Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds
Abstract
:1. Introduction
2. Results
2.1. Laboratory Experiment
Effect of Methanol Extracts on Germination and Initial Growth of C. iria
2.2. Glasshouse Experiment
2.2.1. Effect of Methanol Extract on Plant Height, Leaf Area and Dry Weight of C. iria
2.2.2. Effect of Methanol Extract on Fv/Fm, Photosynthesis Rate, Stomatal Conductance and Transpiration Rate of C. iria
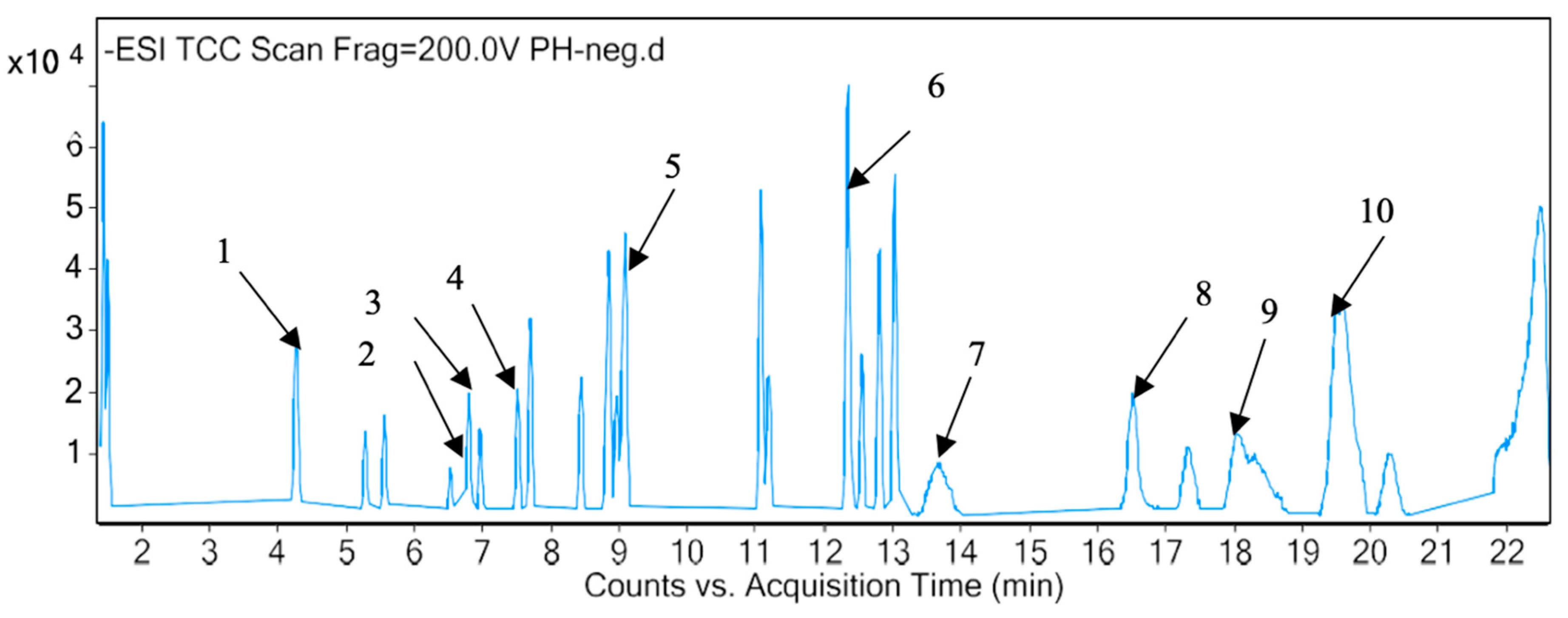
2.3. Identification of Phytotoxic Components from Methanol Extract of P. hysterophorus
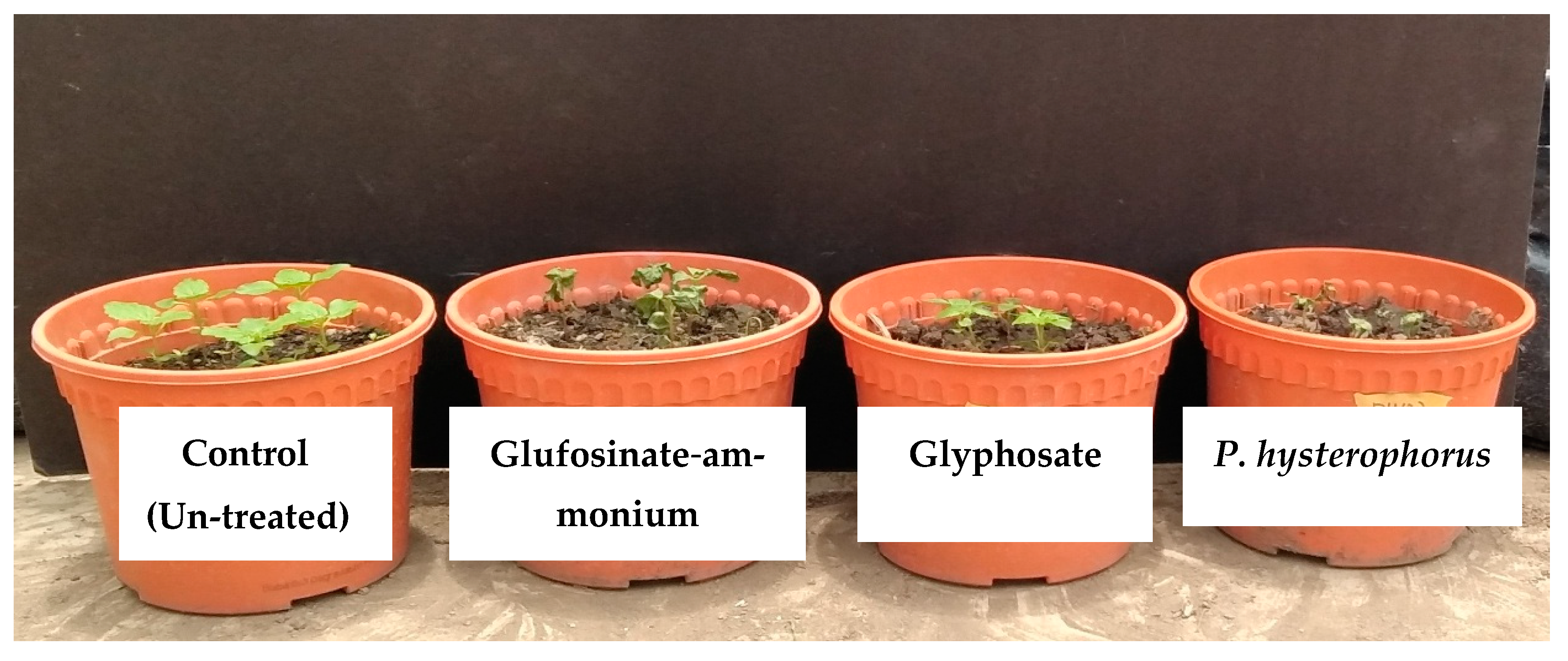
2.4. Efficacy of P. hysterophorus Extract in Comparison with Commercial Herbicides
3. Discussion
4. Materials and Methods
4.1. Test Plants
4.2. Extraction Procedure
4.3. Laboratory Bioassay
4.4. Glasshouse Experiment
4.5. LC-QTOF-MS/MS Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds: Distribution and Biology; The University Press of Hawaii: Malabar, FL, USA, 1991; p. 609. [Google Scholar]
- Rao, A.; Johnson, D.; Sivaprasad, B.; Ladha, J.; Mortimer, A. Weed Management in Direct-Seeded Rice. Adv. Agron. 2007, 93, 153–255. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D. Ecological studies on Cyperus difformis, Cyperus iria and Fimbristylis miliacea: Three troublesome annual sedge weeds of rice. Ann. Appl. Biol. 2009, 155, 103–112. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Johnson, D.E. Responses of Rice Flatsedge (Cyperus iria) and Barnyardgrass (Echinochloa crus-galli) to Rice Interference. Weed Sci. 2010, 58, 204–208. [Google Scholar] [CrossRef]
- Dhammu, H.S.; Sandhu, K.S. Critical period of Cyperus iria L. competition in transplanted rice. In Proceedings of the 13th Australian Weeds Con-ference: Weeds “Threats now and forever?”, Perth, Western Australia, 8–13 September 2002. [Google Scholar]
- Shrestha, A. Weed science as a new discipline and its status in some South Asian universities and colleges: Examples from Bangladesh, Bhutan, Nepal and Pakistan. CAB Rev. 2021, 16, 1–14. [Google Scholar] [CrossRef]
- Heap, I. Herbicide Resistant Weeds. In Integrated Pest Management; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2014; pp. 281–301. [Google Scholar]
- Hussain, M.I.; Reigosa, M.J. Higher peroxidase activity, leaf nutrient contents and carbon isotope composition changes in Arabidopsis thaliana are related to rutin stress. J. Plant Physiol. 2014, 171, 1325–1333. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.K.M.M.; Yeasmin, S.; Qasem, J.R.S.; Juraimi, A.S.; Anwar, P. Allelopathy of Medicinal Plants: Current Status and Future Prospects in Weed Management. Agric. Sci. 2018, 9, 1569–1588. [Google Scholar] [CrossRef] [Green Version]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Assessment of allelopathic compounds to develop new natural herbicides: A review. Allelopathy J. 2021, 52, 19–37. [Google Scholar] [CrossRef]
