Inkjet Printing of Polypyrrole Electroconductive Layers Based on Direct Inks Freezing and Their Use in Textile Solid-State Supercapacitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
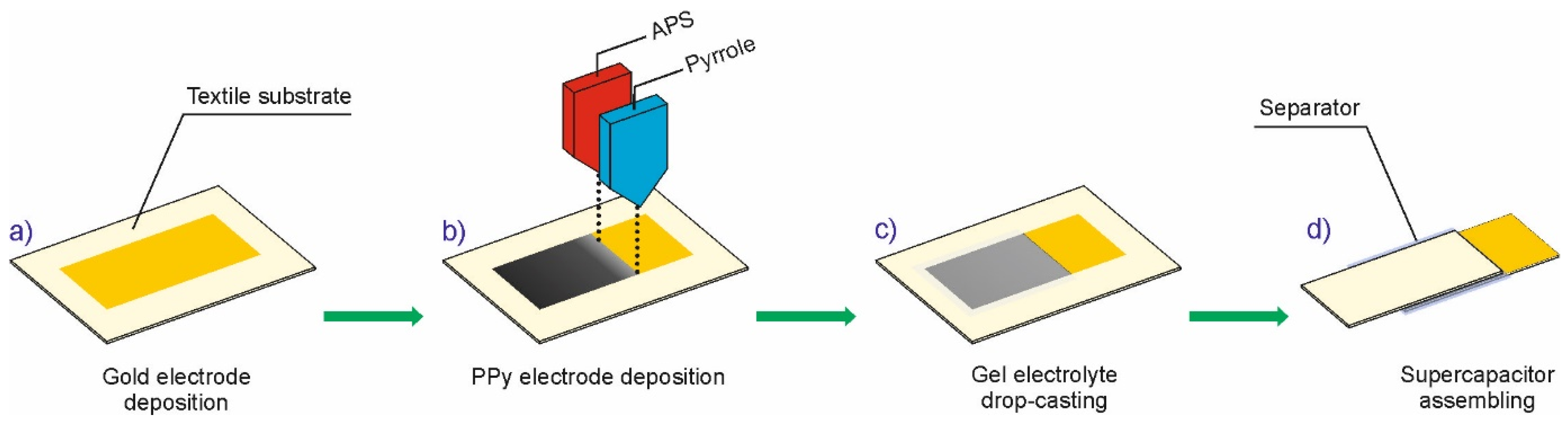
