diffraction
2.6. Morphology Characterization
2.7. Thermal Analysis
2.8. Thermogravimetric Analysis
2.9. Oxygen Barrier Properties
2.10. Water Vapor Barrier Properties
2.11. Contact Angle Test
2.12. Water Absorption
2.13. Degradation Performance Test
3. Results and Discussion
3.1. Fourier Transform Infrared Spectra
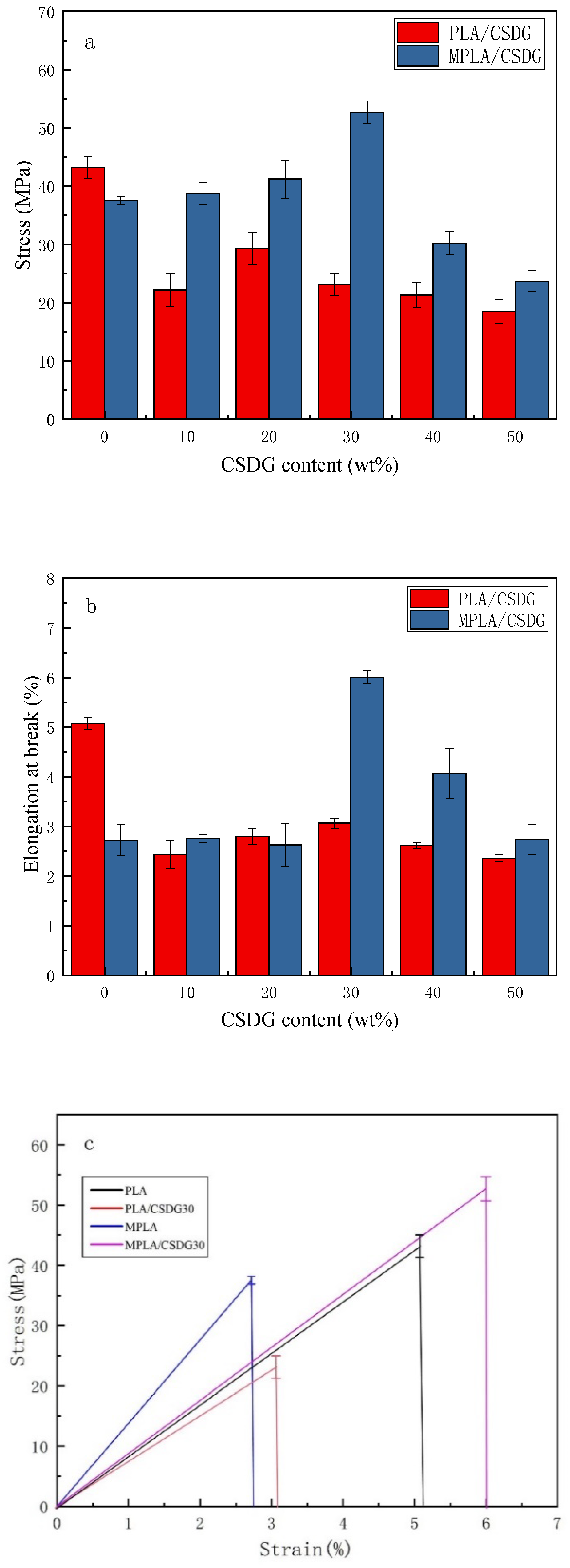
3.2. Data on Mechanical Properties
3.3. X-ray Diffraction Patterns
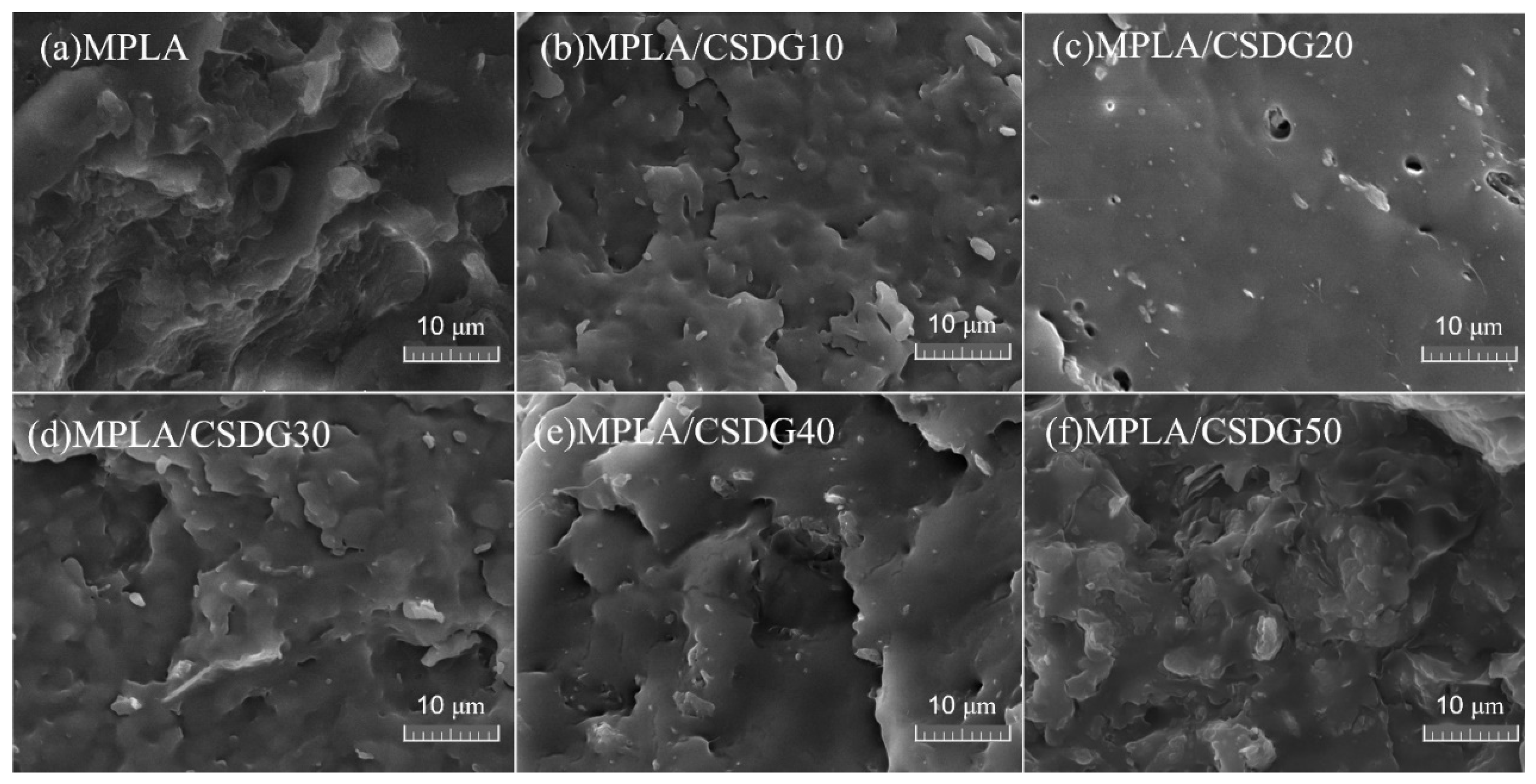
3.4. Morphological Images
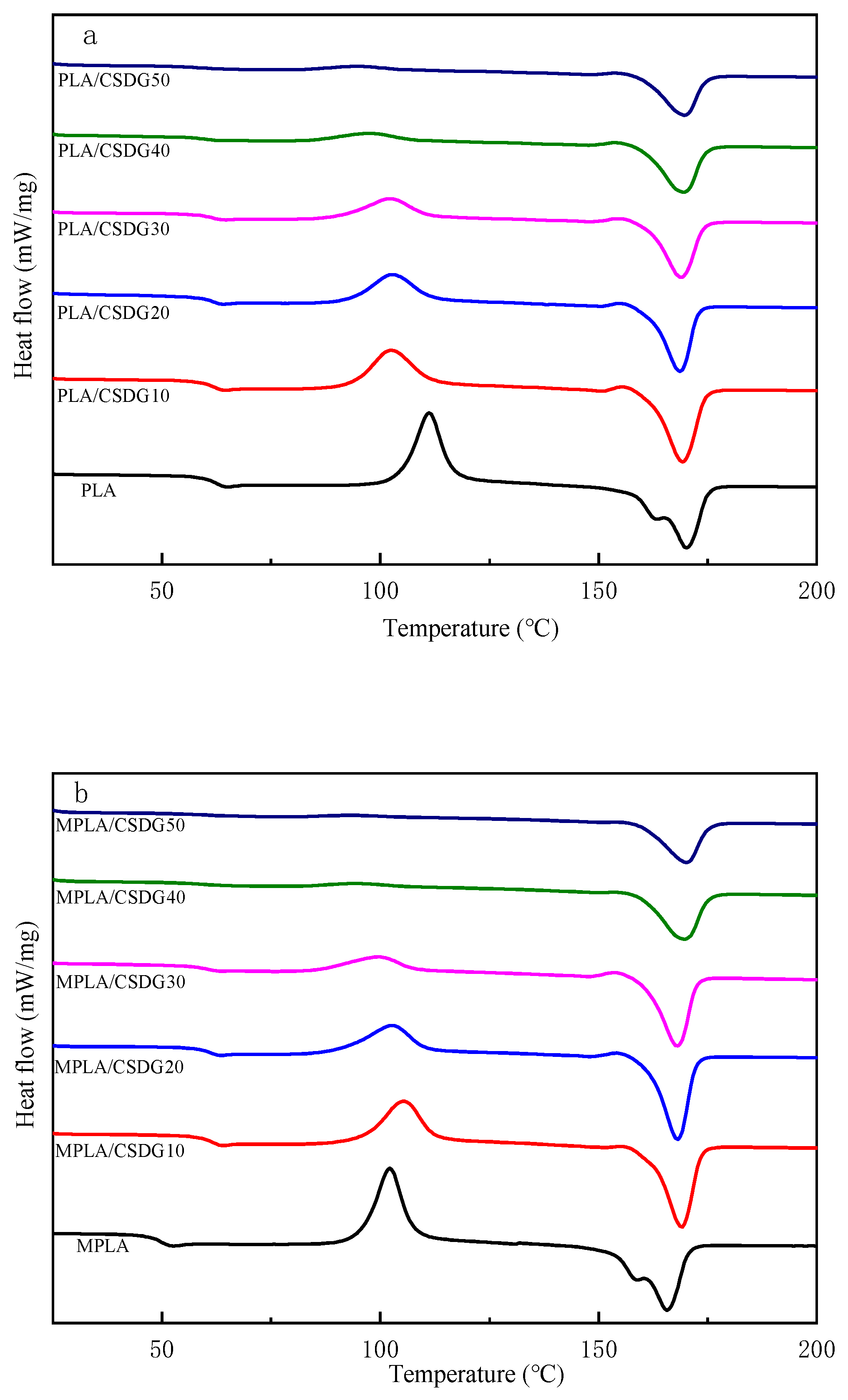
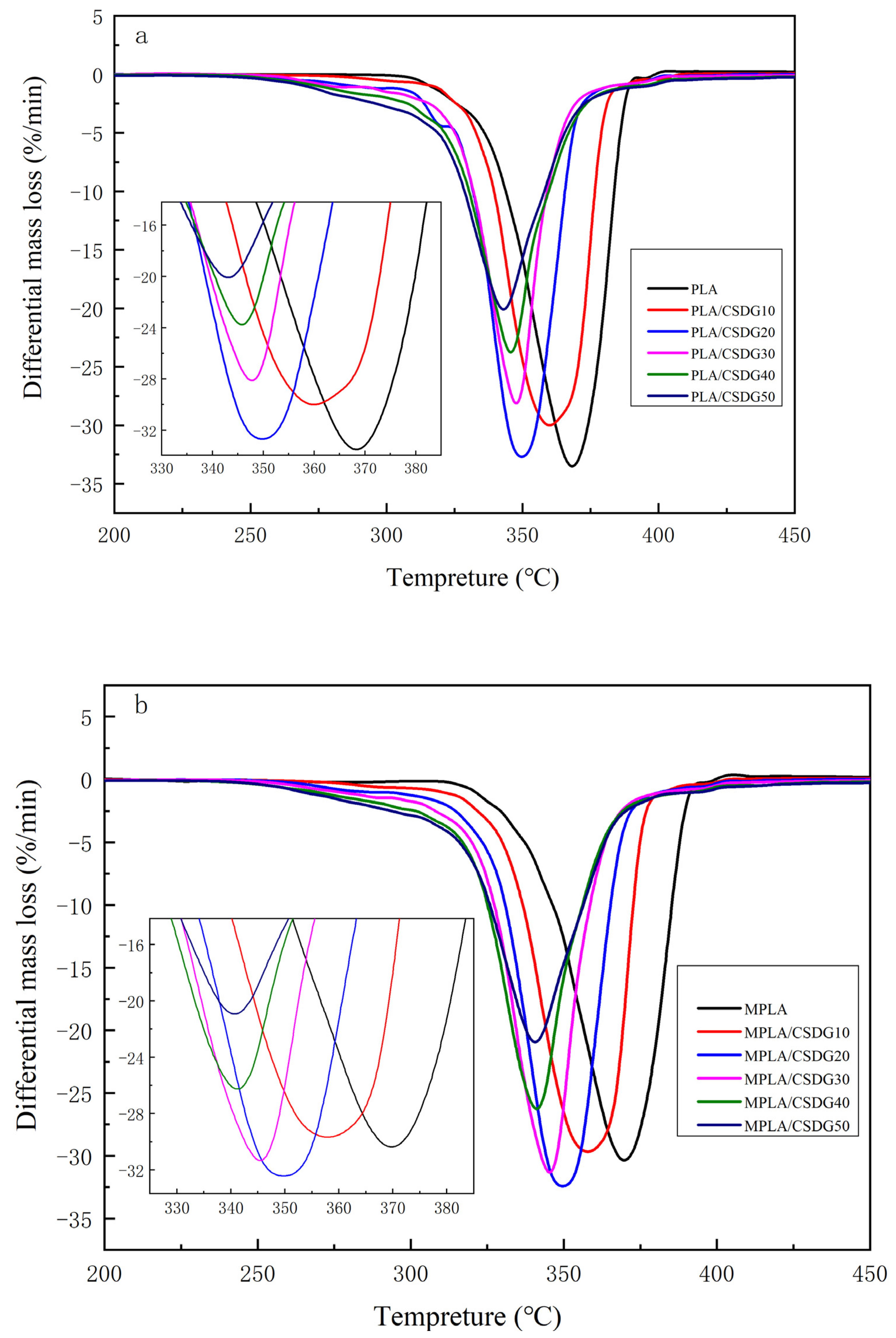
3.5. Thermal Stability Analysis
3.6. Thermogravimetric Analysis
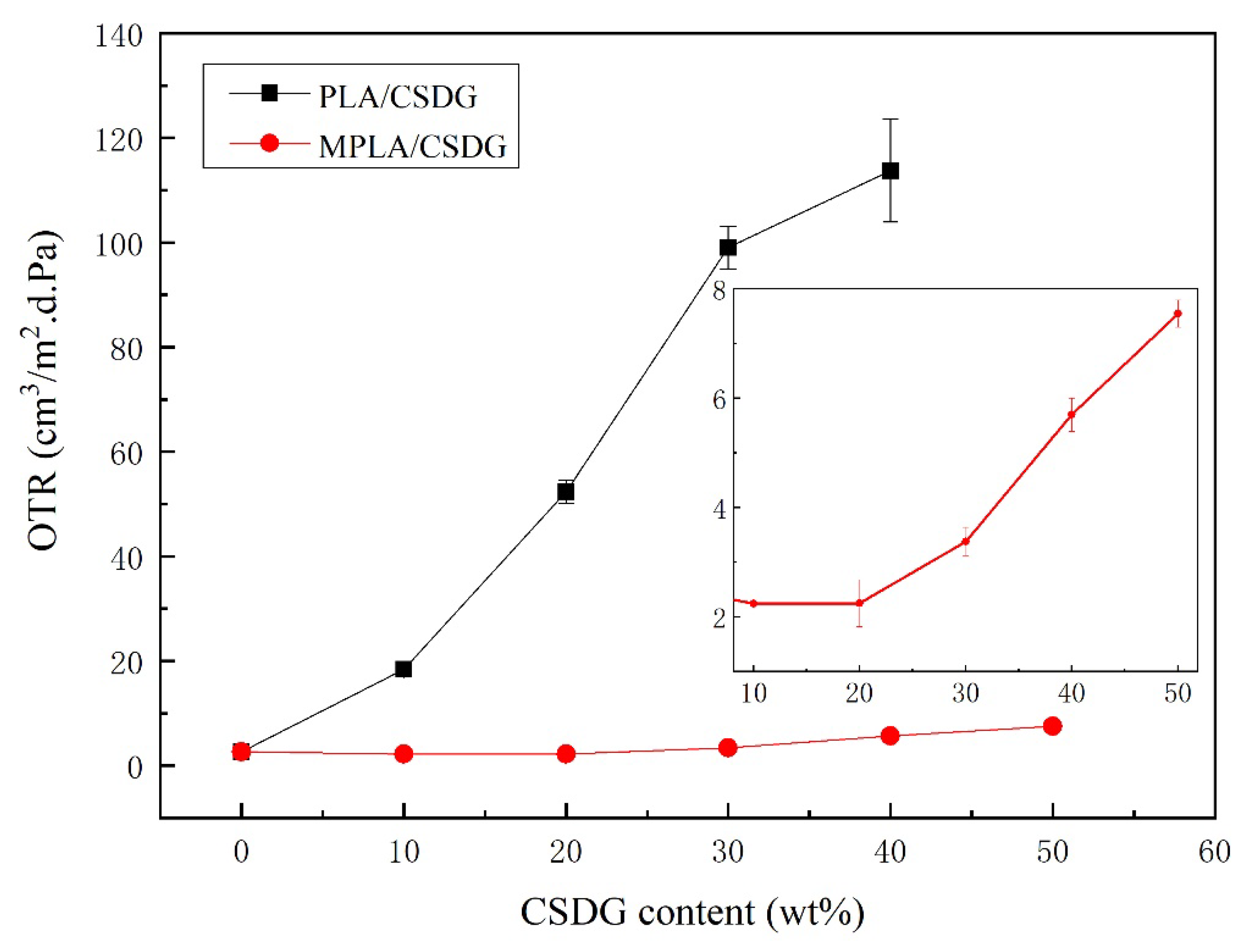
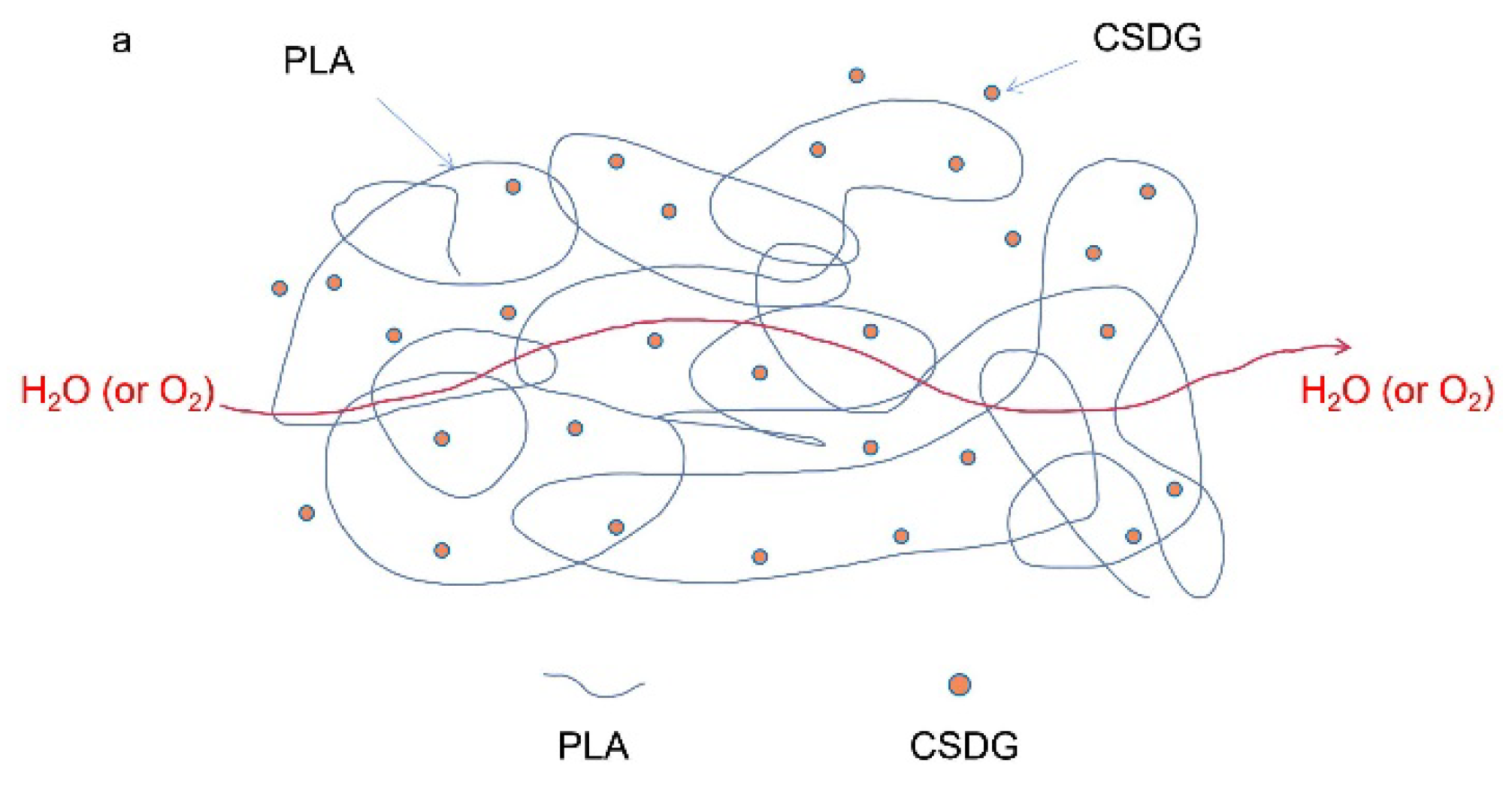
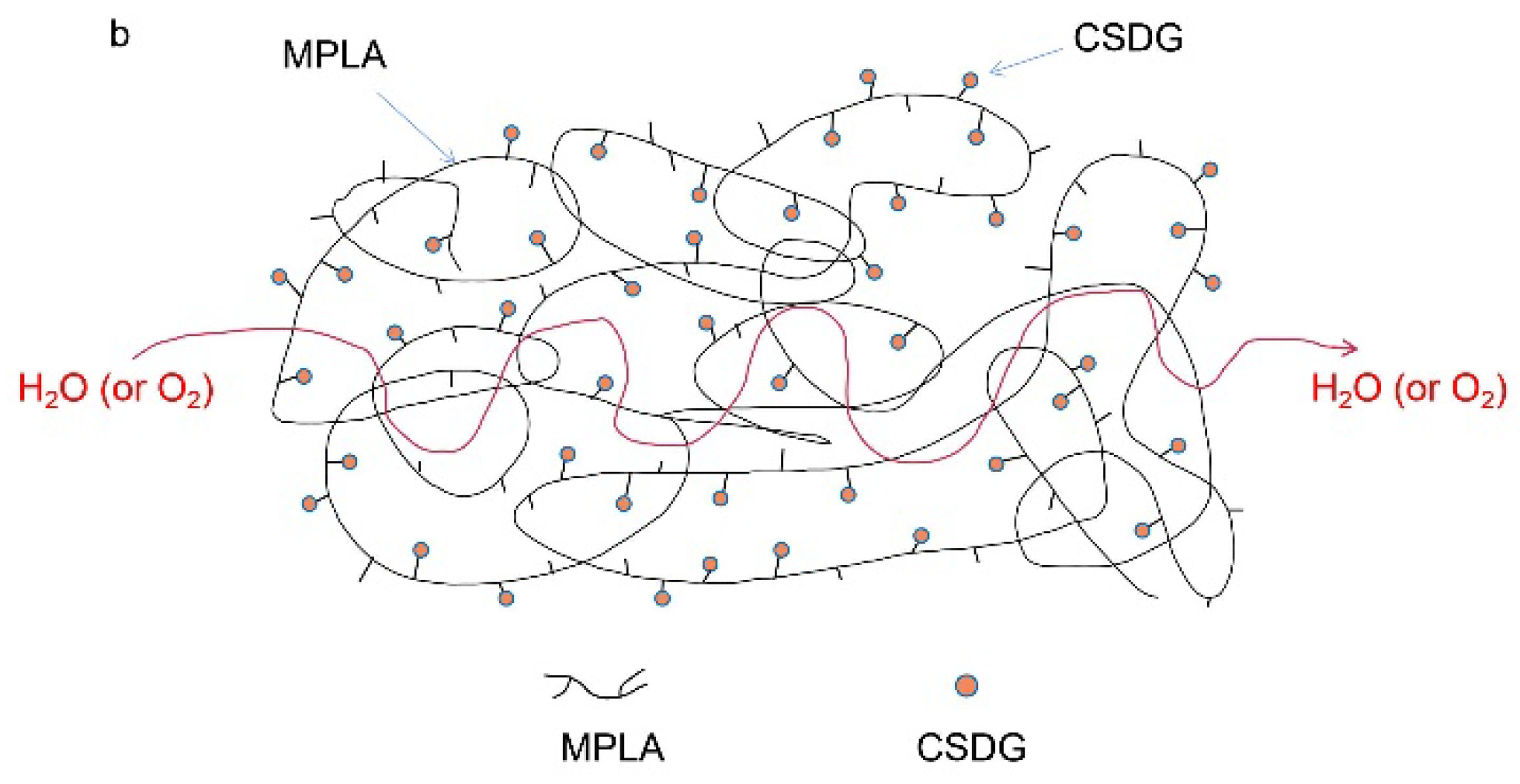
3.7. Analysis of Oxygen Barrier Performance
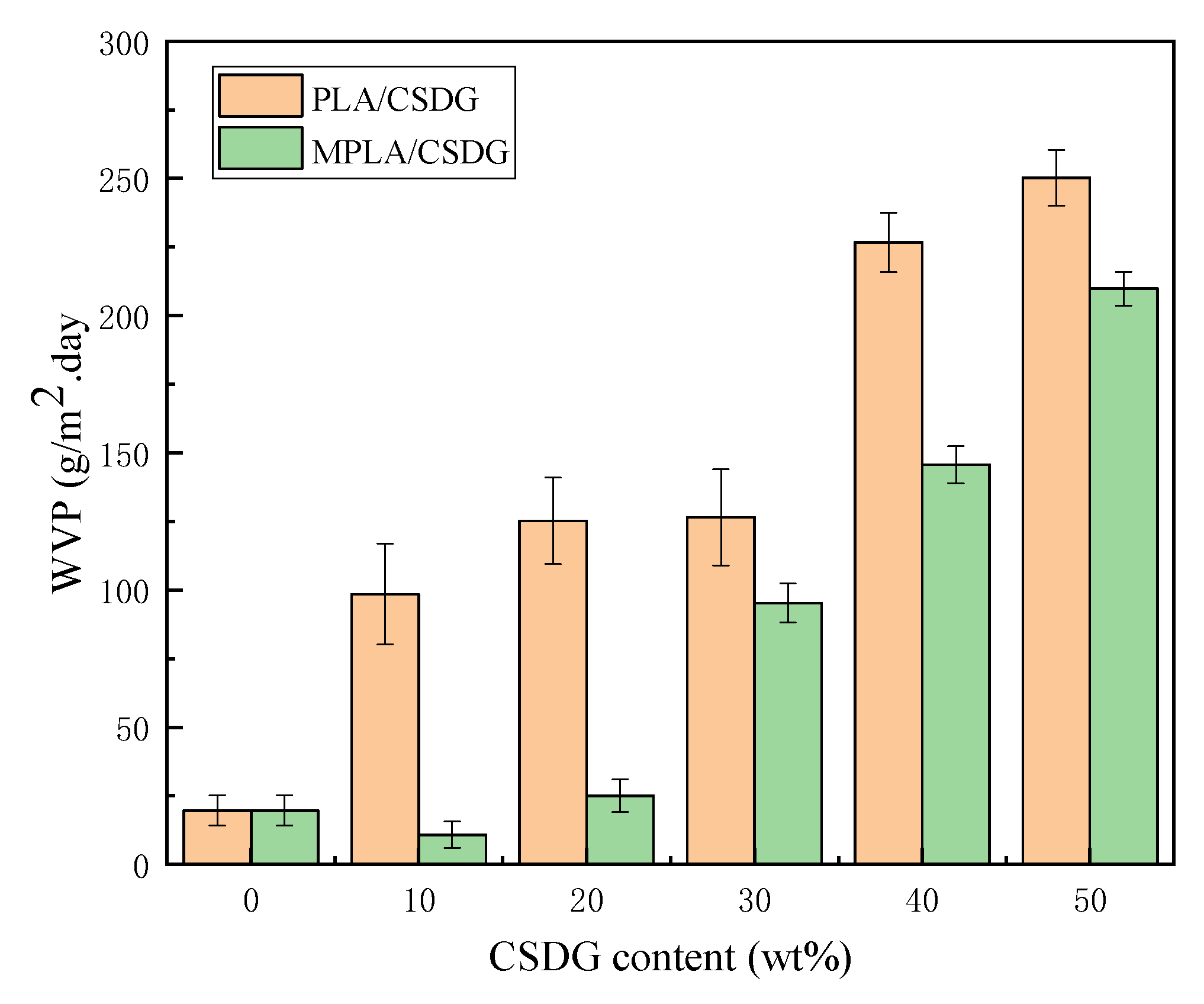
3.8. Analysis of Water Vapor Barrier Properties
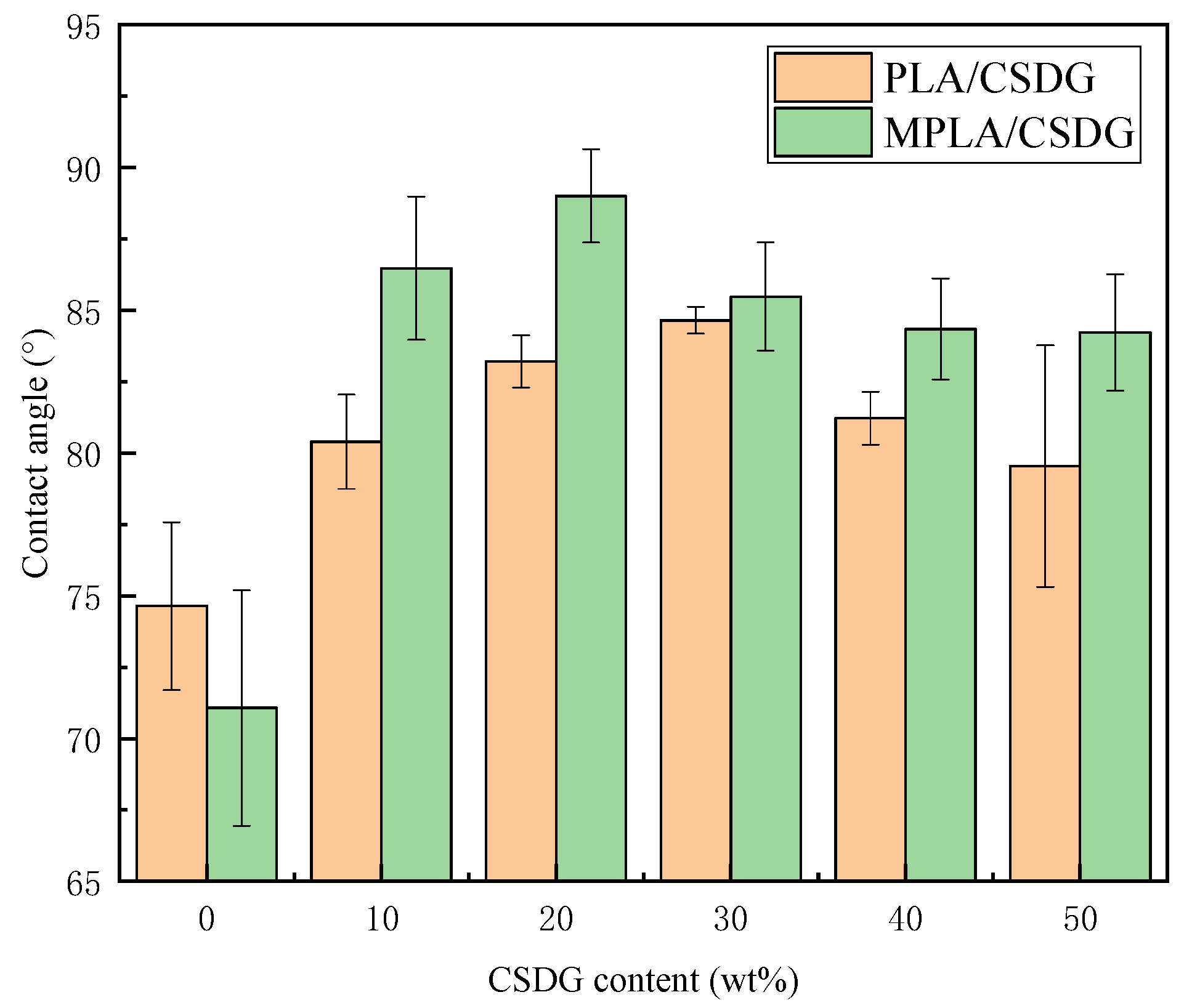
3.9. Contact Angle Data
3.10. Water Absorption Analysis
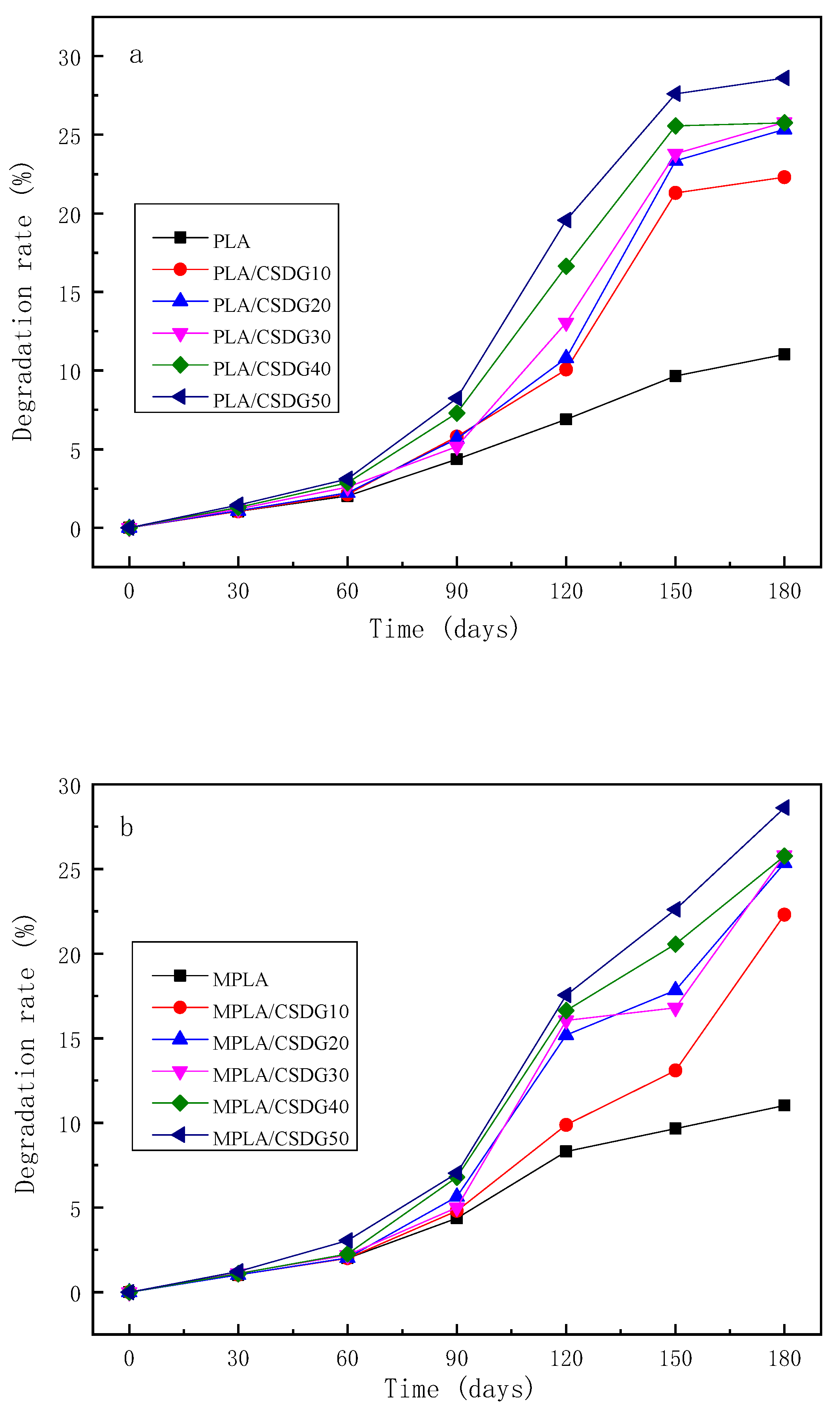
3.11. Biodegradation Rates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalia, S.; Kaith, B.S.; Kaur, I. (Eds.) Cellulose Fibers: Bio-and Nano-Polymer Composites, Green Chemistry and Technology; Springer Science and Business Media: Berlin, Germany, 2011; Volume 13, pp. 209–217. [Google Scholar]
- Guo, J.; Tsou, C.H.; Yu, Y.; Wu, C.S.; Zhang, X.; Chen, Z.; Yang, T.; Ge, F.; Liu, P.; De Guzman, M.R. Conductivity and mechanical properties of carbon black-reinforced poly(lactic acid) (PLA/CB) composites. Iran. Polym. J. 2021. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Y.; Jiang, L.; Huang, Y.; Dan, Y. Polylactide materials with ultraviolet filtering function by introducing natural compound. Polym.-Plast. Technol. Mater. 2021, 60, 1098–1105. [Google Scholar]
- Latos-Brozio, M.; Masek, A. Environmentally friendly polymer compositions with natural amber acid. Int. J. Mol. Sci. 2021, 22, 1556. [Google Scholar] [CrossRef]
- Heidari-Asil, S.A.; Zinatloo-Ajabshir, S.; Amiri, O.; Salavati-Niasari, M. Amino acid assisted-synthesis and characterization of magnetically retrievable ZnCo2O4–Co3O4 nanostructures as high activity visible-light-driven photocatalyst. Int. J. Hydrog. Energy 2020, 45, 22761–22774. [Google Scholar] [CrossRef]
