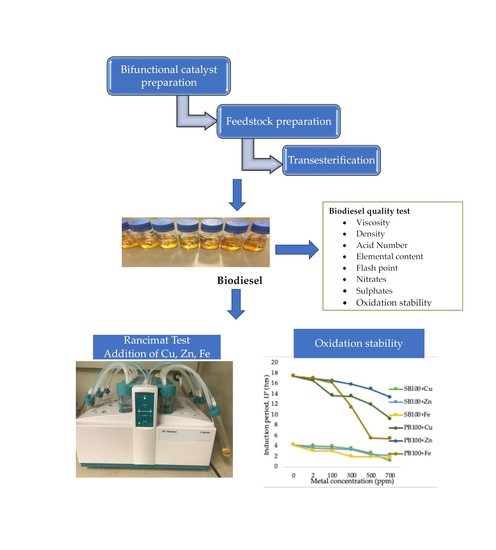
Catalyst and Elemental Analysis Involving Biodiesel from Various Feedstocks
Abstract
1. Introduction
2. Results
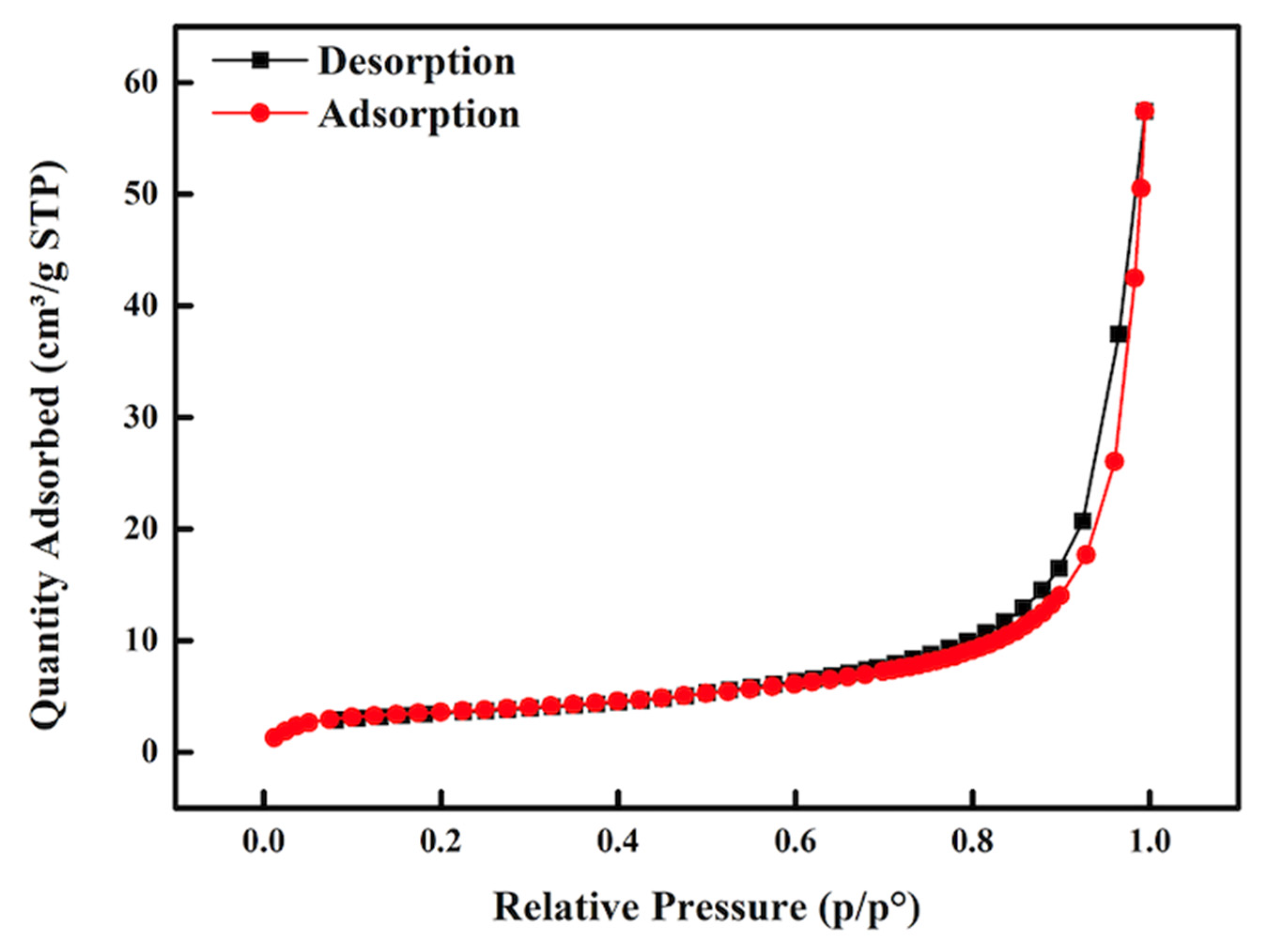
2.1. Bifunctional Catalyst Characterisation
2.2. GC Analysis
2.3. Feedstocks and Biodiesel Quality Analysis
2.4. Elemental Content Analysis
2.4.1. ICP-OES Analysis in Virgin, Waste Sunflower and Palm Oil and Their Biodiesels
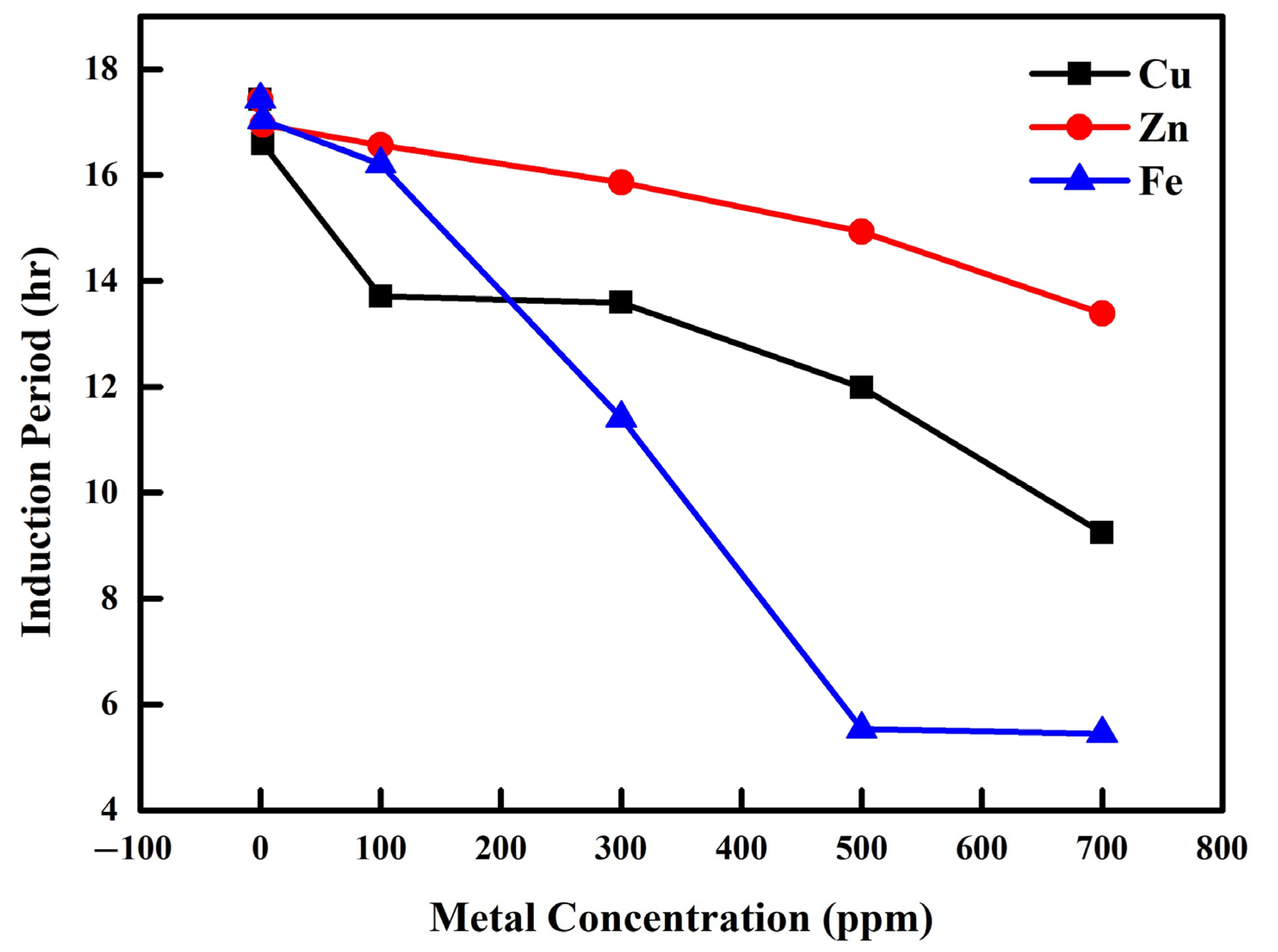
2.4.2. Effect of Cu, Fe and Zn Metals on the Oxidation Stability of Biodiesels Produced from Waste Sunflower and Palm Oils
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Preparation of Bifunctional Catalyst (CaO/Al2O3)
3.3. Transesterification
3.4. Characterisation Techniques
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Green, O.; Studies, G. OECD Green Growth Studies. Director 2012, 9–104. [Google Scholar] [CrossRef]
- Organization of Petroleum Exporting Countries (OPEC). World Oil Outlook 2040; OPEC: Vienna, Austria, 2017; pp. 61–161. ISBN 978-3-9503936-4-4. [Google Scholar] [CrossRef]
- Magsi, H. Industrialization, Environment and Pollution. Dipl. Insight 2015, 7, 24–26. Available online: https://www.researchgate.net/publication/270899735_Industrialization_Environment_and_Pollution (accessed on 2 August 2021).
- Muhammad, M.B.; Aziz, R.A.; Yew, V.W.C. Socio—Economic Effects of Industrialization in the Society. Int. J. Environ. Ecol. Fam. Urban. Stud. 2018, 8, 24–29. Available online: https://www.researchgate.net/publication/327966349_Socio_-_Economic_Effects_of_Industrialization_in_the_Society/citations (accessed on 2 August 2021).
