Screening Papaveraceae as Novel Antibiofilm Natural-Based Agents
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Analysis
2.1.1. Qualitative Analysis
2.1.2. Phytochemical Quantitative Analysis
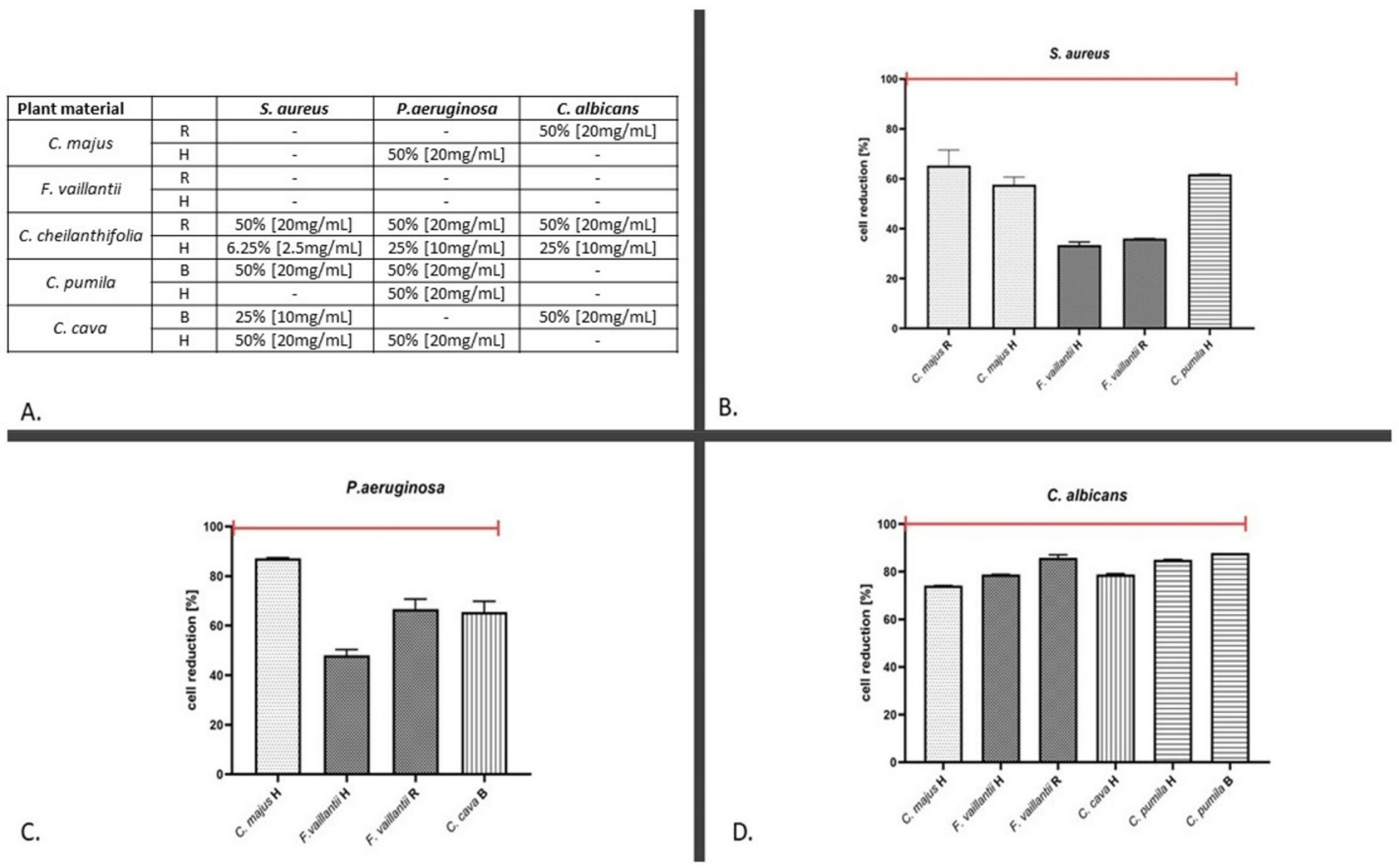
2.2. The Antimicrobial Assays
3. Discussion
4. Materials and Methods
4.1. Plant Material
Plant Material Extraction
4.2. Phytochemical Analysis
4.2.1. Chemical and Reagents
4.2.2. Liquid Chromatography
4.2.3. Mass Spectrometry
4.2.4. Identification and Quantification
4.3. Antimicrobial Assays
4.3.1. Assessment of the Minimal Inhibitory Concentration of the Analyzed Extracts
4.3.2. Assessment of the Antibiofilm Activity of the Analyzed Extracts
4.3.3. Bacterial Cellulose Carrier Preparation
4.3.4. Analysis of the Antimicrobial Activity of Extracts Released from the Bacterial Cellulose Carrier
4.4. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, R.; Guo, Q.; Kennelly, E.J.; Long, C.; Chai, X. Diverse alkaloids and biological activities of Fumaria (Papaveraceae): An ethnomedicinal group. Fitoterapia 2020, 146, 104697. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, S.; Jezierska-Domaradzka, A.; Wójciak-Kosior, M.; Sowa, I.; Junka, A.; Matkowski, A.M. Greater Celandine’s ups and downs—21 Centuries of medicinal uses of Chelidonium majus from the viewpoint of today’s pharmacology. Front. Pharmacol. 2018, 9, 299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinska, S.; Wójciak-Kosior, M.; Dziagwa-Becker, M.; Glensk, M.; Sowa, I.; Fijałkowski, K.; Ruranska-Smutnicka, D.; Matkowski, A.; Junka, A. The Activity of Isoquinoline Alkaloids and Extracts From Chelidonium majus against Pathogenic Bacteria and Candida sp. Toxins 2019, 11, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevens, P.F. Angiosperm Phylogeny Website, Version 14. 2017. Available online: http://www.mobot.org/MOBOT/research/APweb, (accessed on 13 January 2021).
- Jones, W.H.S. Pliny Natural History with an English Translation in Ten Volumes; Libri XXIV–XXVII; Harvard University Press: London, UK, 1966; Volume VII. [Google Scholar]
- Osbaldeston, T.A.; Wood, R.P.A. Dioscorides de Materia Medica, Being a Herbal with Many Other Materials Written in Greek in the First Century of the Common Era; An Indexed Version in Modern English; Ibidis Press: Johannesburg, South Africa, 2000. [Google Scholar]
- Strathdee, S.A.; Davies, S.C.; Marcelin, J.R. Confronting antimicrobial resistance beyond the COVID-19 pandemic and the 2020 US election. Lancet 2020, 396, 1050–1053. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond risk: Bacterial biofilms and their regulating approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Noor Al-Mousawi, U.M.; Al-Waheeb, A.N.; Malik Al-Saadi, S.A.A. Anatomical studies of medicinal plant Fumaria densiflora in Iraq. J. Biol. Agric. Healthc. 2018, 8, 21–26. [Google Scholar]
