Abstract
This paper presents an overview of the principal structural and dynamics characteristics of reverse micelles (RMs) in order to highlight their structural flexibility and versatility, along with the possibility to modulate their parameters in a controlled manner. The multifunctionality in a large range of different scientific fields is exemplified in two distinct directions: a theoretical model for mimicry of the biological microenvironment and practical application in the field of nanotechnology and nano-based sensors. RMs represent a convenient experimental approach that limits the drawbacks of the conventionally biological studies in vitro, while the particular structure confers them the status of simplified mimics of cells by reproducing a complex supramolecular organization in an artificial system. The biological relevance of RMs is discussed in some particular cases referring to confinement and a crowded environment, as well as the molecular dynamics of water and a cell membrane structure. The use of RMs in a range of applications seems to be more promising due to their structural and compositional flexibility, high efficiency, and selectivity. Advances in nanotechnology are based on developing new methods of nanomaterial synthesis and deposition. This review highlights the advantages of using RMs in the synthesis of nanoparticles with specific properties and in nano (bio)sensor design.
1. Introduction
A wide variety of biochemical events, from macromolecular recognition to biocatalysis associated [1] with biological systems, e.g., lipid layers, membranous organelles, and the interior of macromolecular chaperons [2], take place in nano-restricted environments delimited by membranes such as cells and/or cellular compartments [3]. In conjunction with the confinement effect, interactions with the structure of the membrane interface have an essential influence on in vivo molecules of water [4], conferring them special properties in terms of structural organization and dynamics.
Reverse micelles (RMs) consist of nanoscopic droplets of water delimited by a dynamic, but well-defined surfactant layer, and they are uniformly dispersed within a nonpolar organic solvent. With regard to an experimental approach, reverse micelles (RMs) represent an adequate and convenient molecular assembly at nanoscale, with controllable experimental parameters [5,6].
They provide an artificial system that fulfills many of the requirements for a life-mimicking system, such as nanoscopic structures, volume-restricted environments, and interactions with a membrane-like interface. RMs are optically clear solutions, characterized by macroscopic homogeneity but with a microscopically heterogeneous structure. Compared to bulk water, water inside the cell represents an entirely different medium largely reflected by its properties; therefore, in vitro cell models have been proposed to understand the properties of water in confined conditions.
On the other hand, RMs were proposed as experimental media for the synthesis of a large variety of nanoparticles and for the development of nanomaterials. A large variety of nanostructures have been synthesized using reverse micelles, taking into consideration a number of factors for selecting an appropriate surfactant for a specific synthesis reaction in reverse micelles. Since reverse micelles are globular aggregates which are formed by self-assembled surfactants in nonpolar solvents, many different self-assembled structures can be achieved by changing their composition. Thus, spheres, cylinders or interconnected cylinders, and lamellar phase micelle structures can be obtained. Many of these possible nanostructures have been reported in the literature as being grown inside of these shaped templates.
The synthesis of nanoparticles in reverse micelle microemulsions proved to be a useful route in principle due to the possibility to control particle size, thereby ensuring a narrow particle size distribution, as well as a high degree of homogeneity in terms of concentration and morphology.
In the last few decades, reverse micelles have represented a scientific challenge for many domains, especially physical chemistry, as more and more information regarding the structural and dynamics characteristics is being accumulated. Using more innovative techniques and sophisticated equipment, the reverse micellar research domain has been extended, substantial amounts of diversified data and in-depth knowledge have been obtained, and theoretical hypotheses, mathematical models, molecular simulations, etc. have been proposed. Many well-documented reviews and comprehensive books have been published during the years of study, including the latest ones approaching the assessment of current knowledge and dealing with several aspects of reverse micelles [7,8,9,10,11,12,13].
The target of the present review is to demonstrate the unusual versatility of reverse micelles; the same structure has a multifunctional contribution to distinct and totally different scientific fields and diametrically opposed applications, from cell theoretical models to the new and modern nano (bio)technology. Each one of these directions is basically supported by the specific characteristics of reverse micelles, such as the interfacial parameters of the surfactant film, microheterogeneity of the intramicellar structure, multilayered composition of water, redefinition of the pH concept for size-limited systems in the case of biological relevance, mechanisms of reverse micelle formation, composition of the interfacial layer, and experimental significance of intramicellar structure or size for nanomaterial synthesis. Even if some theoretical data mainly sustain one of these applications, they have been treated in a holistic and integrated manner because reverse micelles are a unique, indivisible, and functional whole structure.
In addition to the basic view on the essential physicochemical fundamental characteristics of RMs, special attention is paid to the analysis of the internal cavity characteristics of the RM, such as intramicellar pH, because it is the compartment involved in the development of most applications. Awareness of the particularities of this environment, ignored in most cases, can help to overcome some limitations in the case of existing applications and can extend the potential of RMs to other fields.
2. Fundamental Characteristics of Reverse Micelles
2.1. Microemulsion Origin of Reverse Micelles (RMs)
Generally, different isotropic or non-isotropic ternary systems result from mixing together amphiphilic molecules of surfactants (surface-active agents) with two immiscible solvents, commonly referred to as oil, a term used liberally to refer to any nonpolar water-insoluble solvent, and water, a term describing any polar water-soluble solvent [14]. According to Danielsson and Lindman [15], the thermodynamically stable isotropic liquids obtained from this mixture are usually defined as “microemulsions” and are composed of one or more phases which coexist separately, but are in balance with each other [10], e.g., water-continuous (oil-in-water), oil-continuous (water-in-oil), and bicontinuous (middle phase) [16]. The scientific recognition of microemulsions dates from 1943 (Hoar and Schulman) [17], but the term was first used by the latter author only in 1959 to describe the transparent solution of the multiphase system formed by a water/oil/surfactant (w/o/s) mixture [18,19,20].
RMs are formed in various ternary mixtures and result from spontaneous self-assembly of the surfactant molecules in nonpolar organic solvents, without requesting any high inputs of energy or shear conditions (“dry micelles”). In the presence of water or other polar solvent, RM aggregates are taken up inside the hydrophilic internal region of the two-component cluster, thereby forming isolated droplets uniformly dispersed in the bulk solvent (“swollen micelles”) [21].
Sometimes, RM systems are referred to as water-in-oil microemulsions, with the demarcation line being quite fluid due to some similarities between them: (i) both consist of two immiscible liquids and surfactant molecules forming an interfacial film, (ii) both are thermodynamically stable isotropic systems unlike the usual emulsions, (iii) both contain aggregates in the 5–100 nm size range, and (iv) both have the capacity to solubilize microdroplets dispersed in the solvent continuous phase.
The main difference between microemulsions and reverse micelles principally constitutes the water content inside the core, as well as the physical characteristics of the water molecules. While, in microemulsions, they have the properties of “bulk” water, in reverse micelles water, they either do not exist, i.e., dry micelles, or the volume is very small, i.e., swollen micelles; thus, most or all molecules are more or less tightly related, spatially and/or chemically, to the polar groups of the surfactant. This surfactant–water connection results in special properties of the water inside the core, substantially different from the properties of typical “volume” water from the core of the microemulsion. The progressive swelling of reverse micelles with a small core result in the enlargement of the core and, thus, RM size. Consequently, the water content inside develops characteristics closer to “bulk” water. However, many researches considered that, even at moderate to high degrees of hydration, the different characteristics of water molecules inside RMs remain significant [22].
A distinguishable measurable shift between the two states of water molecules is not available; thus, the terminology is not well defined. A generally accepted differentiation between the two terms may be specified if the term “RM” refers to the nano-aggregates themselves, while the term “RM microemulsion” refers to the whole system which contains the solvents in addition to the surfactant aggregates. The used term “RM system” includes the water content from the water pool of the RMs.
From an experimental point of view, RMs present an extremely important difference compared to other types of microemulsion; they are optically transparent solutions and have a recognized homogeneity, monodispersion, and stability. Therefore, reverse micelles represent a special and distinctive case in the microemulsion domain or colloidal science, and they have the important experimental advantage of allowing advanced study of their structure using any known investigative methods, such as spectrometric methods.
2.2. Formation of Reverse Micelle Microemulsions
The molecular basis of an RM microemulsion formation relies on the particular capacity of a surfactant to be adsorbed spontaneously at the free interface between oil and water. The pellicular property is associated with the amphiphilic structure of surfactants, which confers them a dual affinity: for the hydrophilic groups from the polar phase and re for the hydrophobic groups from the nonpolar phase [23]. It is assumed that repulsive forces characterize the interactions between oil and water, and the absence of cohesiveness at a molecular level generates tension at the w/o/s interface, known as surface tension (γ, expressed in mN/m) [24]. The presence of surfactant molecules modifies the nature of the forces from repulsive toward attractive and minimizes the contact area due to their pellicular ability, reflected in the decrease in γ value below 10−2 mN/m [16,25]. The resulting interfacial energy is exceeded by dispersion entropy and, thus, the dispersion process is facilitated.
The stepwise association of the surfactant is considered to be a model for reverse micelle formation, taking into account the progressive transition from individual dissolved surfactant molecules to the growing micellar aggregate. Experimentally, it the existence of multiple equilibria has been demonstrated, assuming that a stepwise equilibrium characterizes every successive addition of surfactant, which is defined by a corresponding constant. Different association models (closed, open, and Eicke’s) have been considered in the interpretation of the RM formation. In the case of cetyltrimethylammonium bromide (CTAB) in chloroform, the latter model was experimentally validated, according to which a structural reorganization of linear associates results in the formation of cyclic inverse micelles. The process is performed within a narrow range of surfactant concentration, i.e., the so-called critical micellar concentration (CMC) [26,27].
Different basic considerations have been proposed in order to explain the formation and the stability of microemulsions, being concretized in three theories [28]: (i) interfacial/mixed film theory, suggested by Schulman, assumes that the spontaneous formation is the result of a complex dual film constituted at the o/w interface, thus decreasing the tension cumulated in this region to zero at equilibrium [17]; (ii) solubilization theory, proposed by Gillberg, states the relationship between RMs and w/o microemulsions with the help of phase diagrams, treating the microemulsion as a thermodynamically stable monophasic solution of water-swollen micelles (RMs solubilizing water) or oil-swollen micelles (normal micelles solubilizing oil) [29]; (iii) thermodynamic theory, proposed by Paul and Moulik, correlates the process of microemulsion formation with the value of the free energy of the system formed by mixing one phase into another. According to this theory, the resulting negative value governs the microemulsification process in a spontaneous and thermodynamically stable way [30,31].
At low concentrations, surfactant molecules exist independently in solution or are discretely arranged on the o/w surface, while the surface tension is insignificantly modified. The increase in surfactant concentration is accomplished by the addition of more molecules at the o/w surface with a considerable decrease in the surface tension of the solution. This tendency is kept until the surface becomes saturated, whereby the formation of the micelles is initiated, at which point the surface tension no longer changes (Figure 1).
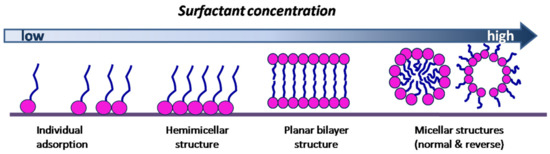
Figure 1.
Concentration influence on typical surfactant structures at the water/oil (w/o) interface.
Experimental studies of the relationship between aggregation and surfactant concentration revealed an evident transition from monomer to aggregate, suggesting the existence of a critical micelle concentration for a surfactant solubilized in nonpolar solvents. However, the nature of the transition may be different as a function of the surfactant characteristics. For example, by decreasing the surfactant concentration, the aggregates formed by an anionic surfactant, such as sodium dioctylsulfosuccinate, do not modify their size and shape until a sharp value of CMC is reached, with no aggregates being detected at a lower value of concentration. In the case of a nonionic surfactant, such as pentaethylene glycol monododecyl ether, a decrease in surfactant concentration reduces the size of the aggregates. The differences in surfactant aggregation are assumed to correlate with the strength of the solvophobic effect in nonaqueous solvents [32].
The micellization process is also characterized by quantitative parameters for self-assembled surfactant molecules. The concentration-dependent parameter is expressed as the critical micellar concentration (CMC), defining the minimum concentration of the surfactant necessary for micelle formation (Figure 1) [16]. It represents the concentration of free or aggregated surfactant molecules in equilibrium with those forming micelles, and it is characteristic for each surfactant and ternary system [33,34].
3. Surfactant Characterization
3.1. Interfacial Film of Surfactant
The properties of water droplets shielded by the surfactant monolayer in RMs, such as their size, shape, polydispersity, and interactions, are governed by the elastic particularities of the interfacial surface, in terms of the preferred spontaneous curvature, e.g., radius of curvature, and the film stiffness, e.g., constants of rigidity for mean and total (Gaussian) curvature that characterize the bending of the surface [16].
The preferred curvature, Co, defined as the difference between the areas occupied by hydrophilic heads and lipophilic tails, is classified by convention as positive curvature (toward oil, corresponding to a prevalent hydrophilic part) (for o/w systems) and negative curvature (toward water, corresponding to a prevalent lipophilic part) (for w/o systems) [4]. The ratio between the radius of the droplets and the radius of spontaneous curvature is correlated with the minimum or “critical” radius of curvature (Rc), which anticipates the shape of aggregates (Figure 2). The direction and the extent of the curvature of the adsorbed interfacial film depend on the composition of the phases it separates, as well as on the surfactant type.
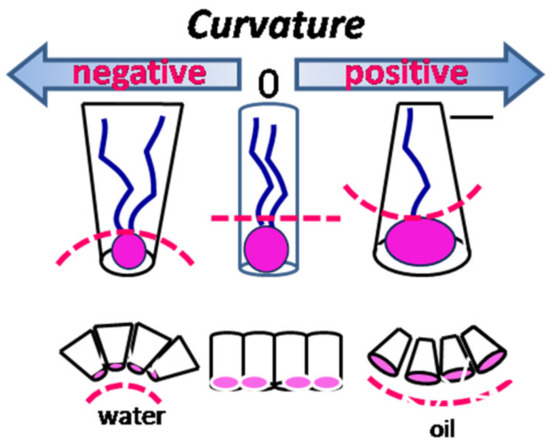
Figure 2.
Correlation between the type of curvature and surfactant morphology.
The amphiphilic structure of a surfactant, which is the critical property for micellization capacity, is described by the hydrophilic–lipophilic balance (HLB), which suggests the type of microemulsion to be formed, e.g., w/o microemulsions for HLB values <10 (predominantly hydrophobic surfactants) and o/w microemulsions for HLB values >10 (predominantly hydrophilic surfactants) [19]. This concept relates the molecular structure to its elastic properties, such as interfacial packing and film curvature, and it empirically expresses the relative proportions of hydrophilic and hydrophobic groups within the molecule of the surfactant [35].
Israelachvili, in the 1970s, stated the concept of molecular packing parameters, which afforded a simple, intuitive, and predictive approach of the self-assembly process according to the contribution of surfactant headgroups [36], while Nagarajan completed this concept by emphasizing the controlling role of tails in predicting the shape and size of equilibrium aggregates [37].
The overall packing shape of surfactant molecules is conveniently described by a geometric parameter, namely, the critical packing parameter (CPP), a dimensionless number, and it can be calculated according to the following formula: CPP = V/(a0∙lc), where a0 is the surface area of the headgroups per molecule and is the result of repulsive hydrophilic forces between them and attractive hydrophobic forces from the hydrocarbon–water interface, V is the effective hydrocarbon volume resulting from chain–chain repulsive steric forces and oil-penetration interactions, and lc is the length of the fully extended chain, which limits the thickness of hydrocarbon layer.
Table 1 summarizes the correlation among the structure of surfactants (e.g., headgroups, tails, and geometry of the surfactant molecule), the interfacial film characteristics (e.g., curvature and CPP values), and the critical packing shape of the surfactant [29,35,36,37,38,39,40].

Table 1.
Relationship between the molecular structure and characteristics of the surfactant and the corresponding phase type. CPP, critical packing parameter.
3.2. Surfactant Types
The quantitative weight of surfactants in the ternary mixtures does not represent the most important contribution to the overall properties of the RMs (Figure 3) (e.g., size and shape of water droplets, polydispersity, and droplet interactions), with their major role being conferred by the elastic properties of the interfacial layer [41]. Most RM systems can be prepared using surfactants with different chemical structures, whether ionic or nonionic, depending on the nature of the hydrophilic and hydrophobic groups.
Figure 3.
Relative proportion of components in a reverse micellar (RM) system.
3.2.1. Conventional Surfactants
Ionic surfactants. The most used surfactants to prepare RMs are anionic surfactants [10,41], which include sodium bis(diethyl hexyl) phosphate(NaDEHP), calcium dodecyl sulfate (CDS), and sodium dodecyl sulphate (SDS); however, in most studies, the first choice for surfactants of any nature is AOT (sodium bis(2-ethyl hexyl) sulfosuccinate), also known as docusate sodium (dioctyl sodium sulfosuccinate).
AOT (Aerosol Orange T or Aerosol OT) is an anionic surfactant with branched twin alkyl chains and a single headgroup (the anionic sulfonate), with Na+ being the usual counterion [2,41]. From a chemical point of view, it is an ester of sulfosuccinate acid (C20H37NaO7S), having three possible rotamers, but only one with appropriate stereochemistry for the most stable conformation of surfactant in nonpolar solvents [42]. The geometry of the AOT molecule is a wedge-like shape, with the polar group sitting at the small cross-section; therefore, it favors aggregates in which the surfactant molecules can keep their concave curvature.
AOT RMs are significantly monodispersed and spherical, retaining their shape over a wide range of water content (w0 from 0 to 60), while the size ranges from radii of less than 1 nm to radii of up to 14 nm.
The special attention received by AOT is related to the particular characteristics of the RM formed, e.g., it enhances the capacity to solubilize large quantities (up to 400,000 molecules) of water inside the cluster and does not need the presence of a cosurfactant [43] (Figure 4a). Its unusual efficiency is attributed to the relationship between structure and property, particularly its inverted truncated cone shape and well-balanced molecular structure [44].
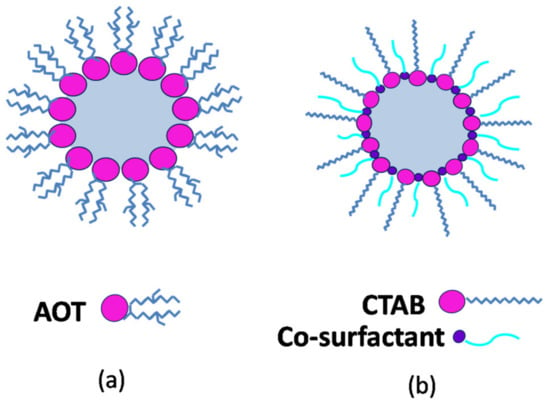
Figure 4.
Schematic cross-section through (a) Aerosol Orange T (AOT)-based RM; (b) cetyltrimethylammonium bromide (CTAB)-based RM.
The cationic group of surfactants with relevance for RM formation typically consists of quaternary ammonium alkyl salts, e.g., alkyl ammonium halides and tetraalkyl-ammonium halides. The most used in different studies and applications is cetyltrimethylammonium bromide (CTAB); however, other cationic surfactants can also be used, e.g., cetylbenzyldimethylammonium chloride (CBAC), benzyl dodecyl bis(hydroxyethyl) ammonium chloride (BDBAC), didodecyl-dimethylammonium bromide (DDAB), tetradecyl trimethyl ammonium bromide (TTAB), tri-octyl methyl ammonium chloride (TOMAC), cetylpyridinum bromide (CPB), and sodium dodecyl-benzenesulfonate (NaDBS) [10,45,46].
Nonionic surfactants. RMs formed with nonionic surfactants have extreme sensitivity to the process conditions and, therefore, are less characterized [10,41]. However, different surfactants from the Tween, Triton (e.g., Triton X-100, polyoxyethylene(10)isooctylphenyl ether), Brij (e.g., Brij30, polyoxyethylene(4) lauryl ether), and Span series, as well as Igepal CO520 or various mixtures of polyoxyethylene(5) nonylphenol ether (NP-5), polyoxyethylene(9) nonylphenol ether (NP-9), and polyoxyethylene(12) nonylphenol ether (NP-12), have been used in various studies [41,47,48].
Mixed surfactants. The simultaneous presence of an ionic and nonionic surfactant results in the formation of mixed RMs; these were specifically proposed in order to reduce some drawbacks encountered in the case of ionic or nonionic surfactants, for instance, to modify the polarity of the surfactant film and, thus, of its surrounding microenvironment [41]. Exemplary mixed RMs typically consist of the well-known anionic surfactant AOT together with Span 60 [45], Tween 85 [49], or Brij 30 [50,51] and the cationic surfactant CTAB with Tween 80 or Triton X-100 [51].
