TNF-α May Exert Different Antitumor Effects in Response to Radioactive Iodine Therapy in Papillary Thyroid Cancer with/without Autoimmune Thyroiditis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Protocol
2.2. Histopathology
2.3. Blood Sampling
2.4. Biomarker Measurements
2.5. Statistics
3. Results
3.1. Clinicopathological Characteristics
3.2. Blood Sample Radioactivity
3.3. Effects of 131I Irradiation on Analyzed Biomarkers
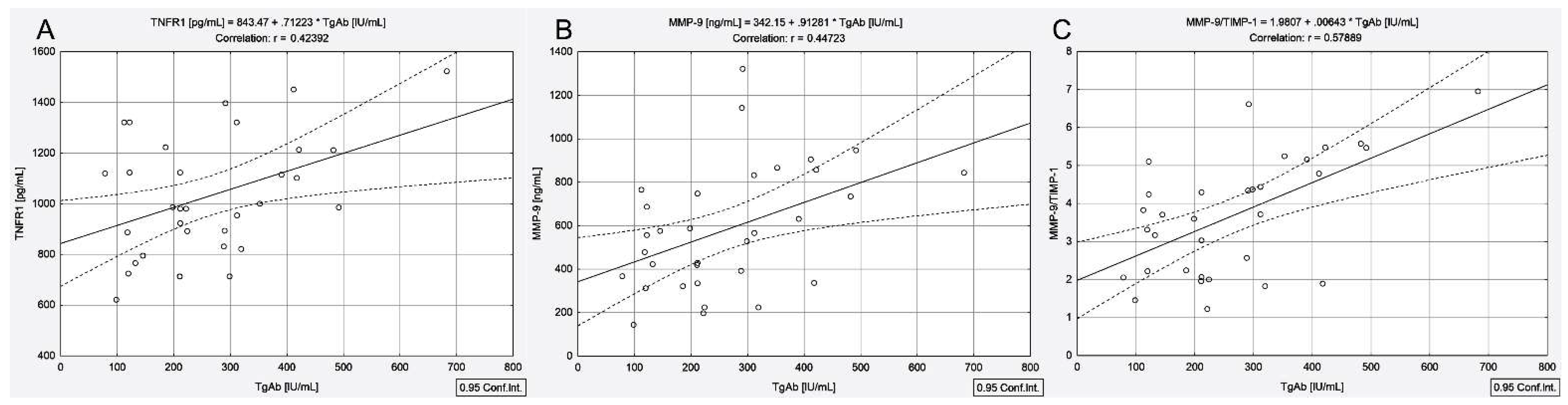
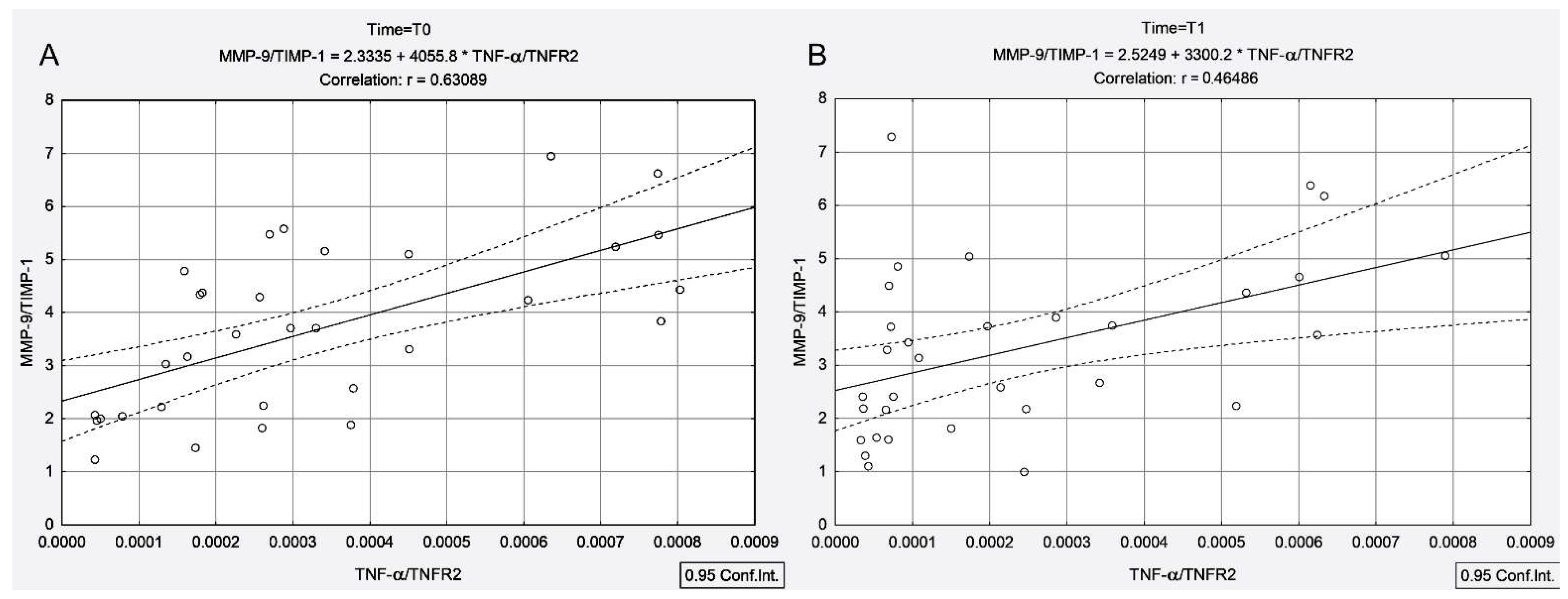
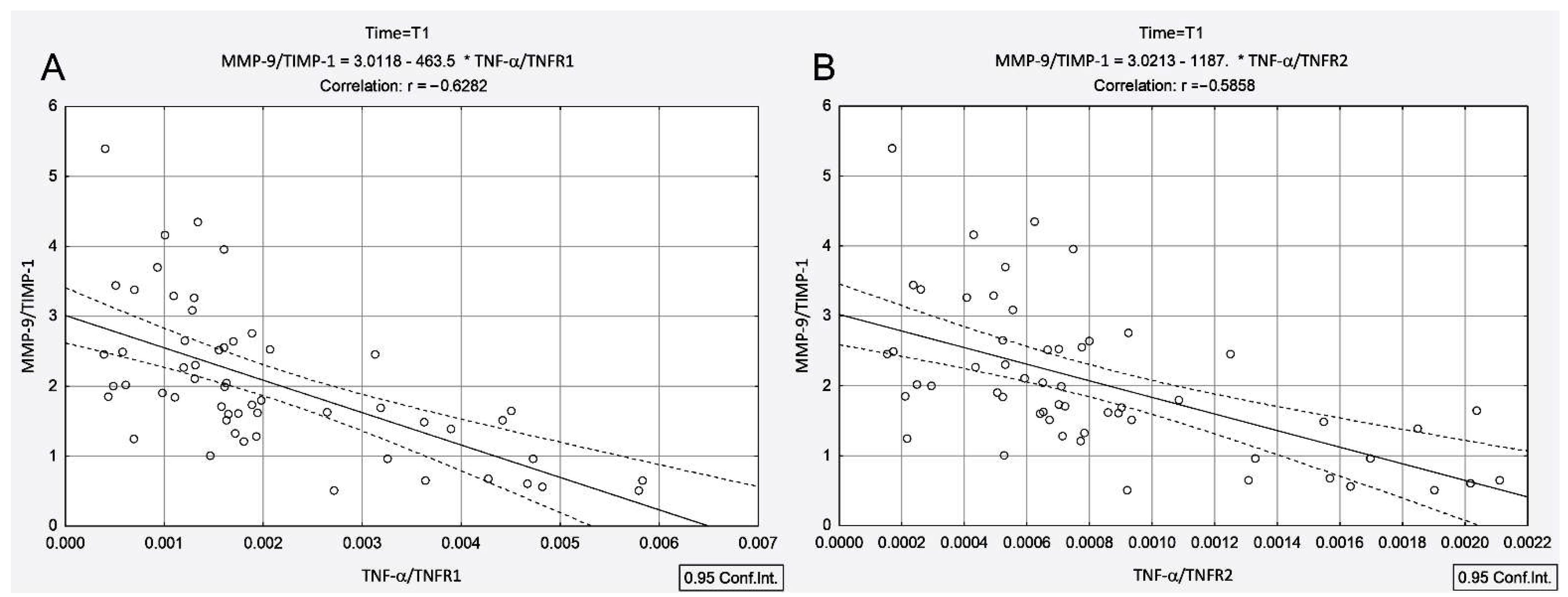
3.4. Correlations
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lerner, A.; Jeremias, P.; Matthias, T. The World Incidence and Prevalence of Autoimmune Diseases is Increasing. Int. J. Celiac Dis. 2015, 3, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Valencia, J.C.; Egbukichi, N.; Erwin-Cohen, R.A. Autoimmunity and Cancer, the Paradox Comorbidities Challenging Therapy in the Context of Preexisting Autoimmunity. J. Interferon Cytokine Res. 2019, 39, 72–84. [Google Scholar] [CrossRef]
- Trovato, M.; Giuffrida, G.; Seminara, A.; Fogliani, S.; Cavallari, V.; Ruggeri, R.M.; Campennì, A. Coexistence of diffuse large B-cell lymphoma and papillary thyroid carcinoma in a patient affected by Hashimoto’s thyroiditis. Arch. Endocrinol. Metab. 2017, 61, 643–646. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, Y.; Choi, J.W.; Kim, Y.S. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: A meta-analysis. Eur. J. Endocrinol. 2013, 168, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.S.; Kim, H.K.; Lee, C.R.; Park, S.; Park, J.H.; Kang, S.W.; Jeong, J.J.; Nam, K.H.; Chung, W.Y.; Park, C.S. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: Clinical manifestation and prognostic outcome. J. Korean Med. Sci. 2012, 27, 883–889. [Google Scholar] [CrossRef] [Green Version]
- Stanciu, A.E. Cytokines in heart failure. Adv. Clin. Chem. 2019, 93, 63–113. [Google Scholar] [CrossRef]
- Lun, Y.; Wu, X.; Xia, Q.; Han, Y.; Zhang, X.; Liu, Z.; Wang, F.; Duan, Z.; Xin, S.; Zhang, J. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol. Head Neck Surg. 2013, 148, 396–402. [Google Scholar] [CrossRef]
