Point Mutations and Cytochrome P450 Can Contribute to Resistance to ACCase-Inhibiting Herbicides in Three Phalaris Species
Abstract
1. Introduction
2. Results
2.1. Dose Response Assay
2.2. 14C-DM Metabolism
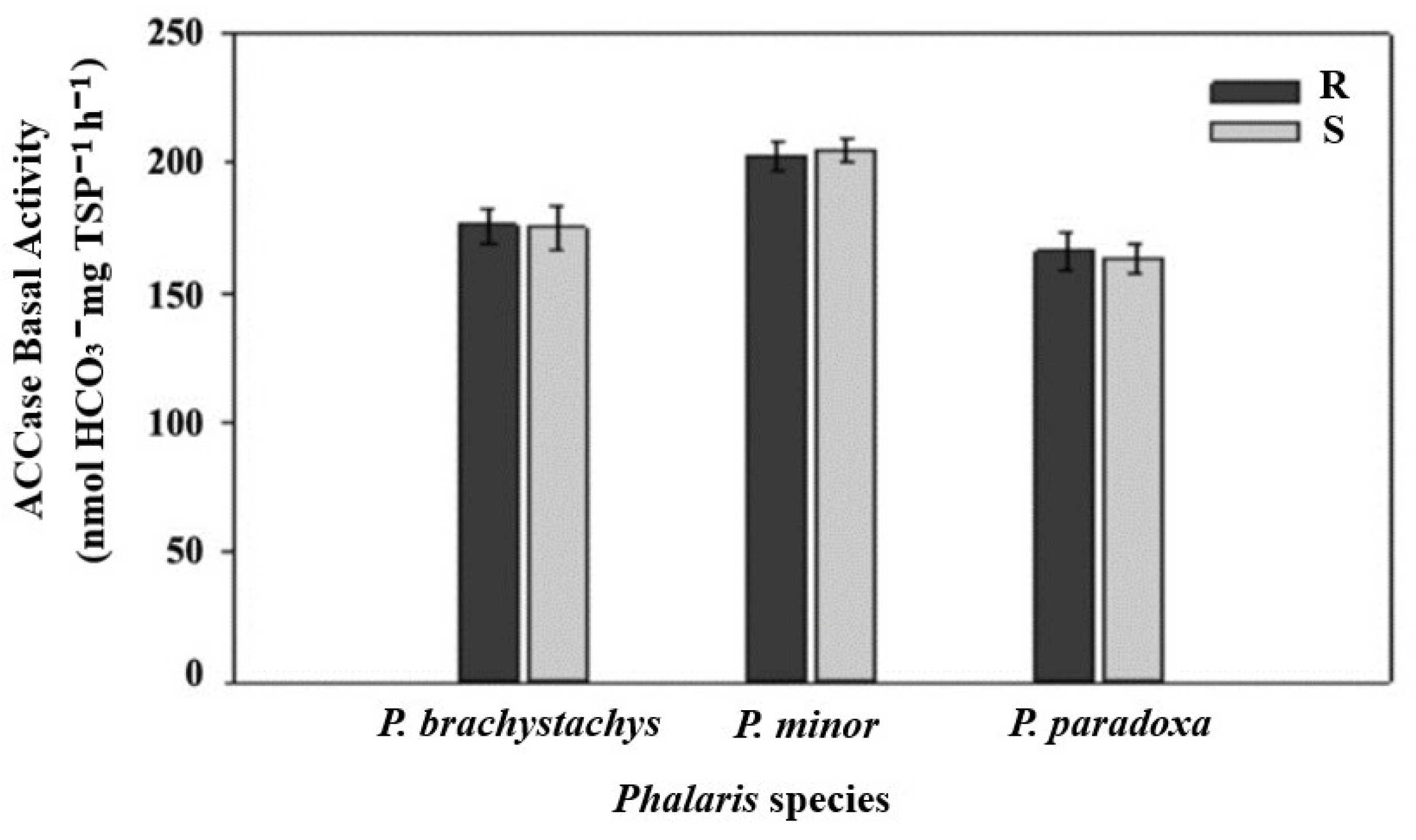
2.3. ACCase Enzyme Activity Assay
2.4. Molecular Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Dose–Response Assays
4.3. 14C-DM Metabolism
4.4. ACCase Enzyme Activity Assay
4.5. Molecular Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alcantara, C.; Jimenez-Hidalgo, M.J.; Saavedra, M. Responses of Phalaris minor Rezt. and Phalaris brachystachys to different levels of soil water availability. Span. J. Agric. Res. 2010, 8, 1074–1083. [Google Scholar] [CrossRef]
- Keshavarzi, M.; Khaksar, M.; Ghadam, P. Biosystematic study of Phalaris L. species (Poaceae) in Iran. Taxon. Biosyst. 2013, 4, 25–30. [Google Scholar]
- Mirkamali, H. Control of Phalaris brachystachys and Phalaris minor in wheat grown in northern Iran. Proc. Br. Crop Prot. Conf. Weeds 1987, 2, 407–412. [Google Scholar]
- Heap, I. International Survey of Herbicide Resistant Weeds. 2021. Available online: http://www.weedscience.org/ (accessed on 15 July 2021).