In addition to synthetic surfactants, some bile salts such as sodium taurocholate (NaTC), 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate hydrate (CHAPS), and sodium cholate (SC) can participate in the formation of mixed RM systems together with ionic surfactants, mostly AOT [52].
3.2.2. Natural Surfactants
Molecules with surface-active properties are naturally found in biological systems, mainly as secondary metabolites, and they have a diverse range of chemical structures. These natural surfactants or biosurfactants, usually of microbial origin, can replace conventional surfactants in the RM composition, with a reduction in negative effects on the environment and a potential contribution to green applications due to their nontoxic nature and biodegradability [53]. Glycolipids, such as long-chain aliphatic acids or hydroxyaliphatic acids, are known nonionic biosurfactant compounds, with the most important members being rhamnolipids [54] produced by Pseudomonas aeruginosa, trehalolipids produced by Rhodococcuserythopolis, and sophorolipids produced by Candida bombicola [55].
3.2.3. Targeted Surfactants
Affinity surfactants. The selective capacity of RMs in extraction and separation applications can be increased, especially through affinity interactions resulting from the presence of affinity ligands in the interfacial film typically formed by ionic or nonionic surfactants. An affinity ligand, such as di(2-ethylhexyl) phosphoric acid, has been used to formulate RMs together with two nonionic surfactants (Triton X-45 and Span 80) [56], while a sugar-binding affinity ligand such as lectin concanavalin A (conA) can be introduced in addition to the anionic AOT surfactant [41,57].
Another approach to improving the selective characteristics of RMs is represented by direct modification of the surfactant structure. In this way, the nonionic Span 85 was modified with Cibacron Blue (CB) F3GA, widely used in dye-ligand affinity chromatography, having structural similarity to natural ligands. The affinity of the CB–Span 85 conjugate was directed toward proteins with cofactor-binding domains, and the corresponding RMs were considered to have promising potential in the biotechnological field [58].
New surfactants. Despite the large range of available commercial surfactants, the broadening of applications and increasing expectations with respect to RM performance have led to the design and synthesis of surfactants according to the new research requests [41]. Accordingly, two new surfactants with relevance for the development of innovative applications were proposed by Goto et al., namely, di(tridecyl) phosphoric acid (DTDPA) and dioleyl phosphoric acid (DOLPA), with their hydrophobic structure adjusted in order to improve the characteristics of RM systems for protein extraction [59].
3.2.4. Cosurfactants
In many cases, the use of a single-chain surfactant is not sufficient to reduce the o/w interfacial tension below 10−2 mN/m in order to compensate for the dispersion entropy, which may lead to inefficient formation and/or stability of RMs. To overcome these inconveniences, addition of a second surfactant with low molecular weight is needed in order to decrease the bending factor of the surfactant layer and enhance the thermodynamic stability. The amphiphilic nature of a cosurfactant allows its accumulation mostly at the interfacial layer, followed by the incorporation of cosurfactant molecules into the layer formed by the main surfactant. Their proposed mechanism of action suggests a buffer action of cosurfactant molecules, resulting in a decrease in both electrostatic repulsive interactions between the charged headgroups and hydrophobic interactions between the hydrophobic tails of the first surfactant molecules [34,60] (Figure 4b). This effect results in a denser packing of surfactant molecules and the formation of a stable RM inner core, as well as increased flexibility of the interfacial film around the droplets of RM and an enhanced interdroplet interaction [47]. On the other hand, the use of a cosurfactant results in modification of the overall HLB of the system, i.e., a cosurfactant with very low HLB is typically added to a surfactant with high HLB.
An advantage of cosurfactant use is the possibility for them to operate as tunable tools for the shape and diameter of RMs (e.g., enlargement of micelle size using a cationic surfactant); however, their presence leads to a system with a higher degree of complexity, with the phase diagram for four components being more difficult to obtain [60].
The efficiency of cosurfactants is correlated with their ability to penetrate the surfactant layer to a greater depth. Generally, the most effective cosurfactants are short- to medium-chain alcohols, for example, n-butanol, n-pentanol, n-hexanol, n-heptanol, n-octanol, n-decanol, isopropanol, and benzyl alcohol, as well as cholesterol, and, in some cases, propylene glycol [60].
4. Characterization of Reverse Micelles
4.1. Structural Characterization of RMs
Reverse micelles are composed of aggregates of surfactant molecules structured in a polar core of hydrophilic headgroups, with their hydrophobic tails directed outside into the organic bulk. The concept of surfactant aggregate formation was initially proposed for an aqueous medium by James William McBain in the early 1910s, while, two decades later, G.S. Hartley named this concept “micelle” (from the Latin “mica” which means small particle), a term borrowed from biology [61]. The term “reverse micelles” originated much later, in the 1970s, specifically defining the reversed/inverted situation of a normal micelle, consisting of an amphiphilic aggregate formed in a nonpolar hydrophobic medium.
Reverse micelles represent dynamic formations [62] that can exchange surfactant molecules with solvent at a high rate, with the residence time of the surfactant molecule being about 10−7 s [63]. Despite the high mobility of surfactant molecules and their continuous vibration, the micellar interface is well defined and not permeable to organic solvents surrounding the micelle aggregates [64].
Most of the structural properties of RMs are induced and controlled by the ratio between the polar phase and surfactant concentration. As water is generally the polar phase used to obtain RMs, this parameter is referred to as the degree of hydration and is considered even more relevant to describe the system features than the water content [65,66,67]. Thus, it is commonly defined as the molar ratio of water to surfactant, i.e., w0 = [H2O]/[surfactant].
Geometrical considerations can be applied in most cases, with droplets typically described as spherical-like structures, and a linear relationship between the average droplet radius Rm and the externally controlled parameter w0 can be set as follows: Rw = 0.175w0, while the hydrodynamic radius, R, is described by the length of the surfactant molecule in addition to Rw: R = Rw + 0.15 [66].
For a particular RM containing the AOT surfactant, this relationship is defined as Rm (nm) = 0.15w0 + 0.44, where 0.44 nm represents the diameter of the dry micellar core delimited by a surfactant film with the optimal interfacial curvature [67].
Generally, the degree of hydration of RM ranges from 1 to 50, a value which can be exceeded in particular systems, corresponding to a diameter size varying from 1 to a few tens of nanometers [68]. RMs are characterized by a surprisingly narrow range of size distribution, with the polydispersion index varying from 10% to 20% depending on the ternary system [69].
The size of RMs is a tunable system parameter, which can be controlled by varying the values of w0, e.g., an increased/decreased diameter of the inner RM cavity is obtained by increasing/decreasing the water content at the same surfactant concentration or by decreasing/increasing the surfactant concentration at a constant water concentration [70]. Furthermore, the concentration of RM aggregates present in bulk solvent can be changed by simultaneous modifying the water and surfactant concentrations, while keeping the w0 value constant.
RMs are characterized by a low interfacial tension (generally less than 10−2 mN∙m−1), but a large surface area (102–103 m2∙cm−3), while their nanometer size (usually no more than 100 nm) confers optical transparency to the colloidal solution (Figure 5). The RM surfactant aggregates generally have a smaller size compared to their hydrophilic micellar counterparts, with an aggregation number less than 50.
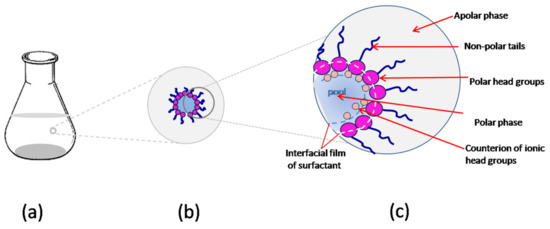
Figure 5.
Schematic representation of RMs at different levels: (a) macroscopic; (b) microscopic; (c) molecular.
As a consequence of the micro-aqueous phase structure in the internal cavity of RMs, the effective polarity and viscosity of the intramicellar water vary radially and differ substantially from those of the solvent water. For example, from fluorescence data, it was concluded that the polarity of the intramicellar medium at low degrees of hydration is closer to the value for methanol, only reaching values equivalent to those of bulk water at high values (w0 = 30). Regarding the viscosity of intramicellar water at low wo values, it was found to be 200 times higher than that of chloroform [71].
The nucleophilic activity of solubilized water in AOT RMs is 103 times higher than that of solvent water [72]. The greatest reaction capacity is presented by water molecules that hydrate the polar groups of the surfactant, as well as those in the immediately adjacent hydration layer. The subsequent addition of water decreases its reaction capacity due to the formation of intermolecular hydrogen bonds.
In contrast to normal micelles from aqueous solutions, reverse micelles are characterized by an electrically neutral outer layer such that Coulomb rejection forces do not occur, thus enabling frequent collisions [63]. It is considered that the exchange of content between RMs is a collision–fusion–fission process [73]; the collision of two RMs results in a dimer containing a single aqueous nucleus obtained via the fusion of two component nuclei, and dimer fission results in the formation of two new RMs with redistributed content [74].
4.2. Water Pool Structure
In RM, the properties of water molecules are generally influenced by the interface, particularly the surfactant heads, modeled as a pair of atomic ions. Their structure inside the water pool is described as a multilayered composition, where the water pool is the term proposed by Menger in 1973 [75,76] and generally accepted to define the water entrapped inside the RMs.
While the typical model takes into consideration two states of water molecules [77], i.e., a layer located nearby the surfactant interface corresponding to the “bound” layer and the bulk-like water in the interior of the water pool considered to be the “free” form, a more complete model identified a structural heterogeneity described by three species of water molecules, with the third specie of water being the molecules “trapped” at the surfactant interface (Figure 6) [78,79,80,81].
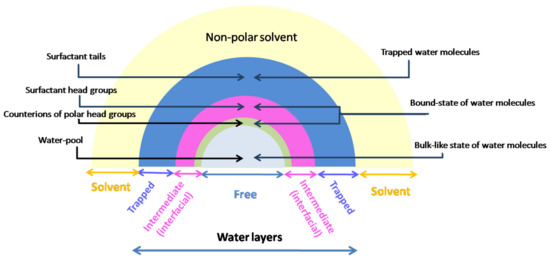
Figure 6.
Correlation between the general structure of RMs and the multilayered structure of water inside RMs.
The region consisting of trapped water is the nearest layer to the surfactant cavity and is composed of molecules which interact directly with the large immobilized headgroups of surfactants, forming a cluster around them, while the H-bonds between water molecules do not exist. In the case of AOT RMs, trapped water is coordinated with the AOT headgroup and/or carbonyl group, with about 13 trapped water molecules corresponding to one surfactant molecule [82].
The ensuing layer is located in the neighboring interface, where its influence is very strong, and it consists of rigidly fixed molecules (“bound” state of water) between the charge groups of surfactant molecule (e.g., 2–3 water molecules rigidly bound by one AOT molecule). In this layer, H-bonds settle either with the headgroup or other water molecules, and ion–dipole interactions between counterions and water molecules also occur. This results in an organization and orientation of the water molecules in this region, corresponding to the intermediate layer in the three-state model or the interfacial layer in the two-state model.
Being protected by the previous water layer, as well as by the counterions, the water molecules inside the water pool of the RM have a similar but not identical structure to bulk water, with each molecule forming approximately four H-bonds [83]. They compose the “free” state of water in the RM, sometimes considered a separate phase, and this behavior is characteristic of large RMs (w0 ≥ 16.5) where the influence of the interface is no longer as powerful in the center of the RM [43].
Generally, the number of water layers in the RM is directly correlated with the interface interaction strength and indirectly related to the RM size and hydration degree [84]. Thus, different percentages of interface-interacting water have been reported, ranging from 100% in very small RMs (w0 = 2) to 36% [43] and 25% [85] in intermediate RMs (w0 = 10 and w0 = 16.5, respectively), and only 8% in very large RMs with w0 = 46 [85].
4.3. pH across the Water Pool
The principal parameter usually used to characterize any aqueous system is the pH, one of the older concepts, defined more than a century ago. The actual standard definition for pH, recommended by the International Union of Pure and Applied Chemistry (IUPAC), is based on hydronium ion concentrations and is applied only for a range of pH between 2 and 12, at an ionic strength of no more than 0.1 mol·L−1 [86]. According to these conditions, the minimum volume necessary to apply the conventional definition of pH in an aqueous solvent is determined by the dissociation of water molecules [87,88]. For instance, at neutral pH, only one in 107 molecules of water is dissociated; however, the number of water molecules in very large RMs is only about 300,000 (w0 = 40), with this number being even lower in smaller RMs (e.g., about 30.8 molecules for w0 = 1.5 or 20,500 molecules for w0 = 10.5) [89]. Therefore, the number of water molecules inside an RM water pool is insufficient to have at least one hydronium ion [90,91]. This argument describes the traditional concept of pH as a bulk macroscopic parameter which cannot be directly applied in the case of size-limited systems with a very small number of water molecules (on average, approximately 103), where the effective proton concentration cannot be calculated. In addition to the abovementioned considerations, a gradient of proton concentrations inside the water pool is expected, related to its structural heterogeneity [86].
The conceptual problems regarding pH in RMs, as well as the experimental difficulties related to the unenforceable use of traditional pH-meters [13,92], were identified as early as 1980 by Luisi and coworkers [83], who emphasized the impossibility of determining the absolute value of pH in RM and recommended two working pH values, the pH inside the water pool, defined as pHwp, and the pH of the starting buffer in bulk water, defined as pHst. Arguing the major differences between the two approaches, the author suggested treating the water inside the RM as a new solvent and proposed the use of an empirical acidity scale based on measurements of 31P-chemical shifts of phosphate buffers [93]. In AOT–isooctane RMs, the experiments reported by Luisi revealed a pHwp/pHst difference of less than 0.4 pH units, a difference which may be variable in other cases or experimental conditions [94].
As the accuracy of the classical definition of pH based on proton activity is at the very least uncertain in RM, researchers have reoriented toward concepts such as “acidity” and “basicity”, which are considered to be more adequate in such systems. These concepts are associated with indirect measurements based on acidity scales using a molecular probe in a pH-sensitive reaction, and they are generally evaluated through a spectroscopic method [95], i.e., either ultraviolet/visible light (UV/Vis) absorption based on the shifts of spectral peaks or (steady-state and time-resolved) fluorescence techniques using a pH-sensitive excitation spectrum [89]. In the latter case, probe molecules presenting excited-state proton transfer may reside in different regions of the water pool [91], e.g., 2-naphthol-6,8-disulfonate sodium salt located in free water [96] or 7-azaindole mostly present in the interfacial zone of AOT RMs [97], and their fluorescence responses suggest/confirm the heterogeneity of the water structure in RMs.
The presence of the probe molecule, even at a very low concentration, can interfere with the formation and nature of the RM, change the overall charge during proton transfer, and/or influence the inside environment [98,99]. For example, in CTAB RMs, the chromophore probe molecule Ru(bpy)32+ affected both the water content and the distribution of droplet size [100]. All of these drawbacks confer a degree of ambiguity to the assay results which can be avoided by using an alternative method such as NMR to evaluate the acidity of the aqueous core [101]. While 31P-chemical shifts, initially proposed, restrict the measurement area to the use of phosphate buffers inside the water pool, other magnetic resonance relaxation measurements have been proposed to measure the local proton activity in RMs. Halliday and coworkers [84] used the effect of pH changes on the relaxation times (T1 and T2) of the aqueous droplets inside RMs (e.g., CTAB–hexanol–water, CTAB–hexane–pentanol–water, and Triton X-100–cyclohexane–hexanol–water). Baruah and coworkers [98,99] used another NMR probe, namely, a highly charged decavanadate (V10) oligomer, for 51V-NMR spectroscopy in AOT–isooctane RMs [99]. The information obtained showed the consistent deprotonation of the probe and indicated the existence of a proton gradient inside the RM (neutral core, but acidic interface) caused by the migration of protons toward the interface and the concentration of counterions in the core of the RM [102].
In addition to the methods using optical pH sensors or molecular probes, Mukherjee and coauthors [92] proposed the acid-catalyzed hydrolysis of sucrose [103], i.e., the inversion of sucrose, and they monitored the reaction via the rotation of polarized light. Their study was based on two working hypotheses: (i) a neutral molecule of sucrose would be located inside the water pool; (ii) the availability of protons inside the water pool would affect the rate of sucrose hydrolysis in the micellar medium compared with the reaction rate in bulk water. In AOT RMs, the slowdown of the inversion rate is correlated, in agreement with the 51V-NMR conclusions, with a deficiency of catalyst, i.e., H+, in the core, while the increased reaction rate in CTAB RMs suggests a higher concentration of protons in this area (Figure 7).
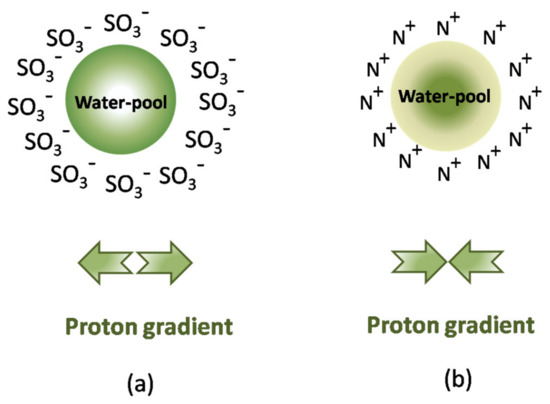
Figure 7.
Proton gradient inside the water pool of the reverse micelle of (a) an anionic surfactant (e.g., AOT), and (b) a cationic surfactant (e.g., CTAB).
A concept proposed by some researchers [104], but not generally accepted [105], is the buffer-like action of water from RMs due to the effect of titratable surfactant headgroups, i.e., the component with a higher concentration in the RM aggregates. This behavior was reported especially for AOT surfactant, which maintains the inside pH values at 5.0–5.5 for any value of bulk buffer used, while the surfactant mixture CTAB/hexanol has a very low buffering capacity in the experimental range of pH from 4 to 10 [92].
All measurements of local pH in RMs are subject to the influence of a diversity of factors, such as ionic strength, viscosity, polarity, and conductivity; therefore, considerable attention must be paid to the accurate description of RM components and experimental conditions, especially for comparative studies. Since 1992, Walde has warned about the pH artefacts caused by high concentrations, acidic/basic substrates, impurities, or products in enzymology experiments [106].
5. Biomimetic Relevance of RMs
A large range of biological systems, e.g., lipid layers and protein pumps, and biochemical functions, e.g., macromolecular recognition and biocatalysis, take place in nano-restricted environments delimited by cellular membranes inside cells and/or cellular organelles. Furthermore, the intracellular environment is characterized by a compact, but highly compartmented structure and a heterogeneous matrix containing various macromolecules with different shapes and sizes.
Conventionally, biological/biochemical studies in vitro confer only a basic view regarding intrinsic information of the biomolecular structure and function resulting from their isolation through extraction procedures and the removal of macromolecules from their natural matrix, accomplished due to the loss of a structured microenvironment and partial alteration of the native structure and functions in vivo [107]. In addition, to avoid aggregation problems and to minimize the nonspecific interactions that occur at high concentrations, studies are generally performed in a diluted buffered solution, which is too simple an environment with respect to the compositional complexity of the intracellular medium.
Addressing these major limitations regarding the relatively idealized thermodynamic conditions of “physiological conditions” used in vitro, so-called “synthetic cells” were proposed, representing simplified mimics of the biological cell environment, including biomembranes, the cytoplasmatic structure, and inside interactions [108]. A successful approach to achieving the correlation of in vitro with in vivo conditions implies the selection of an appropriate model that can simulate, to a high degree of accuracy, the structure and function of the biomolecules, particularly proteins, in biological membrane structures. Currently, inverse micelles are recognized as biomimicking models with biological relevance for in vivo cells, as well as structures such as bilayers, liposomes, and biomembranes [109,110,111].
An additional experimental advantage of RMs is represented by the optical transparency of the system, even at millimolar concentrations of peptides, allowing the spectroscopic analysis of water dynamics and active protein conformation in cell mimics.
The basic principles which make RMs of great interest as a biomimetic model are (i) confinement and the crowded cytoplasmatic environment, (ii) the molecular dynamics of water, and (iii) the biomembrane structure.
5.1. Confinement and Crowding
The confinement concept refers to the accessible volume within a “cage”, in contrast with dispersion throughout space. Starting from the definition of RMs as nano-pockets of water capped by a monolayer of surfactant, it is obvious that they are good candidates for a confinement model of macromolecules included in a restricted volume [112,113]. Even though there are some common features such as the closed geometry and limited size of macromolecules dimensions, many other aspects differentiate RMs from the biological environment, even when referring to the simple interface composed of one or two constituents, which is radically different from the complex multicomponent structure of biological walls/membranes [114].
In addition to the restricted volume, the intracellular environment is characterized by a high concentration of macromolecules which occupy the cellular volume in a range of 5–40% [115]. All macromolecules within this environment have different degrees of contribution to this high value; however, no individual species have a high concentration. Therefore, cytoplasmatic space is generally considered a crowded environment, but not a concentrated one [116,117]. The crowding phenomenon is a closely related concept to confinement, promoting nonspecific interactions between neighboring molecules [118,119].