- Baskar, G.; Aiswarya, R. Trends in catalytic production of biodiesel from various feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Jabade, S.; Sakthivel, M.; Chavan, S. Bio-diesel as an alternative fuel for compression ignition engine: A review. Int. J. Adv. Sci. Technol. 2020, 29, 18–28. [Google Scholar] [CrossRef]
- Torres-García, M.; García-Martín, J.F.; Jiménez-Espadafor Aguilar, F.J.; Barbin, D.F.; Álvarez-Mateos, P. Vegetable oils as renewable fuels for power plants based on low and medium speed diesel engines. J. Energy Inst. 2019, 93, 953–961. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Alhindawi, R.; Nahleh, Y.A.; Kumar, A.; Shiwakoti, N. Projection of greenhouse gas emissions for the road transport sector based on multivariate regression and the double exponential smoothing model. Sustainability 2020, 12, 9152. [Google Scholar] [CrossRef]
- Abbaszaadeh, A.; Ghobadian, B.; Omidkhah, M.R.; Najafi, G. Current biodiesel production technologies: A comparative review. Energy Convers. Manag. 2012, 63, 138–148. [Google Scholar] [CrossRef]
- Harfoot, M.B.J.; Tittensor, D.P.; Knight, S.; Arnell, A.P.; Blyth, S.; Brooks, S.; Butchart, S.H.M.; Hutton, J.; Jones, M.I.; Kapos, V.; et al. Present and future biodiversity risks from fossil fuel exploitation. Conserv. Lett. 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Nguyen, X.P.; Vu, H.N. Corrosion of the metal parts of diesel engines in biodiesel-based fuels. Int. J. Renew. Energy Dev. 2019, 8, 119–132. [Google Scholar] [CrossRef]
- Nair, J.N. Study of Biodiesel Blends and Emission Characteristics of Study of Biodiesel Blends and Emission Characteristics of Biodiesel. (ISO 3297: 2007). Int. J. Innov. Rev. Sci. Eng. Technol. 2013, 2, 3710–3715. Available online: https://www.researchgate.net/publication/281430011_STUDY_OF_BIODIESEL_BLENDS_AND_EMISSION_CHARACTERISTICS_OF_BIODIESEL (accessed on 2 August 2021).
