Magnetic Shielding Study of Bonding and Aromaticity in Corannulene and Coronene
Abstract
:1. Introduction
2. Computational Procedure
3. Results
4. Conclusions
Supplementary Materials
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barth, W.E.; Lawton, R.G. Dibenzo[ghi,mno]fluoranthene. J. Am. Chem. Soc. 1966, 88, 380–381. [Google Scholar] [CrossRef]
- Lawton, R.G.; Barth, W.E. The synthesis of corannulene. J. Am. Chem. Soc. 1971, 93, 1730–1745. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Stuparu, M.C. Corannulene: A curved polyarene building block for the construction of functional materials. Acc. Chem. Res. 2021, 54, 2858–2870. [Google Scholar] [CrossRef]
- Butterfield, A.M.; Gilomen, B.; Siegel, J.S. Kilogram-scale production of corannulene. Org. Process Res. Dev. 2012, 16, 664–676. [Google Scholar] [CrossRef]
- Monaco, G.; Scott, L.T.; Zanasi, R. Magnetic euripi in corannulene. J. Phys. Chem. A 2008, 112, 8136–8147. [Google Scholar] [CrossRef] [PubMed]
- Dunning, T.H., Jr.; Xu, L.T.; Cooper, D.L.; Karadakov, P.B. Spin-coupled generalized valence bond theory: New perspectives on the electronic structure of molecules and chemical bonds. J. Phys. Chem. A 2021, 125, 2021–2050. [Google Scholar] [CrossRef] [PubMed]
- Sironi, M.; Cooper, D.L.; Gerratt, J.; Raimondi, M. The modern valence bond description of naphthalene. J. Chem. Soc. Chem. Commun. 1989, 675–677. [Google Scholar] [CrossRef]
- Sygula, A.; Rabideau, P.W. Structure and inversion barriers of corannulene, its dianion and tetraanion. An ab initio study. J. Mol. Struc. THEOCHEM 1995, 333, 215–226. [Google Scholar] [CrossRef]
- Steiner, E.; Fowler, P.W.; Jenneskens, L.W. Counter-rotating ring currents in coronene and corannulene. Angew. Chem. Int. Ed. 2001, 40, 362–366. [Google Scholar] [CrossRef]
- Bühl, M. The relation between endohedral chemical shifts and local aromaticities in fullerenes. Chem. Eur. J. 1998, 4, 734–739. [Google Scholar] [CrossRef]
- Li, J.; Rogachev, A.Y. Aromatic stabilization of functionalized corannulene cations. Phys. Chem. Chem. Phys. 2016, 18, 11781–11791. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Maerker, C.; Dransfeld, A.; Jiao, H.; van Eikema Hommes, N.J.R. Nucleus-independent chemical shifts: A simple and efficient aromaticity probe. J. Am. Chem. Soc. 1996, 118, 6317–6318. [Google Scholar] [CrossRef] [PubMed]
- Monaco, G.; Zanasi, R. Three contra-rotating currents from a rational design of polycyclic aromatic hydrocarbons: Altan-corannulene and altan-coronene. J. Phys. Chem. A 2012, 116, 9020–9026. [Google Scholar] [CrossRef]
- Monaco, G.; Zanasi, R. Anionic Derivatives of Altan-Corannulene. J. Phys. Org. Chem. 2013, 26, 730–736. [Google Scholar] [CrossRef]
- Dickens, T.K.; Mallion, R.B. Topological ring-currents and bond-currents in hexaanionic altans and iterated altans of corannulene and coronene. J. Phys. Chem. A 2020, 124, 7973–7990. [Google Scholar] [CrossRef]
- Kaipio, M.; Patzschke, M.; Fliegl, H.; Pichierri, F.; Sundholm, D. Effect of fluorine substitution on the aromaticity of polycyclic hydrocarbons. J. Phys. Chem. A 2012, 116, 10257–10268. [Google Scholar] [CrossRef]
- Pelloni, S.; Ligabue, A.; Lazzeretti, P. Ring-current models from the differential Biot-Savart law. Org. Lett. 2004, 6, 4451–4454. [Google Scholar] [CrossRef]
- Karadakov, P.B.; Horner, K.E. Exploring chemical bonds through variations in magnetic shielding. J. Chem. Theory Comput. 2016, 12, 558–563. [Google Scholar] [CrossRef]
- Karadakov, P.B.; Hearnshaw, P.; Horner, K.E. Magnetic shielding, aromaticity, antiaromaticity, and bonding in the low-lying electronic states of benzene and cyclobutadiene. J. Org. Chem. 2016, 81, 11346–11352. [Google Scholar] [CrossRef] [Green Version]
- Lampkin, B.J.; Karadakov, P.B.; VanVeller, B. Detailed visualization of aromaticity using isotropic magnetic shielding. Angew. Chem. Int. Ed. 2020, 59, 19275–19281. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Cubegen. Available online: https://gaussian.com/cubegen/ (accessed on 30 July 2021).
