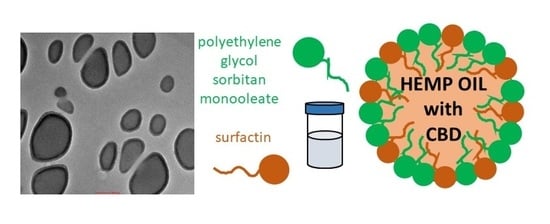
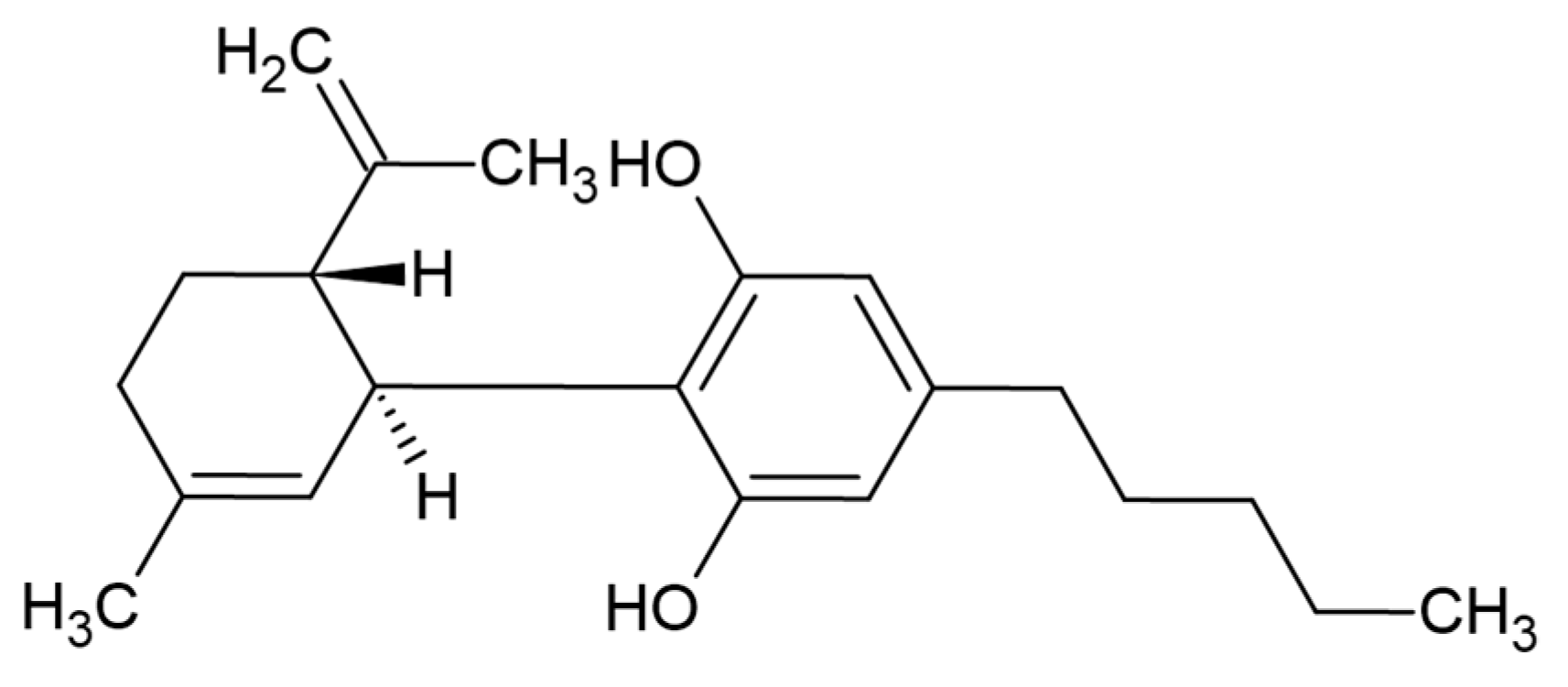
Optimizing the Process Design of Oil-in-Water Nanoemulsion for Delivering Poorly Soluble Cannabidiol Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Nanoemulsion Preparation
2.3. Nanoemulsion Characterization Methods
2.4. Cell Culture
2.5. In Vivo Skin Contact Study
2.6. Statistical Analysis
3. Results
4. Biological Skin Response In Vitro and In Vivo
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Morales, P.; Reggio, P.H. CBD: A New Hope. ACS Med. Chem. Lett. 2019, 694–695. [Google Scholar] [CrossRef] [Green Version]
- McPartland, J.M.; Duncan, M.; Di Marzo, V.; Pertwee, R.G. Are cannabidiol and Δ9-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol. 2015, 172, 737–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duran, M.; Pérez, E.; Abanades, S.; Vidal, X.; Saura, C.; Majem, M.; Arriola, E.; Rabanal, M.; Pastor, A.; Farré, M.; et al. Preliminary efficacy and safety of an oromucosal standardized cannabis extract in chemotherapy-induced nausea and vomiting. Br. J. Clin. Pharmacol. 2010, 70, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.S.; Nogueira Derenusson, G.; Borduqui Ferrari, T.; Wichert-Ana, L.; Duran, F.L.S.; Martin-Santos, R.; Vinícius Simões, M.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef] [PubMed]
- Al Jourdi, H.; Popescu, C.; Udeanu, D.I.; Arsene, A.; Sevastre, A.N.I.; Velescu, B. Ștefan; Lupuliasa, D. Comparative study regarding the physico-chemical properties and microbiological activities of olea europaea l. Oil and cannabis sativa l. seed oil obtained by cold pressing. Farmacia 2019, 67, 759–763. [Google Scholar] [CrossRef] [Green Version]
- Gegotek, A.; Biernacki, M.; Ambrozewicz, E.; Surazyński, A.; Wroński, A.; Skrzydlewska, E. The cross-talk between electrophiles, antioxidant defence and the endocannabinoid system in fibroblasts and keratinocytes after UVA and UVB irradiation. J. Dermatol. Sci. 2016, 81, 107–117. [Google Scholar] [CrossRef]
