Could Japonica Rice Be an Alternative Variety for Increased Global Food Security and Climate Change Mitigation?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey of Consumer Rice Preferences Using a Structured Questionnaire
- knowledge of the rice sub-species and preference of sub-species,
- length of grain, adhesiveness, aroma, and taste after cooking as well as the cooking time,
- willingness to purchase healthier rice at a relatively higher cost (USD 1 = NGN 410).
2.2. Analyses of the Impact of Parboiling on the Properties of Japonica Rice
2.2.1. Rice Parboiling Procedure
2.2.2. Analysis of the Differences in Nutritional and Physical Properties of Milled Rice Samples
- a.
- Analysis of adhesiveness in milled parboiled and non-parboiled rice samples
- b.
- Analysis of amylose content in milled parboiled and non-parboiled rice samples
- c.
- Analysis of alkali digestion in milled parboiled and non-parboiled rice samples
- d.
- Nutritional analyses of milled parboiled and non-parboiled rice samples
- e.
- Color measurement of milled parboiled and non-parboiled rice samples
2.3. Growing of Japonica Rice and the Environmental Impact
2.4. Statistical Analyses
3. Results
3.1. Rice Attributes Preferred by Consumers
3.2. Effects of Parboiling on the Physiochemical Properties of Rice Samples
- a.
- Adhesiveness and amylose content of milled parboiled and non-parboiled rice samples
- b.
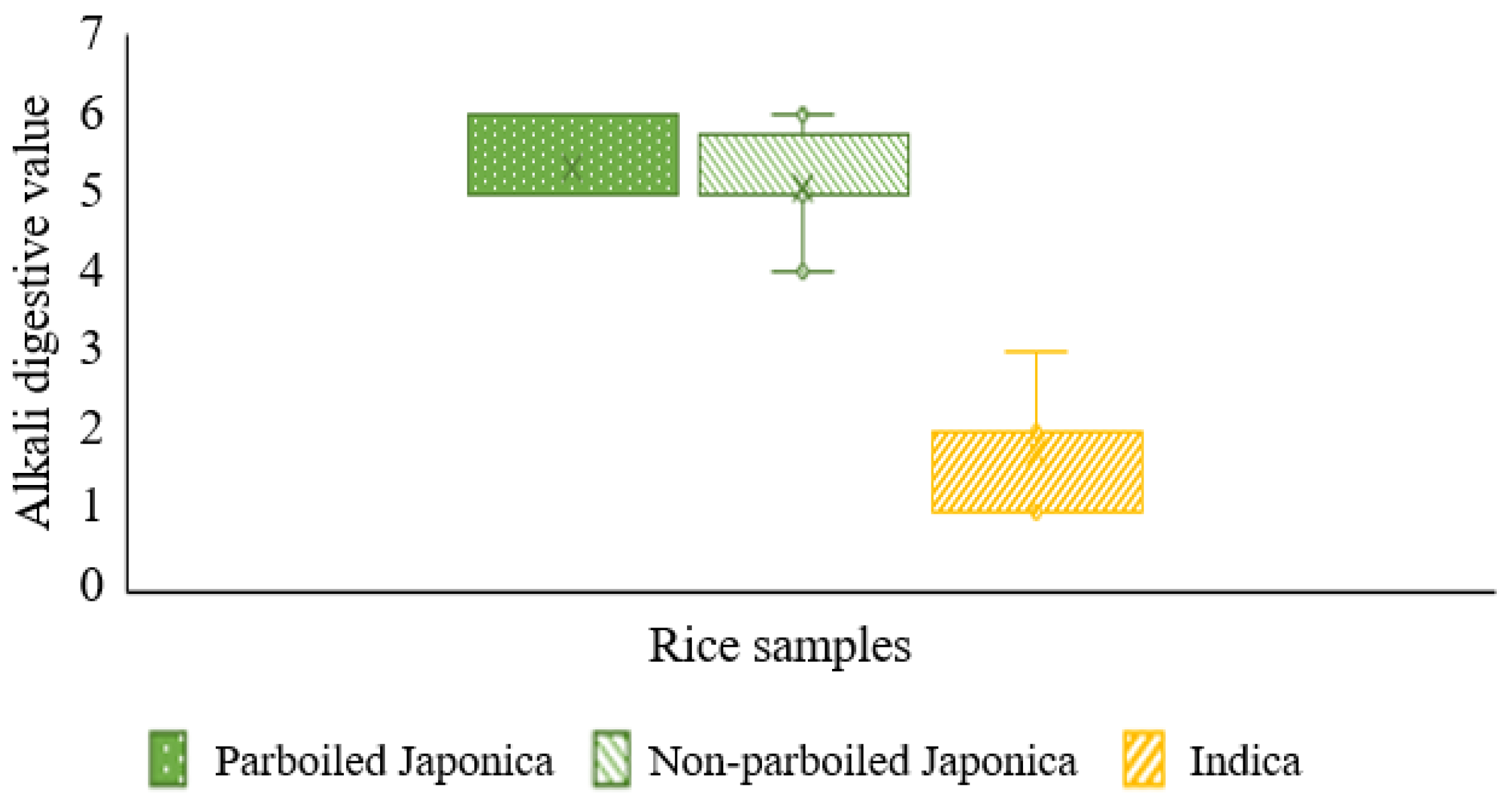
- Alkali digestion in milled parboiled and non-parboiled rice samples
- c.