- Filatov, A.S.; Sumner, N.J.; Spisak, S.N.; Zabula, A.V.; Petrukhina, M.A. Jahn—Teller effect in circulenes: X-ray diffraction study of coronene and corannulene radical anions. Chem. Eur. J. 2012, 18, 15753–15760. [Google Scholar] [CrossRef]
- Petrukhina, M.A.; Andreini, K.W.; Mack, J.; Scott, L.T. X-ray quality geometries of geodesic polyarenes from theoretical calculations: What levels of theory are reliable? J. Org. Chem. 2005, 70, 5713–5716. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Cyrański, M.; Ciesielski, A.; Swirska, B.; Leszczyński, P. Separation of the energetic and geometric contributions to aromaticity. 2. Analysis of the aromatic character of benzene rings in their various topological environments in the benzenoid hydrocarbons. Crystal and molecular structure of coronene. J. Chem. Inf. Comput. Sci. 1996, 36, 1135–1141. [Google Scholar] [CrossRef]
- Seiders, T.J.; Baldridge, K.K.; Grube, G.H.; Siegel, J.S. Structure/energy correlation of bowl depth and inversion barrier in corannulene derivatives: Combined experimental and quantum mechanical analysis. J. Am. Chem. Soc. 2001, 123, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Karadakov, P.B.; Horner, K.E. Magnetic shielding in and around benzene and cyclobutadiene: A source of information about aromaticity, antiaromaticity and chemical bonding. J. Phys. Chem. A 2013, 117, 518–523. [Google Scholar] [CrossRef]