The effects of macromolecular crowding on enzymes were experimentally determined using concentrated solutions of model “crowding agents” (e.g., poly(ethylene glycol), dextran, Ficoll, bovine serum albumin (BSA), or cellulose derivate) [120]. Having in mind that not all crowders are equal with respect to protein activity due to their specific interactions, the replacement of synthetic agents with a native-like medium composed of natural crowders (e.g., intracellular content) will lead in the future to a more realistic model of crowding and confinement inside cells (Figure 8).
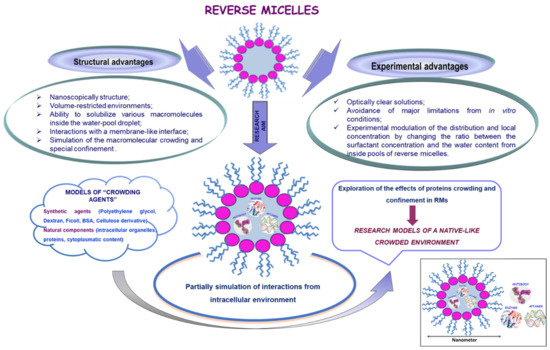
Figure 8.
Reverse Micelles—models of native-like crowded environments.
According to the excluded volume principle, defined as the volume occupied by some macromolecules but unavailable to others, it is considered that confinement has a main effect on the protein stability [121], whereas the crowding contribution is reduced. Instead, the crowded space is involved in the exclusion of volumes (e.g., water) from the proximity of one molecule by the presence of the other macromolecules (e.g., proteins, polysaccharides, or nucleic acids). It is assumed that the basic mechanisms involve (i) a reduction in entropic energy for protein folding cause by the limitation of possible protein conformations and entropy of the unfolded state, and (ii) a modification of water properties due to the vicinity and implication of the interface composed of surfactant and macromolecules, as well of ions [122]. Anticipating interconnected effects, these two phenomena are considered to affect to different extents the essential reactions and events in a living cell, as well as most of the protein features in cellular environments, such as protein folding and stability, conformational behavior, protein dynamics, and biological activity. It is worth mentioning their role in promoting protein conformation in a compact unfolded state and in favoring the dynamic process of transition from the unfolded to folded state of a protein, i.e., the folding rate [123].
5.2. Models for Molecular Dynamics of Water in Restricted Volume
The properties of pure water are generally related to its extended network of hydrogen bonds, while water from natural/biological systems is significantly different, mainly as a consequence of the truncated H-bond network caused by confinement, resulting in a reduction in the cooperative effects. The presence of an interface, which is in the most cases highly charged, and the nanoscale confinement imply the rearrangement of the H-bond network, resulting in the structural dynamics of water molecules in a bioenvironment being significant different from that in bulk water.
Reverse micelles are considered as convenient models for studying the structural organization and dynamics of water molecules in a restricted volume. In addition to their appropriate structure in a natural model, they provide a tunable system for monitoring water dynamics and the interfacial effects in such media [77]. Over the past few decades, the dynamics of water molecules inside RMs have been intensively explored, with a wide range of experimental techniques being applied to measure either the responses of individual molecules of water in RMs or the evolution of the hydrogen-bond network from inside the water pool [111,124,125,126,127]. The progress of physicochemical methods of investigation, especially in recent years, has also had an important impact on research in this area, with some of them contributing to the elucidation of the dynamics of water molecules in restricted media such as RMs. A wide range of experimental techniques have been applied to measure either the responses of individual molecules of water from RMs or the evolution of the hydrogen-bond network from inside the water pool; these include conventional and well-known infrared (IR) and Raman spectroscopy, femtosecond pump-probe, photon-echo, optical Kerr effect, and small-angle X-ray scattering (SAXS) experiments, sum-frequency generation (SFG) spectroscopy, and two-dimensional infrared spectroscopic studies [128,129,130].
The development of new methods based on known principles is aimed at improving the accuracy of the information offered. For instance, by coupling a mid-infrared pulse shaper to an SFG spectrometer, researchers achieved a two-dimensional sum-frequency generation spectrometer combining the advantages of two-dimensional (2D) IR spectra with the monolayer sensitivity of SFG [131,132,133]. Time-resolved sum-frequency generation spectroscopy, a fourth-order extension of conventional SFG spectroscopy, can be used to evidence the structural heterogeneity of water at various interfaces and to define the structural anomalies of interfacial water [134]. While far-infrared (far-IR), Raman, and two-dimensional IR (2D-IR) spectroscopy provide data related to the density of states in the low-frequency range but little related to the hydrogen-bond dynamics, three-dimensional IR (3D-IR) spectroscopy is a nonlinear method with low-frequency degrees of freedom, providing much more data related to intermolecular dynamics, i.e., homogeneously or non-homogeneously broadened, as well as to the hydrogen-bond dynamics in terms of non-Gaussian stochastic processes [135,136]. As a solution to the controversial discussion regarding the structure of water, 2D Raman terahertz spectroscopy has been proposed, which extends the multidimensional vibrational spectroscopy into the far-IR region. Two-dimensional Raman THz spectroscopy is useful for exploring the dynamics of the collective intermolecular modes of liquid water at ambient temperatures, as a consequence of the formation of hydrogen-bond networks. These modes allow elucidating the couplings and inhomogeneities with various degrees of freedom. The echo identified using the technique indicates whether a heterogeneous distribution of hydrogen-bond networks exists, with only a 100 fs timescale.
Molecular dynamics (MD) simulations [137] or MD correlations with vibrational spectroscopy [138], providing fundamental data regarding the physical characteristics of reverse micelles, as well as a newly created web site (http://frequencymap.org (accessed on 2 February 2021)), proposing to define vibrational spectroscopic maps [139], are other examples of approaches directed toward obtaining a clear, coherent, and sustainable image of the water dynamics in restrictive environments.
The experimental observables obtained using different methods do not always lead to the same model of structural organization and dynamics of water, whereby the authors generally explain the discrepancy as a function of the characteristics and particular approaches of the measuring techniques. For instance, absorption spectra and vibrational time decays discriminate various H-bonding environments in RMs, whereas orientational and spectral diffusion functions suggest a size-dependent dynamic which is not strongly influenced by the spatial location inside RMs.
Special steady-state spectra and population relaxation experiments are correlated with the energy level of the system, while spectral diffusion and orientational relaxation are generally governed by the structural evolution of global H-bond networks [139]. Numerous studies concerning water from the interior of RMs revealed an inhomogeneity of water characteristics defined by a multilayered structure, leading to the question whether water from RMs can be evaluated as a single species or as two or more separate populations [140,141,142].
On the basis of these different considerations, many research groups have suggested several models to formulate water inside nanodroplets, which have become landmarks in the complex approach to elucidating water behavior in RMs. The core–shell model [115,143] considers that water properties are influenced only locally, dividing the nanoscopic water pool into two sub-ensembles: the core, located in the center of droplet at some distance from the headgroups and characterized by properties similar to those of bulk water, and the shell, bound to the surfactant layer, modeled using the particular properties of water associated with the surfactants headgroups.
Applying this well-known model in their studies, Fayer [33,103,144] and Levinger [82] reported the results of experiments regarding water in RMs of different size (large, small, and intermediate), concluding that large AOT-based RMs consist of two ensembles of water, whereas only a single water structure was detected in small RMs. They defined the properties of water in the shell layer as corresponding to the characteristics of w0 = 2 RMs, where all water molecules interact with the surfactant interface. According to their conclusions, water in RMs of a medium size (approximately w0 = 10) presents an intermediate state between the collective dynamics (small RMs) represented by a coupled situation and the two-ensemble dynamics related to separation into components (large RMs). In this case, the interface water influenced the dynamics of water molecules situated at a distance from the interface, while the dynamics of all molecules did not correspond to one group, as seen in small RMs. Even if the water dynamics in medium RMs are separable into two components, the small size of the water pool does not allow the interface’s influence to entirely disappear, and a water core similar to bulk water is formed, as seen in large RMs.
Bakker and coworkers calculated the relative fraction of shell and core water in anionic AOT or cationic CTAB RMs and observed a size dependence of orientational dynamics on the distance from the interface [145,146,147]. On the basis of these results, the authors considered that all water molecules encapsulated in RMs, regardless of their spatial location inside the micellar pool, suffer modification of their vibrational relaxation rate according to the alteration of the H-bond network. These nanoconfinement effects are the basis for the so-called “homogeneous droplet” model [126], which considers all water molecules as being identical throughout the micellar droplet due to the rapid transfer of intermolecular energy. According to Piletic, the water inside a small RM shows a more homogeneous dynamic through the water pool due to a collective nature of the H-bond network [143].
The validity of the core–shell model was only experimentally confirmed for cases when the properties of micellar water molecules do not imply an H-bond network. Instead, the measurements of orientational relaxation were in good agreement with investigations of the complex rotational motion of molecules in supramolecular systems performed with the nanosecond fluorescence polarization technique. According to the “wobble-in-a-cone” model [148], the free rotation is restricted to an angular scale range within a cone caused by the orientational constraints imposed by the structural rearrangement of the H-bonds from the surrounding framework. Furthermore, the internal friction decreases the rate of reorientational motion inside organized structures compared to the expected value in an aqueous medium [149]. The model defines a correlation between the timescales required for the orientation of water molecules in a restricted structure and for complete orientational relaxation during the randomization of the whole network of H-bonds. The randomization process assumes the splitting of existing H-bonds and the formation of new ones in a similar way to the Ivanov jump reorientation model [150] and extended jump model proposed by Laage [140]. Thus, in small RMs, a separation of time scales has been detected because the limit of the cone angular scale is achieved before the finalization of total randomization, which is associated with an observed biexponential decay of reorientation. In contrast, no separation in timescales (single-exponential decay) was noticed in large RMs where a reverse situation is encountered (i.e., complete randomization first, followed by the limited cone angular scale being reached).
Moilanen identified two main factors that contribute to the transition from the bimodal nature of dynamics to a collective one: the mechanism of water reorientation and the nature of interface related to RM size [75]. The first is based on the model proposed by Laage and Hynes [140], which considers the reorientation of water molecules as an unlocated process, in which the reorientation of one molecule causes the rearrangement of approximately 16 other molecules from the first and second shells of solvation, thus affecting their dynamics. The most significant effect was observed in small RMs where the dynamics of most water molecules slowed down even when they were at a distance from the interface, while this effect quickly disappeared in the presence of a greater number of water molecules, as seen in large RMs. The second factor refers to the changing structure of the RM interface with the reduction in RM size, particularly with respect to the characteristics of the curvature and surface area per headgroup. Thus, large RMs (w0 ≥ 16.5) had a gentle curvature of the interface, showing an almost planar surface, while a decrease in RM size from w0 16.5 to 6 was associated with a significant surface curvature and an increased rigidity of interface structure caused by the simultaneous interactions of water molecules with multiple headgroups.
Two components of solvation dynamics have been identified in RMs: a fast one (sub-picoseconds) and a slow one (ranging from hundreds to 1000 picoseconds), which are not encountered in bulk water. To understand this special behavior of water in confined geometries, theoretical models have been used for molecular dynamics (MD) simulations such as simplified single-site or simple potential interaction models [67]. Even if not all interactions are taken into account, MD simulations are considered to be a qualitative model for solvation dynamics in a restricted environment. Among the many suggestions, there has been special interest in the MD simulation proposed by Senapathy and Chandra [151], which delimited the interaction contributions of water from the interfacial layer from other effects (e.g., interactions between water and surfactant molecules). In their MD simulations, Faeder and Ladanyi examined the effects of RM size, treating the water and surfactant headgroups at a molecular level only, while the interior was modeled as a rigid spherical cavity, with the surfactant tails and the nonpolar medium as a continuum. The simulations identified distinct water molecular layers independent of the RM size.
5.3. Biomembrane Structure
For a long time, only the protective role of biomembranes was taken into account, and their participation in other life processes was minimized. More recently, the dynamic role of biomembranes was reported, changing the concept of a passive matrix in the case of biomolecular reactions [152,153]. In addition to proteins, a large contribution of lipid bilayers has been identified in cellular activities to the mediation of cellular metabolism or transmission of information [154]. In some cases, the influence of biomembranes on the evolution of cellular life has also been shown, including in the malignancy process where significant differences between normal and malignant cells were detected at this level [10,152].
In living cells, the transmembranal flow of various compounds (e.g., metabolites and nutrients) assumes the rearrangement of the amphiphilic phospholipid bilayer. Different intermediate membranous structures with a carrier role have been identified, including reverse micellar structures. Their similarity with natural structures from the membranes of living cells unequivocally confers them with the status of a membrane-mimetic system.
For example, Hamada et al., constructed a biomimetic water-in-oil microdroplet membrane based on ternary lipids of dioleoyl l-α phosphatidylcholine/saturated dipalmitoyl l-α phosphatidylcholine/cholesterol, to study the lateral localization of the amyloid β peptide at this heterogeneous membrane interface in the presence and absence of a ganglioside lipid raft [155]. Amyloid β peptide was used for selective association on the constructed membrane surface, due to the important role of this peptide in Alzheimer’s disease pathology, where it was considered that the interaction between this peptide and a lipid raft consisting of ganglioside leads to an acceleration of amyloid β peptide aggregation. Thus, the authors proved that the biomimetic lipid raft was achieved using ternary lipid membranes covering microdroplets of olive oil or dodecane, thereby obtaining ordered lipid structures [155].
The molecular diffusion behavior was studied by Harusawa et al. using a highly concentrated solution of polysaccharide dextran and its monomer unit, glucose, inside cell-sized droplets covered with a lipid layer of phosphatidylcholine [156]. The authors modeled an intracellular environment by crowding micrometer-sized polymer droplets covered with a lipid layer in order to investigate the effect of membrane properties on molecular diffusion. Through the addition of poly(ethylene glycol)-conjugated lipids to the phosphatidylcholine membrane, the degree of slow diffusion was altered inside of the small droplets of concentrated dextran. Harusawa et al. demonstrated that droplet size-dependent slow diffusion is unique in polymer solutions; thus, by controlling the surface properties and sizes of membranous and non-membranous organelles, the molecular diffusion can be regulated, also in addition to the polymer concentration and numerous phase behaviors such as liquid–liquid phase separation, with important applications in the fabrication of biopolymer gels/droplets, artificial cells, and other small polymer materials [156].
The effects of droplet size and temperature on the dynamic behaviors of an aqueous solution of hydroxypropyl cellulose (HPC) coated with phospholipids in oil (water-in-oil droplets) were studied by Yoshida et al. [157]. The beginning of phase separation of the HPC solution, one of the most representative biocompatible polymers, with its main component (multi-sugar chain) being found in living cells, was observed by the authors, who demonstrated that the start time of phase separation is decreased with the increase in droplet size [157]. This study of cell-size confinement effects on the dynamic behaviors of biocompatible polymers highlighted the importance of droplet size and of the lower critical solution temperature for the dynamic phase behavior, not only for artificial cell construction, but also for understanding the physicochemical properties of living cells [157].
The well-known RMs consisting of AOT–isooctane–water were also used for studying the insertion of myelinic protein into cell biomembranes [158,159].
With the reconsideration of the role of biomembranes, including their mimics, RMs are no longer treated as simply passive aggregates with interesting structures. A substantial argument in this regard is supported by the discovery of the possible initiation of self-replication of RMs according to the reaction that occurs within micellar structures [160,161].
The recognized status of RMs as a membrane mimetic was valorized in the investigation of different membranal processes such as electron transfer [162], as well as in the evolution of micellar structure or intermicellar interactions. In the latter case, the structure of RMs, in particular AOT RMs, is represented by a hard sphere of repulsive forces (charged surfactant headgroups) [163], operating with a tight attractive outer shell (surfactant tails). Interactions between RMs mainly involve the mechanism of collision–fusion–fission for the exchange of internal content [164]. The first step implies Brownian motion, the second step is characterized by attractive energy and adhesive interactions at the micellar surface level in the absence of Coulomb rejection, and the third step is a result of the short-life of the instable dimer formed [165,166].
Useful information regarding various processes in RM systems, as well as forces and interactions between RMs, has been obtained from examinations performed using simple and rapid electrochemical techniques such as steady-state microelectrode voltametric investigations. Furthermore, a significant scientific contribution allows providing the electroactive probes located at different redox active sites (e.g., aqueous core and internal or external micellar surface) in membrane mimetic structures [167].
Therefore, the importance of RMs as a cell membrane-mimetic system is increasing, being a convenient experimental approach to reproduce, even in part, such a complex supramolecular organization in an artificial system [168]. Some researchers even proposed RMs as a model for the very first membranous structures, i.e., the primitive membranes that supported the self-replication cycles at the beginning of planet [10,169]. Due to their involvement in reactions that precede the process of life, in the opinion of many researchers, RM structures may be placed at the interface between “nonliving” and “living” [160,161].
6. Potential of Reverse Micelles in Innovative Applications
Reverse micelles represent self-organized aggregates with nanometer-sized confined water pools, having great potential for designing and creating subsystems, such as artificial cells, containers, and vehicles from nonliving materials with a wide range of applications (nanotechnology, food, medical science, cosmetics, etc.), due to their characteristics, such as thermodynamic stability, spontaneous formation, large surface area (102–103 m2∙cm−3), low interfacial tension (<10−2 mN∙m−1), transparency, nanometer size (<100 nm), and a highly dynamic nature.
In the past three decades, RMs have been used in different scientific fields for enzymatic catalysis [170], as models of membrane systems for the separation of proteins [171], for enzyme immobilization and storage of their activity [172,173], for studying changes in the protein structure and modeling intracellular crowding [174,175,176,177,178], for encapsulation of drugs and essential oils into hydrophilic water cores [179,180,181], for enhancing and tailoring the luminescent properties of fluorescent compounds [182,183,184,185], for protein refolding [186,187], and as nanoreactors [188,189,190] (Figure 9).
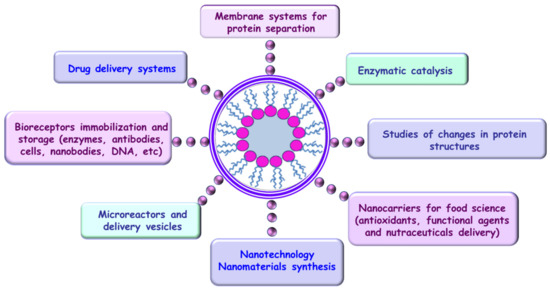
Figure 9.
Self-assembly and application fields of reverse micelles.
Below, the potential of reverse micelles is highlighted for the developments of nanotechnology applied in fields of great interest, in addition to their contribution to opportunities in other domains.
6.1. Development of Nano-Synthesis Using RMs
The large fields of nanotechnology and nano-based sensors have attracted increasing interest in the last few decades. The use of nanotechnology for (bio)sensor production led to an extensive range of applications, allowing miniaturization and enabling their integration in many other devices.
The development of nano (bio)sensors is based on the use of nanomaterials either as directly sensing elements or as associated materials to detect specific molecular interactions occurring at the nanoscale.
Chemical and physical methods have been the most used techniques for the fabrication of well-defined inorganic nanomaterials. Chemical vapor deposition, electrodeposition, and metal–organic vapor deposition based on ionic, atomic, or molecular precursor decomposition also represent viable routes for obtaining ordered nanoparticle networks [191]. Since the special properties of nanomaterials (mechanical, chemical, physical, optical, electrical, magnetic, thermal, and biomedical) are strongly dependent on the size, composition, and shape of the nanostructures, different approaches have been taken into consideration for controlling the nanomaterial fabrication.
The high surface-to-volume ratio and the novel physicochemical properties of the nanomaterials have led to the achievement of high performance in terms of sensitivity, selectivity, and detection limits; therefore, nano (bio)sensors have found application in various fields, including clinical diagnosis, environmental monitoring, food quality control and processing, pharmaceuticals, and agriculture, through onsite, in situ, and online determinations [192].
Thus, in the last few years, considerable attention has been paid to the design and synthesis of nanomaterials (nanoparticles, nanodots, and nanotubes) with specific properties for advance applications by using organized self-assembled microemulsions [37]. Reverse micelles represent nanoscale hydrophilic cavities of microemulsions and an environmentally friendly alternative used for the first time as nano-templates for nanomaterial synthesis, thus resulting in nanostructures of palladium, platinum, and rhodium being obtained for the first time [37].
6.1.1. Reverse Micellar Routes for Nano-Synthesis
While nanostructures with well-defined composition, size, and morphology represent a general aim in synthesis, the formation of a biomimetic structure still represents a challenge in this domain. The re-creation of the relationship between structure and function which characterizes living entities, defined as ”biomimetics” (term proposed by Schmitt in the 1960s) [192], can be achieved via different synthesis approaches. Zan and Wu proposed a classification into functional biomimetic synthesis (FBS), related to mimicking properties, and process biomimetic synthesis (PBS), related to mimicking methods [193].