- Mousavi-Kamazani, M.; Zinatloo-Ajabshir, S.; Ghodrati, M. One-step sonochemical synthesis of Zn(OH)2/ZnV3O8 nanostructures as a potent material in electrochemical hydrogen storage. J. Mater. Sci. Mater. Electron. 2020, 31, 17332–17338. [Google Scholar] [CrossRef]
- Ghodrati, M.; Mousavi-Kamazani, M.; Zinatloo-Ajabshir, S. Zn3V3O8 nanostructures: Facile hydrothermal/solvothermal synthesis, characterization, and electrochemical hydrogen storage. Ceram. Int. 2020, 46, 28894–28902. [Google Scholar] [CrossRef]
- Ashrafi, S.; Mousavi-Kamazani, M.; Zinatloo-Ajabshir, S.; Asghari, A. Novel sonochemical synthesis of Zn2V2O7 nanostructures for electrochemical hydrogen storage. Int. J. Hydrog. Energy 2020, 45, 21611–21624. [Google Scholar] [CrossRef]
- Zinatloo-Ajabshir, S.; Mousavi-Kamazani, M. Effect of copper on improving the electrochemical storage of hydrogen in CeO2 nanostructure fabricated by a simple and surfactant-free sonochemical pathway. Ceram. Int. 2020, 46, 26548–26556. [Google Scholar] [CrossRef]
- Ouyang, W.; Huang, Y.; Luo, H.; Wang, D. Poly (lactic acid) blended with cellulolytic enzyme lignin: Mechanical and thermal properties and morphology evaluation. J. Polym. Environ. 2012, 20, 1–9. [Google Scholar] [CrossRef]
- Mina, M.F.; Beg, M.D.H.; Islam, M.R.; Nizam, A.; Alam, A.K.M.M.; Yunus, R.M. Structures and properties of injection-molded biodegradable poly (lactic acid) nanocomposites prepared with untreated and treated multiwalled carbon nanotubes. Polym. Eng. Sci. 2014, 54, 317–326. [Google Scholar] [CrossRef] [Green Version]
- Jamshidian, M.; Tehrany, E.A.; Imran, M.; Akhtar, M.J.; Cleymand, F.; Desobry, S. Structural, mechanical and barrier properties of active PLA–antioxidant films. J. Food Eng. 2012, 110, 380–389. [Google Scholar] [CrossRef]
- Petinakis, E.; Yu, L.; Edward, G.; Dean, K.; Liu, H.; Scully, A.D. Effect of matrix–particle interfacial adhesion on the mechanical properties of poly (lactic acid)/wood-flour micro-composites. J. Polym. Environ. 2009, 17, 83–94. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Hou, H.; Wang, J.; Hao, C. Ultrasonic method to synthe-size glucan-g-poly(acrylic acid)/sodium lignosulfonate hydrogelsand studies of their adsorption of Cu2+ from aqueous solution. ACS Sustain. Chem. Eng. 2017, 5, 6438–6446. [Google Scholar] [CrossRef]