- Horner, K.E.; Karadakov, P.B. Chemical bonding and aromaticity in furan, pyrrole and thiophene: A magnetic shielding study. J. Org. Chem. 2013, 78, 8037–8043. [Google Scholar] [CrossRef]
- Horner, K.E.; Karadakov, P.B. Magnetic shielding in and around oxazole, imidazole and thiazole: How does the second heteroatom affect aromaticity and bonding? J. Org. Chem. 2015, 80, 7150–7157. [Google Scholar] [CrossRef]
- Karadakov, P.B.; Kirsopp, J. Magnetic shielding studies of C2 and C2H2 support higher than triple bond multiplicity in C2. Chem. Eur. J. 2017, 23, 12949–12954. [Google Scholar] [CrossRef] [PubMed]
- Fedik, N.; Boldyrev, A.I. Insight into the nature of rim bonds in coronene. J. Phys. Chem. A 2013, 117, 8585–8590. [Google Scholar] [CrossRef]
- Schleyer, P.R.; Manoharan, M.; Wang, Z.X.; Kiran, B.; Jiao, H.; Puchta, R.; van Eikema Hommes, N.J.R. Dissected nucleus-independent chemical shift analysis of π-aromaticity and antiaromaticity. Org. Lett. 2001, 3, 2465–2468. [Google Scholar] [CrossRef]
- Fallah-Bagher-Shaidaei, H.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Schleyer, P.v.R. Which NICS aromaticity index for planar π rings is best? Org. Lett. 2006, 8, 863–866. [Google Scholar] [CrossRef]