A large spectrum of biomimetic models is available, but special attention is currently directed toward reverse micelles (RMs), which are generally recognized as a template for cellular structure, including the intracellular medium. On the other hand, RMs are also regarded as ideal templates for nanoparticle synthesis, because each RM can isolate a particle inside its water core, thus preventing particle aggregation. As such, the homogeneity and monodispersion of RMs can be used to control the size of nanostructures, while also maintaining their size distribution, whereas various structures can be used as templates for different morphologies of the same nanomaterial.
The special characteristics of RMs, with respect to their aggregates and the dynamics of the system, have been valorized in the so-called reverse micellar route for the synthesis of nanomaterials [194,195,196], which is considered a bottom-up approach for manufacturing nanomaterials. This approach implies the self-assembly of small components into larger organized systems involving either natural principles or external forces. Synthesis via RMs, sometimes called the microemulsion technique, represents a successful approach allowing the restriction of particle size distribution, as well as prevention of their agglomeration. Generally, it is considered a versatile technique as it is able not only to control the properties of the nanoparticles, but also to vary them across a broad range of sizes and morphologies due to the variety of surfactant aggregate structures that can be used as templates. The RM approach can be considered as a version of biomineralization whereby the biomacromolecules, organized in aggregates, modulate the nucleation, growth, and type of minerals of inorganic origin [197]. Additionally, it is worth mentioning that this route does not imply the use of special equipment, which is an advantage from an economical point of view [198,199].
Two methods have been identified for the application of the technique, according to the number of microemulsions involved in the nanoparticle synthesis and the process governing the particle formation [200].
The first microemulsion method is an additive method where it is assumed that a reactant (precursor for particle formation) is contained inside the water droplets of the microemulsion. The initiation of the reaction leading to particle formation is achieved by using an energy-triggering agent (e.g., pulse radiolysis, laser photolysis, ultrasonication, or temperature elevation [15]) or by subsequently adding, directly into the pre-existing microemulsion, another reactant, usually a precipitating agent in liquid (e.g., a metal-containing solution) or gaseous (e.g., NH3) phase. Both approaches within this first method, i.e., using an energy-triggering agent or subsequently adding a reactant, are based on diffusion processes inside the reactant-containing microemulsion.
The second method involves the mixing of two separate microemulsions, each one containing a different reactant, usually a metal/metal complex and a reducing agent, followed by their reaction and particle formation. This method is based on the dynamic characteristics of RM systems supported by Brownian motion, which enable intermicellar collisions and ensure the energetic level necessary for the fusion–fission events, thus resulting in the mixing of the intramicellar content of two separate microemulsions [6].
A third alternative, less commonly used, involves three microemulsions: two microemulsions with two different metal salts and one microemulsion with the reducing agent. Regardless of the method type, the reaction is finalized with the formation of solid nanoparticles inside the water pool of RMs, and this suspension simultaneously exists with any of the microemulsion that did not take part in the reaction.
It is generally accepted that the overall kinetics of nanoparticle formation is ruled by the rate of intermicellar exchange, implying the transport of reactants through the nonpolar phase and the fusion–fission process. While this overall process is the rate-limiting step of the reaction, the initial size of water droplets has a significant influence on the final size of the formed nanoparticles [201].
In addition to its well-recognized advantages of homogeneity and monodispersion, the RM route for NP synthesis enables the variation of morphology, as well as its control, through the size and shape of RM aggregates. The nature of the RM components (mainly the surfactant structure), the surfactant interfacial properties, and the hydration degree, which are specific parameters of RMs, are generally accepted to be responsible for the fine-tuning of RM structure and, thus, are of great interest for NP synthesis.
6.1.2. Nanomaterial Synthesis and Development of Nano (Bio)Sensors Using RMs
Understanding the molecular basis of these reverse micelle systems is an absolutely necessary condition for the subsequent development of highly pure nanostructured systems, with a well-defined control of their morphology and special properties, leading to advanced applications in various fields of interest.
The design and the synthesis of nanomaterials with specific properties using reverse micelles have been research topics of great since the 1980s, when platinum, palladium, and rhodium metal nanoparticles were first prepared. Reverse micelles represent useful multimolecular structures which offer significant opportunities in designing nanostructured materials with well-defined and uniform properties. However, the desire to use these nanomaterials for advanced applications is limited by the control of their morphology.
For example, the most encountered problems in applications of nanoparticles and nanotubes synthesized using RMs are represented by the impurities (stabilizing ligands and reductants) which must be introduced into the system in large excess. It is well known that these impurities negatively influence the properties of the prepared nanomaterials, especially in relation to their reactivity and stability.
Therefore, the application of nanomaterials synthesized using RMs to the design of nano (bio)sensors is still a new area, with most reported devices being gas sensors and batteries with a limited range of applications in catalysis, pigmentation, detergency, and printing. Nevertheless, over the past 20 years, even if remarkable progress was reported in laboratories, the industrial application of nanomaterials synthesized using RMs remains restricted by the lack of technological advancement in the control of their properties, compatible with large-scale production.
This review highlights the potential of micelles in innovative applications for the development of new nano (bio)sensors, in fields of great interest, such as nanomedicine, food, and life security.
Different approaches to nanoparticle synthesis using RMs have been reported in the literature, using different materials, such as metals [202,203,204], metal oxides [205,206,207], nanocomposites [208], and supported catalysts [209,210].
Microemulsions represent thermodynamically stable dispersions of amphiphilic surfactants, with the reverse micelles microemulsions being formed when water is dispersed in hydrocarbon-based continuous phases. Thus, a hydrophilic headgroup region is formed, surrounding a nanometer-sized water core, with hydrophobic tails extending in the continuous phase [100]. These systems are dynamic, described as nanoreactors, whereby micelles collide due to Brownian motion and coalesce to form dimers, which exchange contents and then break apart again, a process which usually occurs on a very short timescale (less than 1 ms) [211,212]. This process is very important for nanoparticle synthesis, with micelles in these systems providing the appropriate environment for nucleation and growth control, where the last stage is stabilization of the system via the addition of a surfactant, thus preventing the aggregation of nanoparticles [211]. Moreover, the modification and control of RM structures can be achieved via the addition of a cosurfactant [213].
Among the different surfactants used in formulating microemulsions for nanoparticle synthesis, the anionic double-chained sodium bis(2-ethylhexyl) sulfosuccinate (AOT) and nonionic polyoxylethyl-5-nonylphenyl ether are the most used surfactants, due to their ability to form thermodynamically stable reverse micelles and microemulsions, through solubilization of high amounts of water in different hydrophobic organic solvents and without the need for other cosurfactants [214].
The recovery of nanoparticles from reverse micelles represents a key step, with the most commonly used methods being flocculation [215], evaporation to dryness [216], and addition of certain chemical reagents [217], which undergo phase separation and simultaneous precipitation of the surfactant.
Different nano (bio)sensors have been developed using nanoparticles, nanotubes, or quantum dots obtained via the reverse micelle method, for sensitive detection of sugar, amino acids, hydrogen peroxide, hydrogen, nitric oxide, phenolic compounds, or biotin–streptavidin. Their advantages include lowering the overpotentials of many electrochemical reactions, becoming reversible redox reactions that are typically irreversible in conventional unmodified electrodes, thus enhancing the electron transfer between biomolecules. A schematic representation of nano (bio)sensor design using reverse micelles entrapping nanomaterials and bioreceptors is given in Figure 10.
Figure 10.
Nano (bio)sensor design using reverse micelles.
Thus, Ferreira et al., reported an electrochemical sensor based on rhodium nanoparticles for the determination of some phenolic compounds in a cellulose matrix [218]. Rhodium nanoparticles obtained via the reduction of rhodium ions entrapped in reverse micelles, and stabilized in 3-(1-tetradecyl-3-imidazolium) propane-sulfonate surfactant were used for coating the surface of a glassy carbon electrode [218]. These metallic nanoparticles, stabilized with the zwitterionic surfactant, were found to lower the electron transfer resistance at the electrode surfaces by enhancing the electrical conductivity at the electrode surface. By using this sensor, the authors reported the determination of p-coumaric acid in a cellulose matrix, with good reproducibility and reproducibility, at a detection limit of 0.6 µmol·L−1 [218].
Palladium and gold nanoparticles prepared using RMs and stabilized using the same surfactant were applied in the development of electrochemical sensors for hydroquinone and catechol determination [219,220]. Zapp et al. reported the modification of a glassy carbon electrode with a bio-complex based on palladium nanoparticles stabilized with zwitterionic surfactant for simultaneous electrochemical determination of catechol and hydroquinone in cigarette residue samples [219].
A biosensor for the determination of hydroquinone was developed by Fernandes et al., via the immobilization of peroxidase onto gold electrodes, using a solution of well-dispersed gold nanoparticles stabilized with zwitterionic surfactant [220]. The developed biosensor was used for electrochemical determination of hydroquinone in skin-brightening creams, at a low applied potential of 0.09 V vs. Ag/AgCl, with a detection limit of 0.188 µmol·L−1 [220].
A nanocomposite based on PdO-loaded SnO2 nanoparticles was prepared by Yuasa and coauthors, by precipitating Pd(OH)2 and Sn(OH)4 inside an RM, followed by calcination [221]. The obtained nanocomposite was further used to design a thick film sensor device via the screen-printing method for CO gas sensing. Tin oxide (SnO2) represents an attractive material for designing semiconductor gas sensors because of its physicochemical properties, high sensitivity, and chemical stability. The authors showed that the electrical resistance and the response of the designed sensor was highly dependent on the dispersion state of PdO onto SnO2 [221]. By loading PdO onto SnO2, the electric resistance increased, because PdO acted as a stronger acceptor of electrons and removed electrons from the oxide; however, when PdO was reduced to Pd via contact with reducing gases, the electric resistance decreased due to back electron transfer from Pd to SnO2. Usually, a large difference in the electrical resistance of SnO2, due to the oxidized/reduced states of Pd, leads to a high increase in response to reducing gases [222,223].
An oxygen reduction-based electrode was designed via modification with carbon-supported LaMnO3 nanoparticles by Yuasa et al. [224]. The nanoparticles of LaMnO3 were easily prepared via a reduction–oxidation reaction between Mn(NO3)2 and KMnO4 precursors in RMs [224,225]. The black carbon dispersed in cyclohexane was added into reverse micelle solutions containing the precursors, while ethanol was used to break the micelles and to directly precipitate the carbon-supported LaMnO3 precursors. The gas-diffusion-type electrode prepared via modification with LaMnO3 nanoparticles showed high oxygen reduction activity [225].
Nanoparticles of palladium and platinum represent the two most preferred catalytic metals with a high capacity for absorbing hydrogen, thereby considerably improving the sensitivity of hydrogen detection [226,227]. Thus, Grym et al. reported the development of a hydrogen sensor based on semiconductor Schottky barriers and electrophoretic deposition of palladium nanoparticles obtained via the RM method onto InP substrates [228]. Colloidal solutions of spherical palladium nanoparticles with diameters of 7 to 10 nm, dispersed in isooctane, were prepared by mixing two reverse micelle solutions, with the same molar ratio of H2O to AOT, one containing Pd(NH3)4Cl2 and the other containing hydrazine as a reducing agent. Electrophoretic deposition of the obtained palladium nanoparticles was achieved in a cell with two electrodes, by applying an electric field of 2000 V/cm, for about 50 ms [228]. One of the electrodes was fabricated from pure graphite, while the other electrode was prepared using an epi-ready InP substrate, with the distance between electrodes being maintained at 1.5 mm. The detection limits obtained using these diodes for hydrogen was about 1 ppm H2/N2 [228,229].
Copper sulfate was used by Davarpanah et al., as a precursor for obtaining CuO nanoparticles in RMs, using Tween-80 as a surfactant [230]. The gas-sensing properties of the as-obtained CuO nanoparticles were further investigated by the authors, by designing sensors for the detection of NO2 and alcohols. The NO2 detection was performed with a low energy consumption and operation temperature [230].
Optical sensing devices have been developed based on thin films of CdS nanoparticles obtained using the RM technique [231]. The authors succeeded in immobilizing the CdS nanoparticles synthetized in RM solution into films using AOT as a stabilizer, onto solid surfaces (Si wafers), avoiding the modification of the nanoparticle properties as a sensing medium. The obtained nanoparticles were cast into films and immobilized onto solid surfaces using porous polymeric and sol–gel matrices, before being exposed to several organic compounds (hydrocarbon compounds). Since these nanoparticles showed a relatively bright photoemission, the designed sensor was used to identify the target compounds and their specific concentrations on the basis of photoluminescence detection [231].
The development of uniform magnetic nanoparticles represents an important issue in designing ultrahigh-density magnetic sensors. The most used magnetic oxide materials in the development of magnetic nanoparticle-based sensors are magnetite (Fe3O4), cobalt ferrite, nickel ferrite, and zinc ferrite (MFe2O4—where M is a metal such as Co, Ni, or Zn), as it is well known that the magnetic hardness of these materials is defined by the anisotropy of nanoparticles within an exchange length [232]. There are different approaches reported in the literature for the synthesis of magnetic nanoparticles for sensor development, including thermal [233] and sonochemical [234] decomposition of organometallic precursors, high-temperature reduction of metal salts [235], and reduction inside of RMs [236].
Wiedwald et al., reported the preparation of monoatomic (Fe, Co) and bimetallic (Fe–Pt and Co–Pt) nanoparticles with diameters of 2–12 nm, by loading precursors into RMs [237]. The authors used polystyrene-block-poly(2-vinylpyridine) and polystyrene-block-poly(4-vinylpyridine) in anhydrous toluene to form spherical reverse micelles. The pyridine groups of the micelle core allowed selective loading of the precursors, FeCl3 and CoCl2 [237]. In the case of bimetallic nanoparticles, platinum precursors (H2PtCl6, PtCl4, and K[PtCl3C2H4]·H2O) were added first to the micelle solution, which was stirred until loading was complete, followed by the addition of FeCl3 or CoCl2. The deposition of the loaded reverse micelles onto different supports was accomplished by immersing the substrates into the micelle solution and extracting them under ambient conditions with a constant controlled rate [237].
Conducting polymer nanostructures (nanofibers, nanowires, and nanotubes) have also been widely studied for versatile sensor development, due to their properties, such as a reversible signal transduction mechanism, tunable sensitivity, and design flexibility in sensor applications [238,239,240]. Polypyrrole and polyaniline nanotubes have been fabricated inside the cylindrical pores of templates [241,242,243].
Ellipsoidal nanoparticles, nanorods, and nanotubes of poly(3,4-ethylenedioxythiophene) (PEDOT) were fabricated by Yoon et al. on the basis of chemical oxidation polymerization in RMs [244]. The AOT surfactant and an aqueous solution of FeCl3 in hexane were used for the preparation of cylindrical reverse micelles. The obtained PEDOT nanostructures were immobilized onto a microelectrode substrate through a casting solution, with the nanotubes forming networks which provided excellent performance for the developed sensor. The chemical sensors based on PEDOT nanotubes were applied for the detection of alcohol vapors, with their responses being reproducible and reversible [244].
Quantum dots (QD), known as semiconductor nanoparticles, with dimensions ranging from 2 to 20 nm, represent crystalline clusters having great potential for application as fluorescent labels for molecular, cellular, and in vivo imaging determinations. QDs are more stable and cheaper compared to existing labels (fluorescent dyes) [245]; furthermore, due to their special optical and electrochemical properties (such as narrow and size-tunable emission spectra, high stability, excellent resistance to chemical degradation, and broad absorption spectra) [246,247], QDs allow greater flexibility, faster binding kinetics, higher sensitivity, and higher reaction rates for many types of analysis, from immunoassays to DNA assays [248]. QDs can be prepared from different materials, such as metals (gold, silver, or cobalt) [249,250], semiconductors (CdS, CdSe, or InP) [251,252], and insulators (Fe3O4 or SnO2) [253,254], through organometallic thermolysis [251], electrochemical deposition [255], or colloidal self-assembled pattern formation using surfactant micellation [256]. Using different QDs and electrochemical detection methods, multiple analyses can be performed, with applications in gene expression, high-speed screening and medical diagnostics, and environmental and food analysis [248].
Merkoci et al., used QDs based on cadmium, lead, and zinc sulfide nanoparticles for electroanalytical applications. The quantum dots were obtained using the RM method, adding cadmium, lead, or zinc nitrate and sodium sulfide to an AOT/n-heptane water-in-oil microemulsion [257]. Conjugates of CdS, PbS, and ZnS with DNA were prepared by using aqueous solutions of each nanoparticle and thiolated oligonucleotide samples. Subsequently, the detection of DNA was achieved by the authors using streptavidin-coated magnetic beads and CdS QDs [257].
In order to increase the colloidal stability of QDs and to decrease nonspecific adsorption, different strategies have been reported related to the encapsulation of QDs into micelles or other particles. Thus, Dubertret et al. encapsulated fluorescent semiconductor nanocrystals into lipid micelles, comprising n-poly(ethyleneglycol), phosphatidylethanolamine, and posphatidylcholine, with the advantage of these micelles being their very regular size, shape, and structure [258]. Electrochemical applications were developed as a function of the encapsulation of QDs, such as CdS, ZnS, and PbS nanoparticles, within polystyrene beads, in order to create an electrochemical code library [259]. Wang et al. developed encoded redox beads for electrochemical identification, as a function of the encapsulation of different QDs into polystyrene microspheres [204]. The obtained redox-encoded nanoparticles were further used as label sensors, for a large number of recognizable voltametric signatures in the cover-tagging of commercial products [259].
The use of metallic nanoparticles manufactured via the reverse micelle technique in biosensing and biomedical applications has increased in the last few years. Reverse micelles have been used as nanoreactors not only for the synthesis of metal nanoparticles, but also for the preparation of enzyme-immobilized metal nanoparticles in order to increase enzymatic activity and improve their durability [11,260].
Jung et al., reported the synthesis of uniform spherical magnetite nanoparticles, with good size uniformity and a highly crystalline nature (<3 nm), in reverse micelles composed of oleylamine, F127, xylene, and water, in a reaction of iron(III) stearate with hydrazine at 90 °C [260]. The authors evaluated the cytotoxicity of the synthesized magnetite nanoparticles using human adipose-derived stem cells (hADSCs), with the apoptotic activity and cell proliferation behavior of hADSCs being determined at the gene and protein levels after exposure of the cell to magnetite nanoparticles. In this synthesis, oleylamine acted as a major surfactant to produce monodispersed nanoparticles, while F127 ensured the stability of reverse micelles in the xylene solution. By controlling the experimental conditions, the authors succeeded in controlling the size of magnetite nanoparticles.
Preparation of enzyme-immobilized magnetic nanoparticles using the reverse micelle system was reported by Yi and coauthors [261]. Lipase was covalently immobilized on functionalized Fe3O4 magnetic nanoparticles in RMs, with the authors reporting an enhancement of lipase activity in RMs, due to changes in its secondary structure [261].
6.2. Contributions of RMs to Modern Extraction and Purification Techniques
The extraction of proteins from food using RM techniques consists of two steps: a forward extraction that solubilizes proteins in the aqueous core of RMs, and a backward extraction that recovers the solubilized proteins from RMs [262]. Some reports demonstrated the importance of surfactant structures in RMs for forward and backward extraction processes [263,264]. It was reported that gemini surfactant-based RMs showed higher efficiency in extracting proteins, such as egg white ovalbumin and pineapple peel bromelain, considering that the spacer group of gemini surfactant could facilitate its interaction with proteins by increasing the charge densities of headgroups [263,264].
Zhu and coauthors demonstrated that defatted wheat germ proteins extracted using AOT-based RMs possessed better functional properties and a more compact and ordered conformation compared with proteins obtained via alkaline extraction and isoelectric precipitation (AEIP) [265]. Another study performed by Zhao et al. compared the secondary structures of walnut protein fractions (albumin, globulin, prolamin, and glutelin) prepared using two extraction methods, i.e., AOT-based RMs and AEIP [266]. The authors demonstrated the potential of the RM technique to modify the surface properties of all tested protein fractions.
The same author reported in another publication that AOT-based RMs could improve the functional, nutritional, and flavor properties of soyabean proteins compared to the AEIP method [267]. Zhao et al. indicated that improved functional properties included the nitrogen solubility index, oil absorption capacity, foaming and emulsifying capacity, and stability, while better nutritional characteristics included the total and essential amino acid contents, amino acid score, and biological value [267].
The extraction and the recovery of erythromycin and amoxicillin using reverse micelles were carried out by Chuo et al. using an eco-friendly sophorolipid biosurfactant [268]. The use of biosurfactants, such as rhamnolipids and sophorolipids produced by nonpathogenic yeast Candida bombicola, instead of chemical surfactants makes the reverse micelle extraction environmentally friendly [268,269]. The authors demonstrated that the extraction and the recovery of erythromycin and amoxicillin with the sophorolipid reverse micellar system were affected by solution pH, ionic strength, and sophorolipid concentration, with the extraction process being controlled through electrostatic interactions between antibiotics and sophorolipid headgroups [268].