- Oroszlan, P.; Dolejs, L.; Simanek, V.; Preininger, V. Alkaloids of Corydalis cheilanthifolia. Planta Med. 1985, 51, 286. [Google Scholar] [CrossRef]
- Adsersen, A.; Gauguin, B.; Gudiksen, L.; Jager, A.K. Screening of plants used in Danish folk medicine to treat memory dysfunction for acetylcholinesterase inhibitory activity. J. Ethnopharmacol. 2006, 104, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Slavik, J.; Slavikova, L. Alkaloids from Corydalis cava (L.) SCHW. et KOERTE. Collect. Czechoslov. Chem. Commun. 1979, 44, 2261–2274. [Google Scholar] [CrossRef]
- Och, A.; Szewczyk, K.; Pecio, Ł.; Stochmal, A.; Załuski, D.; Bogucka-Kocka, A. UPLC-MS/MS profile of alkaloids with cytotoxic properties of selected medicinal plants of the Berberidaceae and Papaveraceae families. Oxidative Med. Cell. Longev. 2017, 2017, 9369872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, A.; Imming, P. R(-)-Canadaline as first secoberbine alkaloid from Corydalis cava. Phytochem. Lett. 2008, 1, 168–170. [Google Scholar] [CrossRef]
- Gasic, O.; Dragutinovic, A.; Popovic, M. Alkaloids from Corydalis cava. Planta Med. 1981, 42, 135. [Google Scholar] [CrossRef]
- Preininger, V.; Thakur, R.S.; Santavy, F. Isolation and Chemistry of alkaloids from plants of the family Papaveraceae LXVII: Corydalis caua (L.) Sch. et K. (C. tuberosa DC). Pharm. Sci. 1976, 65, 294. [Google Scholar] [CrossRef]
- Novak, Z.; Chlebek, J.; Opletal, L.; Jiros, P.; Macakova, K.; Kunes, J.; Cachlikova, L. Corylucinine, a new alkaloid from Corydalis cava (Fumariaceae), and its cholinesterase activity. Nat. Prod. Commun. 2012, 7, 859–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaźmierczakowa, R.; Zarzycki, K.; Mirek, Z. Polish Red Data Book of Plants; W Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2014; pp. 150–151. [Google Scholar]
- Iranshahy, M.; Javidi-Shirazi, H.; Pham, N.B.; Quinn, R.J.; Sadeghianc, H.; Iranshahi, M. Adlumiceine methyl ester, a new alkaloid from Fumaria vaillanti. J. Asian Nat. Prod. Res. 2014, 16, 1148–1152. [Google Scholar] [CrossRef]
- Gozler, B.; Gozler, T.; Shamma, M. Egenine: A possible intermediate in phthalideisoquinoline biogenesis. Tetrahedron 1983, 39, 577–580. [Google Scholar] [CrossRef]
- Sener, B. Minor Alkaloids of Corydalis rutifolia (Sibth. and Sm.) D. C. subsp. kurdica Cullen and Davis of turkish origin. Int. J. Crude Drug Res. 1988, 26, 155–159. [Google Scholar] [CrossRef]
- Alimova, M.; Israilov, I.A.; Yunusov, M.S.; Yunusov, S.Y. Alkaloids of Fumaria vaillantii. The structure of norjuziphine. Chem. Nat. Compd. 1981, 17, 437–438. [Google Scholar] [CrossRef]