- Ridenour, W.M.; Callaway, R.M. The relative importance of allelopathy in interference: The effects of an invasive weed on a native bunchgrass. Oecologia 2001, 126, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Involvement of Allelopathy in the Invasive Potential of Tithonia diversifolia. Plants 2020, 9, 766. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kobayashi, A.; Ohno, O.; Kimura, F.; Fujii, Y.; Suenaga, K. Phytotoxic substances with allelopathic activity may be central to the strong invasive potential of Brachiaria brizantha. J. Plant Physiol. 2014, 171, 525–530. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.B.; Uddin, K.; Asib, N.B.; Islam, A.M.; Hasan, M. Allelopathic potential of Malaysian invasive weed species to control weedy rice (Oryza sativa f. spontanea Roshev). Allelopath. J. 2021, 53, 53–68. [Google Scholar] [CrossRef]
- Qu, T.; Du, X.; Peng, Y.; Guo, W.; Zhao, C.; Losapio, G. Invasive species allelopathy decreases plant growth and soil microbial activity. PLoS ONE 2021, 16, e0246685. [Google Scholar] [CrossRef] [PubMed]
- Gnanavel, I.; Natarajan, S.K. Parthenium hysterophorus L.: A major threat to natural and agro eco-systems in India. Int. J. Agric. Environ. Biotech. 2013, 6, 261–269. [Google Scholar]
- Masum, S.M.; Hasanuzzaman, M.; Ali, M.H. Threats of Parthenium hysterophorus on agro-ecosystems and its management: A review. Int. J. Agric. Crop Sci. 2013, 6, 684. [Google Scholar]
- Mekonnen, G. Threats and Management Options of Parthenium (Parthenium hysterophorus L.) in Ethiopia. Agric. Res. Technol. Open Access J. 2017, 10, 1–7. [Google Scholar] [CrossRef]
- Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Hasan, M. Bioherbicidal Properties of Parthenium hysterophorus, Cleome rutidosperma and Borreria alata Extracts on Selected Crop and Weed Species. Agronomy 2021, 11, 643. [Google Scholar] [CrossRef]
- Qasem, J.R. Differences in the allelopathy results from field observations to laboratory and glasshouse experiments. Allelopath. J. 2010, 26, 45–58. [Google Scholar]
- Islam, A.H.; Yeasmin, S.; Kader, R.A. Bioassay screening of sawdust obtained from selected tropical tree species for allelopathic properties and their field performance against paddy weeds. Fundam. Appl. Agric. 2019, 4, 906–915. [Google Scholar] [CrossRef]
- El-Mergawi, R.A.; Al-Humaid, A.I. Searching for natural herbicides in methanol extracts of eight plant species. Bull. Natl. Res. Cent. 2019, 43, 22. [Google Scholar] [CrossRef]
- Batish, D. Allelopathic effects of parthenin against two weedy species, Avena fatua and Bidens pilosa. Environ. Exp. Bot. 2002, 47, 149–155. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Pandher, J.K.; Kohli, R. Phytotoxic effects of Parthenium hysterophorus residues on three Brassica species. Weed Biol. Manag. 2005, 5, 105–109. [Google Scholar] [CrossRef]
- Mersie, W.; Singh, M. Allelopathic effect of parthenium (Parthenium hysterophorus L.) extract and residue on some agronomic crops and weeds. J. Chem. Ecol. 1987, 13, 1739–1747. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic Effects of Volatile Monoterpenoids Produced by Salvia leucophylla: Inhibition of Cell Proliferation and DNA Synthesis in the Root Apical Meristem of Brassica campestris Seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Islam, A.M.; Hasan, M.; Musha, M.H.; Uddin, K.; Juraimi, A.S.; Anwar, P. Exploring 55 tropical medicinal plant species available in Bangladesh for their possible allelopathic potentiality. Ann. Agric. Sci. 2018, 63, 99–107. [Google Scholar] [CrossRef]