6.3. Natural Approach Using RMs as Microreactors/Delivery Vesicles
The development of accessible synthetic systems requires a natural approach represented by the self-assembly of small molecular components, such as phospholipids, into discrete, capsule-containing architectures and vesicles. Microdroplets of water in oil have been studied for their potential to be used as cell models or microreactors [270,271,272,273], due to their advantages such as easy manipulation or fusion, good resistance to osmotic and physical stresses, possibility of encapsulation without denaturation of the molecules of interest, and potential to conduct experiments under physiological ionic conditions [274]. A schematic representation of delivery systems which can be developed using reverse micelle molecular components is given in Figure 11.
Figure 11.
Development of delivery systems using reverse micelles.
Hase et al., developed a microstructure using cell-sized phospholipid-coated microdroplets (CPMDs), consisting of a water-in-oil droplet with a phospholipid layer present at the oil/water interface, as a model for living cells [274]. The phospholipid-coated microdroplets were obtained by ultrasonication at 50 °C of a mixture consisting of 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine and mineral oil. The authors described in their report the manipulation of phospholipid-coated microdroplets by using laser tweezers, and monitoring the time of biochemical reactions in a single phospholipid-coated microdroplet obtained via controlled fusion of two CPMDs containing a substrate and a specific enzyme, such as calcein production in the presence of esterase and green fluorescence protein expression [274].
The possibility to create giant phospholipid vesicles that encapsulate long DNA molecules was studied by Kato et al., in order to elucidate the characteristics of the conformational behavior of DNA molecules in a confined space [275]. The authors encapsulated a bacteriophage DNA labeled with fluorescent dye in a water-in-oil microdroplet, and they further investigated the DNA distribution and conformation within the droplet. In their studies, the authors showed that the presence of magnesium ions induced the adsorption of DNA onto the inner surface membrane of a droplet based on phosphatidylethanolamine, while no adsorption was observed onto a phosphatidylcholine membrane. Furthermore, the presence of spermine induced a folded conformation of DNA in bulk solution; however, when the molecules were encapsulated in a microdroplet, DNA was adsorbed onto the membrane surface with concomitant unfolding of its structure [275].
In the last few years, biodegradable amphiphilic polymers, such as polycaprolactone (PCL), polylactic acid (PLA), and polyethylene glycol (PEG), have been used to form micellar structures with internal hydrophobic and external hydrophilic surfaces in aqueous solutions, with the hearts of the micellar structures being able to entrap different compounds with very low solubility in water [276,277,278].
For different biomedical, food, security, and agri-food applications, the development of new techniques for delivering functional ingredients without compromising their functionalities is absolutely necessary. Figure 12 presents the principle of designing an RM system encapsulating an antimicrobial bioactive compound, enriched with photosynthesized metallic nanoparticles, which can be used in different scientific fields.
Figure 12.
Encapsulation of antimicrobial bioactive compounds in reverse micelles.
On the basis of this principle, Jalilzadeh et al. designed self-assembling micelles using biodegradable triblock (PCL–PEG–PCL) and pentablock (PLA–PCL–PEG–PCL–PLA) copolymers, providing an advanced vehicle for the delivery of auraptene, a coumarin therapeutic agent used in breast and colon cancer [279]. The authors investigated the impact of the encapsulated form of this natural compound on the inhibition of colon cancer cell proliferation, demonstrating an improvement in therapeutic activity for the auraptene-loaded pentablock micelles formulated.
Encapsulation of morin, a natural bioflavonoid compound, into water-in-oil reverse micelles was performed by Rahdar et al., investigating the photophysical properties of morin at different concentrations within AOT reverse micelles prepared in a water-in-decane microemulsion system [280]. Morin is a flavonoid found in almond, fig, Indian guava, and other Moraceae family plants, used as food and traditional herbal medicine, showing multiple biological activities such as anti-inflammatory, antifungal, anticancer (breast, lung, and colon) and antibiotic properties [281,282,283,284].
The authors also investigated the optical properties (linear and nonlinear) of morin within water nanodroplets by modifying its concentration at a constant molar ratio of water/AOT/decane [280]. The linear and nonlinear properties of morin were analyzed, indicating a lower stabilization of morin in the microenvironment and the formation of H-bonds in a nano confinement with different structures. The authors demonstrated that an increase in Morin nanodroplet concentration led to an increase in aggregation, as well as an increase in the temperature of the environment [280].
Chen et al., developed reverse micelle-based water-soluble nanoparticles with confined nanoscopic aqueous pools using a versatile arm-cleavable interfacial cross-linked reverse micelle precursor for bioimaging and drug delivery [174]. The authors reported the transfer of RMs from organic media to an aqueous phase via the hydrolysis of acrylamide-based cross-linked water-soluble nanoparticles. The fluorescent reagent 8-hydroxy-1,3,6-pyrenetrisulfonic acid trisodium salt (HPTS) was encapsulated in the core, and gemcitabine, an anticancer drug, was covalently conjugated onto the external surface. This nanoplatform developed by Chen et al. exhibited a high imaging effect and anticancer activity for non-small-cell lung cancer cells [174].
6.4. RMs—An Opportunity in Food Science
The research into and the application of reverse micelles in food science have been accelerated in the last decade [285,286,287], for use in food protein extraction, simultaneous extraction of oils and proteins, utilization and development of effective antioxidants, and enrichment of food-derived components for analysis and quantification [11]. Sun and Bandara in their review presented the important research progress regarding the use of RMs in food science, additionally evaluating the opportunity of RMs as nanocarriers for delivering functional ingredients or nutraceuticals [11].
Nevertheless, the research on applications of reverse micelles as nanocarriers in food science is still limited, but the expertise and knowledge from their applications in drug delivery can be adopted, so that reverse micelles can be developed as nanocarriers for delivering food-derived bioactive compounds, offering a powerful strategy for the food and natural health product industry [11].
6.5. RMs—A Possible New Paradigm for Modern Biocatalysis
Specific chemical and physical properties of the intracellular environment, derived from the real structure of living cells, have a great impact on the particularities of proteins involved in a wide variety of biochemical events inside cells, from macromolecular recognition to biocatalysis associated [1] with biological systems, e.g., lipid layers and membranous organelles [2].
Taking into account the compact and heterogeneous nature of the nano-restricted environment inside living cells, delimited by the cellular membrane, it is obvious that conventional biological/biochemical studies in vitro confer only a basic view [107] regarding the intrinsic information of biomolecular structure and function. The essential differences are caused by their isolation through extraction procedures and the removal of macromolecules from their natural matrix, accomplished due to the loss of a structured microenvironment and partial alteration of the native structure and functions in vivo. In addition, to avoid aggregation problems and to minimize the nonspecific interactions that occur at high concentrations, experiments are generally performed in a diluted buffer, which is much too simple an environment compared to the compositional complexity of the intracellular medium.
In line with the idea that typical experiments in vitro represent only a relative approximation of the real intracellular protein activity and functions, a challenge for modern research in the biocatalysis field is represented by performing enzymatic reactions in a biomimetic medium, using a supramolecular system to model the simple cell-like structure/biological membrane/microsurrounding medium inside the cells [288,289].
Therefore, reverse micelles (RMs) are considered simplified cell mimetics by reproducing a complex supramolecular organization in an artificial system that limits many of the drawbacks. The similitude between their intramicellar and intracellular structure and properties supports RMs as useful models for crowded cytoplasmatic space, which is involved in nonspecific interactions between neighboring molecules and which defines the fundamental reactions and events in a living cell.
Reverse micelles may represent the theoretical answer for a better understanding of the real cellular environment, as well as an appropriate and befitting experimental system for overcoming the major limitations in performing enzymatic reactions at an industrial scale.
According to the research into reverse micelles carried out so far and their applications, the development of value-added applications for bioreceptor (aptamers, nanobodies, cells, etc.) immobilization on nanoparticles using RMs is necessary for biosensing and biomedical applications, in order to overcome the multiple disadvantages regarding their poor activity and specificity after immobilization processes.
7. Conclusions
The aim of the present review was to demonstrate the unusual versatility of reverse micelles, whereby the same structure has a multifunctional contribution to distinct and totally different scientific fields and diametrically opposed applications, namely, cell theoretical models and newly developed methods for advanced and modern nano-biotechnology. Each exemplified direction was supported by the following specific characteristics: (i) the biological relevance as a function of the interfacial parameters of the surfactant film, the microheterogeneity of the intramicellar structure, the multilayered composition of water, and the redefinition of the pH concept for size-limited systems; (ii) nanomaterial synthesis as a function of reverse micelle formation, composition of the interfacial layer, and experimental significance of the intramicellar pH.
In our opinion, the successful development of the reverse micelle field is in strict correlation with the innovative but especially correct use of their unique structure, as well as an understanding of the features that distinguish them from other structures.
Special attention was paid to defining both the pH concept across the water pool of RMs and the experimental significance of water pool pH in order to underline the need for correct methodology describing the preparation/adjustment of the pH from the water pool to a specific pH value appropriate for a particular application (e.g., special requirements for the active conformation of biomolecules or nanoparticle synthesis). The structural and compositional flexibility of RMs supports their significance across a surprisingly large range of functional areas, as well as their versatile approaches from theoretical models to practical applications.
From the available information regarding the biomimetic relevance of RMs as an appropriate and accessible model of cellular structure, this review focused on three aspects identified, in our opinion, as the most interesting and captivating: confinement and the crowded cytoplasmatic environment, water dynamics in a restricted volume, and the dynamic role of membranous structures.
On the other hand, this review was directed toward the applied contribution of RMs to the development of nanotechnologies for the synthesis of nanomaterials for nanodevice design, with a special reference to nano (bio)sensors. A large range of nanomaterials with uniform size have been prepared using RMs as nanoreactors. The nanosized aqueous cores of reverse micelles provide an appropriate stable environment for nanomaterial synthesis. The aggregation of nanomaterials dispersed in RMs is inhibited by the surfactant layers, which act as stabilizers. The main advantages of using RMs in nanoparticle synthesis are that it is a simple technique, operating at room temperature and pressure, and that it does not require special equipment. Moreover, the RM method allows obtaining a large number of nanoparticles with good control of their size and morphology, even in a homogeneous solution.
Author Contributions
M.-L.A., A.-M.G. and M.D. conceived the structure of the paper; M.-L.A., A.-M.G. and M.-L.J. collected the references; I.R., M.C. and M.-L.J. formal analysis investigation; M.-L.A., A.-M.G. and M.-L.J. wrote and edited the paper; M.-L.A., A.-M.G., M.-L.J. and M.D. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CCCDI-UEFISCDI, project number ERANET-MANUNET II -TOX-HAZ-ASSESS no 33/2017.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Acknowledgments
The authors thanks to Ministry of Education and Research of Romania through National Programs PN19.23.03.02/2019 and PNIII-PED-2019-0991/392/2020, and respectively through International Program ERANET-MANUNET-NITRISENS no 216/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tan, H.-S.; Piletic, I.R.; Fayer, M.D. Orientational dynamics of water confined on a nanometer length scale in reverse micelles. J. Chem. Phys. 2005, 122, 174501. [Google Scholar] [CrossRef]
- Yeung, P.S.W.; Eskici, G.; Axelsen, P.H. Infrared spectroscopy of proteins in reverse micelles. Biochim. Biophys. Acta (BBA) Biomembr. 2013, 1828, 2314–2318. [Google Scholar] [CrossRef]
- Martinek, K.; Levashov, A.V.; Klyachko, N.; Khmelnitski, Y.L.; Berezin, I.V. Micellar enzymology. Eur. J. Biochem. 1986, 155, 453–468. [Google Scholar] [CrossRef]
- Van der Loop, T.H.; Panman, M.R.; Lotze, S.; Zhang, J.; Vad, T.; Bakker, H.J.; Sager, W.F.; Woutersen, S. Structure and dynamics of water in nonionic reverse micelles: A combined time-resolved infrared and small angle X-ray scattering study. J. Chem. Phys. 2012, 137, 44503. [Google Scholar] [CrossRef] [PubMed]
- Luisi, P.L.; Giomini, M.; Pileni, M.P.; Robinson, B.H. Reverse micelles as hosts for proteins and small molecules. Biochim. Biophys. Acta (BBA) Rev. Biomembr. 1988, 947, 209–246. [Google Scholar] [CrossRef]
- Singh, K.; Iqubal, M.K.; Shukla, V.K.; Shuaib, M. Microemulsions: Current trends in novel drug delivery systems. J. Pharm. Chem. Biol. Sci. 2014, 1, 39–51. [Google Scholar]
- Groo, A.C.; Matougui, N.; Umerska, A.; Saulnier, P. Reverse micelle-lipid nanocapsules: A novel strategy for drug delivery of the plectasin derivate AP138 antimicrobial peptide. Int. J. Nanomed. 2018, 13, 7565–7574. [Google Scholar] [CrossRef]
- Mohd-Setapar, S.H.; Mohamad-Aziz, S.N.; Chuong, C.S.; Yunus, M.A.C.; Zaini, M.A.A.; Kamaruddin, M.J. A review of mixed reverse micelle system for antibiotic recovery. Chem. Eng. Commun. 2014, 201, 1664–1685. [Google Scholar] [CrossRef]
- Lone, I.H.; Radwan, N.R.E.; Aslam, J.; Akhter, A. Concept of Reverse Micelle Method for the Synthesis of Nano-Structured Material. Curr. Nanosci. 2019, 15, 129. [Google Scholar] [CrossRef]
- UskokoviĆ, V.U.K.; Drofenik, M. Synthesis of materials within reverse micelles. Surf. Rev. Lett. 2005, 12, 239–277. [Google Scholar] [CrossRef]
- Sun, X.; Bandara, N. Applications of reverse micelles technique in food science: A comprehensive review. Trends Food Sci. Technol. 2019, 91, 106–115. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Papavasileiou, K.D.; Papadopoulos, M.G.; Xenakis, A. Reverse Micelles As Antioxidant Carriers: An Experimental and Molecular Dynamics Study. Langmuir 2017, 33, 5077–5085. [Google Scholar] [CrossRef]
- Levinger, N.E.; Swafford, L.A. Ultrafast Dynamics in Reverse Micelles. Annu. Rev. Phys. Chem. 2009, 60, 385–406. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: Terminology, differences, and similarities. Soft Matter 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Langevin, D. Micelles and Microemulsions. Annu. Rev. Phys. Chem. 1992, 43, 341–369. [Google Scholar] [CrossRef]
- Schulman, J.H.; Stoeckenius, W.; Prince, L.M. Mechanism of Formation and Structure of Micro Emulsions by Electron Microscopy. J. Phys. Chem. 1959, 63, 1677–1680. [Google Scholar] [CrossRef]
- Sjöblom, J.; Lindberg, R.; Friberg, S.E. Microemulsions—Phase equilibria characterization, structures, applications and chemical reactions. Adv. Colloid Interface Sci. 1996, 65, 125–287. [Google Scholar] [CrossRef]
- Narang, A.S.; Delmarre, D.; Gao, D. Stable drug encapsulation in micelles and microemulsions. Int. J. Pharm. 2007, 345, 9–25. [Google Scholar] [CrossRef]
- Tovstun, S.A.; Razumov, V. What makes AOT reverse micelles spherical? Colloid Polym. Sci. 2015, 293, 165–176. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Levashov, A.V.; Klyachko, N.L.; Namyotkin, S.N.; Pshezhetsky, A.V.; Martinek, K. Enzymes entrapped in reversed micelles of surfactants in organic solvents: A theoretical treatment of the catalytic activity regulation. J. Theor. Biol. 1988, 133, 327–343. [Google Scholar] [CrossRef]
- Mirgorod, Y.A.; Dolenko, T.A. Liquid Polyamorphous Transition and Self-Organization in Aqueous Solutions of Ionic Surfactants. Langmuir 2015, 31, 8535–8547. [Google Scholar] [CrossRef]
- Katiyar, B.S.; Katiyar, S.S.; Mishra, P.S.; Sailaja, D.L. Microemulsions: A novel drug carrier system. Int. J. Pharm. Sci. Rev. Res. 2013, 20, 138–148. [Google Scholar]
- Thiam, A.R.; Farese, R.V., Jr.; Walther, T.C. The biophysics and cell biology of lipid droplets. Nat. Rev. Mol. Cell Biol. 2013, 14, 775–786. [Google Scholar] [CrossRef]