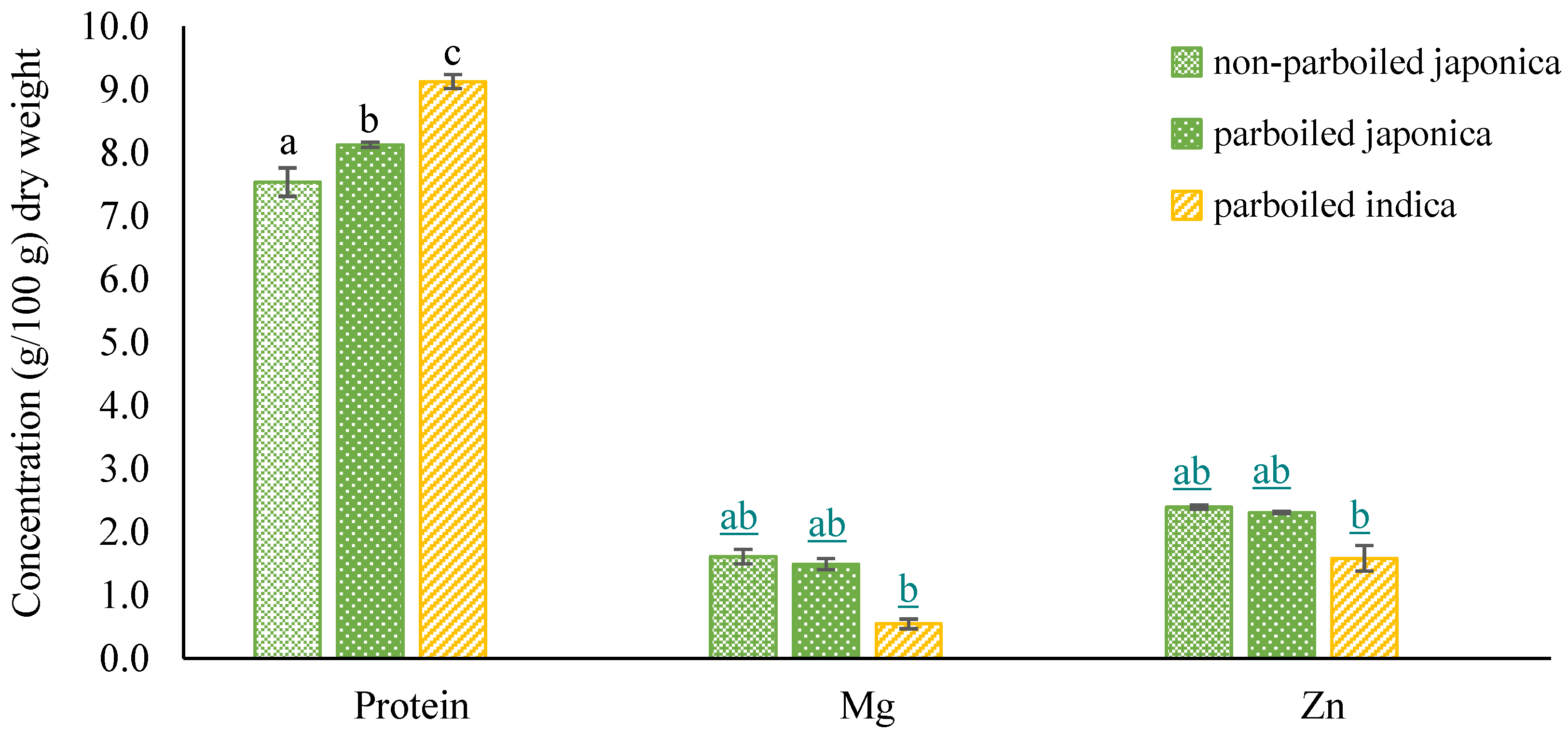
- Nutrient levels observed in milled parboiled and non-parboiled rice samples
- d.
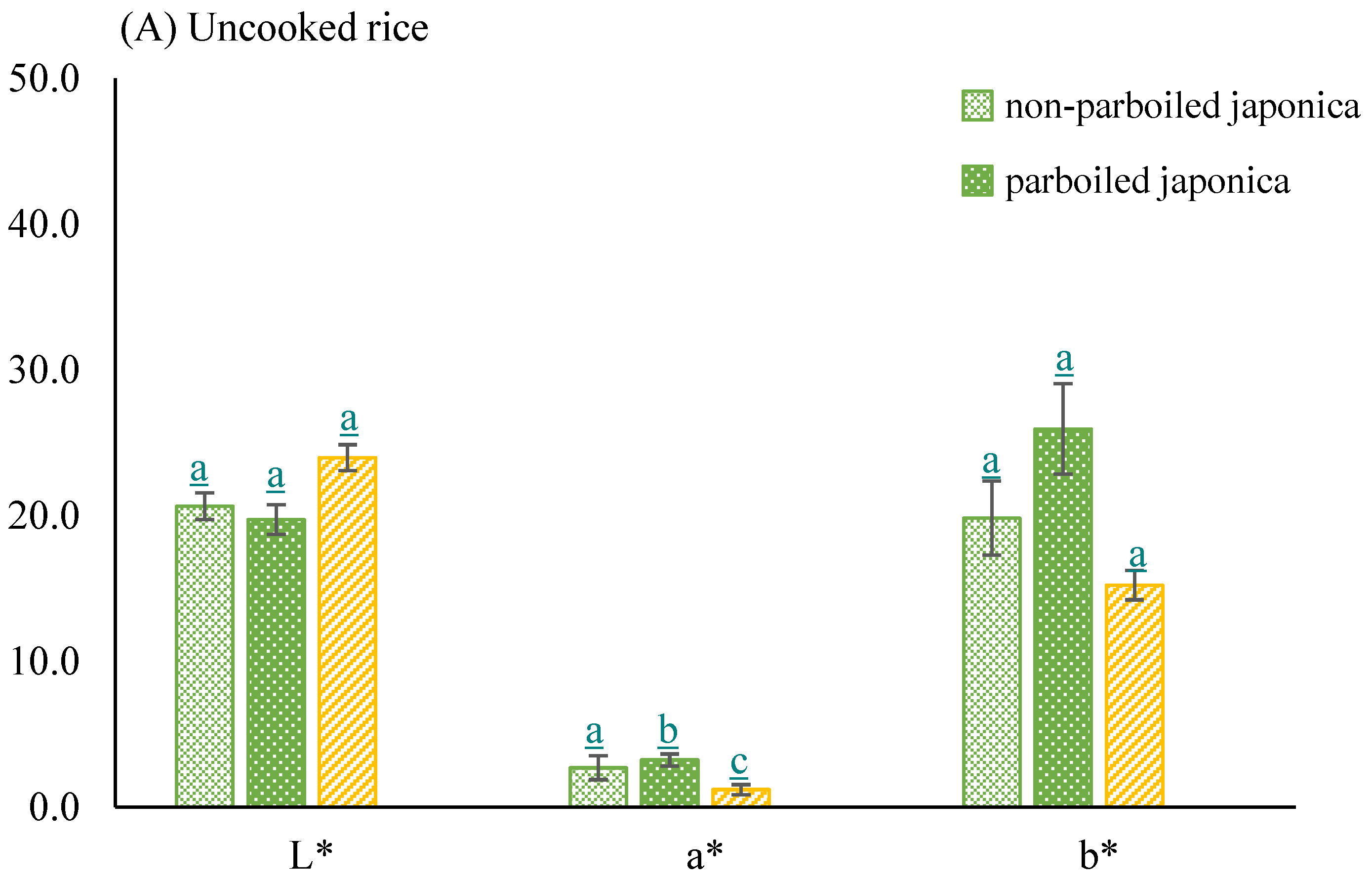
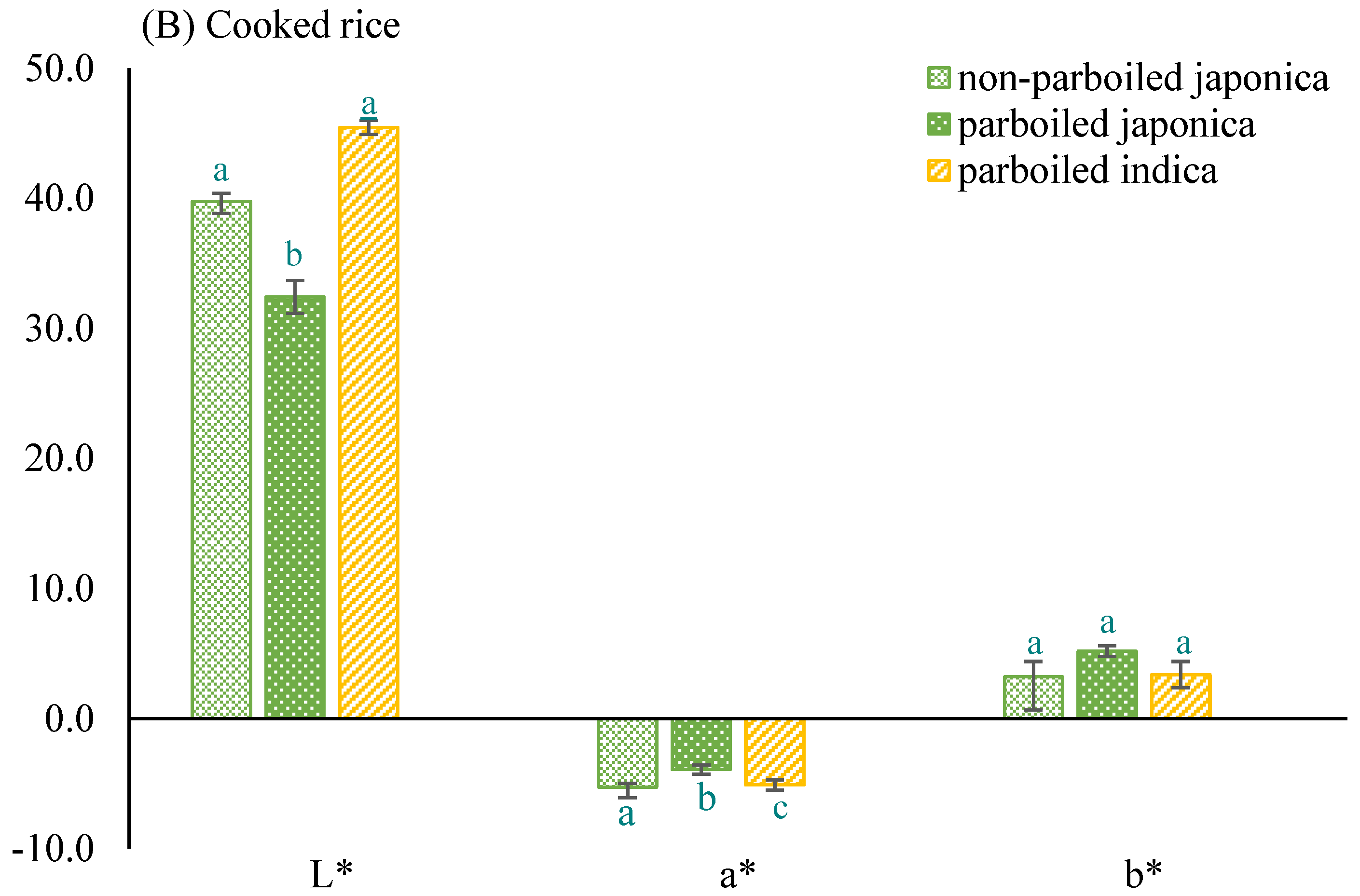
- Color of milled parboiled and non-parboiled rice samples
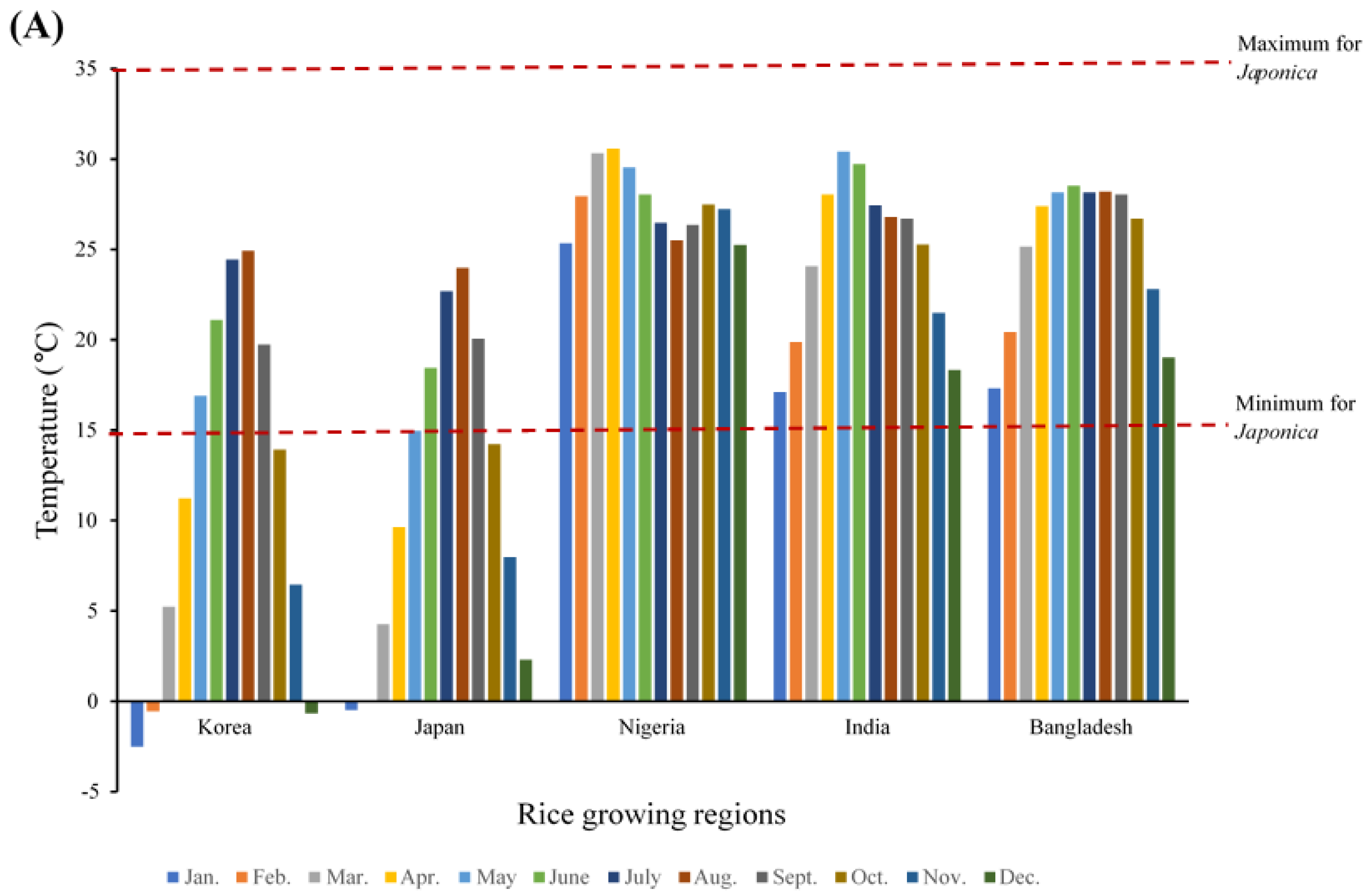
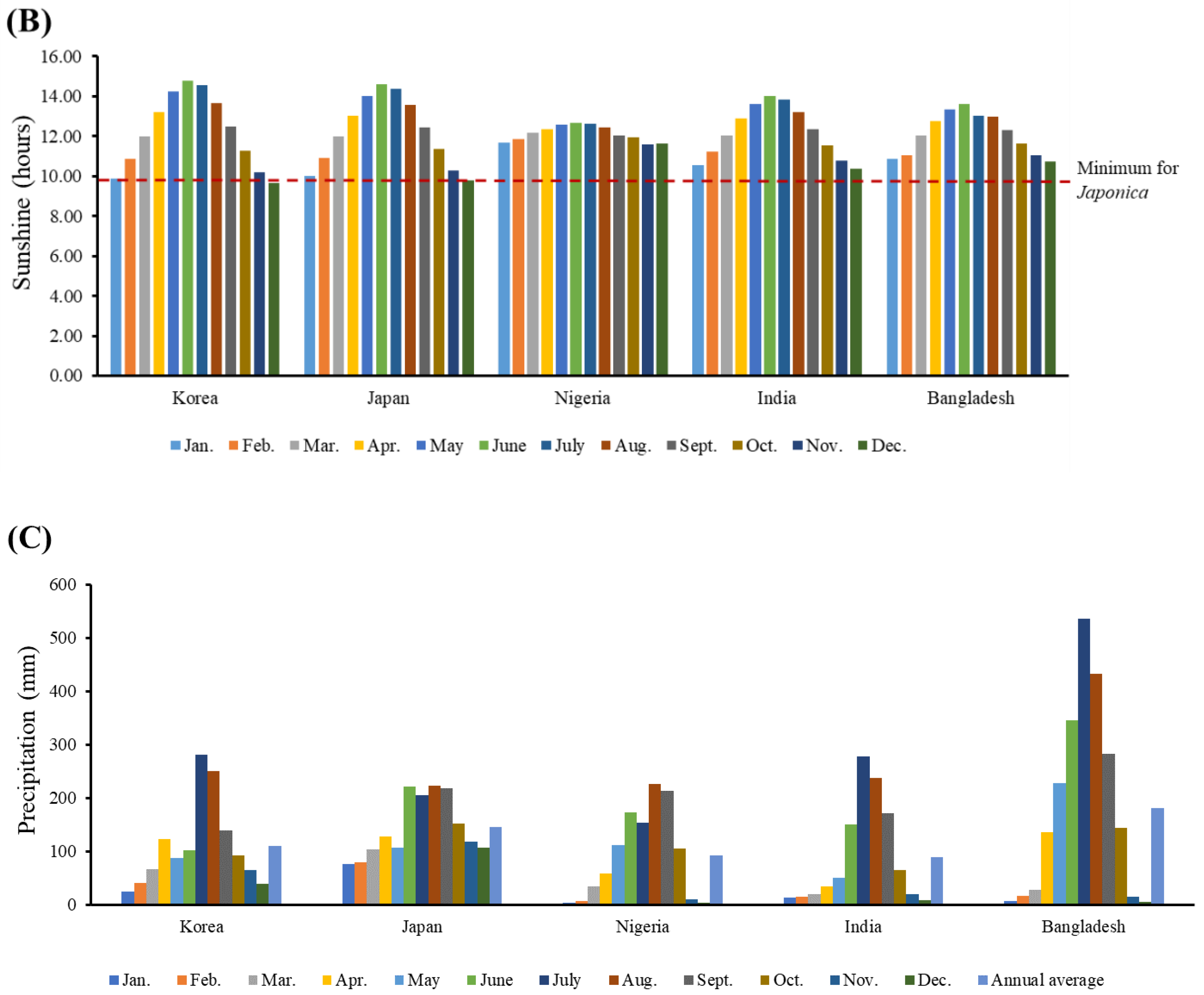
3.3. Growing Conditions of Japonica Rice in Different Rice-Consuming Regions
4. Discussion
4.1. Rice Quality Attributes Preferred by Surveyed Consumers and the Effects of Parboiling on Physicochemical Properties
4.2. Growing Conditions Conducive for Japonica Rice in Different Rice-Consuming Regions
4.3. The Potential Contribution of Japonica Varieties to Greenhouse Gas Reduction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B
- (Survey Questionnaire)
- Age:
- Gender:
- Occupation:
- Country of residence:
- State of residence:
- 1.
- Do you know there are varieties of rice? Yes/No/Not sure
- a.
- If yes, what type(s):
- b.
- Which of the above type do you prefer?
- 2.
- The length of rice is important to me.(a) Strongly Agree (b) Agree (c) Neither Agree nor Disagree (d) Disagree (e) Strongly Disagree