- Ständer, S.; Schmelz, M.; Metze, D.; Luger, T.; Rukwied, R. Distribution of cannabinoid receptor 1 (CB1) and 2 (CB2) on sensory nerve fibers and adnexal structures in human skin. J. Dermatol. Sci. 2005, 38, 177–188. [Google Scholar] [CrossRef]
- Pucci, M.; Pasquariello, N.; Battista, N.; Di Tommaso, M.; Rapino, C.; Fezza, F.; Zuccolo, M.; Jourdain, R.; Agrò, A.F.; Breton, L.; et al. Endocannabinoids stimulate human melanogenesis via type-1 cannabinoid receptor. J. Biol. Chem. 2012, 287, 15466–15478. [Google Scholar] [CrossRef] [Green Version]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016, 7, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Tóth, K.F.; Ádám, D.; Bíró, T.; Oláh, A. Cannabinoid signaling in the skin: Therapeutic potential of the “c(ut)annabinoid” system. Molecules 2019, 24, 918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Lewińska, A.; Jaromin, A.; Jezierska, J. Role of architecture of N-oxide surfactants in the design of nanoemulsions for Candida skin infection. Colloids Surf. B Biointerfaces 2019, 187. [Google Scholar] [CrossRef] [PubMed]
- Fandzloch, M.; Jaromin, A.; Zaremba-Czogalla, M.; Wojtczak, A.; Lewińska, A.; Sitkowski, J.; Wiśniewska, J.; Łakomska, I.; Gubernator, J. Nanoencapsulation of a ruthenium(ii) complex with triazolopyrimidine in liposomes as a tool for improving its anticancer activity against melanoma cell lines. Dalt. Trans. 2020, 49, 1207–1219. [Google Scholar] [CrossRef]
- Lewińska, A.; Domżał-Kędzia, M.; Jaromin, A.; Łukaszewicz, M. Nanoemulsion stabilized by safe surfactin from Bacillus subtilis as a multifunctional, custom-designed smart delivery system. Pharmaceutics 2020, 12, 953. [Google Scholar] [CrossRef]
- Peng, L.C.; Liu, C.H.; Kwan, C.C.; Huang, K.F. Optimization of water-in-oil nanoemulsions by mixed surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2010, 370, 136–142. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kim, S.; Bae, E.K.; Mok, C.K.; Park, J. Formulation of a cosurfactant-free O/W microemulsion using nonionic surfactant mixtures. J. Food Sci. 2008, 73, 115–121. [Google Scholar] [CrossRef]
