Fungal Diversity and Aflatoxins in Maize and Rice Grains and Cassava-Based Flour (Pupuru) from Ondo State, Nigeria
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling of Foods
2.3. Moisture Content Analysis of Ground Grains and Pupuru
2.4. Isolation of Fungi from Ground Grains and Pupuru
2.5. Characterization of Fungal Isolates
2.6. Aflatoxin Analysis of Ground Grains and Pupuru by Enzyme-Linked Immunosorbent Assay
2.7. Data Analysis
3. Results
3.1. Moisture Contents of Ground Grains and Pupuru
3.2. Incidence of Fungi in Ground Grains and Pupuru
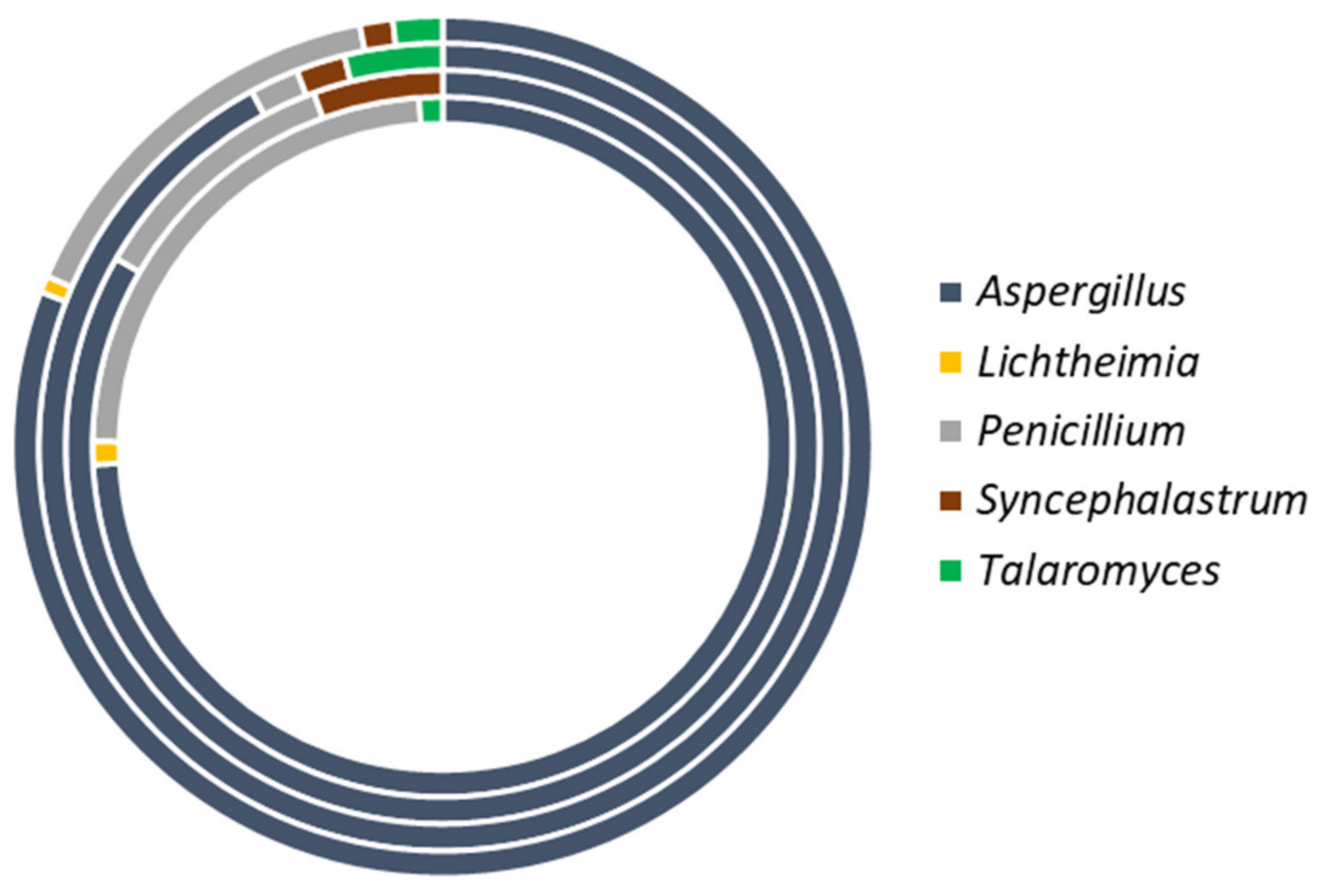
3.2.1. Distribution of Fungi
3.2.2. Species Diversity
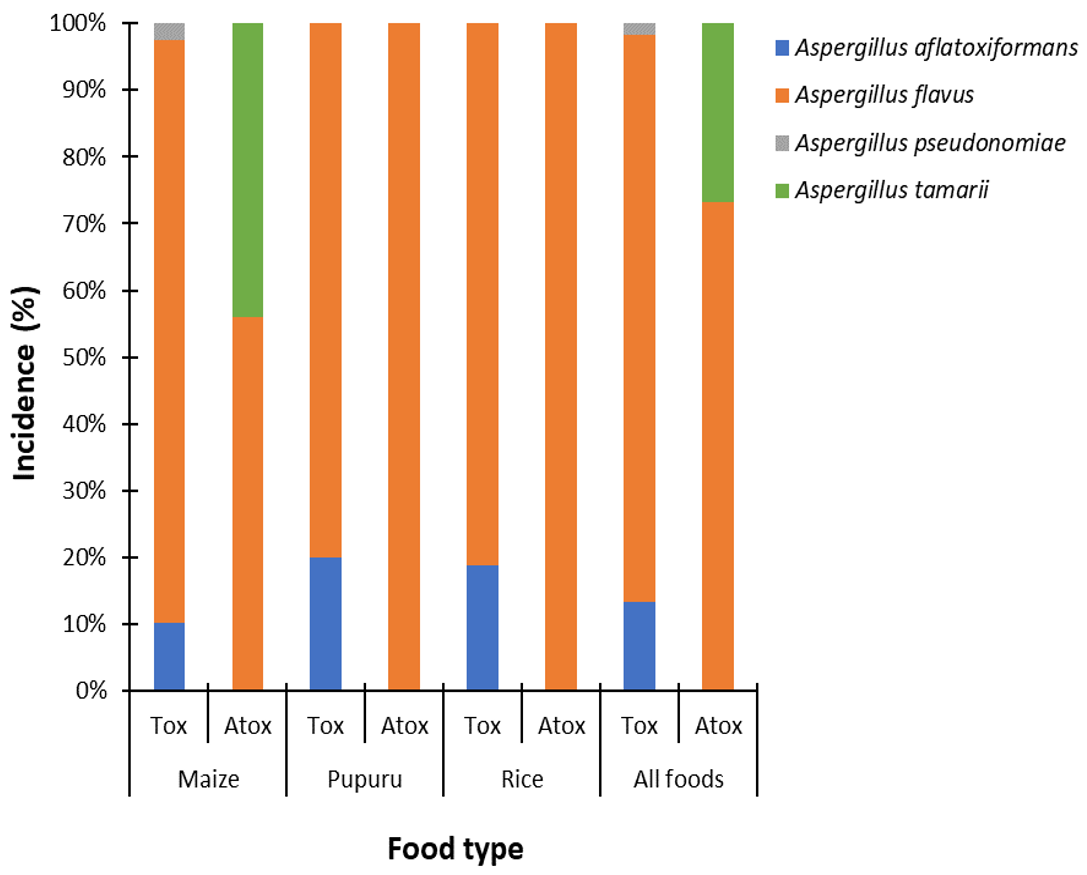
3.2.3. Incidence of Aflatoxigenic and Non-Aflatoxigenic Species of Aspergillus Section Flavi
3.3. Aflatoxin Levels in Ground Grains and Pupuru Samples
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avery, S.V.; Singleton, I.; Magan, N.; Goldman, G.H. The fungal threat to global food security. Fungal Biol. 2019, 123, 555–557. [Google Scholar] [CrossRef] [PubMed]
- Abdus-Salaam, R.; Atanda, O.; Fanelli, F.; Sulyok, M.; Cozzi, G.; Bavaro, S.L.; Krska, R.; Logrieco, A.F.; Ezekiel, C.N.; Salami, W.A. Fungal isolates and metabolites in locally processed rice from five agro-ecological zones of Nigeria. Food Addit. Contam. Part B 2016, 9, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.N.; Kraak, B.; Sandoval-Denis, M.; Sulyok, M.; Oyedele, O.A.; Ayeni, K.I.; Makinde, O.M.; Akinyemi, O.M.; Krska, R.; Crous, P.W.; et al. Diversity and toxigenicity of fungi and description of Fusarium madaense sp. nov. from cereals, legumes and soils in north-central Nigeria. MycoKeys 2020, 67, 95–124. [Google Scholar] [CrossRef]
- Makun, H.A.; Dutton, M.F.; Njobeh, P.B.; Phoku, J.Z.; Yah, C. Incidence, phylogeny and mycotoxigenic potentials of fungi isolated from rice in Niger state, Nigeria. J. Food Saf. 2011, 31, 334–349. [Google Scholar] [CrossRef]
- Shittu, T.; Edema, M.; Dada, O.; Atayese, A. Microorganisms associated with the spoilage of Pupuru. Food Control 2010, 21, 203–206. [Google Scholar] [CrossRef]
- Famurewa, J.; Oluwamukomi, M.O.; Alaba, J.O. Effect of different drying methods on the physicochemical characteristics of cassava flour (“pupuru”). Int. J. Biol. Chem. Sci. 2013, 7, 832–839. [Google Scholar] [CrossRef]
- Akinyele, O.F.; Ikujenlola, A.V.; Omobuwajo, T.O. Physico-chemical and sensory properties of pupuru and pupuru analogues from co-fermented cassava (Manihot esculenta Crantz) and breadfruit (Artocarpus altilis) blends. Acta Univ. Sapientiae Aliment. 2020, 13, 32–50. [Google Scholar] [CrossRef]