- 3.
- Why is the length of rice important to you?
- 4.
- Adhesiveness (stickiness) of rice is important to me.(a) Strongly Agree (b) Agree (c) Neither Agree nor Disagree (d) Disagree (e) Strongly Disagree
- 5.
- Why is the adhesiveness of rice important to you?
- 6.
- Why is the adhesiveness of rice not important to you?
- 7.
- The aroma of rice is important to me.(a) Very Important (b) Important (c) Neither Important nor Unimportant (d) Unimportant (e) Very Unimportant
- 8.
- The price of rice is important in your decision for selecting the brand of rice to buy.(a) Very Important (b) Important (c) Neither Important nor Unimportant (d) Unimportant (e) Very Unimportant
- 9.
- What do you look out for when selecting rice? (Multiple selections is applicable)(a) Rice brand (b) Length of the grain (c) Adhesiveness after cooking (d) Aroma (e) Price (f) Others (please list)
- 10.
- What is the maximum you could pay extra per 10kg bag of rice if you knew it was healthier?(a) Less than ₦1000 (b) ₦1000–2000 (c) ₦3000–4000 (d) More than ₦5000
- 11.
- Which source of rice do you prefer?(a) Foreign (b) Local (c) Any
- 12.
- Why do you prefer your option in question 11 above?
References
- Katayama, R.; Wadhwa, D. Half of the World’s Poor Live in Just 5 Countries. Available online: https://blogs.worldbank.org/opendata/half-world-s-poor-live-just-5-countries (accessed on 1 August 2021).
- Index Mondi. Milled Rice Domestic Consumption by Country in 1000 MT—Country Rankings. 2021. Available online: https://www.indexmundi.com/agriculture/?commodity=milled-rice&graph=domestic-consumption (accessed on 1 August 2021).
- FAOSTAT. Countries by Commodity. Available online: http://www.fao.org/faostat/en/#rankings/countries_by_commodity_imports (accessed on 1 August 2021).
- World Bank. Nutrition Country Profiles. Available online: https://www.worldbank.org/en/topic/health/publication/nutrition-country-profiles (accessed on 1 August 2021).
- FAO. The State of Food Security and Nutrition in the World 2019. Available online: http://www.fao.org/3/ca5162en/ca5162en.pdf (accessed on 1 August 2021).
- FAO. The State of Food Security and Nutrition in the World 2021. Available online: http://www.fao.org/state-of-food-security-nutrition (accessed on 1 August 2021).