- Lim, E.; Shah, S.; Waterhouse, M.; Akker, S.; Drake, W.; Plowman, N.; Berney, D.M.; Richards, P.; Adams, A.; Nowosinska, E.; et al. Impact of thyroiditis on 131I uptake during ablative therapy for differentiated thyroid cancer. Endocr. Connect. 2019, 8, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Rubner, Y.; Kulzer, L.; Werthmöller, N.; Weiss, E.M.; Fietkau, R.; Gaipl, U.S. Antitumor immune responses induced by ionizing irradiation and further immune stimulation. Cancer Immunol. Immunother. 2014, 63, 29–36. [Google Scholar] [CrossRef]
- Menon, H.; Ramapriyan, R.; Cushman, T.R.; Verma, V.; Kim, H.H.; Schoenhals, J.E.; Atalar, C.; Selek, U.; Chun, S.G.; Chang, J.Y.; et al. Role of Radiation Therapy in Modulation of the Tumor Stroma and Microenvironment. Front. Immunol. 2019, 10, 193. [Google Scholar] [CrossRef] [Green Version]
- Pouget, J.P.; Georgakilas, A.G.; Ravanat, J.L. Targeted and Off-Target (Bystander and Abscopal) Effects of Radiation Therapy: Redox Mechanisms and Risk/Benefit Analysis. Antioxid. Redox Signal. 2018, 29, 1447–1487. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, T.; Liu, W.; Zuo, L. Molecular mechanism of bystander effects and related abscopal/cohort effects in cancer therapy. Oncotarget 2018, 9, 18637–18647. [Google Scholar] [CrossRef] [Green Version]
- Widel, M. Radionuclides in radiation-induced bystander effect; may it share in radionuclide therapy? Neoplasma 2017, 64, 641–654. [Google Scholar] [CrossRef] [Green Version]
- Palata, O.; Hradilova Podzimkova, N.; Nedvedova, E.; Umprecht, A.; Sadilkova, L.; Palova Jelinkova, L.; Spisek, R.; Adkins, I. Radiotherapy in Combination with Cytokine Treatment. Front. Oncol. 2019, 9, 367. [Google Scholar] [CrossRef]
- Fischer, R.; Kontermann, R.E.; Pfizenmaier, K. Selective Targeting of TNF Receptors as a Novel Therapeutic Approach. Front. Cell Dev. Biol. 2020, 8, 401. [Google Scholar] [CrossRef]