- Borugadda, V.B.; Paul, A.K.; Chaudhari, A.J.; Kulkarni, V.; Sahoo, N.; Goud, V.V. Influence of Waste Cooking Oil Methyl Ester Biodiesel Blends on the Performance and Emissions of a Diesel Engine. Waste Biomass Valorization 2018, 9, 283–292. [Google Scholar] [CrossRef]
- Peng, D.X. Effect of unleaded gasoline blended with biofuels on gasoline injector wear and exhaust emissions. Ind. Lubr. Tribol. 2017, 69, 208–214. [Google Scholar] [CrossRef]
- Carraretto, C.; Macor, A.; Mirandola, A.; Stoppato, A.; Tonon, S. Biodiesel as alternative fuel: Experimental analysis and energetic evaluations. Energy 2004, 29, 2195–2211. [Google Scholar] [CrossRef]
- Moser, B.R.; Bryan, R. Moser: Biodiesel production, properties, and feedstocks. Vitr. Cell. Dev. Biol. Plant. 2009, 45, 229–266. [Google Scholar] [CrossRef]
- Mandolesi de Araújo, C.D.; de Andrade, C.C.; de Souza, E.; Silva, E.; Dupas, F.A. Biodiesel production from used cooking oil: A review. Renew. Sustain. Energy Rev. 2013, 27, 445–452. [Google Scholar] [CrossRef]
- Rocha, L.D.S.; Corrêa, S.M. Determination of size-segregated elements in diesel-biodiesel blend exhaust emissions. Environ. Sci. Pollut. Res. 2018, 25, 18121–18129. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 2. Economic assessment and sensitivity analysis. Bioresour. Technol. 2003, 90, 229–240. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Dalai, A.K. Waste cooking oil—An economical source for biodiesel: A review. Ind. Eng. Chem. Res. 2006, 45, 2901–2913. [Google Scholar] [CrossRef]
- Boadu, K.O.; Joel, O.F.; Essumang, D.K.; Evbuomwan, B.O. A Review of Methods for Removal of Contaminants in Used Lubricating Oil. Chem. Sci. Int. J. 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Julianto, T.S.; Nurlestari, R. Waste cooking oil as source for renewable fuel in Romania Waste cooking oil as source for renewable fuel in Romania. In Proceedings of the 7th International Conference on Advanced Concept in Mechanical Engineering, Iasi, Romania, 9–10 June 2016; Volume 146, pp. 1–6. [Google Scholar] [CrossRef]
- Agarwal, M.; Chauhan, G.; Chaurasia, S.P.; Singh, K. Journal of the Taiwan Institute of Chemical Engineers Study of catalytic behavior of KOH as homogeneous and heterogeneous catalyst for biodiesel production. J. Taiwan Inst. Chem. Eng. 2012, 43, 89–94. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, Z.; Chen, Y.; Zhang, P.; Duan, S.; Liu, X.; Mao, Z. Preparation of biodiesel catalyzed by solid super base of calcium oxide and its refining process. Chin. J. Catal. 2006, 27, 391–396. [Google Scholar] [CrossRef]
- Calero, J.; Luna, D.; Sancho, E.D.; Luna, C.; Bautista, F.M.; Romero, A.A.; Posadillo, A.; Verdugo, C. Development of a new biodiesel that integrates glycerol, by using CaO as heterogeneous catalyst, in the partial methanolysis of sunflower oil. Fuel 2014, 122, 94–102. [Google Scholar] [CrossRef]
- Alba-Rubio, A.C.; Santamaría-González, J.; Mérida-Robles, J.M.; Moreno-Tost, R.; Martín-Alonso, D.; Jiménez-López, A.; Maireles-Torres, P. Heterogeneous transesterification processes by using CaO supported on zinc oxide as basic catalysts. Catal. Today 2010, 149, 281–287. [Google Scholar] [CrossRef]
- Wen, D.; Jiang, H.; Zhang, K. Supercritical fluids technology for clean biofuel production. Prog. Nat. Sci. 2009, 19, 273–284. [Google Scholar] [CrossRef]
- Banerjee, M.; Dey, B.; Talukdar, J.; Chandra, M. Production of biodiesel from sun fl ower oil using highly catalytic bimetallic gold e silver core e shell nanoparticle. Energy 2014, 69, 695–699. [Google Scholar] [CrossRef]
- Changmai, B.; Vanlalveni, C.; Ingle, A.P.; Bhagat, R.; Rokhum, L. Widely used catalysts in biodiesel production: A review. RSC Adv. 2020, 10, 41625–41679. [Google Scholar] [CrossRef]
- Nogueira, T.; Lucio, C. Determination of Ca, K, Mg, Na, sulfate, phosphate, formate, acetate, propionate, and glycerol in biodiesel by capillary electrophoresis with capacitively coupled contactless conductivity detection. Microchem. J. 2011, 99, 267–272. [Google Scholar] [CrossRef]