- Gherekhloo, J.; Rashed Mohassel, M.H.; Mahalati, M.N.; Zand, E.; Ghanbari, A.; Osuna, M.D.; De Prado, R. Confirmed resistance to aryloxyphenoxypropionate herbicides in Phalaris minor populations in Iran. Weed Biol. Manag. 2011, 11, 29–37. [Google Scholar] [CrossRef]
- Oad, F.C.; Siddiqui, M.H.; Buriro, U.A. Growth and yield losses in wheat due to different weed densities. Asian J. Plant Sci. 2007, 6, 173–176. [Google Scholar] [CrossRef]
- Siddiqui, I.; Bajwa, R.; Huma, Z.E.; Javaid, A. Effect of six problematic weeds on growth and yield of wheat. Pak. J. Bot. 2010, 42, 2461–2471. [Google Scholar]
- Gherekhloo, J.; Alcántara-de la Cruz, R.; Osuna, M.D.; Sohrabi, S.; De Prado, R. Assessing genetic variation and spread of Phalaris minor resistant to ACCase inhibiting herbicides in Iran. Planta Daninha 2020, 38. [Google Scholar] [CrossRef]
- Délye, C.; Zhang, X.Q.; Michel, S.; Matéjicek, A.; Powles, S.B. Molecular bases for sensitivity to Acetyl-Coenzyme A Carboxylase inhibitors in black-grass. Plant Physiol. 2005, 137, 794–806. [Google Scholar] [CrossRef]
- Powles, S.B.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Petit, C.; Duhieu, B.; Boucansaud, K.; Délye, C. Complex genetic control of non-target-site-based resistance to herbicides inhibiting Acetyl-Coenzyme A Carboxylase and Acetolactate-Synthase in Alopecurus myosuroides Huds. Plant Sci. 2010, 178, 501–509. [Google Scholar] [CrossRef]
- Du, L.; Liu, W.; Yuan, G.; Guo, W.; Li, Q.; Wang, J. Cross-resistance patterns to ACCase-inhibitors in American sloughgrass (Beckmannia syzigachne Steud.) homozygous for specific ACCase mutations. Pestic. Biochem. Physiol. 2016, 126, 42–48. [Google Scholar] [CrossRef]
- Gherekhloo, J.; Oveisi, M.; Zand, E.; De Prado, R. A review of herbicide resistance in Iran. Weed Sci. 2016, 64, 551–561. [Google Scholar] [CrossRef]
- Sasanfar, H.; Zand, E.; Baghestani, M.A.; Mirhadi, M.J.; Mesgaran, M.B. Cross-resistance patterns of winter wild oat (Avena ludoviciana) populations to ACCase inhibitor herbicides. Phytoparasitica 2017, 45, 419–428. [Google Scholar] [CrossRef]
- Zangeneh, H.S.; Chamanabad, H.R.M.; Zand, E.; Alcántara-de la Cruz, R.; Travlos, I.S.; De Prado, R.; Alebrahim, M.T. Clodinafop-propargyl resistance genes in Lolium rigidum Guad. populations are associated with fitness costs. Agronomy 2018, 8, 106. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Fernandez, P.; Alcantara, R.; Gherekhloo, J.; Osuna, M.D.; de Prado, R. Ile-1781-Leu and Asp-2078-Gly mutations in ACCase gene, endow cross-resistance to APP, CHD, and PPZ in Phalaris minor from Mexico. Int. J. Mol. Sci. 2015, 14, 415–421. [Google Scholar] [CrossRef] [PubMed]