- Fugazzola, L.; Elisei, R.; Fuhrer, D.; Jarzab, B.; Leboulleux, S.; Newbold, K.; Smit, J. 2019 European Thyroid Association Guidelines for the Treatment and Follow-Up of Advanced Radioiodine-Refractory Thyroid Cancer. Eur. Thyroid J. 2019, 8, 227–245. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Sisson, J.C.; Freitas, J.; McDougall, I.R.; Dauer, L.T.; Hurley, J.R.; Brierley, J.D.; Edinboro, C.H.; Rosenthal, D.; Thomas, M.J.; Wexler, J.A.; et al. Radiation safety in the treatment of patients with thyroid diseases by radioiodine 131I: Practice recommendations of the American Thyroid Association, The American Thyroid Association Taskforce on Radioiodine Safety. Thyroid 2011, 21, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Stanciu, A.E.; Zamfir-Chiru-Anton, A.; Stanciu, M.M.; Stoian, A.P.; Jinga, V.; Nitipir, C.; Bucur, A.; Pituru, T.S.; Arsene, A.L.; Dragoi, C.M.; et al. Clinical significance of serum melatonin in predicting the severity of oral squamous cell carcinoma. Oncol. Lett. 2020, 19, 1537–1543. [Google Scholar] [CrossRef] [Green Version]
- Trovato, M. A historical excursus of diagnostic methods for Hashimoto thyroiditis and Graves’ disease. Gazz. Med. Ital. Arch. Sci. Med. 2020, 179, 479–485. [Google Scholar] [CrossRef]
- Mohamed, S.Y.; Ibrahim, T.R.; Elbasateeny, S.S.; Abdelaziz, L.A.; Farouk, S.; Yassin, M.A.; Embaby, A. Clinicopathological characterization and prognostic implication of FOXP3 and CK19 expression in papillary thyroid carcinoma and concomitant Hashimoto’s thyroiditis. Sci. Rep. 2020, 10, 10651. [Google Scholar] [CrossRef]
- Casella, C.; Ministrini, S.; Galani, A.; Mastriale, F.; Cappelli, C.; Portolani, N. The New TNM Staging System for Thyroid Cancer and the Risk of Disease Downstaging. Front. Endocrinol. 2018, 9, 541. [Google Scholar] [CrossRef] [Green Version]
- Lassmann, M.; Hanscheid, H.; Chiesa, C.; Hindorf, C.; Flux, G.; Luster, M.; EANM Dosimetry Committee. EANM Dosimetry Committee series on standard operational procedures for pretherapeutic dosimetry I: Blood and bone marrow dosimetry in differentiated thyroid cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- WHO International Standard Tumour Necrosis Factor Alpha (Human, Natural). Available online: https://www.nibsc.org/documents/ifu/88-786.pdf (accessed on 6 May 2021).