- Levizou, E.; Karageorgou, P.; Psaras, G.K.; Manetas, Y. Inhibitory effects of water soluble leaf leachates from Dittrichia viscosa on lettuce root growth, statocyte development and graviperception. Flora-Morphol. Distrib. Funct. Ecol. Plants 2002, 197, 152–157. [Google Scholar] [CrossRef] [Green Version]
- Franco, D.M.; Silva, E.M.; Saldanha, L.L.; Adachi, S.A.; Schley, T.R.; Rodrigues, T.M.; Dokkedal, A.L.; Nogueira, F.T.S.; De Almeida, L.F.R. Flavonoids modify root growth and modulate expression of SHORT-ROOT and HD-ZIP III. J. Plant Physiol. 2015, 188, 89–95. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Karami, A.; Haghighi, T.M.; Alizadeh, S.; Maggi, F. Phytotoxic Potential and Phenolic Profile of Extracts from Scrophularia striata. Plants 2021, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elgawad, A.M.; El Gendy, A.E.-N.G.; Assaeed, A.M.; Al-Rowaily, S.L.; Alharthi, A.S.; Mohamed, T.A.; Nassar, M.I.; Dewir, Y.H.; ElShamy, A.I. Phytotoxic Effects of Plant Essential Oils: A Systematic Review and Structure-Activity Relationship Based on Chemometric Analyses. Plants 2020, 10, 36. [Google Scholar] [CrossRef]
- Alam, A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Hakim, M.A. Salinity-induced changes in the morphology and major mineral nutrient composition of purslane (Portulaca oleracea L.) accessions. Biol. Res. 2016, 49, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Algandaby, M.M.; El-Darier, S.M. Management of the noxious weed; Medicago polymorpha L. via allelopathy of some medicinal plants from Taif region, Saudi Arabia. Saudi J. Biol. Sci. 2018, 25, 1339–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renathielly, F.D.S.; Rodrigo, T.B.; Bruno, M.Z.; Maurício, A.P.; Samuel, N.M.D.S.; Reginaldo, F.S.; Da Silva, R.F.; Bressan, R.T.; Zilli, B.M.; Pilatti, M.A.; et al. Allelopathic effect of aqueous extract of fresh leaf castor beans (Ricinus communis L.) applied to the beginning stage of soy (Glycine max L.) and safflower (Carthamus tinctorius L.). Afr. J. Biotechnol. 2016, 15, 2787–2793. [Google Scholar] [CrossRef]
- Dalal, V.K.; Tripathy, B.C. Water-stress induced downsizing of light-harvesting antenna complex protects developing rice seedlings from photo-oxidative damage. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yu, J. Allelochemicals and photosynthesis. In Allelopathy; Springer Science and Business Media LLC: Berlin, Germany, 2006; pp. 127–139. [Google Scholar]
- Lawlor, D.W. Limitation to Photosynthesis in Water-stressed Leaves: Stomata vs. Metabolism and the Role of ATP. Ann. Bot. 2002, 89, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Shigeoka, S. Understanding Oxidative Stress and Antioxidant Functions to Enhance Photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Casson, S.; Gray, J.E. Influence of environmental factors on stomatal development. New Phytol. 2008, 178, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Blum, U.; Gerig, T.M. Relationships between Phenolic Acid Concentrations, Transpiration, Water Utilization, Leaf Area Expansion, and Uptake of Phenolic Acids: Nutrient Culture Studies. J. Chem. Ecol. 2005, 31, 1907–1932. [Google Scholar] [CrossRef]