- Gershoni-Poranne, R.; Stanger, A. The NICS-XY-scan: Identification of local and global ring currents in multi-ring systems. Chem. Eur. J. 2014, 20, 5673–5688. [Google Scholar] [CrossRef] [PubMed]
- Fias, S.; Fowler, P.W.; Delgado, J.L.; Hahn, U.; Bultinck, P. Correlation of delocalization indices and current-density maps in polycyclic aromatic hydrocarbons. Chem. Eur. J. 2008, 14, 3093–3099. [Google Scholar] [CrossRef]
- Fliegl, H.; Taubert, S.; Lehtonen, O.; Sundholm, D. The gauge including magnetically induced current method. Phys. Chem. Chem. Phys. 2011, 13, 20500–20518. [Google Scholar] [CrossRef] [PubMed]
- Geuenich, D.; Hess, K.; Köhler, F.; Herges, R. Anisotropy of the induced current density (ACID), a general method to quantify and visualize electronic delocalization. Chem. Rev. 2005, 105, 3758–3772. [Google Scholar] [CrossRef]
- Kumar, A.; Duran, M.; Solà, M. Is coronene better described by Clar’s aromatic π-sextet model or by the AdNDP representation? J. Comput. Chem. 2017, 38, 1606–1611. [Google Scholar] [CrossRef] [Green Version]
- Merino, G.; Heine, T.; Seifert, G. The induced magnetic field in cyclic molecules. Chem. Eur. J. 2004, 10, 4367–4371. [Google Scholar] [CrossRef]
- Heine, T.; Corminboeuf, C.; Seifert, G. The magnetic shielding function of molecules and pi-electron delocalization. Chem. Rev. 2005, 105, 3889–3910. [Google Scholar] [CrossRef]