- Makinde, O.M.; Ayeni, K.I.; Sulyok, M.; Krska, R.; Adeleke, R.; Ezekiel, C.N. Microbiological safety of ready-to-eat foods in low- and middle-income countries: A comprehensive 10-year (2009 to 2018) review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 703–732. [Google Scholar] [CrossRef]
- Ayeni, K.I.; Atanda, O.O.; Krska, R.; Ezekiel, C.N. Present status and future perspectives of grain drying and storage practices as a means to reduce mycotoxin exposure in Nigeria. Food Control 2021, 126, 108074. [Google Scholar] [CrossRef]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi: CBS Laboratory Manual Series 2, 2nd ed.; Westerdijk Fungal Biodiversity Institute: Utrecht, The Netherlands, 2019; pp. 1–472. [Google Scholar]
- Adetuniji, M.C.; Atanda, O.O.; Ezekiel, C.N.; Dipeolu, A.O.; Uzochukwu, S.V.A.; Oyedepo, J.; Chilaka, C.A. Distribution of mycotoxins and risk assessment of maize consumers in five agro-ecological zones of Nigeria. Eur. Food Res. Technol. 2014, 239, 287–296. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Ogundare, O. Microbiological Quality Assessment of Pupuru and Plantain Flours in an Urban Market in Akure, Ondo State, South Western Nigeria. Open Access Libr. J. 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Jeff-Agboola, Y.A.; Omosanyin, T.R. Occurrence of toxigenic moulds isolated in maize (Zea mays) from Okitipupa metropolis, Ondo State, Nigeria. Int. J. Food Saf. Nutr. Pub. Health Technol. 2017, 9, 28–37. [Google Scholar]
- Makun, H.A.; Gbodi, T.A.; Akanya, O.H.; Salako, E.A.; Ogbadu, G.H. Fungi and some mycotoxins contaminating rice (Oryza sativa) in Niger State, Nigeria. Afr. J. Biotechnol. 2007, 6, 99–108. [Google Scholar] [CrossRef]
- Shehu, K.; Salau, I.A.; Salisu, N. Fungal and mycotoxin contamination of stored maize grains in Kebbi state, north-western Nigeria. J. Adv. Bot. Zool. 2020, 8, 4–8. [Google Scholar]
- Houbraken, J.; Frisvad, J.; Samson, R.A. Taxonomy of Penicillium section Citrina. Stud. Mycol. 2011, 70, 53–138. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; Visagie, C.M.; Houbraken, J.; Hong, S.-B.; Hubka, V.; Klaassen, C.; Perrone, G.; Seifert, K.; Susca, A.; Tanney, J.; et al. Phylogeny, identification and nomenclature of the genus Aspergillus. Stud. Mycol. 2014, 78, 141–173. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Mycotoxin control in low- and middle-income countries. In IARC Working Group Report No. 9; Wild, C.P., Miller, J.D., Groopman, J.D., Eds.; IARC: Lyon, France, 2015; pp. 1–54. [Google Scholar]