- Li, Z.-H.; Wang, Q.; Ruan, X.; Pan, C.-D.; Jiang, D.-A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [Green Version]
- Al-Humaid, A.; El-Mergawi, R.A. Herbicidal activities of seven native plants on the germination and growth of Phalaris minor, Echinochloa crus-galli, Portulaca oleracea and Lactuca sativa. J. Agric. Sci. Technol. 2014, 4, 843–852. [Google Scholar]
- Scognamiglio, M.; D’Abrosca, B.; Esposito, A.; Pacifico, S.; Monaco, P.; Fiorentino, A. Plant growth inhibitors: Allelopathic role or phytotoxic effects? Focus on Mediterranean biomes. Phytochem. Rev. 2013, 12, 803–830. [Google Scholar] [CrossRef]
- Do, Q.-D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez, M.B.; Calderón, N.L.; Croci, C.A. Radiation-induced enhancement of antioxidant activity in extracts of rosemary (Rosmarinus officinalis L.). Food Chem. 2007, 104, 585–592. [Google Scholar] [CrossRef]
- Marchiosi, R.; Dos Santos, W.D.; Constantin, R.P.; De Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; De Oliveira, D.M.; Foletto-Felipe, M.D.P.; Abrahão, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar] [CrossRef]
- Batish, D.R.; Lavanya, K.; Singh, H.P.; Kohli, R.K. Phenolic allelochemicals released by Chenopodium murale affect the growth, nodulation and macromolecule content in chickpea and pea. Plant Growth Regul. 2007, 51, 119–128. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.-Z.; Yan, Z.-Q.; Guo, H.-R.; Qin, B. Phytotoxicity of umbelliferone and its analogs: Structure–activity relationships and action mechanisms. Plant Physiol. Biochem. 2015, 97, 272–277. [Google Scholar] [CrossRef]
- Ma, T.; Zhou, W.; Chen, L.; Wu, L.; Christie, P.; Liu, W. Toxicity of phthalate esters to lettuce (Lactuca sativa) and the soil microbial community under different soil conditions. PLoS ONE 2018, 13, e0208111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batish, D.R.; Singh, H.P.; Pandher, J.K.; Arora, V.; Kohli, R. Phytotoxic effect of Parthenium residues on the selected soil properties and growth of chickpea and radish. Weed Biol. Manag. 2002, 2, 73–78. [Google Scholar] [CrossRef]
- Li, S.; Peng, F.; Xiao, Y.; Gong, Q.; Bao, Z.; Li, Y.; Wu, X. Mechanisms of High Concentration Valine-Mediated Inhibition of Peach Tree Shoot Growth. Front. Plant Sci. 2020, 11, 603067. [Google Scholar] [CrossRef]
- Han, C.-M.; Pan, K.-W.; Wu, N.; Wang, J.-C.; Li, W. Allelopathic effect of ginger on seed germination and seedling growth of soybean and chive. Sci. Hortic. 2008, 116, 330–336. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Suzuki, M.; Noguchi, K.; Ohno, O.; Suenaga, K.; Laosinwattana, C. A Potent Phytotoxic Substance in Aglaia odorata Lour. Chem. Biodivers. 2016, 13, 549–554. [Google Scholar] [CrossRef]
- Blanco, F.M.G.; Ramos, Y.G.; Scarso, M.F.; Jorge, M.F.S.A.L.A.D.C. Determining the Selectivity of Herbicides and Assessing Their Effect on Plant Roots-A Case Study with Indaziflam and Glyphosate Herbicides; IntechOpen: London, UK, 2015; pp. 275–297. [Google Scholar]