- Feldt-Rasmussen, U.; Profilis, C.; Colinet, E.; Black, E.; Bornet, H.; Bourdoux, P.; Carayon, P.; Ericsson, U.B.; Koutras, D.A.; Lamas de Leon, L.; et al. Human thyroglobulin reference material (CRM 457). 2nd Part: Physicochemical characterization and certification. Ann. Biol. Clin. 1996, 54, 343–348. [Google Scholar]
- Swanson, G.P.; Jhavar, S.G.; Hammonds, K. The effect of pelvic radiation alone on lymphocyte subgroups. Clin. Transl. Radiat. Oncol. 2020, 23, 100–102. [Google Scholar] [CrossRef]
- Girardi, F.M.; Barra, M.B.; Zettler, C.G. Papillary thyroid carcinoma: Does the association with Hashimoto’s thyroiditis affect the clinicopathological characteristics of the disease? Braz. J. Otorhinolaryngol. 2015, 81, 283–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widel, M. Radiation induced bystander effect: From in vitro studies to clinical application. Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2016, 5, 63298. [Google Scholar] [CrossRef] [Green Version]
- Lumniczky, K.; Sáfrány, G. The impact of radiation therapy on the antitumor immunity: Local effects and systemic consequences. Cancer Lett. 2015, 356, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Saddala, M.S.; Huang, H. Identification of novel inhibitors for TNFα, TNFR1 and TNFα-TNFR1 complex using pharmacophore-based approaches. J. Transl. Med. 2019, 17, 215. [Google Scholar] [CrossRef]
- Pegoretti, V.; Baron, W.; Laman, J.D.; Eisel, U.L.M. Selective Modulation of TNF-TNFRs Signaling: Insights for Multiple Sclerosis Treatment. Front. Immunol. 2018, 9, 925. [Google Scholar] [CrossRef]

| Variables | PTC | PTC + AIT | ||
|---|---|---|---|---|
| n = 56 | n = 32 | |||
| T0 c | T1 d | T0 c | T1 d | |
| Age (years) a | 43.2 ± 12.7 | 43.2 ± 12.7 | 40.6 ± 12.3 | 40.6 ± 12.3 |
| TgAb (IU/mL) b | 7.9 (6.5–9.4) | 5.2 (3.5–7.2) | 222.7 (138.8–335.9) | 292.0 (194.0–354.4) |
| TNF-α (pg/mL) b | 0.37 (0.20–0.55) | 1.3 (0.9–2.4) | 0.65 (0.35–0.90) | 0.30 (0.20–0.90) |