- Kamala, A.; Shirima, C.; Jani, B.; Bakari, M.; Sillo, H.; Rusibamayila, N.; De Saeger, S.; Kimanya, M.; Gong, Y.; Simba, A.; et al. Outbreak of an acute aflatoxicosis in Tanzania during 2016. World Mycotoxin J. 2018, 11, 311–320. [Google Scholar] [CrossRef]
- Ayeni, K.I.; Akinyemi, O.M.; Kovač, T.; Ezekiel, C.N. Aflatoxin contamination of maize vended in Ondo state, Nigeria, and health risk assessments. Croat. J. Food Sci. Technol. 2020, 12, 123–129. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Donner, M.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 2008, 122, 74–84. [Google Scholar] [CrossRef]
- Ayodele, O.; Akindele, M. Extension Activities for Arable Crops Production in Akure South Local Government Area, Ondo State, Nigeria. J. Agric. Ext. 1970, 22, 1–10. [Google Scholar] [CrossRef]
- Commission of the European Communities. Commission regulation (EC) No 824/2000 of 19 April 2000 establishing procedures for the taking-over of cereals by intervention agencies and laying down methods of analysis for determining the quality of cereals. Off. J. Eur. Commun. 2000, L100, 31–50. [Google Scholar]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.S.; Filtenborg, O. Methods for the detection and isolation of food-borne fungi. In Introduction to Foodborne Fungi; Samson, R.A., Hoekstra, E.S., Frisvad, J.C., Filtenborg, O., Eds.; Centraal Bureau voor Schimmel Cultures: Utretch, The Netherlands, 1995; pp. 235–242. [Google Scholar]
- Frisvad, J.C.; Samson, R.A. Polyphasic taxonomy of Penicillium subgenus Penicillium. A guide to identification of food and air-borne terverticillate Penicillia and their mycotoxins. Stud. Mycol. 2004, 49, 1–174. [Google Scholar]
- Leslie, J.F.; Summerell, B.A.; Bullock, S. The Fusarium Laboratory Manual; Wiley-Blackwell: Oxford, UK, 2006; p. 388. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009; p. 520. [Google Scholar] [CrossRef]
- Samson, R.; Yilmaz, N.; Houbraken, J.; Spierenburg, H.; Seifert, K.; Peterson, S.; Varga, J.; Frisvad, J. Phylogeny and nomenclature of the genus Talaromyces and taxa accommodated in Penicillium subgenus Biverticillium. Stud. Mycol. 2011, 70, 159–183. [Google Scholar] [CrossRef]
- Houbraken, J.; Frisvad, J.; Seifert, K.A.; Overy, D.P.; Tuthill, D.M.; Valdez, J.G.; Samson, R. New penicillin-producing Penicillium species and an overview of section Chrysogena. Persoonia 2012, 29, 78–100. [Google Scholar] [CrossRef]
- Ezekiel, C.; Adetunji, M.; Atanda, O.; Frisvad, J.; Houbraken, J.; Samson, R. Phenotypic differentiation of species from Aspergillus section Flavi on neutral red desiccated coconut agar. World Mycotoxin J. 2014, 7, 335–344. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D.; Mylona, K.; Lambert, R.J. Limiting mycotoxins in stored wheat. Food Addit. Contam. Part A 2010, 27, 644–650. [Google Scholar] [CrossRef]