- Lamba, S.S.; Trottier, R.W. Preliminary phytochemical and pharmacological investigation of Fumaria vaillantii. Q. J. Crude Drug Res. 1977, 15, 25–29. [Google Scholar] [CrossRef]
- Rakhimova, D.A.; Dobronravova, E.K.; Shakirov, T.T. Method for the quantitative determination of protopine in Fumaria vaillantii. Chem. Nat. Compd. 1977, 13, 317–319. [Google Scholar] [CrossRef]
- Shamma, M.; Chinnasamy, P.; Hussain, S.F.; Khan, F. Norpallidine, a new morphinandienone alkaloid from Fumaria vaillantii. Phytochemistry 1976, 15, 1802–1803. [Google Scholar] [CrossRef]
- Ibragimova, M.U.; Israilov, I.A.; Yunusov, M.S.; Yunusov, S.Y. Alkaloids of Fumaria vaillantii, structure of vaillantine. Chem. Nat. Compd. 1974, 10, 481–482. [Google Scholar] [CrossRef]
- Sadikov, A.Z.; Babaev, B.; Shakirov, T.T. Isolation of protopine from Fumaria vaillantii by the ion-exchange method. Chem. Nat. Compd. 1974, 6, 816. [Google Scholar] [CrossRef]
- Davoodi-Roodbordeii, F.; Afshar, M.; Tabrizi, F.H.A.; Choopani, S.; Torkaman, G.; Moayer, F.; Salimi, M. Topical hydrogel containing Fumaria vaillantii Loisel. extract enhances wound healing in rats. BMC Complement. Altern. Med. 2019, 19, 254. [Google Scholar] [CrossRef]
- Jaberian, H.; Piri, K.; Nazari, J. Phytochemical composition and in vitro antimicrobial and antioxidant activities of some medicinal plants. Food Chem. 2013, 136, 237–244. [Google Scholar] [CrossRef]
- Erhardt, W.; Goetz, E.; Boedeker, N.; Seybold, S. Zander. Handwoerterbuch der Pflanzennamen; Eugen Ulmer Verlag: Stuttgart, Germany, 2002. [Google Scholar]
- Zielińska, S.; Wójciak-Kosior, M.; Płachno, B.J.; Sowa, I.; Włodarczyk, M.; Matkowski, A. Quaternary alkaloids in Chelidonium majus in vitro cultures. Ind. Crop. Prod. 2018, 123, 17–24. [Google Scholar] [CrossRef]
- Tome, F.; Colombo, M.L. Distribution of alkaloids in Chelidonium majus and factors affecting their accumulation. Phytochemistry 1995, 40, 37–39. [Google Scholar] [CrossRef]
- Grosso, C.; Ferreres, F.; Gil-Izquierdo, A.; Valentão, P.; Sampaio, M.; Lima, J.; Andrad, P.B. Box-Behnken factorial design to obtain a phenolic-rich extract from the aerial parts of Chelidonium majus L. Talanta 2014, 130, 128–136. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Hahn, R.; Nahrstedt, A. Hydroxycinnamic acid derivatives, caffeoylmalic and new caffeoylaldonic acid esters, from Chelidonium majus. Planta Med. 1993, 59, 71–75. [Google Scholar] [CrossRef] [PubMed]
- The Plant List, Version 1.1. September 2013. Available online: http://www.theplantlist.org/1.1/browse/A/Papaveraceae/Chelidonium/ (accessed on 13 January 2021).