- Azwa, Z.N.; Yousif, B.F.; Manalo, A.C.; Karunasena, W. A review on the degradability of polymeric composites based on natural fibres. Mater. Des. 2013, 47, 424–442. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Fan, M.; Chen, L. Interface and bonding mechanisms of plant fibre composites: An overview. Compos. Part B 2016, 101, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Park, S.; Roh, H.G.; Oh, S.; Kim, S.; Kim, M.; Park, J. Preparation and characterization of super absorbent polymers based on starch aldehyde acrylic es and car-boxymethyl cellulose. Polymers 2018, 10, 605. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Qiu, J.; Feng, H.; Zhang, M.; Lei, L.; Wu, X. Improvement of tensile and thermal properties of poly (lactic acid) composites with admicellar-treated rice straw fiber. Chem. Eng. J. 2011, 173, 659–666. [Google Scholar] [CrossRef]
- Yaacab, N.D.; Ismail, H.; Ting, S.S. Potential Use of Paddy Strawas Filler in Poly Lactic Acid/Paddy Straw Powder Biocomposite: Thermal and Therm alProperties. Procedia Chem. 2016, 19, 757–762. [Google Scholar] [CrossRef] [Green Version]
- Way, C.; Dean, K.; Wu, D.Y.; Palombo, E.A. Palombo Poly(lactic acid) Composites Utilising Sequential Surface Treatments of Lignocellulose: Chemistry, Morphology and Properties. J. Polym. Environ. 2011, 19, 849–862. [Google Scholar] [CrossRef]
- Yusoff, R.B.; Takagi, H.; Nakagaito, A.N. Tensile and flexural properties of poly(lactic acid)-based hybrid green composites reinforced by kenaf, bamboo and coir fibers. Ind. Crop. Prod. 2016, 94, 562–573. [Google Scholar] [CrossRef]
- Sujaritjun, W.; Uawongsuwan, P.; Pivsa-Art, W.; Hamada, H. Mechanic property of surface modified natural fiber reinforced PLA biocomposites. Energy Procedia 2013, 34, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Bourmaud, A.; Pimbert, S. Investigations on Mechanical Properties of Poly(Propylene) and Poly(Lactic Acid)Reinforced by Miscanthus Fibers. Compos. Part A 2008, 39, 1444–1454. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, S.; Sun, C.; Wan, L.; Tan, H.; Zhang, Y. Effect of MAH-g-PLA on the Properties of Wood Fiber/Poly(lactic acid) Composites. Polymers 2017, 9, 591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertini, F.; Canetti, M.; Cacciamani, A.; Elegir, G.; Orlandi, M.; Zoia, L. Effect of ligno-derivatives on thermal properties and degradation behavior of poly (3-hydroxybutyrate)-based biocomposites. Polym. Degrad. Stab. 2012, 97, 1979–1987. [Google Scholar] [CrossRef]