- Food Agricultural Organization of the United Nations (FAO). Rural Structures in the Tropics: Design and Development; Food Agricultural Organization: Rome, Italy, 2011. [Google Scholar]
- Shitu, S.; Macchido, D.A.; Tijjani, M.B. Detection of Aflatoxigenic Moulds and Aflatoxins in Maize and Millet’s Grains Marketed in Zaria Metropolis. J. Adv. Microbiol. 2018, 13, 1–9. [Google Scholar] [CrossRef]
- Getachew, A.; Chala, A.; Hofgaard, I.S.; Brurberg, M.B.; Sulyok, M.; Tronsmo, A.-M. Multimycotoxin and fungal analysis of maize grains from south and southwestern Ethiopia. Food Addit. Contam. Part B 2017, 11, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.N.; Oyedele, O.A.; Kraak, B.; Ayeni, K.I.; Sulyok, M.; Houbraken, J.; Krska, R. Fungal Diversity and Mycotoxins in Low Moisture Content Ready-To-Eat Foods in Nigeria. Front. Microbiol. 2020, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Adetunji, C.; Atanda, O.; Ezekiel, C.N.; Ogara, I.M. The distribution and mycotoxigenicity of fungal isolates of stored maize grains from five agro-ecological zones of Nigeria. Mycotoxicology 2014, 1, 19–28. [Google Scholar]
- Ekwomadu, T.I.; Gopane, R.E.; Mwanza, M. Occurrence of filamentous fungi in maize destined for human consumption in South Africa. Food Sci. Nutr. 2018, 6, 884–890. [Google Scholar] [CrossRef]
- Xing, F.; Liu, X.; Wang, L.; Selvaraj, J.N.; Jin, N.; Wang, Y.; Zhao, Y.; Liu, Y. Distribution and variation of fungi and major mycotoxins in pre- and post-nature drying maize in North China Plain. Food Control 2017, 80, 244–251. [Google Scholar] [CrossRef]
- Avila, C.F.; Moreira, G.M.; Nicolli, C.P.; Gomes, L.B.; Abreu, L.M.; Pfenning, L.H.; Haidukowski, M.; Moretti, A.; Logrieco, A.; Del Ponte, E.M. Fusarium incarnatum-equiseti species complex associated with Brazilian rice: Phylogeny, morphology and toxigenic potential. Int. J. Food Microbiol. 2019, 306, 108267. [Google Scholar] [CrossRef]
- Nicolli, C.P.; Haidukowski, M.; Susca, A.; Gomes, L.B.; Logrieco, A.; Stea, G.; Del Ponte, E.M.; Moretti, A.; Pfenning, L.H. Fusarium fujikuroi species complex in Brazilian rice: Unveiling increased phylogenetic diversity and toxigenic potential. Int. J. Food Microbiol. 2020, 330, 108667. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.R.; Mansouri, M.; Bahonar, A.R.; Shokri, H. Mycoflora of Maize Harvested from Iran and Imported Maize. Pak. J. Biol. Sci. 2007, 10, 4432–4437. [Google Scholar] [CrossRef][Green Version]