- Kho, W.; Kim, M.K.; Jung, M.; Chong, J.P.; Kim, Y.S.; Park, K.-H.; Chong, Y. Strain-specific anti-biofilm and antibiotic-potentiating activity of 3′,4′-difluoroquercetin. Sci. Rep. 2020, 10, 14162. [Google Scholar] [CrossRef] [PubMed]
- Kikowska, M.; Derda, M.; Thiem, B.; Włodarczyk, A.; Długaszewska, J.; Stochmal, A.; Żuchowski, J.; Hadaś, E. Evaluation of antiamoebic and antimicrobial activities in vitro of Chaenomeles japonica (Thunb.) Lindl. Ex Spach extracts. Acta Biol. Crac. Ser. Bot. 2019, 61, 47–58. [Google Scholar] [CrossRef]
- Collins, S.; Wouamba, N.; Happi, G.M.; Pouofo, M.N.; Tchamgoue, J.; Jouda, J.-B.; Longo, F.; Lenta, B.N.; Sewald, N.; Kouam, S.F. Antibacterial Flavonoids and Other Compounds from the Aerial Parts of Vernonia guineensis Benth. (Asteraceae). Chem. Biodivers. 2020, 17, e2000296. [Google Scholar]
- Olszewska, M.A.; Gędas, A.; Simões, M. Antimicrobial polyphenol-rich extracts: Applications and limitations in the food industry. Food Res. Int. 2020, 134, 109214. [Google Scholar] [CrossRef]
- Guzzo, F.; Scognamiglio, M.; Fiorentino, A.; Buommino, E.; D’Abrosca, B. Plant derived natural products against Pseudomonas aeruginosa and Staphylococcus aureus: Antibiofilm activity and molecular mechanisms. Molecules 2020, 25, 5024. [Google Scholar] [CrossRef]
- Hemati, S.; Kouhsari, E.; Sadeghifard, N.; Maleki, A.; Omidi, N.; Mahdavi, Z.; Pakzad, I. Sub-minimum inhibitory concentrations of biocides induced biofilm formation in Pseudomonas aeruginosa. New Microbes New Infect. 2020, 38, 100794. [Google Scholar] [CrossRef] [PubMed]
- Takishita, Y.; Souleimanov, A.; Bourguet, C.; Ohlund, L.B.; Arnold, A.A.; Sleno, L.; Smith, D.L. Pseudomonas entomophila 23S produces a novel antagonistic compound against Clavibacter michiganensis subsp. michiganensis, a pathogen of tomato bacterial canker. Appl. Microbiol. 2021, 1, 60–73. [Google Scholar] [CrossRef]
- Lalucat, J.; Mulet, M.; Gomila, M.; García-Valdés, E. Genomics in bacterial taxonomy: Impact on the genus Pseudomonas. Genes 2020, 11, 139. [Google Scholar] [CrossRef] [Green Version]
- Adetunji, A.I.; Olaniran, A.O. Production and potential biotechnological applications of microbial surfactants: An overview. Saudi J. Biol. Sci. 2021, 28, 669–679. [Google Scholar] [CrossRef]
- Inoue, N.; Terabayashi, T.; Takiguchi-Kawashima, Y.; Fujinami, D.; Matsuoka, S.; Kawano, M.; Tanaka, K.; Tsumura, H.; Ishizaki, T.; Narahara, H.; et al. The benzylisoquinoline alkaloids, berberine and coptisine, act against camptothecin-resistant topoisomerase I mutants. Sci. Rep. 2021, 11, 7718. [Google Scholar] [CrossRef] [PubMed]
- Obiang-Obounou, B.W.; Kang, O.H.; Choi, J.G.; Keum, J.H.; Kim, S.B.; Mun, S.H.; Shin, D.W.; Kim, K.W.; Park, C.B.; Kim, Y.G.; et al. The mechanism of action of sanguinarine against methicillin-resistant Staphylococcus aureus. J. Toxicol Sci. 2011, 36, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonarska-Kujawa, D.; Cyboran-Mikołajczyk, S.; Kleszczyńska, H. Molecular mechanism of action of chlorogenic acid on erythrocyte and lipid membranes. Mol. Membr. Biol. 2015, 32, 46–54. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its main pharmacological activity and potential application in clinical medicine. Oxidative Med. Cell. Longev. 2020, 8825387. [Google Scholar] [CrossRef]