- Lu, H.; Madbouly, S.A.; Schrader, J.A.; Srinivasan, G.; McCabe, K.G.; Grewell, D.; Graves, W.R. Biodegradation Behavior of Poly(lactic acid) (PLA)/Distiller’s Dried Grains with Solubles (DCSDGS) Composites. ACS Sustain. Chem. Eng. 2014, 2, 2699–2706. [Google Scholar] [CrossRef]
- Chi-Hui, T.; Wei-Song, H.; Chin-San, W.; Jui-Chin, C.; Huang, C.; Shih-Hsuan, C.; Chih-Yuan, T.; Wei-Hua, Y.; Shang-Ming, L.; Chih-Kuei, C.; et al. New composition of maleic-anhydride-grafted poly (lactic acid)/rice husk with methylenediphenyl diisocyanate. Mater. Sci. 2014, 20, 446–451. [Google Scholar]
- Tsou, C.Y.; Wu, C.L.; Tsou, C.H.; Chiu, S.H.; Suen, M.C.; Hung, W.S. Biodegradable composition of poly(lactic acid) from renewable wood flour. Polym. Sci. Ser. B 2015, 57, 473–480. [Google Scholar] [CrossRef]
- ASTM D1037-12. Standard Test Methods for Evaluating Properties of Wood-Base Fiber and Particle Panel Materials; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Wu, C.S.; Tsou, C.H. Fabrication, characterization, and application of biocomposites from poly (lactic acid) with renewable rice husk as reinforcement. J. Polym. Res. 2019, 26, 44. [Google Scholar] [CrossRef]
- Tang, G.; Jiang, Z.G.; Li, X.; Zhang, H.B.; Hong, S.; Yu, Z.Z. Electrically conductive rubbery epoxy/diamine-functionalized graphene nanocomposites with improved mechanical properties. Compos. Part B 2014, 67, 564–570. [Google Scholar] [CrossRef]
- Xin, Z.; Qinghua, Z.; Dajun, C. Enhanced mechanical properties of graphene-based poly(vinylalcohol) composites. Macromolecules 2010, 43, 2357–2363. [Google Scholar]
- Faria, D.L.; Júnior, L.M.; de Almeida Mesquita, R.G.; Júnior, M.G.; Pires, N.J.; Mendes, L.M.; Junior, J.B.G. Production of castor oil-based polyurethane resin composites reinforced with coconut husk fibres. J. Polym. Res. 2020, 27, 249. [Google Scholar] [CrossRef]
- Zheng-Lu, M.; Chi-Hui, T.; You-Li, Y.; De Guzman, M.R.; Chin-San, W.; Chen, G.; Tao, Y.; Zhi-Jun, C.; Zeng, R.; Yu, L.; et al. Thermal Properties and Barrier Performance of Antibacterial High-Density Polyethylene Reinforced with Carboxyl Graphene-Grafted Modified High-Density Polyethylene. Ind. Eng. Chem. 2021. [Google Scholar] [CrossRef]