- De Prado, J.L.; Osuna, M.D.; Heredia, A.; De Prado, R. Lolium rigidum, a pool of resistance mechanisms to ACCase inhibitor herbicides. J. Agric. Food Chem. 2005, 53, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Kaundun, S.S. Resistance to acetyl-CoA carboxylase-inhibiting herbicides. Pest Manag. Sci. 2014, 70, 1405–1417. [Google Scholar] [CrossRef]
- Yu, Q.; Collavo, A.; Zheng, M.Q.; Owen, M.; Sattin, M.; Powles, S.B. Diversity of acetyl-coenzyme A carboxylase mutations in resistant Lolium populations: Evaluation using clethodim. Plant Physiol. 2007, 145, 547–558. [Google Scholar] [CrossRef]
- Raghav, N.; Singh, R.; Chhokar, R.S.; Sharma, D.; Kumar, R. Mutations in the plastidic ACCase gene endowing resistance to ACCase-inhibiting herbicide in Phalaris minor populations from India. 3 Biotech 2016, 6, 12. [Google Scholar] [CrossRef][Green Version]
- Laforest, M.; Soufiane, B.; Simard, M.J.; Obeid, K.; Page, E.; Nurse, R.E. Acetyl-CoA carboxylase overexpression in herbicide-resistant large crabgrass (Digitaria sanguinalis). Pest Manag. Sci. 2017, 73, 2227–2235. [Google Scholar] [CrossRef]
- Hochberg, O.; Sibony, M.; Rubin, B. The response of ACCase-resistant Phalaris paradoxa populations involves two different target site mutations. Weed Res. 2009, 49, 37–46. [Google Scholar] [CrossRef]
- Gherekhloo, J.; Osuna, M.D.; De Prado, R. Biochemical and molecular basis of resistance to ACCase-inhibiting herbicides in Iranian Phalaris minor populations. Weed Res. 2012, 52, 367–372. [Google Scholar] [CrossRef]
- Golmohammadzadeh, S.; Rojano-Delgado, A.M.; Vázquez-García, J.G.; Romano, Y.; Osuna-Ruíz, M.D.; Gherekhloo, J.; De Prado, R. Cross-resistance mechanisms to ACCase-inhibiting herbicides in short-spiked canarygrass (Phalaris brachystachys). Plant Phys. Biochem. 2020, 151, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Zand, E.; Baghestani, M.A.; AghaAlikhani, M.; Soufizadeh, S.; Khayami, M.M.; PourAzar, R.; Sabeti, P.; Jamali, M.; Bagherani, N.; Forouzesh, S. Chemical control of weeds in wheat (Triticum aestivum L.) in Iran. Crop Prot. 2010, 29, 1223–1231. [Google Scholar] [CrossRef]
- Collavo, A.; Panozzo, S.; Lucchesi, G.; Scarabel, L.; Sattin, M. Characterisation and management of Phalaris paradoxa resistant to ACCase-inhibitors. Crop Prot. 2011, 30, 293–299. [Google Scholar] [CrossRef]
- Cruz-Hipolito, H.; Domínguez-Valenzuela, J.A.; Osuna, M.D.; De Prado, R. Resistance mechanism to acetyl coenzyme A carboxylase inhibiting herbicides in Phalaris paradoxa collected in Mexican wheat fields. Plant Soil 2012, 355, 121–130. [Google Scholar] [CrossRef]
- Yuan, J.S.; Tranel, P.J.; Stewart, C.N. Non-target-site herbicide resistance: A family business. Trends Plant Sci. 2007, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Owen, M.J.; Goggin, D.E.; Powles, S.B. Non-target-site-based resistance to ALS-inhibiting herbicides in six Bromus rigidus populations from Western Australian cropping fields. Pest Manag. Sci. 2012, 68, 1077–1082. [Google Scholar] [CrossRef]