- Von Grebmer, K.; Bernstein, J.; Prasai, N.; Yin, S.; Yohannes, Y.; Towey, O.; Sonntag, A.; Neubauer, L.; de Waal, A. The Concept of the Global Hunger Index; IFPRI: Washington, DC, USA, 2015; pp. 6–11. [Google Scholar]
- Bhutta, Z.A. Micronutrient needs of malnourished children. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 309–314. [Google Scholar] [CrossRef]
- Fraser, D.R. Vitamin D-deficiency in Asia. J. Steroid Biochem. Mol. Biol. 2004, 89, 491–495. [Google Scholar] [CrossRef]
- Chadha, M.; Oluoch, M. Home-based vegetable gardens and other strategies to overcome micronutrient malnutrition in developing countries. Food Nutr. Agric. 2003, 32, 17–23. [Google Scholar]
- Chauhan, B.S.; Jabran, K.; Mahajan, G. Rice Production Worldwide; Springer: Berlin/Heidelberg, Germany, 2017; Volume 247. [Google Scholar]
- OECD. OECD-FAO Agricultural Outlook 2019–2028; OECD: Paris, France, 2019.
- Kubo, M.; Purevdorj, M. The future of rice production and consumption. J. Food Distrib. Res. 2004, 35, 128–142. [Google Scholar]
- Olivier, J.G.; Schure, K.; Peters, J. Trends in Global CO2 and Total Greenhouse Gas Emissions; PBL Netherlands Environmental Assessment Agency: The Hague, The Netherlands, 2017. [Google Scholar]
- World Bank. Land under Cereal Production. Available online: https://data.worldbank.org/indicator/AG.LND.CREL.HA (accessed on 1 August 2021).
- Statista. Available online: https://www.statista.com/topics/1443/rice/ (accessed on 1 August 2021).
- Singh, M.; Upadhyaya, H.D. Genetic and Genomic Resources for Grain Cereals Improvement; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Seck, P.A.; Tollens, E.; Wopereis, M.C.; Diagne, A.; Bamba, I. Rising trends and variability of rice prices: Threats and opportunities for sub-Saharan Africa. Food Policy 2010, 35, 403–411. [Google Scholar] [CrossRef]
- Lançon, F.; Benz, H.D. Rice Imports in West Africa: Trade Regime and Food Policy Formulation; Flançon and H.David-Benz: Montpellier, France, 2007. [Google Scholar]
- United Nations. Coronavirus: World Risks ‘Biblical’ Famines Due to Pandemic. Available online: https://www.bbc.com/news/world-52373888 (accessed on 1 August 2021).
- Cadoni, P.; Angelucci, F. Analysis of incentives and disincentives for Rice in Nigeria. Gates Open Res. 2019, 3, 643. [Google Scholar]
- Linares, O.F. African rice (Oryza glaberrima): History and future potential. Proc. Natl. Acad. Sci. USA 2002, 99, 16360–16365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NRC. Lost Crops of Africa: Volume I: Grains; National Academies Press: Cambridge, MA, USA, 1996. [Google Scholar]
- OECD-FAO. Cereals. In Agricultural Outlook; OECD: Paris, France, 2018; pp. 109–125. Available online: http://www.fao.org/3/i9166e/i9166e_Chapter3_Cereals.pdf (accessed on 1 August 2021).
- Champagne, E.T.; Bett-Garber, K.L.; Fitzgerald, M.A.; Grimm, C.C.; Lea, J.; Ohtsubo, K.i.; Jongdee, S.; Xie, L.; Bassinello, P.Z.; Resurreccion, A. Important sensory properties differentiating premium rice varieties. Rice 2010, 3, 270–281. [Google Scholar] [CrossRef] [Green Version]
- Yanjie, X.; Yining, Y.; Shuhong, O.; Xiaoliang, D.; Hui, S.; Shukun, J.; Shichen, S.; Jinsong, B. Factors affecting sensory quality of cooked japonica rice. Rice Sci. 2018, 25, 330–339. [Google Scholar] [CrossRef]
- Odenigbo, A.M.; Ngadi, M.; Ejebe, C.; Woin, N.; Ndindeng, S.A. Physicochemical, cooking characteristics and textural properties of TOX 3145 milled rice. J. Food Res. 2014, 3, 82. [Google Scholar] [CrossRef] [Green Version]
- Chemutai, L.; Musyoki, M.; Kioko, W.; Mwenda, N.; Muriira, K.; Piero, N. Physicochemical characterization of selected Rice (Oryza Sativa L.) genotypes based on gel consistency and alkali digestion. Anal. Biochem. 2016, 5, 285. [Google Scholar] [CrossRef] [Green Version]
- Okadome, H. Application of instrument-based multiple texture measurement of cooked milled-rice grains to rice quality evaluation. Jpn. Agric. Res. Q. JARQ 2005, 39, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Oli, P.; Ward, R.; Adhikari, B.; Torley, P. Parboiled rice: Understanding from a materials science approach. J. Food Eng. 2014, 124, 173–183. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D. Effect of soaking temperature on physical and functional properties of parboiled rice cultivars grown in temperate region of India. Food Nutr. Sci. 2013, 4, 282. [Google Scholar] [CrossRef] [Green Version]
- Bello, M.; Baeza, R.; Tolaba, M. Quality characteristics of milled and cooked rice affected by hydrothermal treatment. J. Food Eng. 2006, 72, 124–133. [Google Scholar] [CrossRef]
- da Fonseca, F.A.; Soares Júnior, M.S.; Caliari, M.; Bassinello, P.Z.; da Costa Eifert, E.; Garcia, D.M. Changes occurring during the parboiling of upland rice and in the maceration water at different temperatures and soaking times. Int. J. Food Sci. Technol. 2011, 46, 1912–1920. [Google Scholar] [CrossRef]
- Miah, M.K.; Haque, A.; Douglass, M.P.; Clarke, B. Parboiling of rice. Part II: Effect of hot soaking time on the degree of starch gelatinization. Int. J. Food Sci. Technol. 2002, 37, 539–545. [Google Scholar] [CrossRef]
- Juliano, B.O. The rice grain and its gross composition. In Rice: Chemistry and Technology; American Association of Cereal Chemists: St. Paul, MN, USA, 1985. [Google Scholar]
- Pedersen, B.; Eggum, B. The influence of milling on the nutritive value of flour from cereal grains. 4. Rice. Plant Foods Hum. Nutr. 1983, 33, 267–278. [Google Scholar] [CrossRef]
- Adhikari, B.; Howes, T.; Bhandari, B.; Truong, V. Stickiness in foods: A review of mechanisms and test methods. Int. J. Food Prop. 2001, 4, 1–33. [Google Scholar] [CrossRef]
- Uyeh, D.D.; Woo, S.M.; Hong, D.H.; Ha, Y.S. Optimization of parboiling conditions for enhanced Japonica rice milling. Emir. J. Food Agric. 2016, 28, 764–771. [Google Scholar] [CrossRef] [Green Version]
- Ha, Y.; Park, K.; Kim, H.; Hong, D.; Nah, K.; Seo, S. Milled rice recovery rate of paddy with various moisture contents. J. Korean Soc. Agric. Mach. 2002, 27, 125–132. [Google Scholar]
- Tang, J.; Sokhansanj, S. Determination of moisture content of whole kernel lentil by oven method. Trans. ASAE 1991, 34, 255–0256. [Google Scholar] [CrossRef]
- Sonsanguan, N.; Sirisomboon, P. Determination of stickiness characteristic of cooked rice samples in rice industry. In Proceedings of the 14th TSAE National Conference and the 6th TSAE International Conference, Hua Hin, Thailand, 1–4 April 2013; pp. 151–153. [Google Scholar]
- Choe, Z.R.; Heu, M.H. Optimum conditions for alkali digestibility test in rice. J. Korean Crop. Soc. 1975, 19, 7–13. [Google Scholar]
- IRRI. Standard Evaluation System for Rice; IRRI: Los Baños, Philippines, 1996. [Google Scholar]
- AOAC. Official Methods of Analysis, 21st ed.; AOAC: Rockville, MD, USA, 2019. [Google Scholar]
- Cordero-Lara, K.I. Temperate japonica rice (Oryza sativa L.) breeding: History, present and future challenges. Chil. J. Agric. Res. 2020, 80, 303–314. [Google Scholar] [CrossRef]
- Dhanushka Perera, J. Analysis of the Variability of Cardinal Temperatures in Rice; UM2: 2011. Available online: https://agritrop.cirad.fr/570279/1/document_570279.pdf (accessed on 1 April 2021).