- Aslani, F.; Juraimi, A.S.; Ahmad-Hamdani, M.S.; Omar, D.; Alam, A.; Hashemi, F.S.G.; Hakim, A.; Uddin, M.K. Allelopathic effect of methanol extracts from Tinospora tuberculata on selected crops and rice weeds. Acta Agric. Scand. Sect. B-Plant Soil Sci. 2014, 64, 165–177. [Google Scholar] [CrossRef]
- Mirmostafaee, S.; Azizi, M.; Fujii, Y. Study of Allelopathic Interaction of Essential Oils from Medicinal and Aromatic Plants on Seed Germination and Seedling Growth of Lettuce. Agronomy 2020, 10, 163. [Google Scholar] [CrossRef] [Green Version]
- Wani, A.; Hayat, S.; Ahmad, A.; Tahir, I. Efficacy of brassinosteroid analogues in the mitigation of toxic effects of salt stress in Brassica juncea plants. J. Environ. Biol. 2017, 38, 27–36. [Google Scholar] [CrossRef]
- Burrill, L.C.; Cárdenas, J.; Locatelli, E. Field Manual for Weed Control Research; International Plant Protetion Center, Oregon State University: Corvallis, OR, USA, 1976. [Google Scholar]
- Guiochon, G.; Gritti, F. Shell particles, trials, tribulations and triumphs. J. Chromatogr. A 2011, 1218, 1915–1938. [Google Scholar] [CrossRef]
- Abu Bakar, F.I.; Abu Bakar, M.F.; Abdullah, N.; Endrini, S.; Fatmawati, S. Optimization of Extraction Conditions of Phyto-chemical Compounds and Anti-Gout Activity of Euphorbia hirta L. (Ara Tanah) Using Response Surface Methodology and Liquid Chromatography-Mass Spectrometry (LC-MS) Analysis. Evid. Based Complementary Altern. Med. 2020, 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Dose (g L−1) | Germination (%) | Coleoptile Length (cm) | Radicle Length (cm) |
|---|---|---|---|
| 0.00 | 100.00a (0) | 1.51a (0) | 1.66a (0) |
| 6.25 | 80.00b (20) | 1.20b (20.72) | 1.10b (33.68) |
| 12.5 | 47.00c (53) | 0.86c (43.14) | 0.60c (64.02) |
| 25 | 19.00d (81) | 0.36d (76.24) | 0.24d (85.65) |
| 50 | 0.00e (100) | 0.00e (100) | 0.00e (100) |
| 100 | 0.00e (100) | 0.00e (100) | 0.00e (100) |
| Dose (g L−1) | Plant Height | Leaf Area | Dry Weight |
|---|---|---|---|
| 0 | 64.75a (0) | 151.05a (0) | 5.12a (0) |
| 6.25 | 63.37ab (2.13) | 139.52b (7.63) | 4.89ab (4.46) |
| 12.5 | 62.02ab (4.20) | 132.24c (12.44) | 4.53b (11.44) |
| 25 | 57.42b (11.29) | 115.22d (23.70) | 3.86c (24.55) |
| 50 | 50.31c (22.31) | 91.15e (39.63) | 3.00d (41.20) |
| 100 | 36.00d (44.40) | 72.45f (52.03) | 2.00e (60.81) |
| Dose (g L−1) | Fv/Fm | Photosynthesis Rate | Stomatal Conductance | Transpiration Rate |
|---|---|---|---|---|
| 0 | 1.47a (0) | 45.14a (0) | 0.42a (0) | 11.50a (0) |
| 6.25 | 1.41a (3.90) | 43.50ab (3.64) | 0.41ab (3.43) | 10.83b (5.82) |
| 12.5 | 1.34a (8.56) | 42.50ab (5.86) | 0.40ab (6.04) | 10.41c (9.52) |
| 25 | 1.20ab (17.84) | 40.00b (11.37) | 0.38b (10.07) | 9.35d (18.69) |
| 50 | 1.08ab (26.19) | 35.29c (21.86) | 0.34c (20.31) | 8.20e (28.67) |
| 100 | 0.79b (46.32) | 25.13d (44.41) | 0.25d (39.63) | 6.79f (40.98) |
| Sl. No | RT (min) | Proposed Compound | Molecular Formula | Mass Fragment (m/z) | Polarity |
|---|---|---|---|---|---|
| 1 | 1.436 | Valine | C5H11NO2 | 117.0802 | Positive |
| 2 | 1.418 | Glyceryl sulfoquinovoside | C9H18O10S | 318.063 | Negative |
| 3 | 1.575 | Lotaustralin | C11H19NO6 | 261.1215 | Positive |