| TNFR1 (pg/mL) b | 739.9 (664.5–968.6) | 796.2 (700.4–997.1) | 986.4 (858.8–1212.4) | 1326.9 (1051–1521.5) |
| TNFR2 (pg/mL) b | 1942.9 (1527.3–2301.9) | 2102.9 (1626.7–2498.6) | 2318.5 (2116.8–2610.3) | 2542.8 (2234.7–2794.4) |
| MMP-9 (ng/mL) b | 395.6 (317.4–555.5) | 300.6 (222.9–376.9) | 561.7 (351.6–798.4) | 509.4 (331.4–660.5) |
| TIMP-1 (ng/mL) b | 133.2 (120.5–155.4) | 152.7 (126.5–168.7) | 154.1 (132.4–176.3) | 152.4 (141.2–180.7) |
| TNF-α/TNFR1 b | 4.5 × 10−4 (3 × 10−4–7.5 × 10−4) | 16 × 10−4 (4 × 10−4–58 × 10−4) | 6.1 × 10−4 (4.3 × 10−4–10 × 10−4) | 2.5 × 10−4 (1.3 × 10−4–6.5 × 10−4) |
| TNF-α/TNFR2 b | 2 × 10−4 (1.3 × 10−4–2.7 × 10−4) | 7 × 10−4 (5.1 × 10−4–9.3 × 10−4) | 2.7 × 10−4 (1.6 × 10−4–4.5 × 10−4) | 1.3 × 10−4 (0.7 × 10−4–3.5 × 10−4) |
| MMP-9/TIMP-1 b | 3.1 (2.4–3.7) | 1.8 (1.3–2.5) | 3.7 (2.1–4.9) | 3.2 (2.2–4.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, D.C.; Stanciu, M.M.; Zamfirescu, A.; Stanciu, A.E. TNF-α May Exert Different Antitumor Effects in Response to Radioactive Iodine Therapy in Papillary Thyroid Cancer with/without Autoimmune Thyroiditis. Cancers 2021, 13, 3609. https://doi.org/10.3390/cancers13143609
Gheorghe DC, Stanciu MM, Zamfirescu A, Stanciu AE. TNF-α May Exert Different Antitumor Effects in Response to Radioactive Iodine Therapy in Papillary Thyroid Cancer with/without Autoimmune Thyroiditis. Cancers. 2021; 13(14):3609. https://doi.org/10.3390/cancers13143609
Chicago/Turabian StyleGheorghe, Dan Cristian, Marcel Marian Stanciu, Anca Zamfirescu, and Adina Elena Stanciu. 2021. "TNF-α May Exert Different Antitumor Effects in Response to Radioactive Iodine Therapy in Papillary Thyroid Cancer with/without Autoimmune Thyroiditis" Cancers 13, no. 14: 3609. https://doi.org/10.3390/cancers13143609
APA StyleGheorghe, D. C., Stanciu, M. M., Zamfirescu, A., & Stanciu, A. E. (2021). TNF-α May Exert Different Antitumor Effects in Response to Radioactive Iodine Therapy in Papillary Thyroid Cancer with/without Autoimmune Thyroiditis. Cancers, 13(14), 3609. https://doi.org/10.3390/cancers13143609