- Hu, Y.-J.; Pei, W.; Zhang, H.-C.; Dai, Q.-G.; Huo, Z.-Y.; Ke, X.; Hui, G.; Wei, H.-Y.; Guo, B.-W.; Cui, P.-Y. Comparison of agronomic performance between inter-sub-specific hybrid and inbred japonica rice under different mechanical transplanting methods. J. Integr. Agric. 2018, 17, 806–816. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Gao, L.; Jin, Z.; Chen, H. Simulating the optimal growing season of rice in the Yangtze River Valley and its adjacent area, China. Agric. For. Meteorol. 1998, 91, 251–262. [Google Scholar] [CrossRef]
- Ueno, K.; Miyoshi, K. Difference of optimum germination temperature of seeds of intact and dehusked japonica rice during seed development. Euphytica 2005, 143, 271–275. [Google Scholar] [CrossRef]
- Heinemann, R.J.B.; Fagundes, P.d.L.; Pinto, E.A.; Penteado, M.d.V.C.; Lanfer-Marquez, U. Comparative study of nutrient composition of commercial brown, parboiled and milled rice from Brazil. J. Food Compos. Anal. 2005, 18, 287–296. [Google Scholar] [CrossRef]
- Adu-Kwarteng, E.; Ellis, W.O.; Oduro, I.; Manful, J.T. Rice grain quality: A comparison of local varieties with new varieties under study in Ghana. Food Control 2003, 14, 507–514. [Google Scholar] [CrossRef]
- Islam, M.R.; Roy, P.; Shimizu, N.; Kimura, T. Effect of Processing Conditions on Physical Properties of Parboiled Rice. Food Sci. Technol. Res. 2002, 8, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Chinnaswamy, R.; Hanna, M.A. Expansion, Color and Shear Strength Properties of Com Starches Extrusion-Cooked with Urea and Salts. Starch Stärke 1988, 40, 186–190. [Google Scholar] [CrossRef]
- Ali, N.; Pandya, A.C. Basic concept of parboiling of paddy. J. Agric. Eng. Res. 1974, 19, 111–115. [Google Scholar] [CrossRef]
- Wimberly, J.E.; International Rice Research, I. Technical Handbook for the Paddy Rice Postharvest Industry in Developing Countries; International Rice Research Institute: Los Baños, Philippines, 1983. [Google Scholar]
- Gariboldi, F.; Food and Agriculture Organization of the United. Rice Parboiling; Food and Agriculture Organization of the United Nations: Rome, Italy, 1984. [Google Scholar]
- Adhikaritanayake, T.B.; Noomhorm, A. Effect of continuous steaming on parboiled rice quality. J. Food Eng. 1998, 36, 135–143. [Google Scholar] [CrossRef]
- Sareepuang, K.; Siriamornpun, S.; Wiset, L.; Meeso, N. Effect of soaking temperature on physical, chemical and cooking properties of parboiled fragrant rice. World J. Agric. Sci. 2008, 4, 409–415. [Google Scholar]
- Graham-Acquaah, S.; Manful, J.T.; Ndindeng, S.A.; Tchatcha, D.A. Effects of Soaking and Steaming Regimes on the Quality of Artisanal Parboiled Rice. J. Food Process. Preserv. 2015, 39, 2286–2296. [Google Scholar] [CrossRef]
- Fan, C.; Yu, X.; Xing, Y.; Xu, C.; Luo, L.; Zhang, Q. The main effects, epistatic effects and environmental interactions of QTLs on the cooking and eating quality of rice in a doubled-haploid line population. Theor. Appl. Genet. 2005, 110, 1445–1452. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.L.; Wu, J.G.; Feng, Y.; Yang, X.E.; Shi, C.H. Correlation Analysis of Mineral Element Contents and Quality Traits in Milled Rice (Oryza stavia L.). J. Agric. Food Chem. 2007, 55, 9608–9613. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Peng, S.; Dionisio-Sese, M.L.; Laza, R.C.; Visperas, R.M. Grain filling duration, a crucial determinant of genotypic variation of grain yield in field-grown tropical irrigated rice. Field Crop. Res. 2008, 105, 221–227. [Google Scholar] [CrossRef]
- Vaughan, D.A.; Lu, B.-R.; Tomooka, N. The evolving story of rice evolution. Plant Sci. 2008, 174, 394–408. [Google Scholar] [CrossRef]
- Zhang, Q.; Maroof, M.S.; Lu, T.Y.; Shen, B. Genetic diversity and differentiation of indica and japonica rice detected by RFLP analysis. Theor. Appl. Genet. 1992, 83, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.-R.; Zheng, K.; Qian, H.; Zhuang, J. Genetic differentiation of wild relatives of rice as assessed by RFLP analysis. Theor. Appl. Genet. 2002, 106, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wright, M.; Kimball, J.; Eizenga, G.; McClung, A.; Kovach, M.; Tyagi, W.; Ali, M.L.; Tung, C.-W.; Reynolds, A. Genomic diversity and introgression in O. sativa reveal the impact of domestication and breeding on the rice genome. PLoS ONE 2010, 5, e10780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, D.; Balazs, E.; Heslop-Harrison, J. From crop domestication to super-domestication. Ann. Bot. 2007, 100, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Khush, G.S. Origin, dispersal, cultivation and variation of rice. Plant Mol. Biol. 1997, 35, 25–34. [Google Scholar] [CrossRef]