- Ezekiel, C.N.; Sulyok, M.; Somorin, Y.; Odutayo, F.I.; Nwabekee, S.U.; Balogun, A.T.; Krska, R. Mould and mycotoxin exposure assessment of melon and bush mango seeds, two common soup thickeners consumed in Nigeria. Int. J. Food Microbiol. 2016, 237, 83–91. [Google Scholar] [CrossRef]
- Oyedele, O.A.; Ezekiel, C.N.; Sulyok, M.; Adetunji, M.; Warth, B.; Atanda, O.O.; Krska, R. Mycotoxin risk assessment for consumers of groundnut in domestic markets in Nigeria. Int. J. Food Microbiol. 2017, 251, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Madbouly, A.K.; Ibrahim, M.; Sehab, A.F.; Abdel-Wahhab, M.A. Co-occurrence of mycoflora, aflatoxins and fumonisins in maize and rice seeds from markets of different districts in Cairo, Egypt. Food Addit. Contam. Part B 2012, 5, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Bandyopadhyay, R.; Cotty, P. Diversity of aflatoxin-producing fungi and their impact on food safety in sub-Saharan Africa. Int. J. Food Microbiol. 2014, 174, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Adjovi, Y.; Bailly, S.; Gnonlonfin, B.; Tadrist, S.; Querin, A.; Sanni, A.; Oswald, I.; Puel, O.; Bailly, J.-D. Analysis of the contrast between natural occurrence of toxigenic Aspergilli of the Flavi section and aflatoxin B1 in cassava. Food Microbiol. 2014, 38, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.; Sulyok, M.; Frisvad, J.; Somorin, Y.; Warth, B.; Houbraken, J.; Samson, R.; Krska, R.; Odebode, A. Fungal and mycotoxin assessment of dried edible mushroom in Nigeria. Int. J. Food Microbiol. 2013, 162, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.; Skouboe, P.; Samson, R.A. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. Syst. Appl. Microbiol. 2005, 28, 442–453. [Google Scholar] [CrossRef]
- Iyanyi, N.G.; Ataga, A.E.; Ifegwu, M.K.; Pere, F. Identification of fungal organisms associated with the rhizosphere of maize (Zea mays L.): Basic molecular techniques. Niger. Agric. J. 2020, 51, 399–405. [Google Scholar]
- Frisvad, J.; Hubka, V.; Ezekiel, C.; Hong, S.-B.; Novakova, A.; Chen, A.; Arzanlou, M.; Larsen, T.O.; Sklenář, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef]
- Adejumo, O.; Atanda, O.; Raiola, A.; Somorin, Y.; Bandyopadhyay, R.; Ritieni, A. Correlation between aflatoxin M1 content of breast milk, dietary exposure to aflatoxin B1 and socioeconomic status of lactating mothers in Ogun State, Nigeria. Food Chem. Toxicol. 2013, 56, 171–177. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Warth, B.; Ogara, I.M.; Abia, W.A.; Ezekiel, V.C.; Atehnkeng, J.; Sulyok, M.; Turner, P.C.; Tayo, G.O.; Krska, R.; et al. Mycotoxin exposure in rural residents in northern Nigeria: A pilot study using multi-urinary biomarkers. Environ. Int. 2014, 66, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Ezekiel, C.N.; Oyeyemi, O.; Oyedele, O.A.; Ayeni, K.; Oyeyemi, I.T.; Nabofa, W.; Nwozichi, C.U.; Dada, A. Urinary aflatoxin exposure monitoring in rural and semi-urban populations in Ogun state, Nigeria. Food Addit. Contam. Part A 2018, 35, 1565–1572. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Abia, W.A.; Braun, D.; Sarkanj, B.; Ayeni, K.I.; Oyedele, O.A.; Michael-Chikezie, E.C.; Ezekiel, V.C.; Mark, B.; Ahuchaogu, C.P.; et al. Comprehensive mycotoxin exposure biomonitoring in breastfed and nonexclusively breastfed Nigerian children. MedRxiv 2020, 1–45. [Google Scholar] [CrossRef]
- Šarkanj, B.; Ezekiel, C.N.; Turner, P.C.; Abia, W.A.; Rychlik, M.; Krska, R.; Sulyok, M.; Warth, B. Ultra-sensitive, stable isotope assisted quantification of multiple urinary mycotoxin exposure biomarkers. Anal. Chim. Acta 2018, 1019, 84–92. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsubé, S.; Visagie, C.; Yilmaz, N.; Wang, X.-C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef] [PubMed]
- Susca, A.; Moretti, A.; Stea, G.; Villani, A.; Haidukowski, E.M.; Logrieco, A.F.; Munkvold, G. Comparison of species composition and fumonisin production in Aspergillus section Nigri populations in maize kernels from USA and Italy. Int. J. Food Microbiol. 2014, 188, 75–82. [Google Scholar] [CrossRef]
- Croft, C.A.; Eculibrk, L.; Moore, M.M.; Tebbutt, S.J. Interactions of Aspergillus fumigatus Conidia with Airway Epithelial Cells: A Critical Review. Front. Microbiol. 2016, 7, 472. [Google Scholar] [CrossRef]
- Varga, J.; Houbraken, J.; Van Der Lee, H.A.L.; Verweij, P.; Samson, R.A. Aspergillus calidoustus sp. nov., Causative Agent of Human Infections Previously Assigned to Aspergillus ustus. Eukaryot. Cell 2008, 7, 630–638. [Google Scholar] [CrossRef]
- Varga, J.; Due, M.; Frisvad, J.; Samson, R.A. Taxonomic revision of Aspergillus section Clavati based on molecular, morphological and physiological data. Stud. Mycol. 2007, 59, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Visagie, C.M.; Hirooka, Y.; Tanney, J.; Whitfield, E.; Mwange, K.; Meijer, M.; Amend, A.; Seifert, K.; Samson, R. Aspergillus, Penicillium and Talaromyces isolated from house dust samples collected around the world. Stud. Mycol. 2014, 78, 63–139. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.A.M.P.; Frisvad, J.; Samson, R.A. Taxonomy of Penicillium citrinum and related species. Fungal Divers. 2010, 44, 117–133. [Google Scholar] [CrossRef]