- Krishna, S.H.; Srinivas, N.D.; Raghavarao, K.S.M.S.; Karanth, N.G. Reverse micellar extraction for downstream processing of proteins/enzymes. Hist. Trends Bioprocess. Biotransform. 2002, 75, 119–183. [Google Scholar] [CrossRef]
- Hoffmann, M.M.; Too, M.D.; Vogel, M.; Gutmann, T.; Buntkowsky, G. Breakdown of the Stokes–Einstein Equation for Solutions of Water in Oil Reverse Micelles. J. Phys. Chem. B 2020, 124, 9115–9125. [Google Scholar] [CrossRef] [PubMed]
- Klíčová, L.; Šebej, P.; Štacko, P.; Filippov, S.K.; Bogomolova, A.; Padilla, M.; Klán, P. CTAB/Water/Chloroform Reverse Micelles: A Closed or Open Association Model? Langmuir 2012, 28, 15185–15192. [Google Scholar] [CrossRef]
- Lawrence, M.J.; Rees, G.D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Deliv. Rev. 2000, 45, 89–121. [Google Scholar] [CrossRef]
- Gillberg, G.; Lehtinen, H.; Friberg, S. NMR and IR investigation of the conditions determining the stability of microemulsions. J. Colloid Interface Sci. 1970, 33, 40–53. [Google Scholar] [CrossRef]
- Moulik, S.; Paul, B. Structure, dynamics and transport properties of microemulsions. Adv. Colloid Interface Sci. 1998, 78, 99–195. [Google Scholar] [CrossRef]
- Moulik, S.; Paul, B. Uses and applications of microemulsions. Curr. Sci. 2001, 80, 990–1001. [Google Scholar]
- Smith, G.N.; Brown, P.; Rogers, S.E.; Eastoe, J. Evidence for acritical micelle concentration of surfactants in hydrocarbon solvents. Langmuir 2013, 29, 3252–3258. [Google Scholar] [CrossRef]
- Chaurasiya, R.; Hebbar, U. Reverse Micelles for Nanoparticle Synthesis and Biomolecule Separation. Nanosci. Food Agric. 4 2017, 24, 181–211. [Google Scholar] [CrossRef]
- Kilikian, B.V.; Bastazin, M.R.; Minami, N.M.; Gonçalves, E.M.R.; Junior, A.P. Liquid-liquid extraction by reversed micelles in biotechnological processes. Braz. J. Chem. Eng. 2000, 17, 9. [Google Scholar] [CrossRef]
- Surabhi, K.; Katare, O.P.; Atul, N.; Arun, G. Microemulsions: Developmental Asp. J. Pharm. Biol. Chem. Sci. 2010, 1, 683–706. [Google Scholar]
- Israelachvili, J. The science and applications of emulsions—An overview. Colloids Surf. A Physicochem. Eng. Asp. 1994, 91, 1–8. [Google Scholar] [CrossRef]
- Nagarajan, R. Molecular Packing Parameter and Surfactant Self-Assembly: The Neglected Role of the Surfactant Tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Ballesteros-Gomez, A.; Sicilia, M.D.; Rubio, S. Supramolecular solvents in the extraction of organic compounds. A review. Anal. Chim. Acta 2010, 677, 108–130. [Google Scholar] [CrossRef]
- Manaargadoo-Catin, M.; Ali-Cherif, A.; Pougnas, J.L.; Perrin, C. Hemolysis by surfactants—A review. Adv. Colloid Interface Sci. 2016, 228, 1–16. [Google Scholar] [CrossRef]
- Durand, G.; Abla, M.; Ebel, C.; Breyton, C. New Amphiphiles to Handle Membrane Proteins: “Ménage à Trois” between Chemistry, Physical Chemistry, and Biochemistry. In Membrane Proteins Production for Structural Analysis; Mus-Veteau, I., Ed.; Springer New York: New York, NY, USA, 2014; pp. 205–251. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, X.; Sun, Y. New Development of Reverse Micelles and Applications in Protein Separation and Refolding. Chin. J. Chem. Eng. 2008, 16, 949–955. [Google Scholar] [CrossRef]
- Maitra, A.N.; Eicke, H.F. Effect of rotational isomerism on the water-solubilizing properties of Aerosol OT as studied by hydrogen-1 NMR spectroscopy. J. Phys. Chem. 1981, 85, 2687–2691. [Google Scholar] [CrossRef]
- Fayer, M.D.; Levinger, N.E. Analysis of Water in Confined Geometries and at Interfaces. Annu. Rev. Anal. Chem. 2010, 3, 89–107. [Google Scholar] [CrossRef]
- Nave, S.; Eastoe, J.; Penfold, J. What Is So Special about Aerosol-OT? 1. Aqueous Systems. Langmuir 2000, 16, 8733–8740. [Google Scholar] [CrossRef]
- Basheer, S.A.; Thenmozhi, M. Reverse micellar separation of lipases: A critical review. Int. J. Chem. Sci. 2010, 8, S57–S67. [Google Scholar]
- Fragoso, A.; Pacheco, R.; Karmali, A. Investigation of structural effects and behaviour of Pseudomonas aeruginosa amidase encapsulated in reversed micelles. Process Biochem. 2012, 47, 264–272. [Google Scholar] [CrossRef]
- Eastoe, J.; Hollamby, M.J.; Hudson, L. Recent advances in nanoparticle synthesis with reversed micelles. Adv. Colloid Interface Sci. 2006, 128–130, 5–15. [Google Scholar] [CrossRef]
- Shrestha, L.K.; Dulle, M.; Glatter, O.; Aramaki, K. Structure of Polyglycerol Oleic Acid Ester Nonionic Surfactant Reverse Micelles in Decane: Growth Control by Headgroup Size. Langmuir 2010, 26, 7015–7024. [Google Scholar] [CrossRef]
- Chuo, S.C.; Mohd-Setapar, S.H.; Mohamad-Aziz, S.N.; Starov, V.M. A new method of extraction of amoxicillin using mixed reverse micelles. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 137–144. [Google Scholar] [CrossRef]
- Mitra, R.K.; Sinha, S.S.; Verma, P.K.; Pal, S.K. Modulation of Dynamics and Reactivity of Water in Reverse Micelles of Mixed Surfactants. J. Phys. Chem. B 2008, 112, 12946–12953. [Google Scholar] [CrossRef]
- Bhowal, S.; Priyanka, B.S.; Rastogi, N.K. Mixed reverse micellesfacilitated downstream processing of lipase involving water-oil-water (WOW) liquidemulsion membrane. Biotechnol. Prog. 2014, 30, 1084–1092. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Hazra, P.; Chakraborty, A.; Sarkar, N. Solvation Dynamics of Coumarin 480 in Bile Salt− Cetyltrimethylammonium Bromide (CTAB) and Bile Salt− Tween 80 Mixed Micelles. J. Phys. Chem. B 2003, 107, 13643–13648. [Google Scholar] [CrossRef]
- Mohd-Setapar, S.H.; Mohamad-Aziz, S.N.; Harun, N.H.; Mohd-Azizi, C.Y. Review on the Extraction of Biomolecules by Biosurfactant Reverse Micelles. APCBEE Procedia 2012, 3, 78–83. [Google Scholar] [CrossRef]
- Rodrigues, L.R. Microbial surfactants: Fundamentals and applicability in the formulation of nano-sized drug delivery vectors. J. Colloid Interface Sci. 2015, 449, 304–316. [Google Scholar] [CrossRef]
- Usman, M.; Dadrasnia, A.; Kang, T.; Mahmud, A.; Ismail, S. Application of biosurfactants in environmental biotechnology; remediation of oil and heavy metal. AIMS Bioeng. 2016, 3, 289–304. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Meng, Y.; Feng, X.-D.; Sun, Y. A metal-chelate affinity reverse micellar system for protein extraction. Biotechnol. Prog. 2009, 26, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, V.M.; Dordick, J.S. Affinity-based reverse micellar extraction and separation (ARMES): A facile technique for the purification of peroxidase from soybean hulls. Biotechnol. Prog. 1993, 9, 199–203. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, Y.; Sun, Y. Characterization of reversed micelles of Cibacron Blue F-3GA modified Span 85 for protein solubilization. Colloid Interface Sci. 2005, 290, 259–266. [Google Scholar] [CrossRef]
- Goto, M.; Kuroki, M.; Ono, T.; Nakashio, F. Protein extraction by new reversed micelles with di(tridecyl) phosphoric acid. Sep. Sci. Technol. 1995, 30, 89–99. [Google Scholar] [CrossRef]
- Mathew, D.S.; Juang, R.-S. Role of alcohols in the formation of inverse microemulsions and back extraction of proteins/enzymes in a reverse micellar system. Sep. Purif. Technol. 2007, 53, 199–215. [Google Scholar] [CrossRef]
- Kadam, K.L. Reverse micelles as a bioseparation tool. Enzym. Microb. Technol. 1986, 8, 266–273. [Google Scholar] [CrossRef]
- Munshi, N.; Sarcar, S.; Maitra, A. The effect of droplet dynamics on the kinetics of the horseradish peroxidase catalysed reaction in reverse micelles. Colloids Surf. A Physicochem. Eng. Asp. 1994, 88, 181–189. [Google Scholar] [CrossRef]
- Fletcher, P.D.I.; Howe, A.M.; Robinson, B.H. The kinetics of solubilisate exchange between water droplets of a water-in-oil microemulsion. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1987, 83, 985–1006. [Google Scholar] [CrossRef]
- Lindman, B.; Stilbs, P.; Moseley, M.E. Fourier transform nmr self-diffusion and microemulsion structure. J. Colloid Interface Sci. 1981, 83, 569–582. [Google Scholar] [CrossRef]
- Pomata, M.H.H.; Laria, D.; Skaf, M.S.; Elola, M.D. Molecular dynamics simulations of AOT-water/formamide reverse micelles: Structural and dynamical properties. J. Chem. Phys. 2008, 129, 244503. [Google Scholar] [CrossRef]
- Bru, R.; Sánchez-Ferrer, A.; García-Carmona, F. Kinetic models in reverse micelles. Biochem. J. 1995, 310, 721–739. [Google Scholar] [CrossRef]
- Cringus, D.; Bakulin, A.; Lindner, J.; Vöhringer, P.; Pshenichnikov, M.S.; Wiersma, D. Ultrafast Energy Transfer in Water−AOT Reverse Micelles. J. Phys. Chem. B 2007, 111, 14193–14207. [Google Scholar] [CrossRef]
- Kinugasa, T.; Kondo, A.; Nishimura, S.; Miyauchi, Y.; Nishii, Y.; Watanabe, K.; Takeuchi, H. Estimation for size of reverse micelles formed by AOT and SDEHP based on viscosity measurement. Colloids Surf. A: Physicochem. Eng. Asp. 2002, 204, 193–199. [Google Scholar] [CrossRef]
- Ricka, J.; Borkovec, M.; Uj, H. Coated droplet model of microemulsions: Optical matching and polydispersity. J. Chem. Phys. 1991, 94, 8503–8509. [Google Scholar] [CrossRef]
- Lemyre, J.-L.; Ritcey, A. Characterization of a Reverse Micellar System by H-1 NMR. Langmuir 2010, 26, 6250–6255. [Google Scholar] [CrossRef] [PubMed]
- Zinsli, P.E. Inhomogeneous interior of Aerosol OT microemulsions probed by fluorescence and polarization decay. J. Phys. Chem. 1979, 83, 3223–3231. [Google Scholar] [CrossRef]
- Kondo, H.; Fujiki, K.; Sunamoto, J. Reversed micellar catalysis. Catalysis of dodecylammonium propionate reversed micelles in the hydrolysis of alkyl p-nitrophenyl carbonates. J. Org. Chem. 1978, 43, 3584–3588. [Google Scholar] [CrossRef]
- Gębicki, J.L. Intermicellar material exchange in reverse micelles formed by ionic AOT and nonionic Igepal surfactants studied by means of pulse radiolysis. Influence of the temperature. Cent. Eur. J. Chem. 2004, 2, 371–387. [Google Scholar] [CrossRef]
- Chhaya, U.; Ingale, S. Micellar Enzymology- Chemistry and Applications. Open Biotechnol. J. 2016, 10, 326–334. [Google Scholar] [CrossRef]
- Menger, F.M.; Donohue, J.A.; Williams, R.F. Catalysis in water pools. J. Am. Chem. Soc. 1973, 95, 286–288. [Google Scholar] [CrossRef]
- Menger, F.M.; Saito, G. Adsorption, displacement, and ionization in water pools. J. Am. Chem. Soc. 1978, 100, 4376–4379. [Google Scholar] [CrossRef]
- Venables, D.S.; Huang, K.; Schmuttenmaer, C.A. Effect of Reverse Micelle Size on the Librational Band of Confined Water and Methanol. J. Phys. Chem. B 2001, 105, 9132–9138. [Google Scholar] [CrossRef]
- Onori, G.; D’Angelo, M.; Santucci, A. Structure and state of water in reversed aerosol OT micelles: An infrared study. Trends Colloid Interface Sci. VIII 2007, 97, 158–162. [Google Scholar] [CrossRef]
- Faeder, J.; Ladanyi, B. Molecular Dynamics Simulations of the Interior of Aqueous Reverse Micelles. J. Phys. Chem. B 2000, 104, 1033–1046. [Google Scholar] [CrossRef]
- D’Aprano, A.; Lizzio, A.; Turco Liveri, V.; Aliotta, F.; Vasi, C.; Migliardo, P. Aggregation states of water in reversed AOT micelles: Raman Evidence. J. Phys. Chem. 1988, 92, 4436–4439. [Google Scholar] [CrossRef]
- Rodriguez, J.; Laria, D.; Guardia, E.; Martí, J. Dynamics of water nanodroplets and aqueous protons in non-ionic reverse micelles. Phys. Chem. Chem. Phys. 2009, 11, 1484–1490. [Google Scholar] [CrossRef][Green Version]
- Costard, R.; Elsaesser, T. Femtosecond OH Bending Dynamics of Water Nanopools Confined In Reverse Micelles. J. Phys. Chem. B 2013, 117, 15338–15345. [Google Scholar] [CrossRef]
- Rosenfeld, D.; Schmuttenmaer, C.A. Dynamics of Water Confined Within Reverse Micelles. J. Phys. Chem. B 2006, 110, 14304–14312. [Google Scholar] [CrossRef]
- Onori, G.; Santucci, A. IR investigations of water structure in Aerosol OT reverse micellar aggregates. J. Phys. Chem. 1993, 97, 5430–5434. [Google Scholar] [CrossRef]
- Moilanen, D.E.; Fenn, E.E.; Wong, D.; Fayer, M.D. Water Dynamics at the Interface in AOT Reverse Micelles. J. Phys. Chem. B 2009, 113, 8560–8568. [Google Scholar] [CrossRef]
- Marques, B.; Nucci, N.; Dodevski, I.; Wang, K.; Athanasoula, E.A.; Jorge, C.; Wand, A. Measurement and Control of pH in the Aqueous Interior Micelles. J. Phys. Chem. B 2014, 118, 2020–2031. [Google Scholar] [CrossRef]
- Vodolazkaya, N.A.; McHedlov-Petrossyan, N.O.; Salamanova, N.V.; Surov, Y.N.; Doroshenko, A.O. Molecular spectroscopy studies of solvent properties of dispersed ‘water pools’: Fluorescein and 2,7-dichlorofluorescein in reversed AOT-based microemulsions. J. Mol. Liq. 2010, 157, 105–112. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Chinelatto, A.M.; Shimizu, M.R. Acid—Base indicator equilibria in the presence of aerosol-OT aggregates in heptane. Ion exchange in reversed micelles. J. Colloid Interface Sci. 1982, 88, 420–427. [Google Scholar] [CrossRef]
- Crans, D.; Levinger, N. The Conundrum of pH in Water Nanodroplets: Sensing pH in Reverse Micelle Water Pools. Acc. Chem. Res. 2012, 45, 1637–1645. [Google Scholar] [CrossRef]
- Hasegawa, M.; Sugimura, T.; Suzaki, Y.; Shindo, Y.; Kitahara, A. Microviscosity in Water Pool of Aerosol-OT Reversed Micelle Determined with Viscosity-Sensitive Fluorescence Probe, Auramine O, and Fluorescence Depolarization of Xanthene Dyes. J. Phys. Chem. 1994, 98, 2120–2124. [Google Scholar] [CrossRef]
- da Graça Miguel, M.; Burrows, H.D.; Escaroupa Pereira, M.A.; Varela, A.P. Probing solute distribution and acid-base behaviour in water-in-oil microemulsions by fluorescence techniques. Colloids Surf. A Physicochem. Eng. Asp. 2001, 176, 85–99. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, P.; Gupta, S.; Rafiq, S.; Yadav, R.; Jain, V.K.; Raval, J.; Sen, P. Ramping of pH across the Water-Pool of a Reverse Micelle. Langmuir 2016, 32, 1693–1699. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.L.; Luisi, P. Micellar Solubilization of Biopolymers in Hydrocarbon Solvents III. Empirical Definition of an Acidity Scale in Reverse Micelles. Helv. Chim. Acta 1980, 63, 2302–2311. [Google Scholar] [CrossRef]
- Halliday, N.A.; Peet, A.C.; Britton, M.M. Detection of pH in Microemulsions, without a Probe Molecule, Using Magnetic Resonance. J. Phys. Chem. B 2010, 114, 13745–13751. [Google Scholar] [CrossRef] [PubMed]
- Oshitani, J.; Takashina, S.; Yoshida, M.; Gotoh, K. Water Pool pH of AOT-Based W/O Microemulsions at Various Water Contents Estimated by Absorbance Ratio of Pyranine. J. Chem. Eng. Jpn. 2008, 41, 507–512. [Google Scholar] [CrossRef]
- Cohen, B.; Huppert, D.; Solntsev, K.; Tsfadia, Y.; Nachliel, E.; Gutman, M. Excited State Proton Transfer in Reverse Micelles. J. Am. Chem. Soc. 2002, 124, 7539–7547. [Google Scholar] [CrossRef]
- Kwon, O.-H.; Jang, D.-J. Excited-State Double Proton Transfer of 7-Azaindole in Water Nanopools. J. Phys. Chem. B 2005, 109, 20479–20484. [Google Scholar] [CrossRef]
- Baruah, B.; Swafford, L.; Crans, D.; Levinger, N. Do Probe Molecules Influence Water in Confinement? J. Phys. Chem. B 2008, 112, 10158–10164. [Google Scholar] [CrossRef] [PubMed]
- Baruah, B.; Roden, J.; Sedgwick, M.; Correa, M.; Crans, D.; Levinger, N. When Is Water Not Water? Exploring Water Confined in Large Reverse Micelles Using a Highly Charged Inorganic Molecular Probe. J. Am. Chem. Soc. 2006, 128, 12758–12765. [Google Scholar] [CrossRef]
- Stathatos, E.; Lianos, P.; DelMonte, F.; Levy, D.; Tsiourvas, D. Formation of TiO2 nanoparticles in reverse micelles and their deposition as thin films on glass substrates. Langmuir 1997, 13, 4295–4300. [Google Scholar] [CrossRef]
- Ebert, H.F.K.N. Determination of pH in reversed micelles II. Application of the method proposed previously to other reversed micellar systems. Colloid Polym. Sci. 1982, 260, 697–701. [Google Scholar] [CrossRef]
- Sedgwick, M.; Crans, D.; Levinger, N. What Is Inside a Nonionic Reverse Micelle? Probing the Interior of Igepal Reverse Micelles Using Decavanadate. Langmuir 2009, 25, 5496–5503. [Google Scholar] [CrossRef]
- Pito, D.S.; Fonseca, I.M.; Ramos, A.; Vital, J.; Castanheiro, J.E. Hydrolysis of sucrose using sulfonated poly(vinyl alcohol) as catalyst. Bioresurce Technol. 2009, 100, 4546–4550. [Google Scholar] [CrossRef]
- Hasegawa, M. Buffer-like Action in Water Pool of Aerosol OT Reverse Micelles. Langmuir 2001, 17, 1426–1431. [Google Scholar] [CrossRef]
- Hojo, M.; Ueda, T.; Daike, C.; Takezaki, F.; Furuya, Y.; Miyamoto, K.; Narutaki, A.; Kato, R. Great Enhancement in the Oxidation Ability of Dilute Nitric Acid in Nanoscale Water-Droplets of Reverse Micelle Systems. Bull. Chem. Soc. Jpn. 2006, 79, 1215–1222. [Google Scholar] [CrossRef]
- Walde, P.; Mao, Q.; Bru, R.; Luisi, P.; Kuboi, R. pH artifacts in reverse micellar enzymology: A warning. Pure Appl. Chem. 1992, 64, 1771–1775. [Google Scholar] [CrossRef]
- Küchler, A.; Yoshimoto, M.; Luginbühl, S.; Mavelli, F.; Peter, W. Enzymatic reactions in confined environments. Nat. Nanotechnol. 2016, 11, 409–420. [Google Scholar] [CrossRef]
- Chang, G.G.; Huang, T.M.; Hung, H.-C. Reverse micelles as life-mimicking systems. Proc. Natl. Sci. Counc. Repub. China B 2000, 24, 89–100. [Google Scholar]
- Pileni, M.P. Structure and Reactivity in Reverse Micelles (Studies in Physical and Theoretical Chemistry); Elsevier: Amsterdam, The Netherlands, 1989; Volume 65, ISBN 978-0444881663. [Google Scholar] [CrossRef]
- Pal, N.; Verma, S.D.; Singh, M.; Sen, S. Fluorescence Correlation Spectroscopy: An Efficient Tool for Measuring Size, Size-Distribution and Polydispersity of Microemulsion Droplets in Solution. Anal. Chem. 2011, 83, 7736–7744. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, K. Solvation Dynamics and Proton Transfer in Supramolecular Assemblies. Acc. Chem. Res. 2003, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Spehr, T.L.; Frick, B.; Zamponi, M.; Stühn, B. Dynamics of water confined to reverse AOT micelles. Soft Matter 2011, 7, 5745–5755. [Google Scholar] [CrossRef]
- Fayer, M.D. Water in a Crowd. Physiology 2011, 26, 381–392. [Google Scholar] [CrossRef][Green Version]
- Moilanen, D.; Levinger, N.; Spry, D.B.; Fayer, M.D. Confinement or the Nature of the Interface? Dynamics of Nanoscopic Water. J. Am. Chem. Soc. 2007, 129, 14311–14318. [Google Scholar] [CrossRef]
- Ellis, R.J.; Minton, A.P. Join the crowd. Nature 2003, 425, 27. [Google Scholar] [CrossRef]