- Pan, P.; Liang, Z.; Zhu, B.; Dong, T.; Inoue, Y. Blending Effects on Polymorphic Crystallization of Poly(L-Lactide). Macromolecules 2009, 42, 3374–3380. [Google Scholar] [CrossRef]
- Yang, T.C.; Wu, T.L.; Hung, K.C.; Chen, Y.L.; Wu, J.H. Mechanical properties and extended creep behavior of bamboo fiber reinforced rec ycled poly (lactic acid) composites using thetime-temperature superposition principle. Constr. Build. Mater. 2015, 93, 558–563. [Google Scholar] [CrossRef]
- Guo, J.; Tsou, C.H.; De Guzman, M.R.; Wu, C.S.; Zhang, X.; Chen, Z.; Wen, Y.-H.; Yang, T.; Zhuang, Y.-J.; Ge, F.; et al. Preparation and characterization of bio-based green renewable composites from poly (lactic acid) reinforced with corn stover. J. Polym. Res. 2021, 28, 199. [Google Scholar] [CrossRef]
- Lu, W.; Gramlich, W.M.; Gardner, D.J. Improving the impact strength of Poly(lactic acid) (PLA) in fused layer modeling (FLM). Polymer 2017, 114, 242–248. [Google Scholar]
- Zhou, Y.; Lei, L.; Yang, B.; Li, J.; Ren, J. Preparation of PLA-based nanocomposites modified by nano-CBapulgite with good toughness-strength balance. Polym. Test. 2017, 60, 78–83. [Google Scholar] [CrossRef]
- Qi, Z.; Ye, H.; Xu, J.; Peng, J.; Chen, J.; Guo, B. Synthesis and characterizations of attapulgite reinforced branched poly (butylene succinate) nanocomposites. Colloids Surf. A 2013, 436, 26–33. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, P.; Liang, G.; Gu, A.; Yuan, L.; Guan, Q. The origin of the curing behavior, mechanical and thermal properties of surface functionalized attapulgite/bismaleimide/diallylbisphenol composites. Appl. Surf. Sci. 2014, 288, 435–443. [Google Scholar] [CrossRef]
- Lu, T.; Liu, S.; Jiang, M.; Xu, X.; Wang, Y.; Wang, Z.; Gou, J.; Hui, D.; Zhou, Z. Effects of modifications of bamboo cellulose fibers on the improved mechanical properties of cellulose reinforced poly (lactic acid) composites. Compos. Part B 2014, 62, 191–197. [Google Scholar] [CrossRef]
- Tsou, C.H.; Yao, W.H.; Wu, C.S.; Tsou, C.Y.; Hung, W.S.; Chen, J.C.; De Guzman, M.R. Preparation and characterization of renewable composites from Polylactide and Rice husk for 3D printing applications. J. Polym. Res. 2019, 26, 227–237. [Google Scholar] [CrossRef]