| 4 | 3.162 | Trazolopride | C20H23N5O2 | 365.1851 | Positive |
| 5 | 3.571 | Pirenzepine | C19H21N5O2 | 351.1694 | Positive |
| 6 | 3.92 | 1-Cyclopropyl-3-[[1-(4-hydroxybutyl)benzimidazol-2-yl]methyl]imidazo [4,5-c]pyridin-2-one | C21H23N5O2 | 377.1848 | Positive |
| 7 | 4.239 | Umbelliferone | C9H6O3 | 162.0317 | Positive |
| 8 | 4.244 | Quinic Acid | C7H12O6 | 192.0638 | Negative |
| 9 | 4.941 | Atevirdine | C21H25N5O2 | 379.2002 | Positive |
| 10 | 5.253 | Dihydrophaseic acid 4-O-beta-D-glucoside | C21H32O10 | 444.1998 | Negative |
| 11 | 5.536 | 2-(2-Ethoxyethoxy)ethanol;4-methylbenzenesulfonic acid | C13H22O6S | 306.1136 | Negative |
| 12 | 5.475 | 4-Azidobenzyl benzyl 1,4-butanediylbiscarbamate | C20H23N5O4 | 397.175 | Positive |
| 13 | 5.823 | 4-(N-hydroxyamino)-2r-isobutyl-2S-(2-Thienylthiomethyl)succinyl-L-Phenylalanine-N-Methylamide | C20H31NO3S2 | 397.176 | Positive |
| 14 | 6.08 | Branaplam | C22H27N5O2 | 393.2162 | Positive |
| 15 | 6.257 | Pulchellamine G | C21H31N O6 | 393.2151 | Positive |
| 16 | 6.503 | Hymenoxynin | C21H34O9 | 430.2208 | Negative |
| 17 | 6.939 | Chlorogenic acid | C16H18O9 | 354.0957 | Negative |
| 18 | 7.006 | Parthenin | C15H18O4 | 262.1202 | Positive |
| 19 | 7.006 | Gaillardilin | C17H22O6 | 322.1415 | Positive |
| 20 | 7.006 | Dehydroleucodine | C15H16O3 | 244.1095 | Positive |
| 21 | 7.264 | N-Propyl-3-(1,3-thiazol-2-yl)thian-3-amine | C11H18N2S2 | 242.0928 | Positive |
| 22 | 7.266 | Oleacein | C17H20O6 | 320.1252 | Positive |
| 23 | 7.49 | Bendazac lysine | C22H28N4O5 | 428.2053 | Negative |
| 24 | 7.641 | Lajollamide A | C30H55N5O5 | 565.4206 | Positive |
| 25 | 7.673 | Isochlorogenic acid A | C25H24O12 | 516.127 | Negative |
| 26 | 7.673 | Chlorogenic acid | C16H18O9 | 354.0958 | Negative |
| 27 | 7.897 | 4-[(6-Chloro-2-naphthalenyl)sulfonyl]-1-[[1-(4-pyridinyl)-4-piperidinyl]methyl]-2 piperazinecarboxylic acid | C27H41ClN4O6 | 552.2699 | Positive |
| 28 | 7.905 | N-Chloro-9-(diaminomethylideneamino)-3-hydroxynonanamide | C10H21ClN4O2 | 264.1358 | Positive |
| 29 | 7.908 | 1-(N-6-Amino-n-hexyl)carbamoylimidazole | C10H19ClN4O | 246.1253 | Positive |
| 30 | 8.042 | 2,4-Toluene Diisocyanate Dimer | C18H12N4O4 | 348.0862 | Positive |
| 31 | 8.044 | Alaptide | C9H14N2O2 | 182.1063 | Positive |
| 32 | 8.05 | Carbocyclic-3′-amino-ara-adenosine | C11H16N6O2 | 264.1339 | Positive |
| 33 | 8.054 | Tris(pyrazolyl)ethane | C11H12N6 | 228.1118 | Positive |
| 34 | 8.055 | Descyclopropyl Abacavir | C11H14N6O | 246.1225 | Positive |
| 35 | 8.058 | 1-Boc-3-oxopiperazine | C9H16N2O3 | 200.1162 | Positive |
| 36 | 8.13 | Teroxalene hydrochloride | C28H42Cl2N2OS | 524.2364 | Positive |
| 37 | 8.132 | Ethane;(3-oxo-6′-sulfanylcarbonyloxyspiro [2 -benzofuran-1,9′-xanthene]-3′-yl)oxymethanethioicS-acid;propane | C31H38O7S2 | 586.206 | Positive |
| 38 | 8.133 | (2-Aminoethylamino) 2,2-diaminooxyacetate | C4H12N4O4 | 180.0845 | Positive |
| 39 | 8.134 | N-[(S)-2-Benzo[1,3]dioxol-5-yl-4-(4-phenyl-piperidin-1-yl)-butyl]-N-methyl-benzenesulfonamide | C29H34N2O4S | 506.2237 | Positive |
| 40 | 8.135 | 3-Diazo-1-hexylsulfanyl-1-methylurea | C8H16N4OS | 216.1055 | Positive |
| 41 | 8.135 | Ethylene oxide-b-maleic hydrazide | C6H12N8O3 | 244.103 | Positive |