- Reitermayer, D.; Kafka, T.A.; Lenz, C.A.; Vogel, R.F. Interrelation between Tween and the membrane properties and high pressure tolerance of Lactobacillus plantarum. BMC Microbiol. 2018, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seydlová, G.; Svobodová, J. Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med. 2008, 3, 123–133. [Google Scholar] [CrossRef]
- Wu, Y.S.; Ngai, S.C.; Goh, B.H.; Chan, K.G.; Lee, L.H.; Chuah, L.H.; Vecino, X.; Cruz, J.M.; Moldes, A.B.; Rodrigues, L.R.; et al. Anticancer activities of surfactin potential application of nanotechnology assisted surfactin delivery. Front. Pharmacol. 2017, 21, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.G.; Rangarajan, V. A kinetics study on surfactin production from Bacillus subtilis MTCC 2415 for application in green cosmetics. Biocatal. Agric. Biotechnol. 2021, 33. [Google Scholar] [CrossRef]
- Joe, M.M.; Bradeeba, K.; Parthasarathi, R.; Sivakumaar, P.K.; Chauhan, P.S.; Tipayno, S.; Benson, A.; Sa, T. Development of surfactin based nanoemulsion formulation from selected cooking oils: Evaluation for antimicrobial activity against selected food associated microorganisms. J. Taiwan Inst. Chem. Eng. 2012, 43, 172–180. [Google Scholar] [CrossRef]
- Campelo, M.d.S.; Melo, E.O.; Arrais, S.P.; do Nascimento, F.B.S.A.; Gramosa, N.V.; Soares, S.d.A.; Ribeiro, M.E.N.P.; da Silva, C.R.; Júnior, H.V.N.; Ricardo, N.M.P.S. Clove essential oil encapsulated on nanocarrier based on polysaccharide: A strategy for the treatment of vaginal candidiasis. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610. [Google Scholar] [CrossRef]
- Raju, N.S.; Krishnaswami, V.; Vijayaraghavalu, S.; Kandasamy, R. Nanocosmetics, Fundamentals, Applications and Toxicity, Micro and Nano Technologies. In Transdermal and Bioactive Nanocarriers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 17–33. [Google Scholar] [CrossRef]
- Bergfeld, W.F.; Donald, V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, C.; Slaga, T.J.; Snyder, P.W. Safety Assessment of Polysorbates as Used in Cosmetics. Cosmet. Ingred. Rev. 2015. Available online: https://www.cir-safety.org/sites/default/files/polysorbates_0.pdf (accessed on 22 May 2015).