- Ge, F.F.; Tsou, C.H.; Yuan, S.; De Guzman, M.R.; Zeng, C.Y.; Li, J.; Jia, C.F.; Cheng, B.Y.; Yang, P.C.; Gao, C. Barrier Performance and Biodegradability of Antibacterial Poly (butylene adipate-co-terephthalate) Nanocomposites Reinforced with a New MWCNT-ZnO Nanomaterial. Nanotechnology 2021. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, P.; Haupert, F. Toughening effects of titanium dioxide nanoparticles on TiO2/epoxy resin nanocomposites. Polym. Compos. 2010, 31, 1241–1246. [Google Scholar] [CrossRef]
- Aliotta, L.; Cinelli, P.; Coltelli, M.B.; Righetti, M.C.; Gazzano, M.; Lazzeri, A. Effect of nucleating agents on crystallinity and properties of Poly (lactic acid) (PLA). Eur. Polym. J. 2017, 93, 822–832. [Google Scholar] [CrossRef]
- Lee, S.; Hong, J.Y.; Jang, J. The effect of graphene nanofiller on the crystallization behavior and mechanical properties of poly (vinyl alcohol). Polym. Int. 2013, 62, 901–908. [Google Scholar] [CrossRef]
- Dittanet, P.; Pearson, R.A. Effect of silica nanoparticle size on toughening mechanisms of filled epoxy. Polymer 2012, 53, 1890–1905. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kao, N. PLA based biopolymer reinforced with natural fibre: A review. J. Polym. Environ. 2011, 19, 714. [Google Scholar] [CrossRef]
- Cataldo, V.A.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F. Coffee grounds as filler for pectin: Green composites with competitive performances dependent on the UV irradiation. Carbohydr. Polym. 2017, 170, 198–205. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Super gas barrier and selectivity of graphene oxide-polymer multilayer thin films. Adv. Mater. 2013, 25, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.J.; Tsou, C.H.; Wen, Y.H. A new application of hollow nano-silica added to modified polypropylene to prepare nanocomposite films. NANO Brief Rep. Rev. 2021, 16, 2150117. [Google Scholar] [CrossRef]
- Yao, Y.L.; De Guzman, M.R.; Duan, H.; Gao, C.; Lin, X.; Wen, Y.H.; Du, J.; Lin, L.; Chen, J.C.; Wu, C.S.; et al. Infusing high-density polyethylene with graphene-zinc oxide to produce antibacterial nanocomposites with improved properties. Chin. J. Polym. Sci. 2020, 38, 898–907. [Google Scholar] [CrossRef]