| 42 | 8.136 | N-[3-(1H-Imidazol-4-yl)propyl]-N′-methylthiourea | C8H14N4S | 198.0952 | Positive |
| 43 | 8.136 | 1-Methylpiperazine-1,4-Diium Bis | C5H14N4O6 | 226.0914 | Positive |
| 44 | 8.136 | 3-(2-Methylpropylthio)-1H-1,2,4-triazol-5-amine | C6 H12N4S | 172.0801 | Positive |
| 45 | 8.136 | Benzylamidinoisothiourea | C9H12N4S | 208.0792 | Positive |
| 46 | 8.136 | 1-Amino-3-(propylamino)thiourea | C4H12N4S | 148.0798 | Positive |
| 47 | 8.136 | 9-hydroxyellipticine | C17H14N2O | 262.1122 | Positive |
| 48 | 8.136 | 4-Phenylamino-3-quinolinecarbonitrile deriv. 28 | C27H30Cl2N4O4 | 544.16 | Positive |
| 49 | 8.136 | 1-(3-ethyl-1,2,4-thiadiazol-5-yl)azetidin-3-amine | C7H12N4S | 184.0793 | Positive |
| 50 | 8.413 | 1,8,15,22,29,36-Hexaazacyclodotetracontane-2,7,16,21,30,35-hexone | C36H66N6O6 | 678.504 | Positive |
| 51 | 8.415 | 2,4,6-tris(3-methylbutoxy)-1,3,5-triazine | C18H33N3O3 | 339.2522 | Positive |
| 52 | 8.435 | Arginyl-tyrosyl-aspartic acid | C19H28N6O7 | 452.2022 | Positive |
| 53 | 8.636 | 8-(2,4,6-Trimethoxyphenyl)-9H-purine-2,6-diamine | C14H16N6O3 | 316.1282 | Positive |
| 54 | 8.818 | Dimethyl 2-(heptane-1-sulfonyl)butanedioate | C13H24O6S | 308.1298 | Negative |
| 55 | 8.721 | AC-Ala-gln-ala-pna | C19H26 N6O7 | 450.1864 | Positive |
| 56 | 9.065 | Laciniatin | C17H14O8 | 346.0693 | Positive |
| 57 | 9.067 | 2-[(3,5-Dinitrobenzoyl)amino]benzoic acid | C14H9N3O7 | 331.0461 | Negative |
| 58 | 9.243 | 3-Ethyl-1-propyl-8-(1H-pyrazol-4-yl)-1H-purine-2,6(3H,7H)-dione | C13H16N6O2 | 288.134 | Positive |
| 59 | 11.645 | Apnea | C18H22N6O4 | 386.1696 | Positive |
| 60 | 11.844 | Thyroliberin N-ethylamide | C18H26N6O4 | 390.2011 | Positive |
| 61 | 11.996 | Hexadecasphinganine | C16H35NO2 | 273.2672 | Positive |
| 62 | 12.034 | Phytosphingosine | C18H39NO3 | 317.2935 | Positive |
| 63 | 12.176 | Dihydroxyethyllauramine oxide | C16H35NO3 | 289.262 | Positive |
| 64 | 12.193 | Lauramine oxide | C14H31NO | 229.2405 | Positive |
| 65 | 12.308 | Rishitin | C14H22O2 | 222.161 | Negative |
| 66 | 12.316 | Dioctylnitrosamine | C16H34N2O | 270.2673 | Positive |
| 67 | 12.343 | Dodecylacrylamide | C15H29NO | 239.2251 | Positive |
| 68 | 12.349 | Tetrabutylurea | C17H36N2O | 284.2832 | Positive |
| 69 | 12.703 | Aminopregnane | C21H37N | 303.2934 | Positive |
| 70 | 12.778 | Tridecylglycerol | C16H34O3 | 274.2512 | Positive |
| 71 | 13.164 | 2,3,3-Tris(1,2-diaminoethyl)-2-ethylhexanoic acid | C14H34N6O2 | 318.2769 | Positive |
| 72 | 13.633 | 4-dodecylbenzenesulfonic acid | C18H30O3S | 326.1916 | Negative |
| 73 | 14.691 | Angoletin | C18H20O4 | 300.1357 | Positive |
| 74 | 14.694 | Phthalic anhydride | C8H4O3 | 148.069 | Positive |
| 75 | 15.406 | Eicosasphinganine | C20H43NO2 | 329.3298 | Positive |
| 76 | 16.483 | Lauryl sulfate | C12H26O4S | 266.1551 | Negative |
| 77 | 16.957 | Dodecandial-disemicarbazon | C14H28N6O2 | 312.2282 | Positive |
| 78 | 18.267 | Benzenesulfonic acid, tridecyl- | C19H32O3S | 340.2072 | Negative |
| 79 | 19.135 | 3-[5-(3-Dimethylamino-1,2,4-thiadiazol)-yl] quinuclidine | C11H18N4S | 238.125 | Positive |