- Dydak, K.; Junka, A.; Dydak, A.; Brożyna, M.; Paleczny, J.; Fijalkowski, K.; Kubielas, G.; Aniołek, O.; Bartoszewicz, M. In vitro efficacy of bacterial cellulose dressings chemisorbed with antiseptics against biofilm formed by pathogens isolated from chronic wounds. Int. J. Mol. Sci. 2021, 22, 3996. [Google Scholar] [CrossRef]
- Brożyna, M.; Żywicka, A.; Fijałkowski, K.; Gorczyca, D.; Oleksy-Wawrzyniak, M.; Dydak, K.; Migdał, P.; Dudek, B.; Bartoszewicz, M.; Junka, A. The novel quantitative qssay for measuring the antibiofilm activity of volatile compounds (AntiBioVol). Appl. Sci. 2020, 10, 7343. [Google Scholar] [CrossRef]
- Krasowski, G.; Junka, A.; Paleczny, J.; Czajkowska, J.; Makomaska-Szaroszyk, E.; Chodaczek, G.; Majkowski, M.; Migdał, P.; Fijałkowski, K.; Kowalska-Krochmal, B.; et al. In vitro evaluation of polihexanide, octenidine and NaClO/HClO-based antiseptics against biofilm formed by wound pathogens. Membranes 2021, 11, 62. [Google Scholar] [CrossRef]
- Tasse, J.; Cara, A.; Saglio, M.; Villet, R.; Laurent, F. A steam-based method to investigate biofilm. Sci. Rep. 2018, 8, 13040. [Google Scholar] [CrossRef]
- Latka, A.; Drulis-Kawa, Z. Advantages and limitations of microtiter biofilm assays in the model of antibiofilm activity of Klebsiella phage KP34 and its depolymerase. Sci. Rep. 2020, 10, 20338. [Google Scholar] [CrossRef]
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef]
- Sowa, I.; Zielinska, S.; Sawicki, J.; Bogucka-Kocka, A.; Staniak, M.; Bartusiak-Szczesniak, E.; Podolska-Fajks, M.; Kocjan, R.; Wojciak-Kosior, M. Systematic evaluation of chromatographic parameters for isoquinoline alkaloids on XB-C18 core-shell column using different mobile phase compositions. J. Anal. Methods Chem. 2018, 3, 9624327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staniak, M.; Wójciak-Kosior, M.; Sowa, I.; Strzemski, M.; Sawicki, J.; Dresler, S.; Tyszczuk-Rotko, K. Applicability of a monolithic column for separation of isoquinoline alkalodis from Chelidonium majus extract. Molecules 2019, 24, 3612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bylund, D.; Norstrom, S.H.; Essen, S.A.; Lundstrom, U.S. Analysis of low molecular mass organic acids in natural waters by ion exclusion chromatography tandem mass spectrometry. J. Chromatogr. A 2007, 1176, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Erk, T.; Bergmann, H.; Richling, E. A novel method for the quantification of quinic acid in food using stable isotope dilution analysis. J. AOAC Int. 2009, 92, 730–733. [Google Scholar] [CrossRef] [Green Version]