- Elkadi, M.; Pillay, A.; Manuel, J.; Khan, M.Z.; Stephen, S.; Molki, A. Sustainability study on heavy metal uptake in neem biodiesel using selective catalytic preparation and hyphenated mass spectrometry. Sustainability 2014, 6, 2413–2423. [Google Scholar] [CrossRef]
- Isis, A.; de Oliveira, A.P.; de Magalhães, M.R.L.; Villa, R.D. Determination of sodium and potassium in biodiesel by flame atomic emission spectrometry, with dissolution in ethanol as a single sample preparation step. Fuel 2012, 93, 381–384. [Google Scholar] [CrossRef]
- Chaves, E.S.; de Loos-vollebregt, M.T.C.; Curtius, A.J.; Vanhaecke, F. Spectrochimica Acta Part B Determination of trace elements in biodiesel and vegetable oil by inductively coupled plasma optical emission spectrometry following alcohol dilution. Spectrochim. Acta Part B At. Spectrosc. 2011, 66, 733–739. [Google Scholar] [CrossRef]
- Mendil, D.; Uluözlü, Ö.D.; Tüzen, M.; Soylak, M. Investigation of the levels of some element in edible oil samples produced in Turkey by atomic absorption spectrometry. J. Hazard. Mater. 2009, 165, 724–728. [Google Scholar] [CrossRef]
- Pillay, A.E.; Elkadi, M.; Fok, S.C.; Stephen, S.; Manuel, J.; Khan, M.Z.; Unnithan, S. A comparison of trace metal profiles of neem biodiesel and commercial biofuels using high performance ICP-MS. Fuel 2012, 97, 385–389. [Google Scholar] [CrossRef]
- Sánchez, R.; Sánchez, C.; Lienemann, C.P.; Todolí, J.L. Metal and metalloid determination in biodiesel and bioethanol. J. Anal. At. Spectrom. 2015, 30, 64–101. [Google Scholar] [CrossRef]
- Waynick, J.A. Characterization of biodiesel oxidation and oxidation products. Tech. Lit. Rev. 2005, 1–51. [Google Scholar] [CrossRef][Green Version]
- Fazal, M.A.; Haseeb, A.S.M.A.; Masjuki, H.H. Degradation of automotive materials in palm biodiesel. Energy 2012, 40, 76–83. [Google Scholar] [CrossRef]
- Garrido, M.D.; Frías, I.; Díaz, C.; Hardisson, A. Concentrations of metals in vegetable edible oils. Food Chem. 1994, 50, 237–243. [Google Scholar] [CrossRef]
- Santos, D.C.M.B.; Guida, M.A.B.; Barbosa, I.S.; Passos, M.L.C. Evaluation of Digestion Procedures for Simultaneous Determination of Ca, P, Mg, K and Na in Biodiesel by Inductively Coupled Plasma Optical Emission Spectrometry. J. Braz. Chem. Soc. 2010, 21, 2278–2284. [Google Scholar] [CrossRef][Green Version]
- Health Effects Institute: Understanding the Health Effects of Components of the Particulate Matter Mix: Progress and Next Steps. HEI Perspect. 2002, 1–20. Available online: https://www.healtheffects.org/publication/understanding-health-effects-components-particulate-matter-mix-progress-and-next-steps (accessed on 2 August 2021).
- Ruzinska, E.; Stollmann, V.; Hagara, V.; Jablonski, M. Analysis of selected heavy metals in biomass for preparation of biofuels—Part II. Determination of heavy metals content. Ann. Wars. Univ. Life Sci. SGGW. For. Wood Technol. 2015, 92, 383–389. [Google Scholar]
- Black, L.T. Comparison of three atomic absorption techniques for determining metals in soybean oil. J. Am. Oil Chem. Soc. 1975, 52, 88–91. [Google Scholar] [CrossRef]
- Sompech, S.; Dasri, T.; Thaomola, S. Preparation and characterization of amorphous silica and calcium oxide from agricultural wastes. Orient. J. Chem. 2016, 32, 1923–1928. [Google Scholar] [CrossRef]
- Aitlaalim, A.; Ouanji, F.; Benzaouak, A.; El Mahi, M.; Lotfi, E.M.; Kacimi, M.; Liotta, L.F. Utilization of waste grooved razor shell (Grs) as a catalyst in biodiesel production from refined and waste cooking oils. Catalysts 2020, 10, 703. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Niaei, A.; Salari, D. Production of γ-Al2O3 from Kaolin. Open J. Phys. Chem. 2011, 1, 23–27. [Google Scholar] [CrossRef]
- Kesserwan, F.; Ahmad, M.N.; Khalil, M.; El-Rassy, H. Hybrid CaO/Al2O3 aerogel as heterogeneous catalyst for biodiesel production. Chem. Eng. J. 2020, 385, 2–11. [Google Scholar] [CrossRef]
- Ruan, G.; Zhang, Z.; Yin, M.; Xu, G. Effect of aluminum powder on the synthesis of corundum-mullite composites. Ceram. Silikaty. 2013, 57, 133–137. [Google Scholar]