- Hatami, Z.M.; Gherekhloo, J.; Rojano-Delgado, A.M.; Osuna, M.D.; Alcántara, R.; Fernández, P.; Sadeghipour, H.R.; de Prado, R. Multiple mechanisms increase levels of resistance in Rapistrum rugosum to ALS herbicides. Front. Plant Sci. 2016, 7, 169. [Google Scholar] [CrossRef]
- Ghanizadeh, H.; Harrington, K.C. Non-target site mechanisms of resistance to herbicides. Crit. Rev. Plant Sci. 2017, 36, 24–34. [Google Scholar] [CrossRef]
- Nakka, S.; Jugulam, M.; Peterson, D.; Asif, M. Herbicide resistance: Development of wheat production systems and current status of resistant weeds in wheat cropping systems. Crop J. 2019, 7, 750–760. [Google Scholar] [CrossRef]
- Fernández, P.; Alcántara de la Cruz, R.; Cruz-Hipólito, H.; Osuna, M.D.; De Prado, R. Underlying resistance mechanisms in the Cynosurus echinatus biotype to acetyl CoA carboxylase-inhibiting herbicides. Front. Plant Sci. 2016, 7, 449. [Google Scholar] [CrossRef]
- Preston, C.; Tardif, F.J.; Christopher, J.T.; Powles, S.B. Multiple resistance to dissimilar herbicide chemistries in a biotype of Lolium rigidum due to enhanced activity of several herbicide degrading enzymes. Pestic. Biochem. Physiol. 1996, 54, 123–134. [Google Scholar] [CrossRef]
- Letouzé, A.; Gasquez, J. Enhanced activity of several herbicide-degrading enzymes: A suggested mechanism responsible for multiple resistance in blackgrass (Alopecurus myosuroides Huds.). Agronomie 2003, 23, 601–608. [Google Scholar] [CrossRef]
- Yun, M.S.; Yogo, Y.; Miura, R.; Yamasue, Y.; Fischer, A.J. Cytochrome P-450 monooxygenase activity in herbicide-resistant and -susceptible late watergrass (Echinochloa phyllopogon). Pestic. Biochem. Physiol. 2005, 83, 107–114. [Google Scholar] [CrossRef]
- Jugulam, M.; Shyam, C. Non-target-site resistance to herbicides: Recent developments. Plants 2019, 8, 417. [Google Scholar] [CrossRef]
- Liu, W.; Harrison, D.K.; Chalupska, D.; Gornicki, P.; O’Donnell, C.C.; Adkins, S.W.; Haselkorn, R.; Williams, R.R. Single-site mutations in the carboxyltransferase domain of plastid acetyl-CoA carboxylase confer resistance to grass-specific herbicides. Proc. Natl. Acad. Sci. USA 2007, 104, 3627–3632. [Google Scholar] [CrossRef]
- Délye, C.; Matéjicek, A.; Michel, S. Cross-resistance patterns to ACCase-inhibiting herbicides conferred by mutant ACCase isoforms in Alopecurus myosuroides Huds. (black-grass), re-examined at the recommended herbicide field rate. Pest Manag. Sci. 2008, 64, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Gronwald, J.W.; Eberlein, C.V.; Betts, K.J.; Baerg, R.J.; Ehlke, N.J.; Wyse, D.L. Mechanism of diclofop resistance in an Italian ryegrass (Lolium multiflorum Lam.). Pestic. Biochem. Physiol. 1992, 44, 126–139. [Google Scholar] [CrossRef]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R Software Package for Dose–response Studies: The Concept and Data Analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]