- Akinwande, M.; Dikko, H.; Samson, A. Variance inflation factor: As a condition for the inclusion of suppressor variable(s) in regression analysis. Open J. Stat. 2015, 5, 754–767. [Google Scholar] [CrossRef] [Green Version]

| Number | Compound | Parent Ion (m/z) | Production (m/z) | Ion Mode | Content [µg/g D.W. ± SD] | ||||
|---|---|---|---|---|---|---|---|---|---|
| ALKALOIDS | C. majus | C. cava | C. pumila | C. cheilanthifolia | F. vaillantii | ||||
| 1. | Protopine derivative | 354 | 320, 260, 196 | + | p | p | nd | p | p |
| 2. | Allocryptopine | 369.6 | 352, 187.9, 290 | + | 22.77 ± 1.75 a | 6.46 ± 0.70 b | 49.14 ± 1.41 c | 265.06 ± 3.73 d | 5.96 ± 0.50 b |
| 3. | Coptisine | 320.1 | 291.9, 204, 262 | + | 5455.79 ± 22.97 a | 1820.82 ± 9.90 b | 2.75 ± 0.05 c | 5794.90 ± 22.72 d | 76.73 ± 1.04 e |
| 4. | Berberine | 336.4 | 320, 292, 321.1 | + | 83.02 ± 1.77 a | 1.21 ± 0.07 b | 60.70 ± 0.88 c | 2696.04 ± 12.05 d | 1.82 ± 0.05 b |
| 5. | Chelidonine derivative | 370 | 356, 339 | + | p | nd | nd | p | Nd |
| 6. | Chelidonine | 353.8 | 275, 189, 247 | + | 130.77 ± 2.80 a | 1.85 ± 0.06 b | 0.24 ± 0.01 b | ndb | 8.03 ± 0.07 c |
| 7. | Chelerythrine | 348.1 | 332, 304, 333 | + | 22.55 ± 0.54 a | 1.25 ± 0.09 b | 4.78 ± 0.1 c | 21.15 ± 0.25 d | 2.04 ± 0.08 b |
| 8. | Tetrahydroberberine | 340 | 176, 149 | + | p | p | p | p | p |
| 9. | Tetrahydrocoptisine | 324 | 176, 149 | + | p | p | p | p | p |
| 10. | Coptisine derivative | 324 | 190 | + | p | p | p | p | p |
| 11. | Sanguinarine | 332.1 | 274.1, 316.9, 246 | + | 33.24 ± 1.67 a | 2.53 ± 0.06 b | 6.23 ± 0.06 c | 57.22 ± 0.44 d | 22.71 ± 0.3 e |
| 12. | Protopine | 320.2 | 303.2, 107, 123.8 | + | 315.67 ± 6.06 a | 136.52 ± 2.80 b | 890.77 ± 1.97 c | 748.43 ± 4.88 d | 1083.21 ± 10.83 e |
| MISCELLANEOUS | |||||||||
| 13. | Malic acid | 133.1 | 115, 71 | − | p | p | p | p | p |
| 14. | Trans-aconitic acid | 172.9 | 85, 129 | − | p | p | p | p | p |
| 15. | Quinic acid | 191 | 85, 93 | − | p | p | p | p | p |
| 16. | Salicylic acid | 137.3 | 93, 65, 44.8 | − | 3.05 ± 0.10 | nd | nd | LOQ | nd |
| 17. | Trans-caffeic acid | 179.2 | 135, 134, 89 | − | 130.37 ± 4.06 a | 52.89 ± 0.92 b | 27.17 ± 1.27 c | 16.05 ± 0.20 d | 50.98 ± 1.84 b |
| 18. | Chlorogenic acid | 353 | 191, 85, 92.9 | − | 159.84 ± 1.04 a | 750.97 ± 2.68 b | nd c | 558.65 ± 11.03 d | 82.37 ± 2.25 e |
| 19. | p-Coumaric acid | 163.2 | 119.1, 93.1, 117 | − | 78.89 ± 2.58 a | 10.04 ± 0.53 b | 11.56 ± 0.66 b | 1.05 ± 0.08 c | 42.51 ± 1.65 d |
| 20. | Vanillin | 151.2 | 136, 91.8, 108 | − | 15.54 ± 0.93 | nd | nd | nd | nd |
| 21. | Quercetin | 301.1 | 151, 65, 121 | − | 1.75 ± 0.11 a | 1.23 ± 0.02 a | 26.20 ± 0.32 b | 206.01 ± 1.13 c | 262.36 ± 1.04 d |
| 22. | Kaempferol | 285 | 169 | − | nd | nd | nd | LOQ | nd |
| Number | Compound | Parent Ion (m/z) | Production (m/z) | Ion Mode | Content [µg/g D.W. ± SD] | ||||
|---|---|---|---|---|---|---|---|---|---|
| ALKALOIDS | C. majus | C. cava | C. pumila | C. cheilanthifolia | F. vaillantii | ||||
| 1 | Protopine derivative | 354 | 320, 260, 196 | + | p | LOQ | p | p | p |