- Granados-Correa, F.; Bonifacio-Martínez, J.; Hernández-Mendoza, H.; Bulbulian, S. Capture of CO2 on γ-Al2O3 materials prepared by solution-combustion and ball-milling processes. J. Air Waste Manag. Assoc. 2016, 66, 643–654. [Google Scholar] [CrossRef]
- Mirghiasi, Z.; Bakhtiari, F.; Darezereshki, E.; Esmaeilzadeh, E. Preparation and characterization of CaO nanoparticles from Ca(OH)2 by direct thermal decomposition method. J. Ind. Eng. Chem. 2014, 20, 113–117. [Google Scholar] [CrossRef]
- Yang, W.; Feng, Y.; Chu, W. Promotion Effect of CaO Modification on Mesoporous Al2O3-Supported Ni Catalysts for CO2 Methanation. Int. J. Chem. Eng. 2016, 2016, 2041821. [Google Scholar] [CrossRef]
- Intarasiri, S.; Ratana, T.; Sornachamni, T.; Tungkamani, S.; Phongaksorn, M. Pore size effect of mesoporous support on metal particle size of Co/SiO2 catalyst in Fischer-Tropsch synthesis. Int. J. Adv. Appl. Sci. 2018, 5, 80–85. [Google Scholar] [CrossRef]
- Imtiaz, A.; Farrukh, M.A.; Khaleeq-Ur-Rahman, M.; Adnan, R. Micelle-assisted synthesis of Almiddot; CaO nanocatalyst: Optical properties and their applications in photodegradation of 2,4,6-trinitrophenol. Sci. World J. 2013, 2013, 641420. [Google Scholar] [CrossRef]
- Teo, S.H.; Taufiq-Yap, Y.H.; Rashid, U.; Islam, A. Hydrothermal effect on synthesis, characterization and catalytic properties of calcium methoxide for biodiesel production from crude Jatropha curcas. RSC Adv. 2015, 5, 4266–4276. [Google Scholar] [CrossRef]
- Nayebzadeh, H.; Saghatoleslami, N.; Haghighi, M.; Tabasizadeh, M. Catalytic Activity of KOH-CaO-Al2O3 Nanocomposites in Biodiesel Production: Impact of Preparation Method. Int. J. Self Propagating High Temp. Synth. 2019, 28, 18–27. [Google Scholar] [CrossRef]
- Marinković, D.M.; Avramović, J.M.; Stanković, M.V.; Stamenković, O.S.; Jovanović, D.M.; Veljković, V.B. Synthesis and characterization of spherically-shaped CaO/Γ-Al2O3 catalyst and its application in biodiesel production. Energy Convers. Manag. 2017, 144, 399–413. [Google Scholar] [CrossRef]
- Wacharasindhu, S.; Likitmaskul, S.; Punnakanta, L.; Chaichanwatanakul, K.; Angsusingha, K.; Tuchinda, C. Serum IGF-I and IGFBP-3 Levels for Normal Thai Children and their Usefulness in Clinical Practice. J. Med. Assoc. Thail. 1998, 81, 420–430. [Google Scholar]
- Yang, F.; Ning, Z.; Liu, H. Fractal characteristics of shales from a shale gas reservoir in the Sichuan Basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore structure and fractal characteristics of different shale lithofacies in the dalong formation in the western area of the lower yangtze platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Zhu, L.; Cao, M.; Zhou, H.; Zhang, N.; Zheng, J.; Li, Y.; Chen, B.H. A highly stable and active CaO/Al2O3 base catalyst in the form of calcium aluminate phase for oxidation of cyclohexanone to ε-caprolactone. Catal. Lett. 2014, 144, 1188–1196. [Google Scholar] [CrossRef]
- Huang, B.; Bartholomew, C.H.; Woodfield, B.F. Improved calculations of pore size distribution for relatively large, irregular slit-shaped mesopore structure. Microporous Mesoporous Mater. 2014, 184, 112–121. [Google Scholar] [CrossRef]
- Ljupković, R.B.; Radulović, N.S.; Bojić, A.L.; Zarubica, A.R.; Mićić, R.D.; Tomić, M.D. Značaj strukturnih karakteristika CaO katalizatora za proizvodnju biodizela: Uticaj na smanjenje emisije gasova staklene bašte. Hem. Ind. 2014, 68, 399–412. [Google Scholar] [CrossRef]
- Stankovic, M. Preparation of CaO/γ -Al2O3 catalyst for biodiesel fuels. The catalytic activity in relation to thermal. In Proceedings of the 12th International Conference on Fundamental and Applied Aspects of Physical Chemistry, Belgrade, Serbia, 22–26 September 2014; pp. 2–3. [Google Scholar] [CrossRef]
- Elias, S.; Rabiu, A.M.; Okeleye, B.I.; Okudoh, V.; Oyekola, O. Bifunctional heterogeneous catalyst for biodiesel production from waste vegetable oil. Appl. Sci. 2020, 10, 3153. [Google Scholar] [CrossRef]