- Huang, X.; Kurata, N.; Wang, Z.-X.; Wang, A.; Zhao, Q.; Zhao, Y.; Liu, K.; Lu, H.; Li, W.; Guo, Y. A map of rice genome variation reveals the origin of cultivated rice. Nature 2012, 490, 497. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.-X.; Nguyen, H.T. Genetic variation detected with RAPD markers among upland and lowland rice cultivars (Oryza sativa L.). Theor. Appl. Genet. 1994, 87, 668–672. [Google Scholar] [CrossRef] [PubMed]
- Olsen, K.M.; Caicedo, A.L.; Polato, N.; McClung, A.; McCouch, S.; Purugganan, M.D. Selection under domestication: Evidence for a sweep in the rice waxy genomic region. Genetics 2006, 173, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Z.; Zhang, S.; Wang, Y.; Ford-Lloyd, B.V.; Tu, M.; Jin, X.; Wu, Y.; Yan, H.; Yang, X.; Liu, P. Differentiation and distribution of indica and japonica rice varieties along the altitude gradients in Yunnan Province of China as revealed by InDel molecular markers. Genet. Resour. Crop. Evol. 2010, 57, 891–902. [Google Scholar] [CrossRef]
- Lu, B.-R.; Cai, X.; Xin, J. Efficient indica and japonica rice identification based on the InDel molecular method: Its implication in rice breeding and evolutionary research. Prog. Nat. Sci. 2009, 19, 1241–1252. [Google Scholar] [CrossRef]
- Morishima, H.; Oka, H.-I. Phylogenetic differentiation of cultivated rice, XXII. Numerical evaluation of the indica-japonica differentiation. Jpn. J. Breed. 1981, 31, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.; Laza, R.C.; Visperas, R.M.; Khush, G.S.; Virk, P.; Zhu, D. Rice: Progress in breaking the yield ceiling. In Proceedings of the 4th International Crop Science Congress, Brisbane, Australia, 26 September–1 October 2004. [Google Scholar]
- Xiong, Z.; Zhang, S.; Ford-Lloyd, B.; Jin, X.; Wu, Y.; Yan, H.; Liu, P.; Yang, X.; Lu, B.-R. Latitudinal distribution and differentiation of rice germplasm: Its implications in breeding. Crop Sci. 2011, 51, 1050–1058. [Google Scholar] [CrossRef] [Green Version]
- Dubey, A.; Verma, S.; Goswami, S.; Devedee, A. Effect of Temperature on Different Growth Stages and Physiological Process of Rice crop—A Review. Bull. Environ. Pharmacol. Life Sci. 2018, 7, 129–136. [Google Scholar]
- Kondo, M.; Okamura, T. Growth response of rice plant to water temperature. Agric. Hortic. 1931, 6, 517–530. [Google Scholar]
- Osada, A.; Sasiprapa, V.; Rahong, M.; Dhammanuvong, S.; Chakrabndhu, H. Abnormal occurrence of empty grains of indica rice plants in the dry, hot season in Thailand. Jpn. J. Crop. Sci. 1973, 42, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Ghadirnezhad, R.; Fallah, A. Temperature Effect on Yield and Yield Components of Different Rice Cultivars in Flowering Stage. Int. J. Agron. 2014, 2014, 846707. [Google Scholar] [CrossRef]
- Ikeda, K. Photoperiodic flower induction in rice plants as influenced by light intensity and quality. JARQ. Jpn. Agric. Res. Q. 1985, 18, 164–170. [Google Scholar]
- Smith, P.; Martino, D.; Cai, Z.; Gwary, D.; Janzen, H.; Kumar, P.; McCarl, B.; Ogle, S.; O’Mara, F.; Rice, C.; et al. Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/ar4-wg3-chapter8-1.pdf (accessed on 1 August 2021).
- Geetha Thanuja, K.; Karthikeyan, S. Exploring bio-mitigation strategies to reduce carbon footprint in wetland paddy system. Bioresour. Technol. Rep. 2020, 12, 100557. [Google Scholar] [CrossRef]
- Epule, E.T.; Peng, C.; Mafany, N.M. Methane emissions from paddy rice fields: Strategies towards achieving a win-win sustainability scenario between rice production and methane emission reduction. J. Sustain. Dev. 2011, 4, 188. [Google Scholar] [CrossRef]
- Yan, X.; Akiyama, H.; Yagi, K.; Akimoto, H. Global estimations of the inventory and mitigation potential of methane emissions from rice cultivation conducted using the 2006 Intergovernmental Panel on Climate Change Guidelines. Glob. Biogeochem. Cycles 2009, 23. [Google Scholar] [CrossRef]
- Zou, J.; Huang, Y.; Zheng, X.; Wang, Y. Quantifying direct N2O emissions in paddy fields during rice growing season in mainland China: Dependence on water regime. Atmos. Environ. 2007, 41, 8030–8042. [Google Scholar] [CrossRef]