| 2 | Allocryptopine | 369.6 | 352, 187.9, 290 | + | 147.96 ± 5.53 a | 36.52 ± 1.25 b | 699.72 ± 3.72 c | 330.01 ± 2.46 d | 9.26 ± 0.94 e |
| 3 | Coptisine | 320.1 | 291.9, 204, 262 | + | 2744.21 ± 14.02 a | 143.26 ± 1.66 b | 10.24 ± 0.25 c | 3605.56 ± 12.92 d | 134.24 ± 2.22 b |
| 4 | Berberine | 336.4 | 320, 292, 321.1 | + | 345.45 ± 5.76 a | 16.85 ± 0.3 b | 739.54 ± 3.41 c | 176.03 ± 3.32 d | 1.81 ± 0.03 e |
| 5 | Chelidonine derivative | 370 | 356, 339 | + | p | LOQ | p | nd | nd |
| 6 | Chelidonine | 353.8 | 275, 189, 247 | + | 1936.11 ± 15.48 a | 13.69 ± 0.85 b | nd c | 6.74 ± 0.07 bc | 48.58 ± 1.28 d |
| 7 | Chelerythrine | 348.1 | 332, 304, 333 | + | 742.36 ± 4.63 a | 11.26 ± 0.24 b | 36.14 ± 0.89 c | 48.28 ± 1.10 d | 2.07 ± 0.06 e |
| 8 | Tetrahydroberberine | 340 | 176, 149 | + | p | p | p | p | p |
| 9 | Tetrahydrocoptisine | 324 | 176, 149 | + | p | p | p | p | p |
| 10 | Coptisine derivative | 324 | 190 | + | p | p | p | p | p |
| 11 | Sanguinarine | 332.1 | 274.1, 316.9, 246 | + | 915.56 ± 3.45 a | 17.46 ± 0.49 b | 28.30 ± 0.42 c | 493.20 ± 3.87 d | 45.56 ± 0.67 e |
| 12 | Protopine | 320.2 | 303.2, 107, 123.8 | + | 586.48 ± 12.67 a | 131.79 ± 1.56 b | 1260.62 ± 13.93 c | 6529.64 ± 13.88 c | 3610.09 ± 9.71 d |
| MISCELLANEOUS | |||||||||
| 13. | Malic acid | 133.1 | 115, 71 | − | p | p | p | p | p |
| 14. | Trans-aconitic acid | 172.9 | 85, 129 | − | p | p | p | p | p |
| 15. | Quinic acid | 191 | 85, 93 | − | p | p | p | p | p |
| 16. | Salicylic acid | 137.3 | 93, 65, 44.8 | − | nd | nd | nd | nd | LOQ |
| 17. | Trans-caffeic acid | 179.2 | 135, 134, 89 | − | nd | LOQ | nd | nd | nd |
| 18. | Chlorogenic acid | 353 | 191, 85, 92.9 | − | nd a | 1198.03 ± 8.63 b | nd a | 51.18 ± 2.75 c | 1.22 ± 0.06 a |
| 19. | Vanillin | 151.2 | 136, 91.8, 108 | − | nd a | nd a | 12.4 ± 0.28 b | 28.93 ± 1.03 c | 18.71 ± 0.94 d |
| 20. | Quercetin | 301.1 | 151, 65, 121 | − | nd a | nd a | nd a | 205.54 ± 1.19 b | 305.65 ± 1.78 c |
| Plant Material | Reduction of Biofilm-forming Cell Number [TTC Assessment] | Reduction of Biofilm Biomass [Crystal Violet Assessment] | |||||
|---|---|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | C. albicans | S. aureus | P. aeruginosa | C. albicans | ||
| C. majus | R | 71.79 | −179.81 | 95.69 | 10.84 | n.s | 66.63 |
| H | 26.11 | −200.74 | 93.72 | 20.41 | n.s | 65.02 | |
| F. vaillantii | R | 67.01 | −97.61 | 92.92 | 24.29 | n.s | 66.20 |
| H | 52.42 | −94.27 | 95.04 | 20.81 | n.s | 65.27 | |
| C. cheilanthifolia | R | 70.02 | −177.17 | 93.55 | 7.63 | n.s | 74.64 |
| H | 89.10 | −194.71 | 93.85 | 10.99 | n.s | 68.92 | |
| C. pumila | B | 66.84 | −395.46 | 92.06 | 11.21 | n.s | 54.99 |
| H | 82.74 | −276.47 | 94.40 | 25.20 | n.s | 58.86 | |
| C. cava | B | 67.61 | −105.26 | 95.78 | 11.04 | n.s | 62.54 |
| H | 74.53 | −84.47 | 95.70 | 22.12 | n.s | 67.14 | |
| S. aureus | P. aeruginosa | C. albicans | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R2 | Beta | p | Compound | R2 | Beta | p | Compound | R2 | Beta | p | ||