- Marinkovic, D.; Stankovic, M.; Velickovic, A.; Avramovic, J.; Cakic, M.; Veljkovic, V. The synthesis of CaO loaded onto Al2O3 from calcium acetate and its application in transesterification of the sunflower oil. Savrem. Tehnol. 2015, 4, 26–32. [Google Scholar] [CrossRef]
- Capuano, D.; Costa, M.; Di Fraia, S.; Massarotti, N.; Vanoli, L. Direct use of waste vegetable oil in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 759–770. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Adam, A.A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Chhetri, A.; Watts, K.; Islam, M. Waste Cooking Oil as an Alternate Feedstock for Biodiesel Production. Energies 2008, 1, 3. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. JAOCS J. Am. Oil Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Axelsson, L.; Franzén, M.; Ostwald, M.; Berndes, G.; Lakshmi, G.; Ravindranath, N.H. Perspective: Jatropha cultivation in southern India: Assessing farmers’ experiences. Biofuels Bioprod. Biorefin. 2012, 6, 246–256. [Google Scholar] [CrossRef]
- Yasin, M.H.M.; Mamat, R.; Yusop, A.F.; Rahim, R.; Aziz, A.; Shah, L.A. Fuel physical characteristics of biodiesel blend fuels with alcohol as additives. Procedia Eng. 2013, 53, 701–706. [Google Scholar] [CrossRef]
- Bukkarapu, K.R.; Rahul, T.S.; Kundla, S.; Vardhan, G.V. Effects of blending on the properties of diesel and palm biodiesel. IOP Conf. Ser. Mater. Sci. Eng. 2018, 330, 012092. [Google Scholar] [CrossRef]
- Giakoumis, E.G. Analysis of 22 vegetable oils’ physico-chemical properties and fatty acid composition on a statistical basis, and correlation with the degree of unsaturation. Renew. Energy 2018, 126, 403–419. [Google Scholar] [CrossRef]
- Yaşar, F. Comparision of fuel properties of biodiesel fuels produced from different oils to determine the most suitable feedstock type. Fuel 2020, 264, 2–6. [Google Scholar] [CrossRef]
- Barabas, I.; Todoru, I.A. Biodiesel Quality, Standards and Properties; Montero, G., Ed.; Technical Univeraity of Cluj-Napoca: Cluj-Napoca, Romania, 2011; pp. 1–28. ISBN 978-953-307-784-0. [Google Scholar] [CrossRef]
- Al-Abdullah, M.H.; Kalghatgi, G.T.; Babiker, H. Flash points and volatility characteristics of gasoline/diesel blends. Fuel 2015, 153, 67–69. [Google Scholar] [CrossRef]
- Senthil, R.; Silambarasan, R. Annona: A new biodiesel for diesel engine: A comparative experimental investigation. J. Energy Inst. 2015, 88, 459–469. [Google Scholar] [CrossRef]
- Canesin, E.A.; de Oliveira, C.C.; Matsushita, M.; Felicidade Dias, L.; Reghiany Pedrão, M.; de Souza, N.E. Characterization of residual oils for biodiesel production. Electron. J. Biotechnol. 2014, 17, 39–45. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Moein, P. Biodiesel synthesis from waste vegetable oil via transesterification reaction in supercritical methanol. J. Supercrit. Fluids 2013, 76, 24–31. [Google Scholar] [CrossRef]
- Palash, S.M.; Kalam, M.A.; Masjuki, H.H.; Masum, B.M.; Rizwanul Fattah, I.M.; Mofijur, M. Impacts of biodiesel combustion on NOx emissions and their reduction approaches. Renew. Sustain. Energy Rev. 2013, 23, 473–490. [Google Scholar] [CrossRef]
- Sadeghzadeh, B. A review of zinc nutrition and plant breeding. J. Soil Sci. Plant Nutr. 2013, 13, 907–927. [Google Scholar] [CrossRef]
- Avila Orozco, F.D.; Kovachev, N.; Aguirre Pastor, M.Á.; Domini, C.E.; Fernández Band, B.S.; Hernández, A.C. Analysis of metals and phosphorus in biodiesel B100 from different feedstock using a Flow Blurring®multinebulizer in inductively coupled plasma-optical emission spectrometry. Anal. Chim. Acta. 2014, 827, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lyra, F.H.; Tereza, M.; Dias, W.; Brandão, G.P.; Pessoa, H.M.; de Castro, E.V. Determination of Na, K, Ca and Mg in biodiesel samples by flame atomic absorption spectrometry (FAAS) using microemulsion as sample preparation. Microchem. J. 2010, 96, 180–185. [Google Scholar] [CrossRef]