- Heine, T.; Islas, R.; Merino, G. σ and π contributions to the induced magnetic field: Indicators for the mobility of electrons in molecules. J. Comput. Chem. 2007, 28, 302–309. [Google Scholar] [CrossRef]
- Matito, E.; Poater, J.; Duran, M.; Solà, M. An analysis of the changes in aromaticity and planarity along the reaction path of the simplest diels–alder reaction. Exploring the validity of different indicators of aromaticity. J. Mol. Struct. THEOCHEM 2005, 727, 165–171. [Google Scholar] [CrossRef]
- Dobrowolski, J.C.; Lipiński, P.F.J. On splitting of the NICS(1) magnetic aromaticity index. RSC Adv. 2016, 6, 23900–23904. [Google Scholar] [CrossRef]
- Scott, L.T.; Cheng, P.-C.; Hashemi, M.M.; Bratcher, M.S.; Meyer, D.T.; Warren, H.B. Corannulene. A three-step synthesis. J. Am. Chem. Soc. 1997, 119, 10963–10968. [Google Scholar] [CrossRef]
- Zanasi, R.; Lazzeretti, P.; Malagoli, M.; Piccinini, F. Molecular magnetic properties within continuous transformations of origin of the current density. J. Chem. Phys. 1995, 102, 7150–7157. [Google Scholar] [CrossRef]
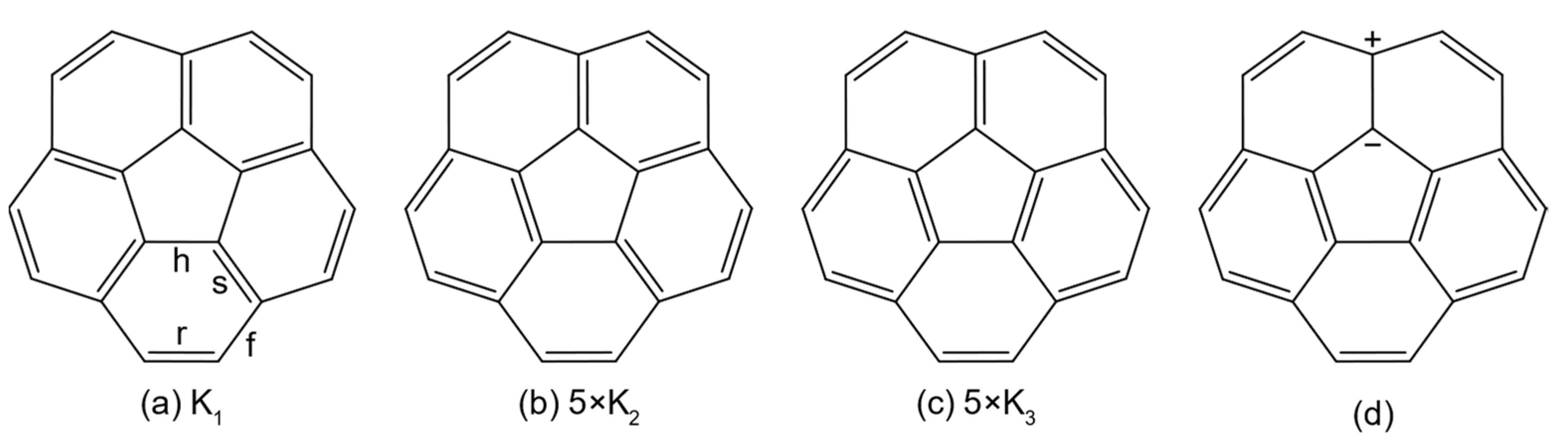
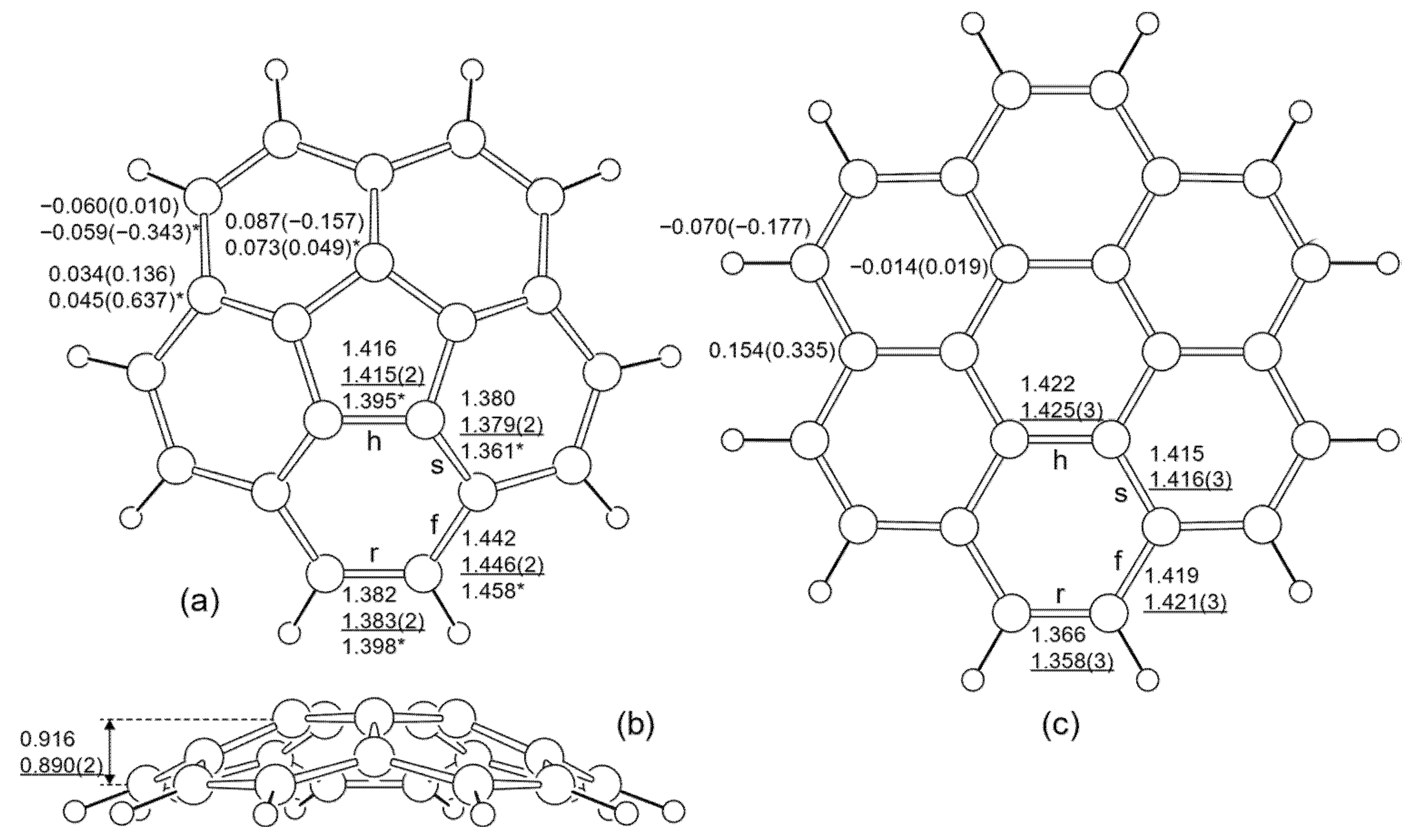

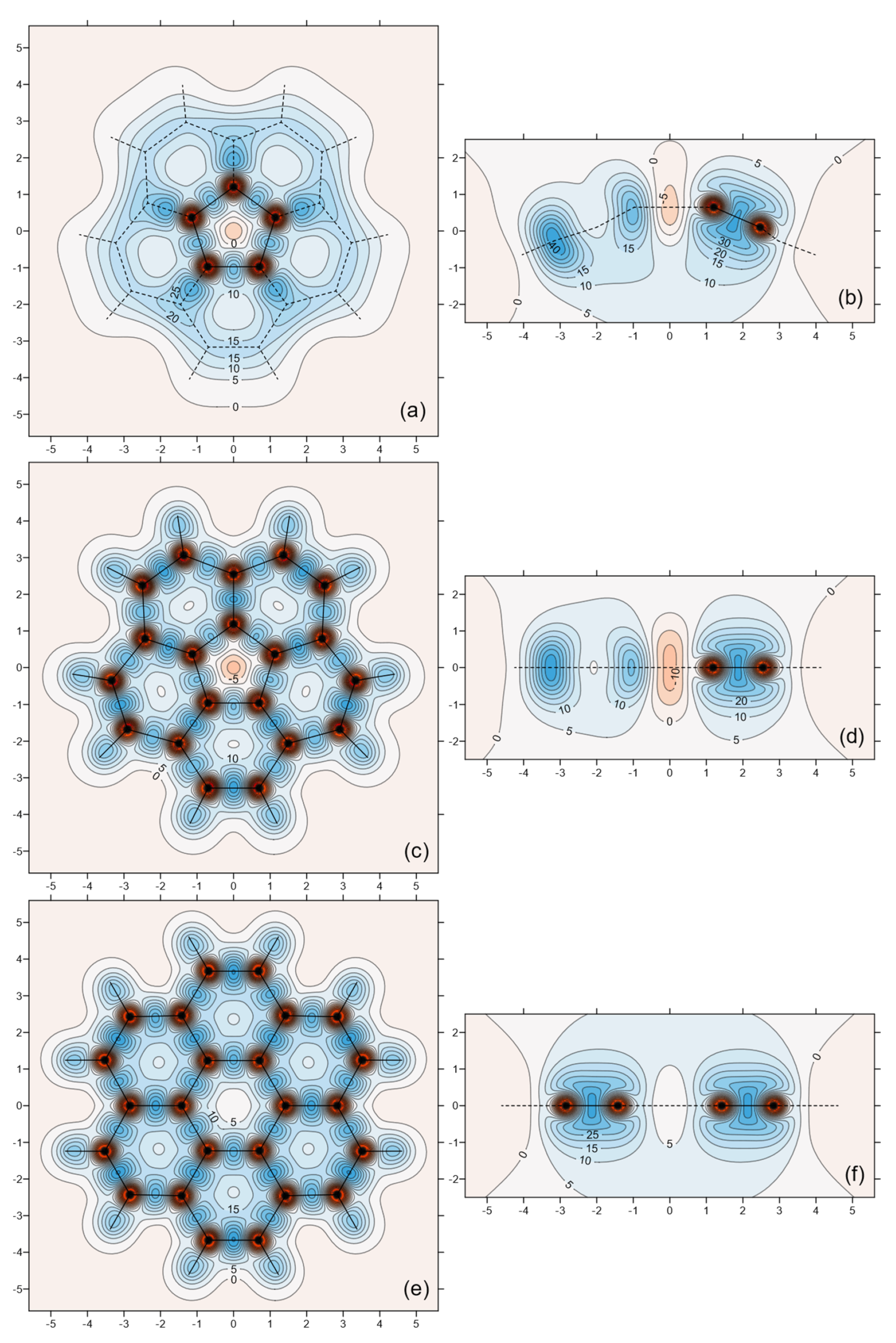

| Property | C5v Corannulene | D5h Corannulene | D6h Coronene |
|---|---|---|---|
| σiso(Chub) | 41.6 | 47.1 | 57.0 |