- Neue, H.-U.; Sass, R.L. Trace gas emissions from rice fields. In Global Atmospheric-Biospheric Chemistry; Springer: Berlin/Heidelberg, Germany, 1994; pp. 119–147. [Google Scholar]
- Conrad, R. Control of methane production in terrestrial ecosystems. In Exchange of Trace Gases between Terrestrial Ecosystems and the Atmosphere; Andreae, M.O., Schimel, D.S., Eds.; Wiley Chichester: Chichester, UK, 1989; pp. 39–58. [Google Scholar]
- Seiler, W.; Holzapfel-Pschorn, A.; Conrad, R.; Scharffe, D. Methane emission from rice paddies. J. Atmos. Chem. 1983, 1, 241–268. [Google Scholar] [CrossRef]
- Schütz, H.; Seiler, W.; Conrad, R. Processes involved in formation and emission of methane in rice paddies. Biogeochemistry 1989, 7, 33–53. [Google Scholar] [CrossRef]
- Zheng, H.; Huang, H.; Yao, L.; Liu, J.; He, H.; Tang, J. Impacts of rice varieties and management on yield-scaled greenhouse gas emissions from rice fields in China: A meta-analysis. Biogeosci. Discuss. 2013, 10, 19045–19069. [Google Scholar]
- van Beek, C.L.; Meerburg, B.G.; Schils, R.L.; Verhagen, J.; Kuikman, P.J. Feeding the world’s increasing population while limiting climate change impacts: Linking N2O and CH4 emissions from agriculture to population growth. Environ. Sci. Policy 2010, 13, 89–96. [Google Scholar] [CrossRef]
- Van Groenigen, K.J.; Van Kessel, C.; Hungate, B.A. Increased greenhouse-gas intensity of rice production under future atmospheric conditions. Nat. Clim. Chang. 2013, 3, 288. [Google Scholar] [CrossRef]



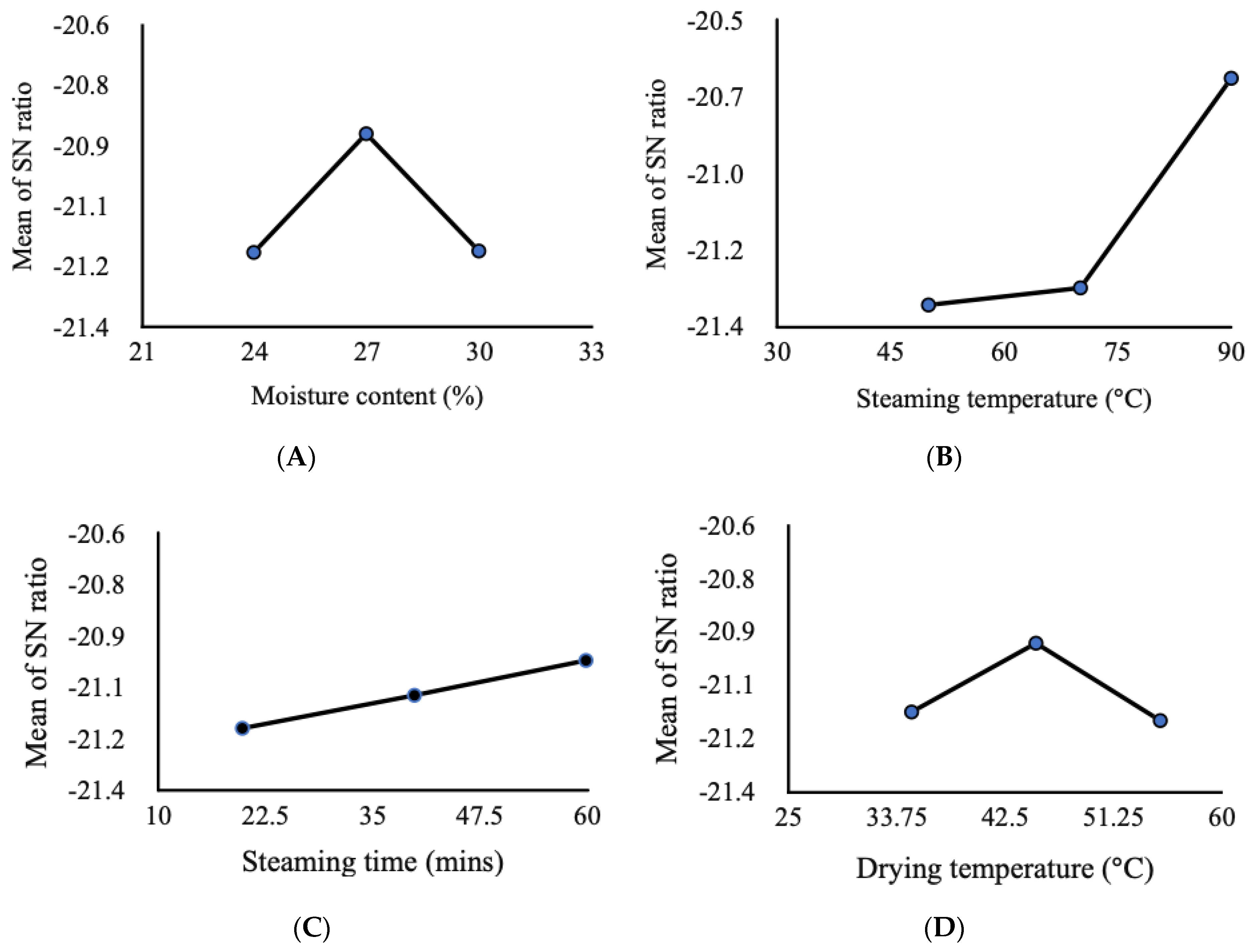
| Exp. No. | Moisture Content after Soaking (% Wet Basis) | Steaming Temperature (°C) | Steaming Duration (min) | Drying Temperature (°C) |
|---|---|---|---|---|
| 1 | 24 | 50 | 20 | 35 |
| 2 | 24 | 70 | 40 | 45 |
| 3 | 24 | 90 | 60 | 55 |
| 4 | 27 | 70 | 20 | 55 |
| 5 | 27 | 90 | 40 | 35 |
| 6 | 27 | 50 | 60 | 45 |
| 7 | 30 | 90 | 20 | 45 |
| 8 | 30 | 50 | 40 | 55 |
| 9 | 30 | 70 | 60 | 35 |
| Degree of Digestion | Alkali Digestion Classification | Alkali Digestion Value |
|---|---|---|
| Grain not affected | Low | 1 |
| Grain swollen | Low | 2 |
| Grain swollen, collar incomplete and narrow | Low | 3 |
| Grain swollen, collar complete and wide | Intermediate | 4 |
| Grain split or segmented, collar complete and wide | Intermediate | 5 |
| Grain dispersed, merging with collar | High | 6 |
| Grain completely dispersed and intermingled | High | 7 |
| Treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Amylose (%) | 17.93 | 18.33 | 19.03 | 18.53 | 19.43 | 18.20 | 19.37 | 18.37 | 18.87 |
| S/N Value | 25.07 | 25.26 | 25.59 | 25.36 | 25.77 | 25.20 | 25.74 | 25.28 | 25.51 |
| Constituent | Units | Non-Parboiled Japonica (n = 3) | Parboiled Japonica (n = 3) | Parboiled Indica (n = 3) | Reported Values in Literature (Range) |
|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |||
| Crude Protein | g/100 g | 7.5 (0.23) | 8.1 (0.11) | 9.1 (0.03) | 5.71–6.71 a; 7.21–8.53 b |
| Crude Fat | g/100 g | 0.4 (0.23) | 0.4 (0.23) | 0.5 (0.28) | 0.31–0.47 a |
| Ash | g/100 g | 0.5 (0.24) | 0.5 (0.26) | 0.5 (0.19) | 0.49–0.60 a |
| Carbohydrates | g/100 g | 91.6 (0.28) | 90.9 (0.54) | 89.9 (0.44) | |
| Fe | mg/100 g | 0.8 (0.18) | 0.9 (0.23) | 1.0 (0.13) | 0.12–0.78 a |
| Mg | mg/100 g | 1.6 (0.04) | 1.5 (0.09) | 0.5 (0.02) | |
| Zn | mg/100 g | 2.4 (0.11) | 2.3 (0.08) | 1.6 (0.20) | 0.84–1.46 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyeh, D.D.; Asem-Hiablie, S.; Park, T.; Kim, K.; Mikhaylov, A.; Woo, S.; Ha, Y. Could Japonica Rice Be an Alternative Variety for Increased Global Food Security and Climate Change Mitigation? Foods 2021, 10, 1869. https://doi.org/10.3390/foods10081869
Uyeh DD, Asem-Hiablie S, Park T, Kim K, Mikhaylov A, Woo S, Ha Y. Could Japonica Rice Be an Alternative Variety for Increased Global Food Security and Climate Change Mitigation? Foods. 2021; 10(8):1869. https://doi.org/10.3390/foods10081869
Chicago/Turabian StyleUyeh, Daniel Dooyum, Senorpe Asem-Hiablie, Tusan Park, Kyungmin Kim, Alexey Mikhaylov, Seungmin Woo, and Yushin Ha. 2021. "Could Japonica Rice Be an Alternative Variety for Increased Global Food Security and Climate Change Mitigation?" Foods 10, no. 8: 1869. https://doi.org/10.3390/foods10081869
APA StyleUyeh, D. D., Asem-Hiablie, S., Park, T., Kim, K., Mikhaylov, A., Woo, S., & Ha, Y. (2021). Could Japonica Rice Be an Alternative Variety for Increased Global Food Security and Climate Change Mitigation? Foods, 10(8), 1869. https://doi.org/10.3390/foods10081869