2.2. Deposition of PPy Layers on Textile Substrates with Direct Inks Freezing
2.3. Fabrication of Textile Solid-State Supercapacitor
2.4. Characterization
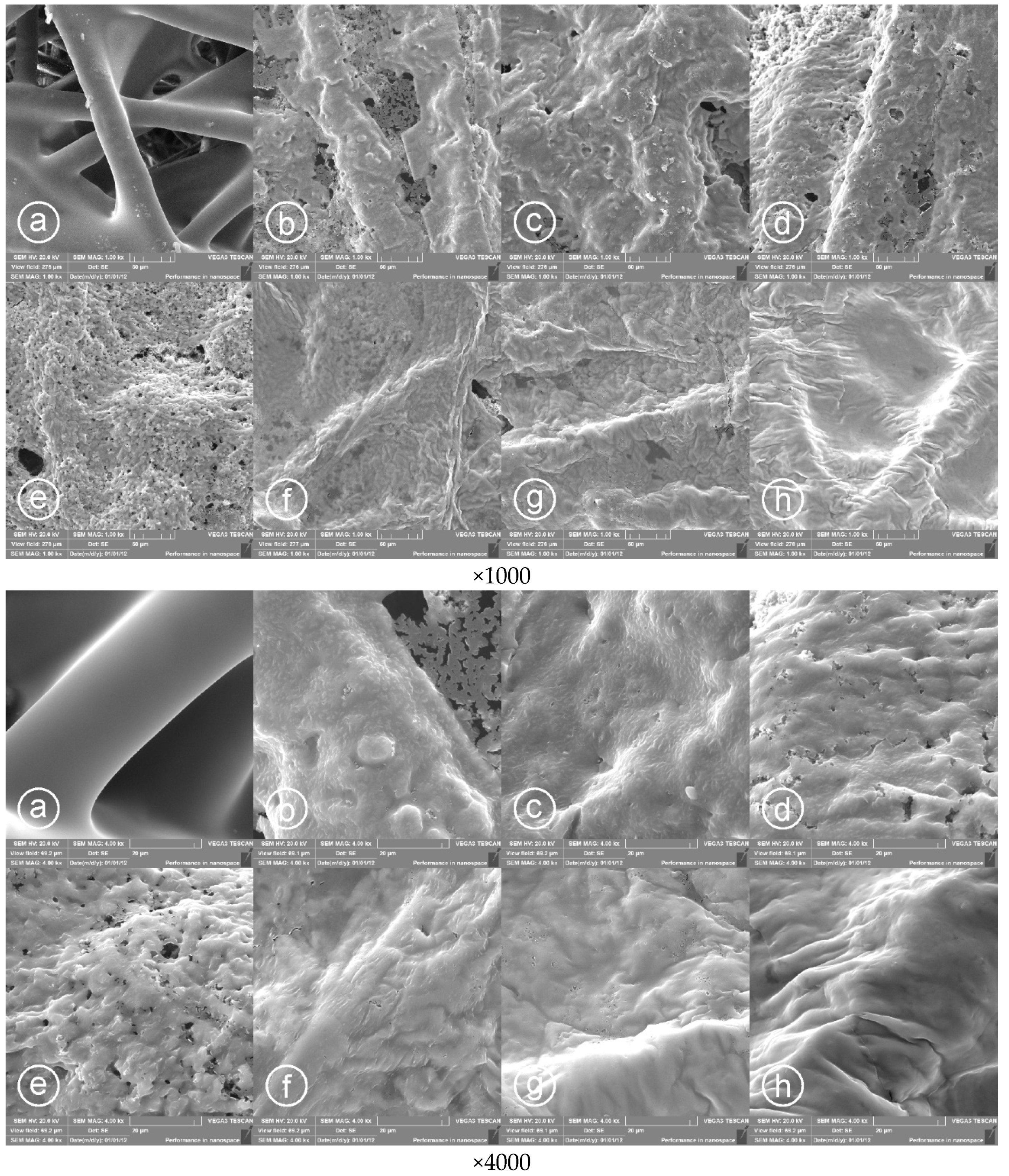
2.4.1. Scanning Electron Microscopy