| σiso(Cflank) | 46.8 | 51.4 | 50.1 |
| σiso(Crim) | 51.4 | 50.2 | 52.9 |
| σiso(H) | 24.0 | 23.9 | 22.7 |
| NICS(0)hub | 8.2 | 11.9 | −0.1 |
| NICS(+1)hub | 2.9 | 5.3 | −4.6 |
| NICS(−1)hub | −4.2 | ||
| NICS(0)rim | −6.5 | −4.7 | −9.7 |
| NICS(+1)rim | −5.4 | −7.5 | −12.0 |
| NICS(−1)rim | −13.0 |
| Property | C5v Corannulene | D6h Coronene |
|---|---|---|
| δ(Chub) δ(Cflank) | 5.0 a, 6.4 b | −6.1 a, −4.7 b |
| σiso(Cflank) σiso(Chub) | 5.2 | −6.9 |
| δ(Chub) δ(Crim) | 8.6 a, 10.7 b | −3.6 a, −2.2 b |
| σiso(Crim) σiso(Chub) | 9.8 | −4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karadakov, P.B. Magnetic Shielding Study of Bonding and Aromaticity in Corannulene and Coronene. Chemistry 2021, 3, 861-872. https://doi.org/10.3390/chemistry3030063
Karadakov PB. Magnetic Shielding Study of Bonding and Aromaticity in Corannulene and Coronene. Chemistry. 2021; 3(3):861-872. https://doi.org/10.3390/chemistry3030063
Chicago/Turabian StyleKaradakov, Peter B. 2021. "Magnetic Shielding Study of Bonding and Aromaticity in Corannulene and Coronene" Chemistry 3, no. 3: 861-872. https://doi.org/10.3390/chemistry3030063
APA StyleKaradakov, P. B. (2021). Magnetic Shielding Study of Bonding and Aromaticity in Corannulene and Coronene. Chemistry, 3(3), 861-872. https://doi.org/10.3390/chemistry3030063