- Akinfala, T.O.; Houbraken, J.; Sulyok, M.; Adedeji, A.R.; Odebode, A.C.; Krska, R.; Ezekiel, C.N. Moulds and their secondary metabolites associated with the fermentation and storage of two cocoa bean hybrids in Nigeria. Int. J. Food Microbiol. 2020, 316, 108490. [Google Scholar] [CrossRef]
- Ezekiel, C.N.; Ayeni, K.I.; Akinyemi, M.O.; Sulyok, M.; Oyedele, O.A.; Babalola, D.A.; Ogara, I.M.; Krska, R. Dietary risk assessment and consumer awareness of mycotoxins among household consumers of cereals, nuts and legumes in north-central Nigeria. Toxins 2021. under review. [Google Scholar]
- Okeke, C.A.; Ezekiel, C.N.; Nwangburuka, C.C.; Sulyok, M.; Ezeamagu, C.; Adeleke, R.; Dike, K.; Krska, R. Bacterial Diversity and Mycotoxin Reduction During Maize Fermentation (Steeping) for Ogi Production. Front. Microbiol. 2015, 6, 1402. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on the risks for public and animal health related to the presence of citrinin in food and feed. EFSA J. 2012, 10, 1–82. [Google Scholar] [CrossRef]
- Peterson, S.W.; Jurjević, Ž. The Talaromyces pinophilus species complex. Fungal Biol. 2019, 123, 745–762. [Google Scholar] [CrossRef]
- Neelaveni, V.; Tupaki-Sreepurna, A.; Thanneru, V.; Kindo, A.J. Lichtheimia ramosa isolated from a young patient from an infected wound after a road traffic accident. J. Acad. Clin. Microbiol. 2017, 19, 59–61. [Google Scholar]
- Baby, S.; Ramya, T.; Geetha, R. Onychomycosis by Syncephalastrum racemosum: Case report from Kerala, India. Dermatol. Rep. 2015, 7, 5527. [Google Scholar] [CrossRef]
- Fapohunda, S.O.; Moore, G.G.; Aroyeun, S.O.; Ayeni, K.I.; Aduroja, D.E.; Odetunde, S.K. Isolation and characterization of fungi isolated from Nigerian cocoa samples. Curr. Life Sci. 2018, 4, 46–52. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC Monographs on Evaluation of Carcinogenic Risk to Humans, No. 56; International Agency for Research on Cancer: Lyon, France, 1993. [Google Scholar]
- Onyedum, S.; Adefolalu, F.; Muhammad, H.; Apeh, D.; Agada, M.; Imienwanrin, M.; Makun, H. Occurrence of major mycotoxins and their dietary exposure in North-Central Nigeria staples. Sci. Afr. 2020, 7, e00188. [Google Scholar] [CrossRef]
- Nduti, N. Technical University of Kenya Aflatoxin variations in maize flour and grains collected from various regions of Kenya. Afr. J. Food Agric. Nutr. Dev. 2017, 17, 11743–11756. [Google Scholar] [CrossRef]
- Adetuwo, O.J. Evaluation of the microbiological quality and safety of pupuru and garri on sale at Okitipupa main market in Ondo state, Nigeria. Am. J. Food Sci. Nutr. 2020, 2, 32–44. [Google Scholar]
- European Union EUR-Lex. Access to European Union Law. Available online: https://eur-lex.europa.eu (accessed on 4 January 2021).