- Minton, A.P. Implications of macromolecular crowding for protein assembly. Curr. Opin. Struct. Biol. 2000, 10, 34–39. [Google Scholar] [CrossRef]
- Minton, A.P. Influence of excluded volume upon macromolecular structure and associations in ‘crowded” media. Curr. Opin. Biotechnol. 1997, 8, 65–69. [Google Scholar] [CrossRef]
- Zhou, H.-X.; Rivas, G.; Minton, A. Macromolecular Crowding and Confinement: Biochemical, Biophysical, and Potential Physiological Consequences. Annu. Rev. Biophys. 2008, 37, 375–397. [Google Scholar] [CrossRef]
- Zhou, H.-X. Protein Folding in Confined and Crowded Environments. Arch. Biochem. Biophys. 2008, 469, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Honegger, P.; Steinhauser, O. Towards capturing cellular complexity: Combining encapsulation and macromolecular crowding in a reverse micelle. Phys. Chem. Chem. Phys. 2019, 21, 8108–8120. [Google Scholar] [CrossRef] [PubMed]
- Murakami, H. Protein and Water Confined in Nanometer-Scale Reverse Micelles Studied by Near Infrared, Terahertz, and Ultrafast Visible Spectroscopies. Adv. Protein Chem. Struct. Biol. 2013, 93, 183–211. [Google Scholar] [CrossRef]
- Kuznetsova, I.M.; Zaslavsky, B.; Breydo, L.; Turoverov, K.; Uversky, V. Beyond the Excluded Volume Effects: Mechanistic Complexity of the Crowded Milieu. Molecules 2015, 20, 1377–1409. [Google Scholar] [CrossRef]
- Bakulin, A.A.; Cringus, D.; Pieniazek, P.A.; Skinner, J.L.; Jansen, T.L.C.; Pshenichnikov, M.S. Dynamics of Water Confined in Reversed Micelles: Multidimensional Vibrational Spectroscopy Study. J. Phys. Chem. B 2013, 117, 15545–15558. [Google Scholar] [CrossRef] [PubMed]
- Quist, P.-O.; Halle, B. Water dynamics and aggregate structure in reversed micelles at sub-zero temperatures. A deuteron spin relaxation study. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1988, 84, 1033–1046. [Google Scholar] [CrossRef]
- Piletic, I.; Tan, H.-S.; Fayer, M.D. Dynamics of Nanoscopic Water: Vibrational Echo and Infrared Pump−Probe Studies of Reverse Micelles. J. Phys. Chem. B 2005, 109, 21273–21284. [Google Scholar] [CrossRef] [PubMed]
- Harpham, M.; Ladanyi, B.; Levinger, N.; Herwig, K. Water motion in reverse micelles studied by quasielastic neutron scattering and molecular dynamics simulations. J. Chem. Phys. 2004, 121, 7855–7868. [Google Scholar] [CrossRef] [PubMed]
- Abel, S.; Sterpone, F.; Bandyopadhyay, S.; Marchi, M. Molecular Modeling and Simulations of AOT− Water Reverse Micelles in Isooctane: Structural and Dynamic Properties. J. Phys. Chem. B 2004, 108, 19458–19466. [Google Scholar] [CrossRef]
- Perakis, F.; De Marco, L.; Shalit, A.; Tang, F.; Kann, Z.R.; Kühne, T.D.; Torre, R.; Bonn, M.; Nagata, Y. Vibrational Spectroscopy and Dynamics of Water. Chem. Rev. 2016, 116, 7590–7607. [Google Scholar] [CrossRef]
- Kikhney, G.; Svergun, D.I. A practical guide to small angle X-ray scattering (SAXS) of flexible and intrinsically disordered proteins. FEBS Lett. 2015, 589, 2570–2577. [Google Scholar] [CrossRef]
- Oliver, T.A.A. Recent advances in multidimensional ultrafast spectroscopy. R. Soc. Open Sci. 2018, 5, 171425. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Ho, J.-J.; Serrano, A.L.; Skoff, D.R.; Zhang, T.; Zanni, M.T. Two-dimensional sum-frequency generation (2D SFG) spectroscopy: Summary of principles and its application to amyloid fiber monolayers. Faraday Discuss. 2015, 177. [Google Scholar] [CrossRef]
- Segarra-Martí, J.; Jaiswal, V.K.; Pepino, A.J.; Giussani, A.; Nenov, A.; Mukamel, S.; Garavelli, M.; Rivalta, I. Two-dimensional electronic spectroscopy as a tool for tracking molecular conformations in DNA/RNA aggregates. Faraday Discuss. 2018, 207, 233. [Google Scholar] [CrossRef]
- Kraack, J.P.; Hamm, P. Surface-Sensitive and Surface-Specific Ultrafast Two-Dimensional Vibrational Spectroscopy. Chem. Rev. 2017, 117, 10623. [Google Scholar] [CrossRef]
- Cyran, J.D.; Backus, E.H.G.; Nagata, Y.; Bonn, M. Structure from Dynamics: Vibrational Dynamics of Interfacial Water as a Probe of Aqueous Heterogeneity. Phys. Chem. B 2018, 14, 14–3679. [Google Scholar] [CrossRef]
- Garrett-Roe, S.; Hamm, P. What Can We Learn from Three-Dimensional Infrared Spectroscopy? Acc. Chem. Res. 2009, 42, 1412–1422. [Google Scholar] [CrossRef]
- Garrett-Roe, S.; Perakis, F.; Rao, F.; Hamm, P. Three-dimensional infrared spectroscopy of isotope-substituted liquid water reveals heterogeneous dynamics. J. Phys. Chem. B 2011, 115, 6976–6984. [Google Scholar] [CrossRef][Green Version]
- Faramarzi, S.; Bonnett, B.; Scaggs, C.A.; Hoffmaster, A.; Grodi, D.; Harvey, E.; Mertz, B. Molecular Dynamics Simulations as a Tool for Accurate Determination of Surfactant Micelle Properties. Langmuir 2017, 33, 9934–9943. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, P.A.; Lin, Y.-S.; Chowdhary, J.; Ladanyi, B.M.; Skinner, J.L. Vibrational Spectroscopy and Dynamics of Water Confined inside Reverse Micelles. J. Phys. Chem. B 2009, 113, 15017–15028. [Google Scholar] [CrossRef] [PubMed]
- Hirose, Y.; Yui, H.; Sawada, T. The ultrafast relaxation dynamics of a viscosity probe molecule in an AOT-reversed micelle: Contribution of the specific interactions with the local environment. J. Phys. Chem. B 2004, 108, 9070–9076. [Google Scholar] [CrossRef]
- Laage, D.; Hynes, J.T. A Molecular Jump Mechanism of Water Reorientation. Science 2006, 311, 832. [Google Scholar] [CrossRef] [PubMed]
- Kitchens, C.L.; Bossev, D.P.; Roberts, C.B. Solvent Effects on AOT Reverse Micelles in Liquid and Compressed Alkanes Investigated by Neutron Spin−Echo Spectroscopy. J. Phys. Chem. B 2006, 110, 20392–20400. [Google Scholar] [CrossRef]
- Chattopadhyay, A.; Mukherjee, S.; Raghuraman, H. Reverse micellar organization and dynamics: A wavelength-selective fluorescence approach. J. Phys. Chem. B 2002, 106, 13002–13009. [Google Scholar] [CrossRef]
- Piletic, I.R.; Moilanen, D.E.; Spry, D.B.; Levinger, N.E.; Fayer, M.D. Testing the Core/Shell Model of Nanoconfined Water in Reverse Micelles Using Linear and Nonlinear IR Spectroscopy. J. Phys. Chem. A 2006, 110, 4985–4999. [Google Scholar] [CrossRef] [PubMed]
- Fenn, E.E.; Wong, D.B.; Fayer, M.D. Water dynamics in small reverse micelles in two solvents: Two-dimensional infrared vibrational echoes with two-dimensional background subtraction. J. Chem. Phys. 2011, 134, 54512. [Google Scholar] [CrossRef] [PubMed]
- Dokter, A.M.; Petersen, C.; Woutersen, S.; Bakker, H.J. Vibrational dynamics of ice in reverse micelles. J. Chem. Phys. 2008, 128, 44509. [Google Scholar] [CrossRef] [PubMed]
- Dokter, A.; Woutersen, S.; Bakker, H. Inhomogeneous dynamics in confined water nanodroplets. Proc. Natl. Acad. Sci. USA 2006, 103, 15355–15358. [Google Scholar] [CrossRef]
- Dokter, A.; Woutersen, S.; Bakker, H. Anomalous Slowing Down of the Vibrational Relaxation of Liquid Water Upon Nanoscale Confinement. Phys. Rev. Lett. 2005, 94, 178301. [Google Scholar] [CrossRef]
- Lipari, G.; Szabo, A. Effect of librational motion on fluorescence depolarization and nuclear magnetic resonance relaxation in macromolecules and membranes. Biophys. J. 1980, 30, 489–506. [Google Scholar] [CrossRef]
- Tan, H.-S.; Piletic, I.R.; Riter, R.E.; Levinger, N.E.; Fayer, M.D. Dynamics of Water Confined on a Nanometer Length Scale in Reverse Micelles: Ultrafast Infrared Vibrational Echo Spectroscopy. Phys. Rev. Lett. 2005, 94, 57405. [Google Scholar] [CrossRef]
- Ivanov, E.N. Theory of Rotational Brownian Motion. J. Exptl. Theor. Phys. 1963, 45, 1509–1517. [Google Scholar]
- Senapati, S.; Chandra, A. Dielectric Constant of Water Confined in a Nanocavity. J. Phys. Chem. B 2001, 105, 5106–5109. [Google Scholar] [CrossRef]
- Pollack, G.H. The role of aqueous interfaces in the cell. Adv. Colloid Interface Sci. 2003, 103, 173–196. [Google Scholar] [CrossRef]
- Martinek, K.; Klyachko, N.L.; Kabanov, A.V.; Khmelnitsky, Y.L.; Levashov, A.V. Micellar enzymology: Its relation to membranology. Biochim. Biophys. Acta (BBA) Biomembr. 1989, 981, 161–172. [Google Scholar] [CrossRef]
- Siegel, D.P. Inverted micellar structures in bilayer membranes. Formation rates and half-lives. Biophys. J. 1984, 45, 399–420. [Google Scholar] [CrossRef]
- Hamada, T.; Morita, M.; Kishimoto, Y.; Komatsu, Y.; Vestergaard, M.C.; Takagi, M. Biomimetic Microdroplet Membrane Interface: Detection of the Lateral Localization of Amyloid Beta Peptides. J. Phys. Chem. Lett. 2010, 1, 170–173. [Google Scholar] [CrossRef]
- Harusawa, K.; Watanabe, C.; Kobori, Y.; Tomita, K.; Kitamura, A.; Kinjo, M.; Yanagisawa, M. Membrane Surface Modulates Slow Diffusion in Small Crowded Droplets. Langmuir 2021, 37, 437–444. [Google Scholar] [CrossRef]
- Yoshida, K.; Horii, K.; Saito, A.; Takashima, A.; Nishio, I. Confinement Effects on Polymer Dynamics: Thermo-Responsive Behaviours of Hydroxypropyl Cellulose Polymers in Phospholipid-Coated Droplets (Water-in-Oil Emulsion). Polymers 2017, 9, 680. [Google Scholar] [CrossRef] [PubMed]
- Vacher, M.; Waks, M.; Nicot, C. Myelin Proteins in Reverse Micelles: Tight Lipid Association Required for Insertion of the Folch-Pi Proteolipid into a Membrane-Mimetic System. J. Neurochem. 1989, 52, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Delahodde, A.; Vacher, M.; Nicot, C.; Waks, M. Solubilization and insertion into reverse micelles of the major myelin transmembrane proteolipid. FEBS Lett. 1984, 172, 343–347. [Google Scholar] [CrossRef]
- Bachmann, P.A.; Walde, P.; Luisi, P.L.; Lang, J. Self-replicating micelles: Aqueous micelles and enzymatically driven reactions in reverse micelles. J. Am. Chem. Soc. 1991, 113, 8204–8209. [Google Scholar] [CrossRef]
- Bachmann, P.A.; Walde, P.; Luisi, P.L.; Lang, J. Self-replicating reverse micelles and chemical autopoiesis. J. Am. Chem. Soc. 1990, 112, 8200–8201. [Google Scholar] [CrossRef]
- Doherty, I.D.C.P. Simultaneous Observation of Attractive Interaction, Depletion Forces, and “Sticky” Encounters between AOT Reverse Micelles in Isooctane Using Microelectrode Voltammetry. J. Phys. Chem. B 2000, 104, 8061–8067. [Google Scholar] [CrossRef]
- Koper, G.J.M.; Bedeaux, D. The Percus-Yevick approximation for repulsive hard spheres with surface adhesion. Phys. A Stat. Mech. Appl. 1992, 187, 489–502. [Google Scholar] [CrossRef]
- Jain, T.K.; Cassin, G.; Badiali, J.P.; Pileni, M.P. Relation between Exchange Process and Structure of AOT Reverse Micellar System. Langmuir 1996, 12, 2408–2411. [Google Scholar] [CrossRef]
- Molina, P.G.; Silber, J.J.; Correa, N.M.; Sereno, L. Electrochemistry in AOT Reverse Micelles. A Powerful Technique To Characterize Organized Media. J. Phys. Chem. C 2007, 111, 4269–4276. [Google Scholar] [CrossRef]
- D’Angelo, M.; Fioretto, D.; Onori, G.; Santucci, A. Micellar interactions in water-in-oil microemulsions. J. Mol. Struct. 1996, 383, 157–163. [Google Scholar] [CrossRef]
- Doherty, I.C. Voltammetry as a Tool for Monitoring Micellar Structural Evolution? Anal. Chem. 2000, 72, 687–695. [Google Scholar] [CrossRef]
- Park, H.-R.; Im, S.-E.; Seo, J.-J.; Bark, K.-M. Spectroscopic Properties of Quercetin in AOT Reverse Micelles. Bull. Korean Chem. Soc. 2014, 35, 828–832. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Khilar, K.C. Effects of intermicellar exchange rate on the formation of silver nanoparticles in reverse microemulsions of AOT. Langmuir 2000, 16, 905–910. [Google Scholar] [CrossRef]
- Zhang, D.H.; Guo, Z.; Dong, X.Y.; Sun, Y. Characterization of lipase in reversed micelles formulated with Cibacron Blue F-3GA modified Span 85. Biotechnol. Prog. 2007, 23, 108–115. [Google Scholar] [CrossRef]
- Nishii, Y.; Kinugasa, T.; Nii, S.; Takahashi, K. Transport behavior of protein in bulk liquid membrane using reversed micelles. J. Membr. Sci. 2002, 195, 11–21. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Zhang, C.; Feng, X.; Han, B.; Yang, G. Synthesis of alpha-chymotrypsin/polymer composites by a reverse micelle/gas antisolvent method. Colloids Surf. B Biointerfaces 2007, 59, 11–15. [Google Scholar] [CrossRef]
- Wu, X.; Ge, J.; Zhu, J.; Zhang, Y.; Yong, Y.; Liu, Z. A general method for synthesizing enzyme–polymer conjugates in reverse emulsions using Pluronic as a reactive surfactant. Chem. Commun. 2015, 51, 9674. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, L.; Yao, Y.; Zhang, S.; Gu, Z. Reverse micelle-based water-soluble nanoparticles for simultaneous bioimaging and drug delivery. Org. Biomol. Chem. 2017, 15, 3232–3238. [Google Scholar] [CrossRef]
- Wand, A.J.; Ehrhardt, M.; Flynn, P. High-resolution NMR of encapsulated proteins dissolved in low-viscosity fluids. Proc. Natl. Acad. Sci. USA 1998, 95, 15299–15302. [Google Scholar] [CrossRef]
- Kielec, J.; Valentine, K.; Babu, C.; Wand, A.J. Reverse micelles in integral membrane protein structural biology by solution NMR spectroscopy. Structure 2009, 17, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Nucci, N.; Pometun, M.; Wand, A.J. Mapping the hydration dynamics of ubiquitin. J. Am. Chem. Soc. 2011, 133, 12326–12329. [Google Scholar] [CrossRef]
- Dodevski, I.; Nucci, N.; Valentine, K.; Sidhu, G.; O′Brien, E.; Pardi, A.; Wand, A.J. Optimized Reverse Micelle Surfactant System for High-Resolution NMR Spectroscopy of Encapsulated Proteins and Nucleic Acids Dissolved in Low Viscosity Fluids. J. Am. Chem. Soc. 2014, 136, 3465–3474. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Tewari, P.; Blei, C.; Hales, K.; Pochan, D.; Leroux, J. Self-Assembled Nanocages for Hydrophilic Guest Molecules. J. Am. Chem. Soc. 2006, 128, 14599–14605. [Google Scholar] [CrossRef]
- Jones, M.; Gao, H.; Leroux, J. Reverse polymeric micelles for pharmaceutical applications. J. Control. Release Off. J. Control. Release Soc. 2008, 132, 208–215. [Google Scholar] [CrossRef]
- Wang, T.; Wang, N.; Hao, A.; He, X.; Li, T.; Deng, Y. Lyophilization of water-in-oil emulsions to prepare phospholipid-based anhydrous reverse micelles for oral peptide delivery. Eur. J. Pharm. Sci. 2010, 39, 373–379. [Google Scholar] [CrossRef]
- Bayraktutan, T.; Meral, K.; Onganer, Y. Photophysical properties of pyronin dyes in reverse micelles of AOT. J. Lumin. 2014, 145, 925–929. [Google Scholar] [CrossRef]
- Singh, P.; Kumbhakar, M.; Pal, H.; Nath, S. Ultrafast Torsional Dynamics of Protein Binding Dye Thioflavin-T in Nanoconfined Water Pool. J. Phys. Chem. B 2009, 113, 8532–8538. [Google Scholar] [CrossRef]
- Chatterjee, A.; Seth, D. Effect of nanocavities on the torsional dynamics of thioflavin T in various non-aqueous reverse micelles. Photochem. Photobiol. Sci. 2013, 12, 369–383. [Google Scholar] [CrossRef]
- Banerjee, C.; Ghatak, C.; Mandal, S.; Ghosh, S.; Kuchlyan, J.; Sarkar, N. Curcumin in Reverse Micelle: An Example to Control Excited-State Intramolecular Proton Transfer (ESIPT) in Confined Media. J. Phys. Chem. B 2013, 117, 6906–6916. [Google Scholar] [CrossRef]
- Ono, T.; Nagatomo, M.; Nagao, T.; Ijima, H.; Kawakami, K. Nonaggregating refolding of Ribonuclease A using reverse micellar dialysis. Biotechnol. Bioeng. 2005, 89, 290–295. [Google Scholar] [CrossRef]
- Wu, X.Y.; Liu, Y.; Dong, X.Y.; Sun, Y. Protein refolding mediated by reverse micelles of Cibacron Blue F-3GA modified nonionic surfactant. Biotechnol. Prog. 2006, 22, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; He, Y.; Wan, L.; Cui, Z.; Jessop, P. Synthesis of CdS nanoparticles in switchable surfactant reverse micelles. Chem. Commun. 2013, 49, 1912–1914. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gunderson, W.; Weitz, A.; Hendrich, M.; Ryabov, A.; Collins, T. Activation of Dioxygen by a TAML Activator in Reverse Micelles: Characterization of an Fe(III)Fe(IV) Dimer and Associated Catalytic Chemistry. J. Am. Chem. Soc. 2015, 137, 9704–9715. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Ryabov, A.; Collins, T. Kinetic Evidence for Reactive Dimeric TAML Iron Species in the Catalytic Oxidation of NADH and a Dye by O2 in AOT Reverse Micelles. ACS Catal. 2016, 6, 3713–3718. [Google Scholar] [CrossRef]
- Wang, J. Carbon-nanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar] [CrossRef]
- Schmitt, O.H. Some interesting and useful biomimetic transforms. In Proceedings of the Third International Biophysics Congress, Boston, MA, USA, 29 August–3 September 1969; p. 297. [Google Scholar]
- Zan, G.; Wu, Q. Biomimetic and Bioinspired Synthesis of Nanomaterials/Nanostructures. Adv. Mater. 2016, 28, 2099–2147. [Google Scholar] [CrossRef]
- Pileni, M.P.; Motte, L.; Petit, C. Synthesis of cadmium sulfide in situ in reverse micelles: Influence of the preparation modes on size, polydispersity, and photochemical reactions. Chem. Mater. 1992, 4, 338–345. [Google Scholar] [CrossRef]
- Ganguli, A.K.; Vaidya, S.; Ahmad, T. Synthesis of nanocrystalline materials through reverse micelles: A versatile methodology for synthesis of complex metal oxides. Bull. Mater. Sci. 2008, 31, 415–419. [Google Scholar] [CrossRef]
- Fernández, L.; Garro, N.; Morales, J.M.; Burguete, P.; Latorre, J.; Guillem, C.; Beltrán, A.; Beltrán, D.; Amorós, P. Supramolecular capping-ligand effect of lamellar silica mesostructures for the one-pot synthesis of highly dispersed ZnO nanoparticles. Nanotechnology 2006, 17, 4456–4463. [Google Scholar] [CrossRef]
- Qi, L. Synthesis of Inorganic Nanostructures in Reverse Micelle. Encycl. Surf. Colloid Sci. 2006, 6183–6207. [Google Scholar] [CrossRef]
- Hu, A.; Yao, Z.; Yu, X. Phase behavior of a sodium dodecanol allyl sulfosuccinic diester/n-pentanol/methyl acrylate/butyl acrylate/water microemulsion system and preparation of acrylate latexes by microemulsion polymerization. J. Appl. Polym. Sci. 2009, 113, 2202–2208. [Google Scholar] [CrossRef]
- Pileni, M.-P. Nanocrystals: Fabrication, organization and collective properties. C. R. Chim. 2003, 6, 965–978. [Google Scholar] [CrossRef]
- Ganguli, D.; Ganguli, M. Inorganic Particle Synthesis via Macro and Microemulsions: A Micrometer to Nanometer Landscape, 2003rd ed.; Springer Science + Business Media: New York, NY, USA, 2003; Originally published by Kluwer Academic/Plenum Publishers. [Google Scholar] [CrossRef]
- Quintanilla-Carvajal, M.; Matiacevich, S. Role of Surfactants and Their Applications in Structured Nanosized Systems. In Food Nanoscience and Nanotechnology; Food Engineering Series; Hernández-Sánchez, H., Gutiérrez-López, G., Eds.; Springer: Cham, Switzerland, 2015; pp. 177–186. [Google Scholar] [CrossRef]