2.4.2. Raman Spectroscopy
2.4.3. Surface Resistance
2.4.4. Supercapacitor Characterization
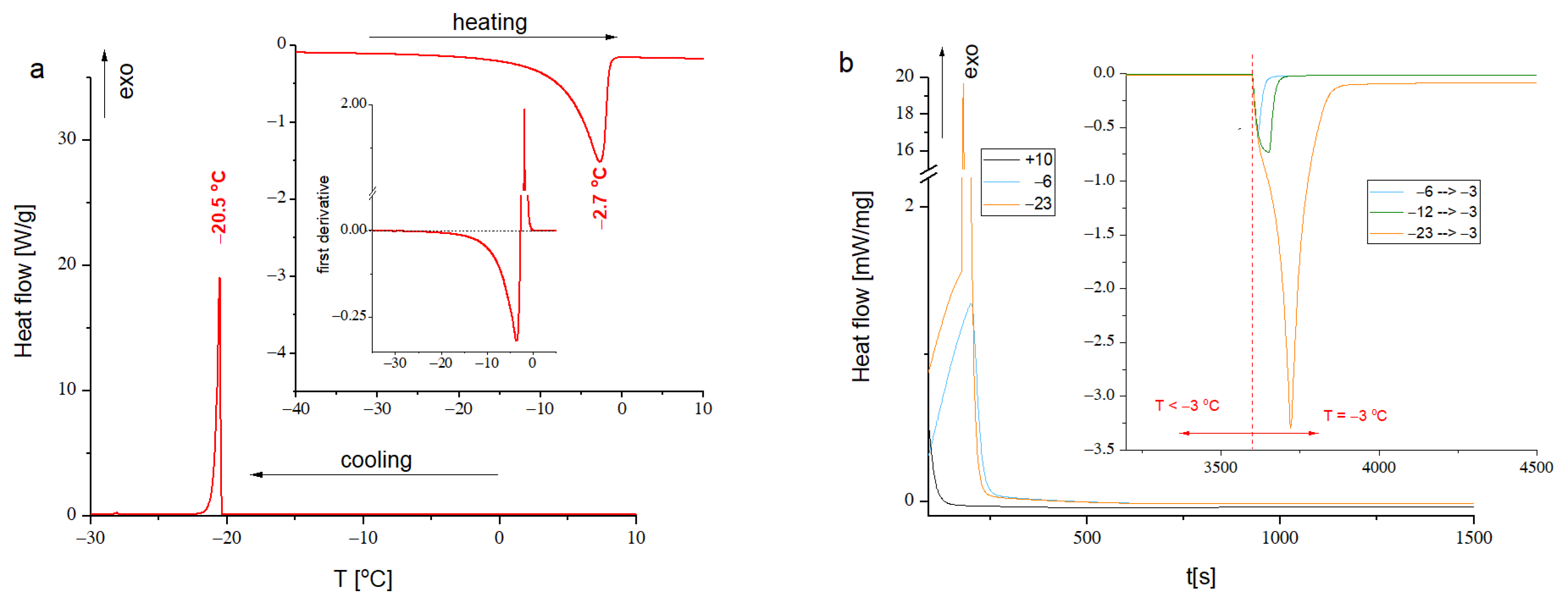
2.4.5. Differential Scanning Calorimetry (DSC)
3. Results