- Ramos, M.; Jiménez, A.; Peltzer, M.; Garrigós, M.C. Development of novel nano-biocomposite antioxidant films based on poly (lactic acid) and thymol for active packaging. Food Chem. 2014, 162, 149–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Y.; Tsou, C.H.; Gao, C.; Chen, J.C.; Guzman, M. Evaluating distillers grains as bio-fillers for high-density polyethylene. J. Polym. Res. 2020, 27, 167. [Google Scholar] [CrossRef]
- Liu, H.; Song, W.; Chen, F.; Guo, L.; Zhang, J. Interaction of microstructure and interfacial adhesion on impact performance of polylactide (PLA) ternary blends. Macromolecules 2011, 44, 1513–1522. [Google Scholar] [CrossRef]





| Sample | PLA (%) | CSDG (%) | MPLA (%) |
|---|---|---|---|
| PLA/CSDG10 | 90 | 10 | 0 |
| PLA/CSDG20 | 80 | 20 | 0 |
| PLA/CSDG30 | 70 | 30 | 0 |
| PLA/CSDG40 | 60 | 40 | 0 |
| PLA/CSDG50 | 50 | 50 | 0 |
| MPLA/CSDG10 | 0 | 10 | 90 |
| MPLA/CSDG20 | 0 | 20 | 80 |
| MPLA/CSDG30 | 0 | 30 | 70 |
| MPLA/CSDG40 | 0 | 40 | 60 |
| MPLA/CSDG50 | 0 | 50 | 50 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PLA | 43.20 ± 1.9 | 5.08 ± 0.1 |
| PLA/CSDG10 | 22.18 ± 2.9 | 2.43 ± 0.3 |
| PLA/CSDG20 | 29.33 ± 2.8 | 2.80 ± 0.2 |
| PLA/CSDG30 | 23.11 ± 1.9 | 3.05 ± 0.1 |
| PLA/CSDG40 | 21.32 ± 2.2 | 2.60 ± 0.06 |
| PLA/CSDG50 | 18.47 ± 2.1 | 2.35 ± 0.07 |
| MPLA | 37.08 ± 0.7 | 2.71 ± 0.3 |
| MPLA/CSDG10 | 38.72 ± 1.9 | 2.75 ± 0.08 |
| MPLA/CSDG20 | 41.13 ± 3.3 | 2.61 ± 0.4 |
| MPLA/CSDG30 | 52.65 ± 2.0 | 6.02 ± 0.1 |
| MPLA/CSDG40 | 30.30 ± 2.0 | 4.05 ± 0.5 |
| MPLA/CSDG50 | 23.73 ± 1.8 | 2.72 ± 0.3 |
| Sample | Glass Transition Temperature (°C) | Recrystallization Temperature (Tc) (°C) | Enthalpy of Crystallization (J/g) | Melting Temperature (°C) | Melting Enthalpy (J/g) | Crystallinity (%) |
|---|---|---|---|---|---|---|
| PLA | 61.7 | 111.2 | 29.79 | 170.1 | 31.84 | 37.0 |
| PLA/CSDG10 | 61.2 | 102.5 | 21.67 | 169.3 | 32.67 | 38.74 |
| PLA/CSDG20 | 61.1 | 102.8 | 16.77 | 168.2 | 26.12 | 34.84 |
| PLA/CSDG30 | 61.1 | 102.2 | 11.82 | 169 | 24.6 | 37.51 |
| PLA/CSDG40 | 59.9 | 97.9 | 7.692 | 169.5 | 23.1 | 40.93 |
| PLA/CSDG50 | 58.6 | 94.8 | 3.198 | 169.7 | 19.4 | 41.43 |
| MPLA | 48.8 | 102.3 | 21.27 | 165.7 | 30.3 | 32.31 |
| MPLA/SDG10 | 61.1 | 105.4 | 21.92 | 169 | 32.3 | 38.33 |
| MPLA/SDG20 | 60.6 | 102.7 | 15.94 | 168.7 | 31.2 | 41.58 |
| MPLA/SDG30 | 60.5 | 99.6 | 8.66 | 169.5 | 26.8 | 43.78 |
| MPLA/SDG40 | 59.2 | 94.2 | 2.87 | 169.8 | 24.5 | 41.54 |