| Herbicides | Species | Biotype | d | b | GR50 (g ai ha−1) | RF a |
|---|---|---|---|---|---|---|
| Diclofop-methyl (FOP) | P. brachystachys | R | 99.04 ± 2.09 | 3.66 ± 0.79 | 1336.45 ± 109.29 | 10.31 ± 0.68 |
| S | 98.13 ± 2.10 | 1.21 ± 0.57 | 129.56 ± 7.52 | - | ||
| P. minor | R | 95.21 ± 3.09 | 3.85 ± 0.77 | 1832.34 ± 103.98 | 7.48 ± 0.73 | |
| S | 96.18 ± 3.81 | 2.88 ± 0.75 | 244.90 ± 19.71 | - | ||
| P. paradoxa | R | 95.69 ± 11.15 | 2.74 ± 0.93 | 2831.61 ± 129.18 | 11.87 ± 1.57 | |
| S | 92.17 ± 7.43 | 1.63 ± 0.45 | 238.62 ± 31.87 | |||
| Cyclocydim (DIM) | P. brachystachys | R | 99.94 ± 2.14 | 1.73 ± 0.49 | 39.61 ± 2.12 | 4.48 ± 0.25 |
| S | 95.48 ± 1.10 | 1.42 ± 0.57 | 8.84 ± 0.95 | - | ||
| P. minor | R | 97.3 ± 5.55 | 1.77 ± 0.30 | 198.44 ± 25.13 | 19.65 ± 1.89 | |
| S | 98.98 ± 5.73 | 1.33 ± 0.19 | 10.10 ± 1.63 | |||
| P. paradoxa | R | 96.19 ± 4.44 | 2.09 ± 0.37 | 236.85 ± 22.67 | 24.05 ± 2.74 | |
| S | 99.44 ± 4.67 | 1.47 ± 0.18 | 9.85 ± 1.21 | - | ||
| Pinoxaden (DEN) | P. brachystachys | R | 98.50 ± 4.74 | 0.69 ± 0.11 | 41.02 ± 3.89 | 5.38 ± 0.74 |
| S | 99.71 ± 2.57 | 1.23 ± 0.46 | 7.63 ± 0.41 | - | ||
| P. minor | R | 97.21 ± 5.88 | 1.48 ± 0.25 | 56.87 ± 9.25 | 6.81 ± 1.58 | |
| S | 96.87 ± 9.25 | 1.34 ± 0.21 | 8.35 ± 1.38 | - | ||
| P. paradoxa | R | 96.81 ± 4.56 | 1.72 ± 0.37 | 116.95 ± 17.28 | 17.12 ± 1.62 | |
| S | 97.37 ± 6.04 | 1.63 ± 0.28 | 6.83 ± 1.03 | - |
| Phalaris Species | % Extracted Radioactivity | |||||
|---|---|---|---|---|---|---|
| DM | D-Acid | D-Conjugates | ||||
| R | S | R | S | R | S | |
| P. brachystachys† | 12.53 ± 2.13 b | 26.72 ± 2.48 a | 22.43 ± 1.83 b | 67.50 ± 3.17 a | 65.12 ± 3.42 a | 16.83 ± 1.31 a |
| P. brachystachys‡ | 30.41 ± 4.24 a | 28.71 ± 3.52 a | 59.87 ± 1.05 a | 58.65 ± 2.19 a | 16.80 ± 0.81 b | 15.61 ± 0.54 a |
| P. minor† | 33.33 ± 1.45 b | 35.18 ± 1.73 a | 55.33 ± 4.33 a | 56.66 ± 2.60 a | 11.33 ± 2.90 b | 12.37 ± 2.40 a |
| P. minor‡ | 34.33 ± 2.02 b | 33.33 ± 1.45 a | 62.04 ± 2.30 a | 62.13 ± 1.52 a | 12.66 ± 1.45 b | 11.33 ± 0.66 a |
| P. paradoxa† | 29.11 ± 0.47 b | 32.33 ± 2.22 a | 57.33 ± 2.37 a | 56.33 ± 1.18 a | 16.43 ± 1.88 b | 14.56 ± 1.08 a |
| P. paradoxa‡ | 29.66 ± 1.18 b | 30.66 ± 1.18 a | 67.66 ± 1.65 a | 66.66 ± 0.72 a | 13.66 ± 0.54 b | 12.39 ± 0.82 a |
| Herbicide | Species | Biotype | d | b | I50 (µM) | RF a |
|---|---|---|---|---|---|---|