- Vieira, M.A.; Castro, C.; Ara, R. Determination of As in Vegetable Oil and Biodiesel by Graphite Furnace Atomic Absorption Spectrometry. Energy Fuels 2009, 23, 5942–5946. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Iqbal, J.; Carney, W.A.; Lacaze, S.; Theegala, C.S. Metals Determination in Biodiesel (B100) by ICP-OES with Microwave Assisted Acid Digestion. Open Anal. Chem. J. 2010, 4, 18–26. [Google Scholar] [CrossRef][Green Version]
- Woods, G.D.; Fryer, F.I. Direct elemental analysis of biodiesel by inductively coupled plasma—mass spectrometry. Anal. Bioanal. Chem. 2007, 389, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Szyczewski, P.; Frankowski, M.; Zioła-Frankowska, A.; Siepak, J.; Szyczewski, T.; Piotrowski, P.; Pillay, A.E.; Elkadi, M.; Fok, S.C.; Stephen, S.; et al. Metals Determination in Biodiesel (B100) by ICP-OES with Microwave Assisted Acid Digestion. Microchem. J. 2010, 96, 18121–18129. [Google Scholar] [CrossRef][Green Version]
- Henrique Lyra, F.; Weitzel Dias Carneiro, M.T.; Pedrini Brandão, G.; Moura Pessoa, H.; Ribeiro De Castro, E.V. Direct determination of phosphorus in biodiesel samples by graphite furnace atomic absorption spectrometry using a solid sampling accessory. J. Anal. At. Spectrom. 2009, 24, 1262–1266. [Google Scholar] [CrossRef]
- Ramadhas, A.S.; Jayaraj, S.; Muraleedharan, C. Biodiesel: Production and Properties; The Royal Society of Chemistry: Cambridge, UK, 2016; pp. 41–80. ISBN 978-1-84973-470-7. [Google Scholar]
- de Goede, S.; Wilken, C.; Ajam, M.; Roets, P.; Engelbrecht, P.; Woolard, C. A Comparison of the Stability Performance of Blends of Paraffinic Diesel and Petroleum-Derived Diesel, with RME Biodiesel Using Laboratory Stability Measurement Techniques. J. Fuels 2015, 2015, 528497. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Baena, L.; Rios, L.A.; Calderón, J.A. The oxidative stability of biodiesel and its impact on the deterioration of metallic and polymeric materials: A review. J. Braz. Chem. Soc. 2012, 23, 2159–2175. [Google Scholar] [CrossRef]
- Qiu, F.; Li, Y.; Yang, D.; Li, X.; Sun, P. Biodiesel production from mixed soybean oil and rapeseed oil. Appl. Energy 2011, 88, 2050–2055. [Google Scholar] [CrossRef]
- Komariah, L.N.; Dewi, T.K.; Ramayanti, C. Study on corrosion behavior of storage tanks filled with biodiesel and the blends. IOP Conf. Ser. Mater. Sci. Eng. 2019, 543, 1–7. [Google Scholar] [CrossRef]
- Yeşilyurt, M.K.; Öner, İ.V.; Yılmaz, E.Ç. Biodiesel Induced Corrosion and Degradation: A Review. Pamukkale Univ. J. Eng. Sci. 2019, 25, 60–70. [Google Scholar] [CrossRef]
- Chaves, E.S.; José, E.; Araujo, R.G.O.; Vladimir, J.; Lúcia, V.; Frescura, A.; Curtius, A.J. Metals and phosphorus determination in vegetable seeds used in the production of biodiesel by ICP OES and ICP-MS. Microchem. J. 2010, 96, 71–76. [Google Scholar] [CrossRef]
- Kivevele, T. Storage and thermal stability of biodiesel produced from manketti nut oil of Southern Africa origin with the influence of metal contaminants and antioxidants. SN Appl. Sci. 2020, 2, 930. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P. Effect of metal contents on oxidation stability of biodiesel/diesel blends. Fuel 2014, 116, 14–18. [Google Scholar] [CrossRef]
- Shiotani, H.; Goto, S. Studies of fuel properties and oxidation stability of biodiesel fuel. SAE Tech. Pap. 2007, 116, 70–75. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. The effect of metals and metal oxides on biodiesel oxidative stability from promotion to inhibition. Fuel Process. Technol. 2018, 177, 75–80. [Google Scholar] [CrossRef]
- Sarin, A.; Arora, R.; Singh, N.P.; Sarin, R.; Sharma, M.; Malhotra, R.K. Effect of metal contaminants and antioxidants on the oxidation stability of the methyl ester of pongamia. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 567–572. [Google Scholar] [CrossRef]
- Baena, L.M.; Calderón, J.A. Effects of palm biodiesel and blends of biodiesel with organic acids on metals. Heliyon 2020, 6, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sentanuhady, J.; Saputro, W.; Muflikhun, M.A. Metals and chemical compounds contaminants in diesel engine lubricant with B20 and B100 biofuels for long term operation. Sustain. Energy Technol. Assess. 2021, 45, 101161. [Google Scholar] [CrossRef]