- Arechabala, B.; Coiffard, C.; Rivalland, P.; Coiffard, L.J.M.; De Roeck-Holtzhauer, Y. Comparison of cytotoxicity of various surfactants tested on normal human fibroblast cultures using the neutral red test, MTT assay and LDH release. J. Appl. Toxicol. 1999, 19, 163–165. [Google Scholar] [CrossRef]
- Md Saari, N.H.; Chua, L.S.; Hasham, R.; Yuliati, L. Curcumin-loaded nanoemulsion for better cellular permeation. Sci. Pharm. 2020, 88, 44. [Google Scholar] [CrossRef]
- Teo, S.Y.; Yew, M.Y.; Lee, S.Y.; Rathbone, M.J.; Gan, S.N.; Coombes, A.G.A. In Vitro Evaluation of Novel Phenytoin-Loaded Alkyd Nanoemulsions Designed for Application in Topical Wound Healing. J. Pharm. Sci. 2017, 106, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Wooster, T.J.; Moore, S.C.; Chen, W.; Andrews, H.; Addepalli, R.; Seymour, R.B.; Osborne, S.A. Biological fate of food nanoemulsions and the nutrients they carry-internalisation, transport and cytotoxicity of edible nanoemulsions in Caco-2 intestinal cells. RSC Adv. 2017, 7, 40053–40066. [Google Scholar] [CrossRef] [Green Version]
- Petrosino, S.; Verde, R.; Vaia, M.; Allará, M.; Iuvone, T.; Di Marzo, V. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J. Pharmacol. Exp. Ther. 2018, 365, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phyther. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef] [PubMed]
- Lewińska, A.; Domżał-Kędzia, M.; Kierul, K.; Bochynek, M.; Pannert, D.; Nowaczyk, P.; Łukaszewicz, M. Targeted Hybrid Nanocarriers as a System Enhancing the Skin Structure. Molecules 2021, 26, 1063. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.D.; Acquaviva, A.; et al. Metabolomic profile and antioxidant/anti-inflammatory effects of industrial hemp water extract in fibroblasts, keratinocytes and isolated mouse skin specimens. Antioxidants 2021, 10, 44. [Google Scholar] [CrossRef] [PubMed]
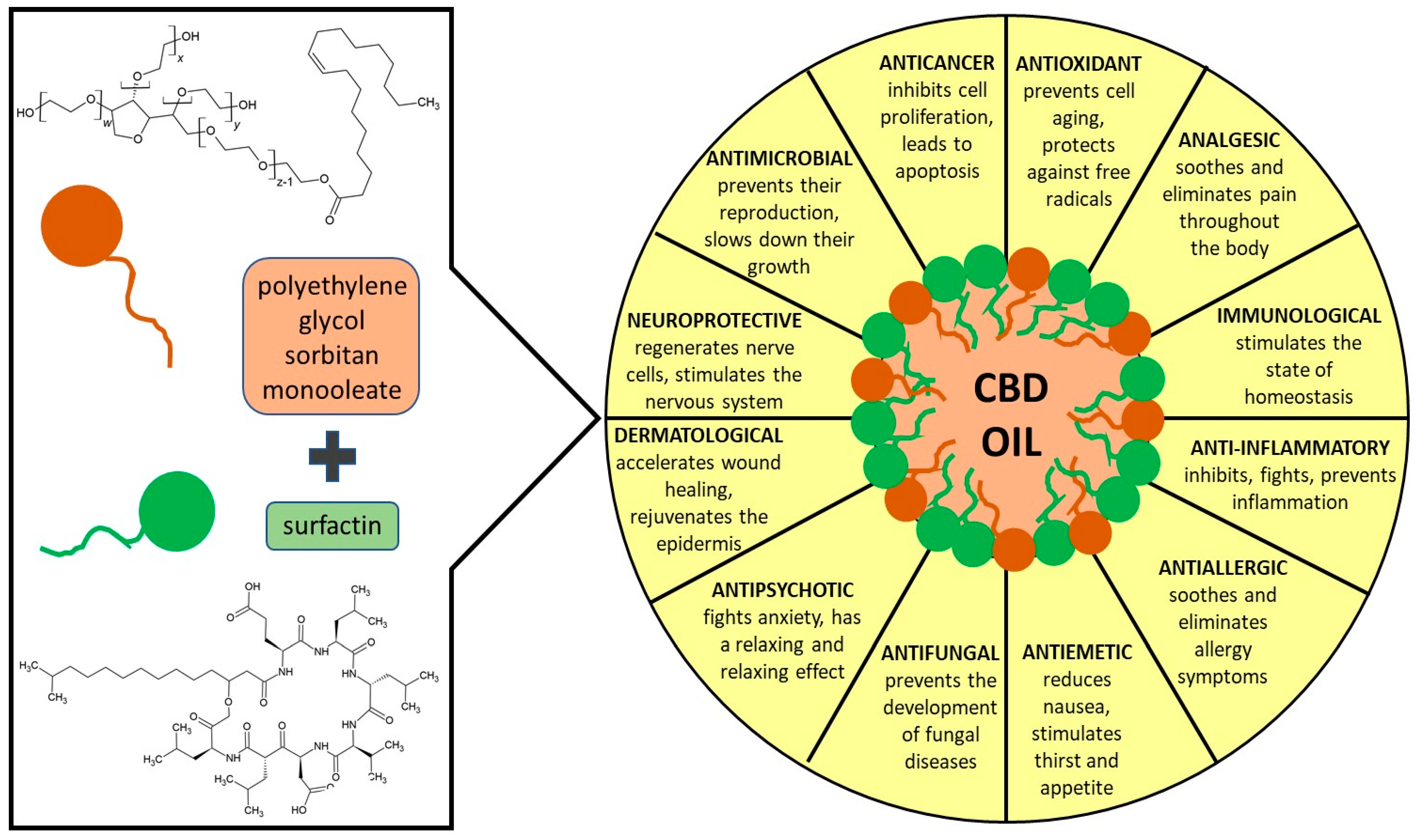



 1:50,
1:50,  1:100,
1:100,  1:200.