| MPLA/CSDG50 | 59.5 | 93.6 | 1.51 | 170.1 | 20.3 | 41.22 |
| Sample | Initial Degradation Temperature (°C) | Temperature at Maximum Mass Loss (°C) | Mass Loss Rate (%) |
|---|---|---|---|
| PLA | 311.98 ± 1.7 | 368.38 ± 0.9 | 67.33 ± 2.2 |
| PLA/CSDG10 | 290.79 ± 1.5 | 360.21 ± 1.3 | 72.37 ± 3.5 |
| PLA/CSDG20 | 269.85 ± 3.6 | 349.86 ± 1.1 | 60.70 ± 3.3 |
| PLA/CSDG30 | 265.58 ± 0.9 | 347.89 ± 1.3 | 65.18 ± 4.1 |
| PLA/CSDG40 | 257.58 ± 1.7 | 345.77 ± 0.8 | 56.52 ± 2.0 |
| PLA/CSDG50 | 255.88 ± 1.9 | 342.99 ± 1.5 | 57.77 ± 2.9 |
| MPLA | 307.83 ± 0.4 | 368.70 ± 0.5 | 64.60 ± 1.6 |
| MPLA/CSDG10 | 286.28 ± 1.4 | 357.61 ± 1.0 | 60.33 ± 3.0 |
| MPLA/CSDG20 | 266.40 ± 1.0 | 349.25 ± 0.6 | 57.22 ± 2.1 |
| MPLA/CSDG30 | 264.09 ± 0.7 | 345.19 ± 0.5 | 60.67 ± 2.8 |
| MPLA/CSDG40 | 254.96 ± 1.8 | 341.13 ± 0.9 | 57.30 ± 2.5 |
| MPLA/CSDG50 | 249.77 ± 0.9 | 340.52 ± 1.4 | 55.32 ± 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-J.; Tsou, C.-H.; Tsai, M.-L.; Guo, J.; De Guzman, M.R.; Yang, T.; Gao, C.; Lei, Y.; Gan, P.-W.; Chen, S.; et al. Barrier Properties and Hydrophobicity of Biodegradable Poly(lactic acid) Composites Reinforced with Recycled Chinese Spirits Distiller’s Grains. Polymers 2021, 13, 2861. https://doi.org/10.3390/polym13172861
Chen Z-J, Tsou C-H, Tsai M-L, Guo J, De Guzman MR, Yang T, Gao C, Lei Y, Gan P-W, Chen S, et al. Barrier Properties and Hydrophobicity of Biodegradable Poly(lactic acid) Composites Reinforced with Recycled Chinese Spirits Distiller’s Grains. Polymers. 2021; 13(17):2861. https://doi.org/10.3390/polym13172861
Chicago/Turabian StyleChen, Zhi-Jun, Chi-Hui Tsou, Meng-Lin Tsai, Jipeng Guo, Manuel Reyes De Guzman, Tao Yang, Chen Gao, Yan Lei, Pei-Wen Gan, Shuang Chen, and et al. 2021. "Barrier Properties and Hydrophobicity of Biodegradable Poly(lactic acid) Composites Reinforced with Recycled Chinese Spirits Distiller’s Grains" Polymers 13, no. 17: 2861. https://doi.org/10.3390/polym13172861
APA StyleChen, Z.-J., Tsou, C.-H., Tsai, M.-L., Guo, J., De Guzman, M. R., Yang, T., Gao, C., Lei, Y., Gan, P.-W., Chen, S., Tu, L.-J., Qu, C.-L., Wang, R.-Y., & Wu, C.-S. (2021). Barrier Properties and Hydrophobicity of Biodegradable Poly(lactic acid) Composites Reinforced with Recycled Chinese Spirits Distiller’s Grains. Polymers, 13(17), 2861. https://doi.org/10.3390/polym13172861