- Thangavelu, S.K.; Ahmed, A.S.; Ani, F.N. Impact of metals on corrosive behavior of biodiesel-diesel-ethanol (BDE) alternative fuel. Renew. Energy 2016, 94, 1–9. [Google Scholar] [CrossRef]
- Hu, E.; Xu, Y.; Hu, X.; Pan, L.; Jiang, S. Corrosion behaviors of metals in biodiesel from rapeseed oil and methanol. Renew. Energy 2012, 37, 371–378. [Google Scholar] [CrossRef]
- Nurul, L.; Arita, S.; Prianda, B.E.; Dewi, T.K. Engineering Sciences Technical assessment of biodiesel storage tank; A corrosion case study. J. King Saud Univ. Eng. Sci. 2021, 33, 1–6. [Google Scholar] [CrossRef]
- Marinković, D.M.; Miladinović, M.R.; Avramović, J.M.; Krstić, I.B.; Stanković, M.V.; Stamenković, O.S.; Jovanović, D.M.; Veljković, V.B. Kinetic modeling and optimization of sunflower oil methanolysis catalyzed by spherically-shaped CaO/Γ-Al2O3 catalyst. Energy Convers. Manag. 2018, 163, 122–133. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Optimization of the activity of CaO/Al2O3 catalyst for biodiesel production using response surface methodology. Appl. Catal. A Gen. 2009, 366, 154–159. [Google Scholar] [CrossRef]


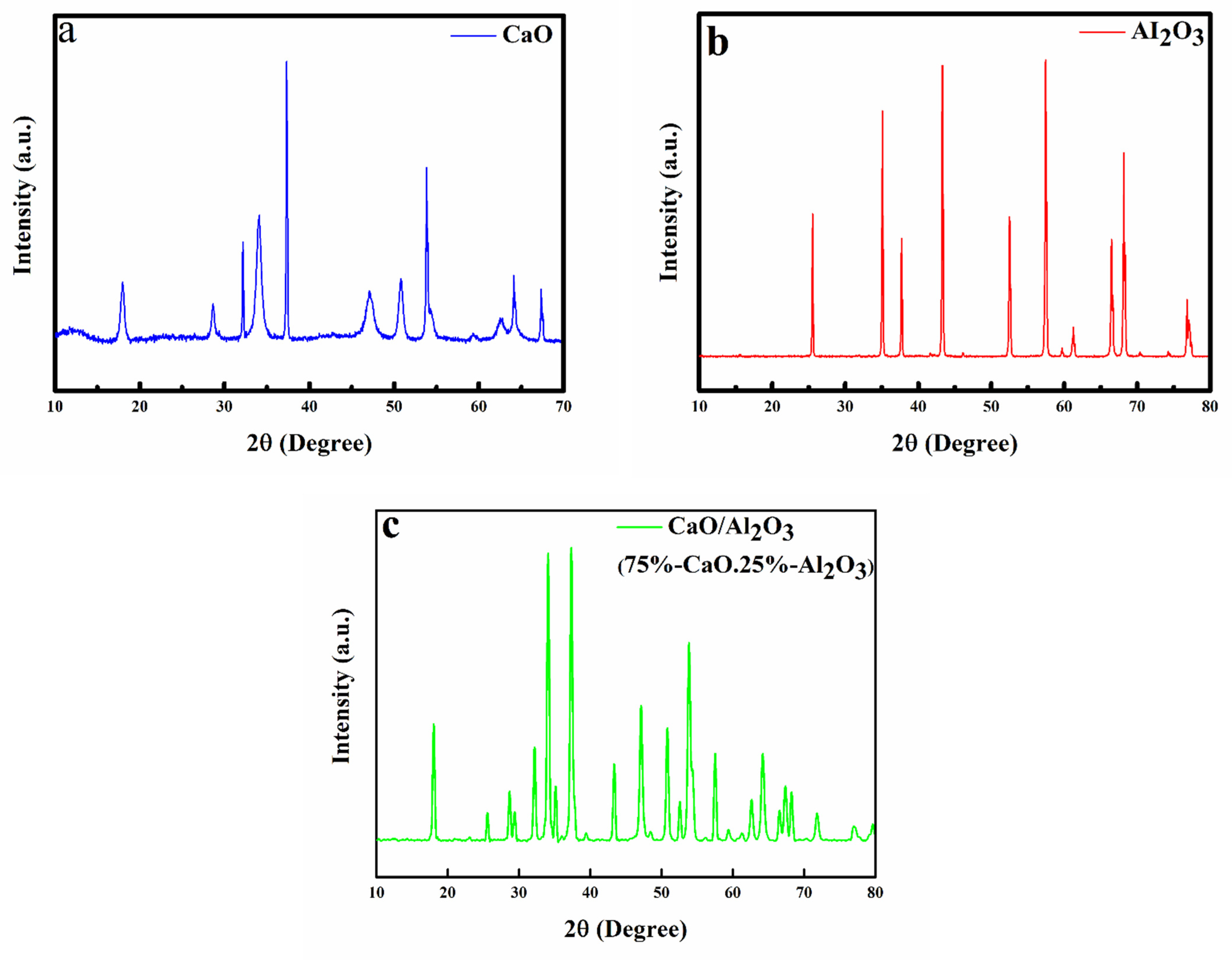

| Material | Compound | Formula | Composition (%) |
|---|---|---|---|
| CaO | Portlandite | Ca (OH)2 | 50 |
| Lime | CaO | 50 | |
| Al2O3 | Aluminium Oxide | Al2O3 | 0.69 |
| Oxonium Aluminium Oxide | H3O2Al22O34 | 75 | |
| 75CaO-25 Al2O3 | Portlandite | Ca (OH)2 | 92.08 |
| Lime | CaO | 63.79 | |
| Calcite | CaCO3 | 8.73 | |
| Aluminium Oxide | Al2O3 | 22.92 |
| Catalyst | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| Al2O3 | 0.6239 | 0.000857 | 5.9147 |
| 75%CaO/25%Al2O3 | 13.0006 | 0.079732 | 24.0371 |
| FFA Acid Types | Carbon Chain | WPO (wt%) | WSO (wt%) |
|---|---|---|---|
| Lauric | C12:0 | 0.42 | - |
| Myrisitc | C14:0 | 0.53 | -- |
| Palmitic | C16:0 | 16.25 | 4.36 |
| Stearic | C18:0 | 1.50 | 3.39 |
| Oleic | C18:1 | 13.82 | 9.45 |
| Linoleic | C18:3 | 3.04 | 27.25 |
| Properties | WPO | PB100 | WSO | SB100 | ASTM | EN&SA |
|---|---|---|---|---|---|---|
| Flash Point (°C) | - | 170 | 200 | 175 | >93 | >120 |
| Oxidation Stability (h) | - | 17.43 | - | 4.20 | >3 | >6 |
| Density at 15 °C (g/cm3) | 0.9168 | 0.892 | 0.9212 | 0.8871 | 0.9 | 0.86–0.9 |
| Viscosity at 40 °C (cSt) | 47.5 | 11.1 | 37.1 | 6 | 1.9–6 | 3.5–5 |
| Acid Value (mg of KOH/g of oil) | 3.23 | 0.55 | 1.26 | 0.25 | <0.5 | <0.5 |
| Nitrates | 3 | 11 | 4 | 11 | - | - |
| Sulphates | 153 | 118 | 160 | 121 | - | - |
| Glycol | 0 | 10 | 12 | 11 | - | - |
| Soot | 11 | 11 | 11 | 12 | - | - |
| Elements (mg/L) | VSO | WSO | SB100 | VPO | WPO | PB100 |
|---|---|---|---|---|---|---|
| Na | 0.971 | 1.133 | 0.333 | 1.648 | 2.399 | 0.383 |
| Mg | 0 | 0 | 0.125 | 0 | 0.068 | 0.266 |
| K | 0 | 0 | 0 | 0 | 0.012 | 0 |
| Ca | 0.024 | 0.025 | 27.692 | 0.206 | 0.345 | 23.534 |
| Fe | 3.654 | 4.054 | 2.686 | 5.199 | 6.229 | 2.810 |
| P | 17.625 | 21.264 | 8.239 | 31.937 | 44.453 | 8.913 |
| Al | 1.682 | 1.969 | 0.870 | 2.861 | 4.109 | 0.921 |
| Cu | 0 | 0 | 0 | 0 | 0 | 0 |
| Zn | 1.185 | 1.216 | 1.623 | 1.407 | 1.601 | 1.666 |
| S | - | - | 4 | - | - | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simbi, I.; Aigbe, U.O.; Oyekola, O.; Osibote, O.A. Catalyst and Elemental Analysis Involving Biodiesel from Various Feedstocks. Catalysts 2021, 11, 971. https://doi.org/10.3390/catal11080971
Simbi I, Aigbe UO, Oyekola O, Osibote OA. Catalyst and Elemental Analysis Involving Biodiesel from Various Feedstocks. Catalysts. 2021; 11(8):971. https://doi.org/10.3390/catal11080971
Chicago/Turabian StyleSimbi, Ines, Uyiosa Osagie Aigbe, Oluwaseun Oyekola, and Otolorin Adelaja Osibote. 2021. "Catalyst and Elemental Analysis Involving Biodiesel from Various Feedstocks" Catalysts 11, no. 8: 971. https://doi.org/10.3390/catal11080971
APA StyleSimbi, I., Aigbe, U. O., Oyekola, O., & Osibote, O. A. (2021). Catalyst and Elemental Analysis Involving Biodiesel from Various Feedstocks. Catalysts, 11(8), 971. https://doi.org/10.3390/catal11080971