| Aerial parts | Planktonic cells/MIC | Quercetin | 0.699 | −0.614 | 0.000 | Quercetin | 0.965 | −0.984 | 0.000 | Berberine | 0.960 | −0.748 | 0.000 |
| Chlorogenic Acid | 0.219 | 0.524 | 0.000 | Coptisine | 0.021 | −0.268 | 0.003 | ||||||
| Sanguinarine | 0.072 | −0.322 | 0.001 | Quercetin | 0.010 | −0.131 | 0.036 | ||||||
| Biofilm/TTC | p-coumaric Acid | 0.781 | −0.525 | 0.030 | Exclusion of Data from Model | Chelerythrine | 0.628 | −0.818 | 0.004 | ||||
| Biofilm/Crystal Violet | Statistical Model Insignificant | Statistical Model Insignificant | No Correlation p ˂ 0.25 | ||||||||||
| Underground parts | Planktonic Cells/MIC | Allocryptopine | 0.340 | 0.892 | 0.001 | Chelidonine | 0.537 | −0.487 | 0.020 | Coptisine | 0.825 | −0.817 | 0.002 |
| Chlorogenic Acid | 0.478 | 0.757 | 0.004 | Allocryptopine | 0.318 | 0.537 | 0.007 | ||||||
| Biofilm/TTC | Sanguinarine | 0.533 | 0.690 | 0.015 | Allocryptopine | 0.869 | −0.869 | 0.002 | Vanillin | 0.478 | −0.308 | 0.003 | |
| Quercetin | 0.033 | 0.192 | 0.000 | Berberine | 0.233 | −0.233 | 0.005 | ||||||
| Sanguinarine | 0.160 | 0.667 | 0.000 | ||||||||||
| Chlorogenic Acid | 0.121 | 0.615 | 0.000 | ||||||||||
| Biofilm/Crystal Violet | Statistical Model Insignificant | Exclusion of Data from Model | Statistical Model Insignificant | ||||||||||
| Plant Material | Volume of Extract [mL] | Concentration of Extract [mg/mL] | Zone of Growth Inhibition [mm] | ||
|---|---|---|---|---|---|
| S. aureus | P. aeruginosa | C. albicans | |||
| C. cava H | 0.9 | 21.76 | 19 | 0 | 0 |
| C. cheilanthifolia H | 1 | 22.8 | 21 | 22 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielińska, S.; Dziągwa-Becker, M.; Junka, A.; Piątczak, E.; Jezierska-Domaradzka, A.; Brożyna, M.; Paleczny, J.; Sobiecka, A.; Słupski, W.; Mess, E.; et al. Screening Papaveraceae as Novel Antibiofilm Natural-Based Agents. Molecules 2021, 26, 4778. https://doi.org/10.3390/molecules26164778
Zielińska S, Dziągwa-Becker M, Junka A, Piątczak E, Jezierska-Domaradzka A, Brożyna M, Paleczny J, Sobiecka A, Słupski W, Mess E, et al. Screening Papaveraceae as Novel Antibiofilm Natural-Based Agents. Molecules. 2021; 26(16):4778. https://doi.org/10.3390/molecules26164778
Chicago/Turabian StyleZielińska, Sylwia, Magdalena Dziągwa-Becker, Adam Junka, Ewelina Piątczak, Anna Jezierska-Domaradzka, Malwina Brożyna, Justyna Paleczny, Aleksandra Sobiecka, Wojciech Słupski, Eleonora Mess, and et al. 2021. "Screening Papaveraceae as Novel Antibiofilm Natural-Based Agents" Molecules 26, no. 16: 4778. https://doi.org/10.3390/molecules26164778
APA StyleZielińska, S., Dziągwa-Becker, M., Junka, A., Piątczak, E., Jezierska-Domaradzka, A., Brożyna, M., Paleczny, J., Sobiecka, A., Słupski, W., Mess, E., Kucharski, M., Çiçek, S. S., Zidorn, C., & Matkowski, A. (2021). Screening Papaveraceae as Novel Antibiofilm Natural-Based Agents. Molecules, 26(16), 4778. https://doi.org/10.3390/molecules26164778