1:200.
 1:50,
1:50,  1:100,
1:100,  1:200.
1:200.
 30s,
30s,  40s,
40s,  50s.
50s.
 30s,
30s,  40s,
40s,  50s.
50s.

 30s,
30s,  40s,
40s,  50s.
50s.
 30s,
30s,  40s,
40s,  50s.
50s. 
| NE | Nanoemulsion Composition % | DH [nm] | PdI | ζ [mV] | pH | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S | P | O * | W | HIU | SON | HIU | SON | HIU | SON | ||
| A | 1 | 4 | 1 | 94 | 173.7 ± 4.3 | 333.5 ± 6.9 | 0.347 ± 0.009 | 0.450 ± 0.018 | 0.0 ± 0.2 | −59.9 ± 0.9 | 3.47 |
| B | 1 | 8 | 1 | 90 | 325.2 ± 18.0 | 497.2 ± 17.5 | 0.481 ± 0.059 | 0.633 ± 0.026 | −36.7 ± 1.2 | −36.9 ± 0.7 | 4.00 |
| C | 8 | 4 | 1 | 87 | 254.8 ± 22.3 | 331.0 ± 8.1 | 0.347 ± 0.029 | 0.479 ± 0.066 | −90.7 ± 1.0 | −81.1 ± 0.8 | 6.26 |
| D | 8 | 8 | 1 | 83 | 689.4 ± 72 | 1418.0 ± 105.0 | 0.691 ± 0.062 | 0,987 ± 0.021 | −72.6 ± 0.3 | −55.6 ± 0.6 | 5.64 |
| E | 5 | 5 | 1 | 89 | 175.0 ± 5.3 | 621.5 ± 20.7 | 0.350 ± 0.040 | 0.625 ± 0.075 | −94.7 ± 3.9 | −85.1 ± 0.1 | 5.30 |
| F | 10 | 10 | 1 | 80 | 222.5 ± 2.9 | 749.3 ± 33.5 | 0.355 ± 0.046 | 0.720 ± 0.042 | −82.7 ± 2.01 | −86.5 ± 0.9 | 6.48 |
| G | 10 | 0 | 1 | 89 | 168.0 ± 4.9 | 586.8 ± 32.4 | 0.337 ± 0.037 | 0.658 ± 0.017 | −110.3 ± 3.5 | −60.2 ± 0.1 | 6.67 |
| H | 20 | 0 | 1 | 79 | 880.1 ± 21.1 | 526.7 ± 35.9 | 0.829 ± 0.149 | 0.749 ± 0.008 | −119.3 ± 1.2 | −35.7 ± 0.4 | 6.70 |
| I | 0 | 10 | 1 | 89 | 128.6 ± 1.2 | 216.7 ± 4.5 | 0.180 ± 0.017 | 0.409 ± 0.016 | −55.5 ± 2.7 | −81.7 ± 0.2 | 2.93 |
| J | 0 | 20 | 1 | 79 | 133.0 ± 2.1 | 286.2 ± 14.9 | 0.363 ± 0.008 | 0.574 ± 0.133 | −19.3 ± 0.4 | −55.6 ± 0.0 | 3.60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewińska, A. Optimizing the Process Design of Oil-in-Water Nanoemulsion for Delivering Poorly Soluble Cannabidiol Oil. Processes 2021, 9, 1180. https://doi.org/10.3390/pr9071180
Lewińska A. Optimizing the Process Design of Oil-in-Water Nanoemulsion for Delivering Poorly Soluble Cannabidiol Oil. Processes. 2021; 9(7):1180. https://doi.org/10.3390/pr9071180
Chicago/Turabian StyleLewińska, Agnieszka. 2021. "Optimizing the Process Design of Oil-in-Water Nanoemulsion for Delivering Poorly Soluble Cannabidiol Oil" Processes 9, no. 7: 1180. https://doi.org/10.3390/pr9071180
APA StyleLewińska, A. (2021). Optimizing the Process Design of Oil-in-Water Nanoemulsion for Delivering Poorly Soluble Cannabidiol Oil. Processes, 9(7), 1180. https://doi.org/10.3390/pr9071180