- Barnickel, P.; Wokaun, A.; Sager, W.; Eicke, H.F. Size tailoring of silver colloids by reduction in WO microemulsions. J. Colloid Interface Sci. 1992, 148, 80–90. [Google Scholar] [CrossRef]
- Lisiecki, I.; Pileni, M.P. Synthesis of copper metallic clusters using reverse micelles as microreactors. J. Am. Chem. Soc. 1993, 115, 3887–3896. [Google Scholar] [CrossRef]
- Solla-Gullón, J.; Montiel, V.; Aldaz, A.; Clavilier, J. Electrochemical characterisation of platinum nanoparticles prepared by microemulsion: How to clean them without loss of crystalline surface structure. J. Electroanal. Chem. 2000, 491, 69–77. [Google Scholar] [CrossRef]
- Chhabra, V.; Pillai, V.; Mishra, B.K.; Morrone, A.; Shah, D.O. Synthesis, Characterization, and Properties of Microemulsion-Mediated Nanophase TiO2 Particles. Langmuir 1995, 11, 3307–3311. [Google Scholar] [CrossRef]
- Osseo-Asare, K.; Arriagada, F.J. Preparation of SiO2 nanoparticles in a non-ionic reverse micellar system. Colloids Surf. 1990, 50, 321–339. [Google Scholar] [CrossRef]
- Song, K.C.; Kim, J.H. Preparation of Nanosize Tin Oxide Particles from Water-in-Oil Microemulsions. J. Colloid Interface Sci. 1999, 212, 193–196. [Google Scholar] [CrossRef]
- Kida, T.; Guan, G.; Minami, Y.; Ma, T.; Yoshida, A. Photocatalytic hydrogen production from water over a LaMnO3/CdS nanocomposite prepared by the reverse micelle method. J. Mater. Chem. 2003, 13, 1186–1191. [Google Scholar] [CrossRef]
- Towata, A.; Uwamino, Y.; Sando, M.; Iseda, K.; Taoda, H. Synthesis of titania photocatalysts dispersed with nickel nanosized particles. Nanostructured Mater. 1998, 10, 1033–1042. [Google Scholar] [CrossRef]
- Toshiyuki, M.; Kazuyasu, F.; Yumin, P.; Ken-ichi, M.; Gin-ya, A. Carbon Monoxide Oxidation Characteristics Over the Al2O3-supported CeO2-ZrO2 Catalysts Prepared by the Microemulsion Method. Chem. Lett. 1997, 26, 1285–1286. [Google Scholar] [CrossRef]
- López-Quintela, M.A.; Tojo, C.; Blanco, M.C.; García Rio, L.; Leis, J.R. Microemulsion dynamics and reactions in microemulsions. Curr. Opin. Colloid Interface Sci. 2004, 9, 264–278. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Holzwarth, J.F.; Wang, D.I.C.; Hatton, T.A. Coalescence and solubilizate exchange in a cationic four-component reversed micellar system. J. Phys. Chem. 1990, 94, 7232–7239. [Google Scholar] [CrossRef]
- Zhang, J.; Han, B.; Liu, J.; Zhang, X.; He, J.; Liu, Z.; Jiang, T.; Yang, G. Recovery of Silver Nanoparticles Synthesized in AOT/C12E4 Mixed Reverse Micelles by Antisolvent CO2. Chem. A Eur. J. 2002, 8, 3879–3883. [Google Scholar] [CrossRef]
- Borsarelli, C.D.; Braslavsky, S.E. The partial molar volume of the proton in water determined by laser-induced optoacoustic studies. J. Photochem. Photobiol. B Biol. 1998, 43, 222–228. [Google Scholar] [CrossRef]
- Dutta, P.K.; Jakupca, M.; Reddy, K.S.N.; Salvati, L. Controlled growth of microporous crystals nucleated in reverse micelles. Nature 1995, 374, 44. [Google Scholar] [CrossRef]
- Steigerwald, M.L.; Alivisatos, A.P.; Gibson, J.M.; Harris, T.D.; Kortan, R.; Muller, A.J.; Thayer, A.M.; Duncan, T.M.; Douglass, D.C.; Brus, L.E. Surface derivatization and isolation of semiconductor cluster molecules. J. Am. Chem. Soc. 1988, 110, 3046–3050. [Google Scholar] [CrossRef]
- Chen, D.-H.; Wu, S.-H. Synthesis of Nickel Nanoparticles in Water-in-Oil Microemulsions. Chem. Mater. 2000, 12, 1354–1360. [Google Scholar] [CrossRef]
- Moreira Ferreira, L.; Dutra de Souza, F.; Nome Aguilera, F.J.; Cruz Vieira, I. Electrochemical sensor based on rhodium nanoparticles stabilized in zwitterionic surfactant for p-coumaric acid analysis. Can. J. Chem. 2016, 95, 113–119. [Google Scholar] [CrossRef]
- Zapp, E.; Souza, F.D.; Souza, B.S.; Nome, F.; Neves, A.; Vieira, I.C. A bio-inspired sensor based on surfactant film and Pd nanoparticles. Analyst 2013, 138, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Cadorin Fernandes, S.; Dutra de Souza, F.; Silveira de Souza, B.; Nome, F.; Cruz Vieira, I. Gold nanoparticles dispersed in zwitterionic surfactant for peroxidase immobilization in biosensor construction. Sens. Actuators B Chem. 2012, 173, 483–490. [Google Scholar] [CrossRef]
- Yuasa, M.; Masaki, T.; Kida, T.; Shimanoe, K.; Yamazoe, N. Nano-sized PdO loaded SnO2 nanoparticles by reverse micelle method for highly sensitive CO gas sensor. Sens. Actuators B Chem. 2009, 136, 99–104. [Google Scholar] [CrossRef]
- Matsushima, S.; Yasutake, T.; Norio, M.; Noboru, Y. Electronic Interaction between Metal Additives and Tin Dioxide in Tin Dioxide-Based Gas Sensors. Jpn. J. Appl. Phys. 1988, 27, 1798. [Google Scholar] [CrossRef]
- Shigenori, M.; Jun, T.; Norio, M.; Noboru, Y. TEM Observation of the Dispersion State of Pd on SnO2. Chem. Lett. 1989, 18, 1651–1654. [Google Scholar] [CrossRef]
- Yuasa, M.; Sakai, G.; Shimanoe, K.; Teraoka, Y.; Yamazoe, N. Reverse Micelle-Based Preparation of Carbon-Supported La1−xSrxMn1−yFeyO3+δ for Oxygen Reduction Electrode. J. Electrochem. Soc. 2004, 151, A1690–A1695. [Google Scholar] [CrossRef]
- Yuasa, M.; Shimanoe, K.; Teraoka, Y.; Yamazoe, N. Preparation of carbon-supported nano-sized LaMnO3 using reverse micelle method for energy-saving oxygen reduction cathode. Catal. Today 2007, 126, 313–319. [Google Scholar] [CrossRef]
- Sato, T.; Uno, S.; Hashizume, T.; Hasegawa, H. Large Schottky Barrier Heights on Indium Phosphide-Based Materials Realized by In-Situ Electrochemical Process. Jpn. J. Appl. Phys. 1997, 36, 1811–1817. [Google Scholar] [CrossRef]
- Kimura, T.; Hideki, H.; Taketomo, S.; Tamotsu, H. Sensing Mechanism of InP Hydrogen Sensors Using Pt Schottky Diodes Formed by Electrochemical Process. Jpn. J. Appl. Phys. 2006, 45, 3414. [Google Scholar] [CrossRef]
- Grym, J.; Prochazkova, O.; Yatskiv, R.; Piksova, K. Hydrogen sensors based on electrophoretically deposited Pd nanoparticles onto InP. Nanoscale Res. Lett. 2011, 6, 5. [Google Scholar] [CrossRef]
- Zdansky, K. Highly sensitive hydrogen sensor based on graphite-InP or graphite-GaN Schottky barrier with electrophoretically deposited Pd nanoparticles. Nanoscale Res. Lett. 2011, 6, 490. [Google Scholar] [CrossRef] [PubMed]
- Davarpanah, S.J.; Karimian, R.; Goodarzi, V.; Piri, F. Synthesis of Copper (II) Oxide (CuO) Nanoparticles and Its Application as Gas Sensor. J. Appl. Biotechnol. Rep. 2016, 322, 329–332. [Google Scholar]
- Guliants, E.A.; Schwarb, R.; Bearbower, H.; Gord, J.R.; Bunker, C.E. Functional nanoparticles in thin films as sensing media. Rev. Adv. Mater. Sci. 2005, 10, 289–294, ISSN 1606-5131. [Google Scholar]
- Kalantar-zadeh, K.; Fry, B.N. Inorganic Nanotechnology Enabled Sensors. In Nanotechnology-Enabled Sensors; Springer US: Boston, MA, USA, 2008; pp. 283–370. [Google Scholar] [CrossRef]
- Collins, P.G.; Arnold, M.S.; Avouris, P. Engineering carbon nanotubes and nanotube circuits using electrical breakdown. Science 2001, 292, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Avouris, P. Carbon nanotube electronics. Chem. Phys. 2002, 281, 429–445. [Google Scholar] [CrossRef]
- Hu, J.; Ouyang, M.; Yang, P.; Lieber, C.M. Controlled growth and electrical properties of heterojunctions of carbon nanotubes and silicon nanowires. Nature 1999, 399, 48. [Google Scholar] [CrossRef]
- Dai, H.; Hafner, J.H.; Rinzler, A.G.; Colbert, D.T.; Smalley, R.E. Nanotubes as nanoprobes in scanning probe microscopy. Nature 1996, 384, 147. [Google Scholar] [CrossRef]
- Wiedwald, U.; Han, L.Y.; Biskupek, J.; Kaiser, U.; Ziemann, P. Preparation and characterization of supported magnetic nanoparticles prepared by reverse micelles. Beilstein J. Nanotechnol. 2010, 1, 24–47. [Google Scholar] [CrossRef]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated Polymer-Based Chemical Sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.J.; Lewis, N.S.; Schauer, C.L.; Sotzing, G.A.; Stitzel, S.E.; Vaid, T.P.; Walt, D.R. Cross-Reactive Chemical Sensor Arrays. Chem. Rev. 2000, 100, 2595–2626. [Google Scholar] [CrossRef]
- Swager, T.M. The Molecular Wire Approach to Sensory Signal Amplification. Acc. Chem. Res. 1998, 31, 201–207. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Facile fabrication of polypyrrole nanotubes using reverse microemulsion polymerization. Chem. Commun. 2003, 720–721. [Google Scholar] [CrossRef]
- Jang, J.; Yoon, H. Formation mechanism of conducting polypyrrole nanotubes in reverse micelle systems. Langmuir 2005, 21, 11484–11489. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Goux, W.J.; Manohar, S.K. Synthesis of polyaniline nanofibers by “nanofiber seeding”. J. Am. Chem. Soc. 2004, 126, 4502–4503. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.; Chang, M.; Jang, J. Formation of 1D poly(3,4-ethylenedioxythiophene) nanomaterials in reverse microemulsions and their application to chemical sensors. Adv. Funct. Mater. 2007, 17, 431–436. [Google Scholar] [CrossRef]
- Murphy, C.J. Optical sensing with quantum dots. Anal. Chem. 2002, 74, 520A–526A. [Google Scholar] [CrossRef] [PubMed]
- Empedocles, S.; Bawendi, M. Spectroscopy of Single CdSe Nanocrystallites. Acc. Chem. Res. 1999, 32, 389–396. [Google Scholar] [CrossRef]
- Kuno, M.; Lee, J.K.; Dabbousi, B.O.; Mikulec, F.V.; Bawendi, M.G. The band edge luminescence of surface modified CdSe nanocrystallites: Probing the luminescing state. J. Chem. Phys. 1997, 106, 9869–9882. [Google Scholar] [CrossRef]
- Marin, S.; Merkoci, A. Direct electrochemical stripping detection of cystic-fibrosis-related DNA linked through cadmium sulfide quantum dots. Nanotechnology 2009, 20, 55101. [Google Scholar] [CrossRef]
- Brown, L.O.; Hutchison, J.E. Controlled growth of gold nanoparticles during ligand exchange. J. Am. Chem. Soc. 1999, 121, 882–883. [Google Scholar] [CrossRef]
- Ershov, B.G.; Sukhov, N.L.; Janata, E. Formation, Absorption Spectrum, and Chemical Reactions of Nanosized Colloidal Cobalt in Aqueous Solution. J. Phys. Chem. B 2000, 104, 6138–6142. [Google Scholar] [CrossRef]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Guzelian, A.A.; Katari, J.E.B.; Kadavanich, A.V.; Banin, U.; Hamad, K.; Juban, E.; Alivisatos, A.P.; Wolters, R.H.; Arnold, C.C.; Heath, J.R. Synthesis of Size-Selected, Surface-Passivated InP Nanocrystals. J. Phys. Chem. 1996, 100, 7212–7219. [Google Scholar] [CrossRef]
- Rockenberger, J.; Scher, E.C.; Alivisatos, A.P. A New Nonhydrolytic Single-Precursor Approach to Surfactant-Capped Nanocrystals of Transition Metal Oxides. J. Am. Chem. Soc. 1999, 121, 11595–11596. [Google Scholar] [CrossRef]
- Trentler, T.J.; Denler, T.E.; Bertone, J.F.; Agrawal, A.; Colvin, V.L. Synthesis of TiO2 Nanocrystals by Nonhydrolytic Solution-Based Reactions. J. Am. Chem. Soc. 1999, 121, 1613–1614. [Google Scholar] [CrossRef]
- Penner, R.M. Hybrid electrochemical/chemical synthesis of quantum dots. Acc. Chem. Res. 2000, 33, 78–86. [Google Scholar] [CrossRef]
- Tonucci, R.J.; Justus, B.L.; Campillo, A.J.; Ford, C.E. Nanochannel array glass. Science 1992, 258, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Merkoci, A.; del Valle, M.; Alegret, S. Bionanostructures and their integration into electrochemical sensing system. A review of DNA applications. Rev. Mex. Fis. 2006, 52, 1–5, ISSN 0035-001X. [Google Scholar]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.D.; Rivas, G. Encoded beads for electrochemical identification. Anal. Chem. 2003, 75, 4667–4671. [Google Scholar] [CrossRef]
- Jung, E.; Kim, S.W.; Cho, A.; Kim, Y.J.; Jeong, G.J.; Kim, J.; Bhang, S.H.; Yu, T. Synthesis of Sub 3 nm-Sized Uniform Magnetite Nanoparticles Using Reverse Micelle Method for Biomedical Application. Materials 2019, 12, 3850. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Dai, F.; Zhao, C.; Si, Y. A reverse micelle strategy for fabricating magnetic lipase-immobilized nanoparticles with robust enzymatic activity. Sci. Rep. 2017, 7, 9806. [Google Scholar] [CrossRef] [PubMed]
- Leser, M.E.; Mrkoci, K.; Luisi, P.L. Reverse micelles in protein separation: The use of silica for the back-transfer process. Biotechnol. Bioeng. 1993, 41, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Cai, J.; Guo, X. Extraction of ovalbumin with gemini surfactant reverse micelles-effect of gemini surfactant structure. Sep. Purif. Technol. 2016, 158, 367–373. [Google Scholar] [CrossRef]
- Wan, J.; Guo, J.; Miao, Z.; Guo, X. Reverse micellar extraction of bromelain from pineapple peel–effect of surfactant structure. Food Chem. 2016, 197, 450–456. [Google Scholar] [CrossRef]
- Zhu, K.X.; Sun, X.H.; Chen, Z.C.; Peng, W.; Qian, H.F.; Zhou, H.M. Comparison of functional properties and secondary structures of defatted wheat germ proteins separated by reverse micelles and alkaline extraction and isoelectric precipitation. Food Chem. 2010, 123, 1163–1169. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, H.; Zhang, X.; Zhu, H. Comparison of structures of walnut protein fractions obtained through reverse micelles and alkaline extraction with isoelectric precipitation. Int. J. Biol. Macromol. 2019, 125, 1214–1220. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Liu, H.; Zhang, G.; Ao, Q. Functional, nutritional and flavor characteristic of soybean proteins obtained through reverse micelles. Food Hydrocoll. 2018, 74, 358–366. [Google Scholar] [CrossRef]
- Chuo, S.C.; Abd-Talib, N.; Mohd-Setapar, S.H.; Hassan, H.; Nasir, H.M.; Ahmad, A.; Lokhat, D.; Ashraf, G.M. Reverse micelle Extraction of Antibiotics using an Eco-friendly Sophorolipids Biosurfactant. Sci. Rep. 2018, 8, 477. [Google Scholar] [CrossRef]
- Deshpande, M.; Daniels, L. Evaluation of sophorolipid biosurfactant production by Candida bombicola using animal fat. Bioresour. Technol. 1995, 54, 143–150. [Google Scholar] [CrossRef]
- Pietrini, A.V.; Luisi, P.L. Cell-free Protein Synthesis through Solubilisate Exchange in Water/Oil Emulsion Compartments. ChemBioChem 2004, 5, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, A.D.; Tawfik, D.S. Directed evolution of an extremely fast phosphotriesterase by in vitro compartmentalization. EMBO J. 2003, 22, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Katsura, S.; Yamaguchi, A.; Inami, H.; Matsuura, S.; Hirano, K.; Mizuno, A. Indirect micromanipulation of single molecules in water-in-oil emulsion. Electrophoresis 2001, 22, 289–293. [Google Scholar] [CrossRef]
- Atencia, J.; Beebe, D.J. Controlled microfluidic interfaces. Nature 2005, 437, 648–655. [Google Scholar] [CrossRef]
- Hase, M.; Yamada, A.; Hamada, T.; Baigl, D.; Yoshikawa, K. Manipulation of cell-sized phospholipid-coated microdroplets and their use as biochemical microreactors. Langmuir 2007, 23, 348–352. [Google Scholar] [CrossRef]
- Kato, A.; Shindo, E.; Sakaue, T.; Tsuji, A.; Yoshikawa, K. Conformational transition of giant DNA in a confined space surrounded by a phospholipid membrane. Biophys. J. 2009, 97, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Guo, S.R.; Li, H.Q.; Liu, L.; Li, H.L.; Gu, J.G. Synthesis of Three Types of Amphiphilic Poly (ethylene glycol)-block-Poly (sebacic anhydride) Copolymers and Studies of their Micellar Solutions. Macromol. Chem. Phys. 2006, 27, 1359–1367. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Wang, H.; Wu, S.; Zhao, K.; Sun, H.; Kong, D.; Wang, C.; Leng, X.; Zhu, D. Preparation and evaluation of PCL-PEG-PCL polymeric nanoparticles for doxorubicin delivery against breast cancer. RSC Adv. 2016, 6, 54727–54737. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Jalilzadeh, N.; Samadi, N.; Salehi, R.; Dehghan, G.; Iranshahi, M.; Dadpour, M.R.; Hamishehkar, H. Novel nano-vehicle for delivery and efficiency of anticancer auraptene against colon cancer cells. Sci. Rep. 2020, 10, 1606. [Google Scholar] [CrossRef] [PubMed]
- Rahdar, A.; Sanchooli, E.; Karimzadeh, R.; Sahoo, D. Investigation on the Linear and Nonlinear Properties of Morin in Presence of Reverse Micelle and Different Oil Content in Reverse Micelle. J. Fluoresc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Höfener, S.; Kooijman, P.C.; Groen, J.; Ariese, F.; Visscher, L. Fluorescence behavior of (selected) flavonols: A combined experimental and computational study. Phys. Chem. Chem. Phys. 2013, 15, 12572–12581. [Google Scholar] [CrossRef]
- Dhanasekar, C.; Rasool, M. Morin, a dietary bioflavonol suppresses monosodium urate crystal-induced inflammation in an animal model of acute gouty arthritis with reference to NLRP3 inflammasome, hypo-xanthine phospho-ribosyl transferase, and inflammatory mediators. Eur. J. Pharm. 2016, 786, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, K.F.S.; Oliveira, T.T.D.; Nagem, T.J.; Pinto, A.D.S.; Oliveira, M.G.A.; Soares, J.F. Effect of flavonoids morin; quercetin and nicotinic acid on lipid metabolism of rats experimentally fed with triton. Braz. Arch. Biol. Technol. 2001, 44, 263–267. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Contents and antibacterial activity of flavonoids extracted from leaves of Psid guajava. J. Med. Plants Res. 2010, 4, 393–396. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Papadimitriou, K.; Alexandraki, V.; Balkiza, F.; Georgalaki, M.; Papadimitriou, V.; Tsakalidou, E.; Xenakis, A. Reverse micelles as nanocarriers of nisin against foodborne pathogens. Food Chem. 2018, 255, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Kittipongpittaya, K.; Mcclements, D.J.; Decker, E.A. Impact of phosphoethanolamine reverse micelles on lipid oxidation in bulk oils. J. Am. Oil Chem. Soc. 2014, 91, 1931–1937. [Google Scholar] [CrossRef]
- Gu, W.X.; Zhu, M.; Song, N.; Du, X.; Yang, Y.W.; Gao, H. Reverse micelles based on biocompatible b-cyclodextrin conjugated polyethylene glycol block polylactide for protein delivery. J. Mater. Chem. B 2015, 3, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Tonova, K.; Lazarova, Z. Reversed micelle solvents as tools of enzyme purification and enzyme-catalyzed conversion. Biotechnol. Adv. 2008. [Google Scholar] [CrossRef] [PubMed]
- Orlich, B.; Schomäcker, R. Enzyme Catalysis in Reverse Micelles. Adv. Biochem. Eng. Biotechnol. 2002, 75, 185–208. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).