| Diclofop-methyl (FOP) | P. brachystachys | R | 99.64 ± 0.83 | 0.67 ± 0.03 | 6.65 ± 0.78 | 20.78 |
| S | 101.99 ± 0.23 | 0.72 ± 0.02 | 0.32 ± 0.05 | - | ||
| P. minor | R | 94.90 ± 3.68 | 0.56 ± 0.09 | 11.04 ± 4.57 | 12.99 | |
| S | 96.49 ± 4.56 | 0.49 ± 0.07 | 0.85 ± 0.03 | - | ||
| P. paradoxa | R | 93.98 ± 3.46 | 0.55 ± 0.08 | 9.73 ± 3.80 | 16.37 | |
| S | 97.91 ± 4.26 | 0.48 ± 0.06 | 0.59 ± 0.23 | - | ||
| Cyclocydim (DIM) | P. brachystachys | R | 103.11 ± 1.40 | 0.52 ± 0.03 | 2.40 ± 0.34 | 10.91 |
| S | 100.53 ± 1.58 | 0.69 ± 0.04 | 0.22 ± 0.02 | - | ||
| P. minor | R | 96.98 ± 2.90 | 1.76 ± 0.43 | 3.46 ± 1.87 | 5.16 | |
| S | 97.87 ± 2.81 | 0.98 ± 0.57 | 0.67 ± 0.91 | - | ||
| P. paradoxa | R | 98.76 ± 0.76 | 0.87 ± 0.32 | 2.86 ± 0.98 | 6.21 | |
| S | 96.67 ± 0.67 | 1.11 ± 0.12 | 0.46 ± 0.06 | - | ||
| Pinoxaden (DEN) | P. brachystachys | R | 100.60 ± 1.40 | 0.90 ± 0.09 | 14.43 ± 1.90 | 18.50 |
| S | 102.93 ± 2.22 | 0.62 ± 0.06 | 0.78 ± 0.01 | |||
| P. minor | R | 97.76 ± 2.76 | 0.78 ± 0.98 | 8.09 ± 2.80 | 13.71 | |
| S | 96.56 ± 1.98 | 0.87 ± 0.19 | 0.59 ± 0.07 | - | ||
| P. paradoxa | R | 97.87 ± 2.98 | 0.76 ± 0.09 | 12.78 ± 2.87 | 19.36 | |
| S | 95.56 ± 3.98 | 1.09 ± 0.09 | 0.66 ± 0.09 | - |
| Herbicides | Rate (g a.i. ha−1) | |
|---|---|---|
| Biotype S | Biotype R | |
| Diclofop-methyl a | 0, 45, 90, 180, 360, 720, 1080 | 0, 1000, 1500, 2000, 3000, 3500, 4000, |
| Cycloxydim b | 0, 5, 10, 20, 40, 60, 100, 200 | 0, 50, 100, 200, 300, 400, 800,1200 |
| Pinoxaden c | 0, 4, 8, 16, 32, 64, 128, 256 | 0, 25, 50, 100, 200, 400, 600, 800 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-García, J.G.; Torra, J.; Palma-Bautista, C.; Alcántara-de la Cruz, R.; Prado, R.D. Point Mutations and Cytochrome P450 Can Contribute to Resistance to ACCase-Inhibiting Herbicides in Three Phalaris Species. Plants 2021, 10, 1703. https://doi.org/10.3390/plants10081703
Vázquez-García JG, Torra J, Palma-Bautista C, Alcántara-de la Cruz R, Prado RD. Point Mutations and Cytochrome P450 Can Contribute to Resistance to ACCase-Inhibiting Herbicides in Three Phalaris Species. Plants. 2021; 10(8):1703. https://doi.org/10.3390/plants10081703
Chicago/Turabian StyleVázquez-García, José G., Joel Torra, Candelario Palma-Bautista, Ricardo Alcántara-de la Cruz, and Rafael De Prado. 2021. "Point Mutations and Cytochrome P450 Can Contribute to Resistance to ACCase-Inhibiting Herbicides in Three Phalaris Species" Plants 10, no. 8: 1703. https://doi.org/10.3390/plants10081703
APA StyleVázquez-García, J. G., Torra, J., Palma-Bautista, C., Alcántara-de la Cruz, R., & Prado, R. D. (2021). Point Mutations and Cytochrome P450 Can Contribute to Resistance to ACCase-Inhibiting Herbicides in Three Phalaris Species. Plants, 10(8), 1703. https://doi.org/10.3390/plants10081703