| 80 | 19.496 | Benzenesulfonic acid, undecyl- | C17H28O3S | 312.176 | Negative |
| 81 | 19.918 | N,N-bis(2-hydroxyethyl)stearylamine | C22H47NO2 | 357.3609 | Positive |
| 82 | 20.245 | Benzoyl benzenecarboperoxoate;dodecane-1-thiol;toluene | C33H44O4S | 536.2965 | Positive |
| Tested Weeds | P. hysterophorus | Synthetic Herbicides | |||||
|---|---|---|---|---|---|---|---|
| 0 g L−1 | 20 g L−1 | 40 g L−1 | 80 g L−1 | Glyphosate | Glufosinate-Ammonium | ||
| A. conyzoides | 1.00d | 2.75c | 5.50b | 9.00a | 9.00a | 9.00a | |
| Visual injury (Scale) | C. iria | 1.00e | 2.50d | 4.00c | 5.25b | 9.00a | 9.00a |
| O. sativa | 1.00e | 2.25d | 3.00c | 4.50b | 9.00a | 9.00a | |
| A. conyzoides | 32.00a (0) | 24.62b (23.02) | 14.62c (54.32) | 0.00d (100) | 0.00d (100) | 0.00d (100) | |
| Plant height (cm) | C. iria | 64.75a (0) | 55.75b (13.58) | 44.25c (37.71) | 37.00d (42.97) | 0.00e (100) | 0.00e (100) |
| O. sativa | 67.00a (0) | 58.50b (12.68) | 49.50c (26.08) | 39.53d (41.02) | 0.00e (100) | 0.00e (100) | |
| A. conyzoides | 26.45a (0) | 18.34b (30.66) | 3.14c (88.10) | 0.45d (98.28) | 0.22d (99.17) | 0.27d (98.96) | |
| Fresh weight (g pot−1) | C. iria | 25.95a (0) | 20.21b (22.10) | 15.70c (39.45) | 12.80d (50.60) | 0.30e (98.86) | 0.50e (98.08) |
| O. sativa | 12.70a (0) | 8.89b (29.97) | 6.99c (44.93) | 5.44d (57.13) | 0.14e (98.92) | 0.19e (98.48) | |
| A. conyzoides | 5.13a (0) | 3.04b (40.78) | 0.50c (90.36) | 0.07c (98.63) | 0.03c (99.42) | 0.05c (99.08) | |
| Dry weight (g pot−1) | C. iria | 6.29a (0) | 4.95b (21.12) | 3.98c (36.53) | 2.28d (63.80) | 0.06e (98.97) | 0.10e (98.43) |
| O. sativa | 3.36a (0) | 2.25b (32.27) | 1.75bc (47.49) | 1.24c (62.76) | 0.03d (99.05) | 0.04d (98.77) | |
| Scale | Injury (%) | Effects on Weeds |
|---|---|---|
| 1 | 0 | No effect (all foliage green and alive) |
| 2 | 1–10 | Very light symptoms |
| 3 | 11–30 | Light symptoms |
| 4 | 31–49 | Symptoms not reflected in yield |
| 5 | 50 | Medium |
| 6 | 51–70 | Fairly heavy damage |
| 7 | 71–90 | Heavy damage |
| 8 | 91–99 | Very heavy damage |
| 9 | 100 | Complete kill (dead) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motmainna, M.; Juraimi, A.S.; Uddin, M.K.; Asib, N.B.; Islam, A.K.M.M.; Ahmad-Hamdani, M.S.; Hasan, M. Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds. Plants 2021, 10, 1445. https://doi.org/10.3390/plants10071445
Motmainna M, Juraimi AS, Uddin MK, Asib NB, Islam AKMM, Ahmad-Hamdani MS, Hasan M. Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds. Plants. 2021; 10(7):1445. https://doi.org/10.3390/plants10071445
Chicago/Turabian StyleMotmainna, Mst., Abdul Shukor Juraimi, Md. Kamal Uddin, Norhayu Binti Asib, A. K. M. Mominul Islam, Muhammad Saiful Ahmad-Hamdani, and Mahmudul Hasan. 2021. "Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds" Plants 10, no. 7: 1445. https://doi.org/10.3390/plants10071445
APA StyleMotmainna, M., Juraimi, A. S., Uddin, M. K., Asib, N. B., Islam, A. K. M. M., Ahmad-Hamdani, M. S., & Hasan, M. (2021). Phytochemical Constituents and Allelopathic Potential of Parthenium hysterophorus L. in Comparison to Commercial Herbicides to Control Weeds. Plants, 10(7), 1445. https://doi.org/10.3390/plants10071445