| Food Type | N | Mean (%) ± SE | Range (%) | |
|---|---|---|---|---|
| Min | Max | |||
| Maize | 46 | 7.72 ± 0.30 c | 3.51 | 12.3 |
| Pupuru | 20 | 11.3 ± 0.35 a | 9.10 | 14.6 |
| Rice | 40 | 9.44 ± 0.46 b | 5.08 | 16.1 |
| Total | 106 | 9.04 ± 0.26 | 3.51 | 16.1 |
| Food Type | N | % | Incidence (%) of Contaminated Food Samples | Mean (μg/kg) ± SE | Range (μg/kg) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤4 μg/kg * | ≤ 10 μg/kg | ≤20 μg/kg ** | >20–<100 μg/kg | ≥100 μg/kg | Min | Max | ||||
| Maize | 12 | 100 | 8.3 | 16.7 | 25.0 | 16.7 | 58.3 | 101 ± 19.3 | 3.5 | 173.3 |
| Pupuru | 10 | 40.0 | 50.0 | 75.0 | 75.0 | 25.0 | 0.00 | 7.9 ± 4.4 | 1.75 | 21 |
| Rice | 20 | 75.0 | 40.0 | 86.7 | 93.3 | 6.7 | 0.00 | 6.5 ± 1.6 | 1.75 | 22.8 |
| Total | 42 | 73.8 | 29.0 | 58.1 | 64.5 | 12.9 | 22.6 | 43.1 ± 11.1 | 1.75 | 173.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekpakpale, D.O.; Kraak, B.; Meijer, M.; Ayeni, K.I.; Houbraken, J.; Ezekiel, C.N. Fungal Diversity and Aflatoxins in Maize and Rice Grains and Cassava-Based Flour (Pupuru) from Ondo State, Nigeria. J. Fungi 2021, 7, 635. https://doi.org/10.3390/jof7080635
Ekpakpale DO, Kraak B, Meijer M, Ayeni KI, Houbraken J, Ezekiel CN. Fungal Diversity and Aflatoxins in Maize and Rice Grains and Cassava-Based Flour (Pupuru) from Ondo State, Nigeria. Journal of Fungi. 2021; 7(8):635. https://doi.org/10.3390/jof7080635
Chicago/Turabian StyleEkpakpale, Daniella O., Bart Kraak, Martin Meijer, Kolawole I. Ayeni, Jos Houbraken, and Chibundu N. Ezekiel. 2021. "Fungal Diversity and Aflatoxins in Maize and Rice Grains and Cassava-Based Flour (Pupuru) from Ondo State, Nigeria" Journal of Fungi 7, no. 8: 635. https://doi.org/10.3390/jof7080635
APA StyleEkpakpale, D. O., Kraak, B., Meijer, M., Ayeni, K. I., Houbraken, J., & Ezekiel, C. N. (2021). Fungal Diversity and Aflatoxins in Maize and Rice Grains and Cassava-Based Flour (Pupuru) from Ondo State, Nigeria. Journal of Fungi, 7(8), 635. https://doi.org/10.3390/jof7080635