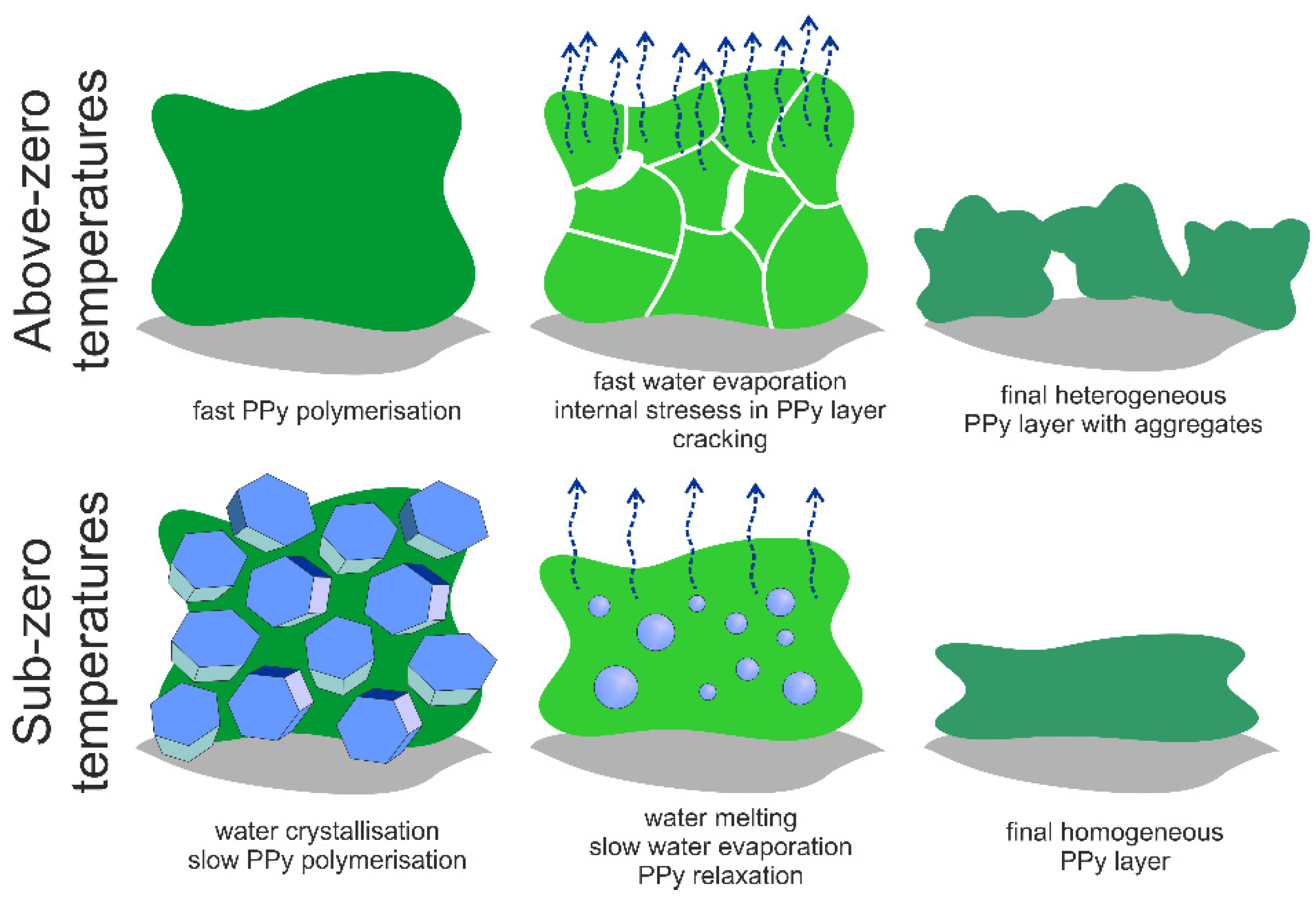
3.1. Surface Morphology of Printed PPy Layers
3.2. DSC Analysis
3.3. Surface Resistance of Printed PPy Layers
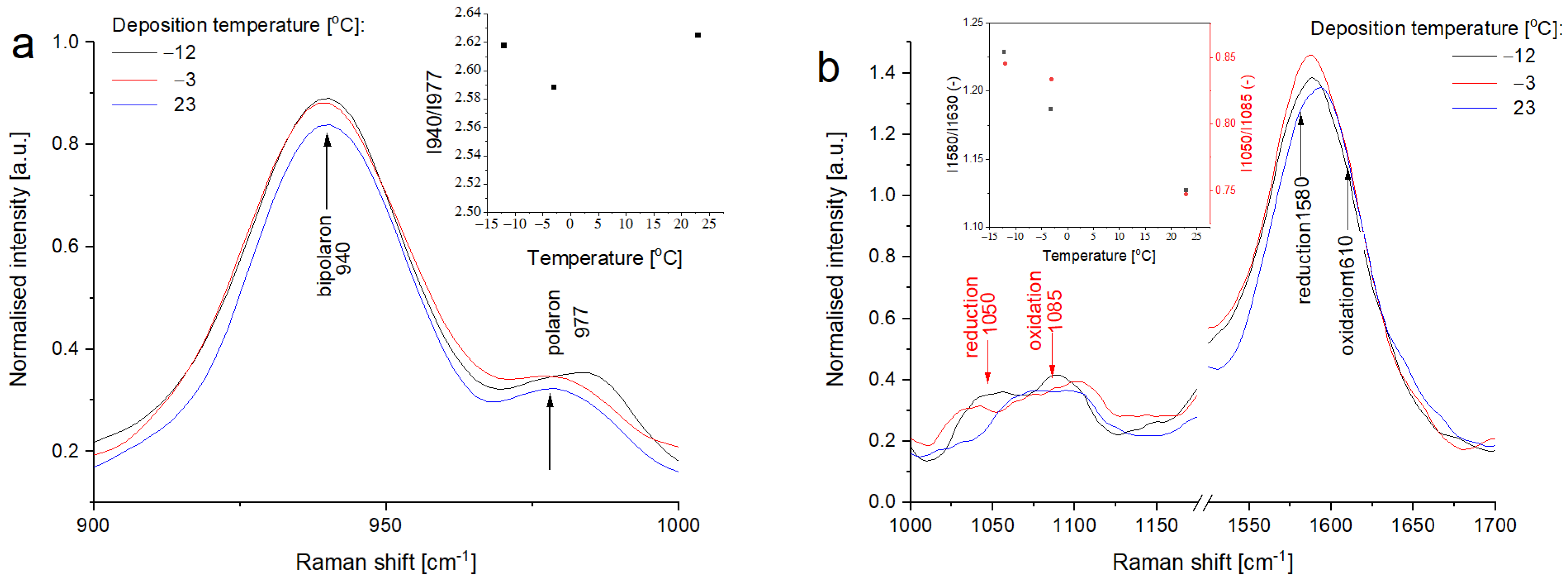
3.4. Raman Spectroscopy of Printed PPy Layers
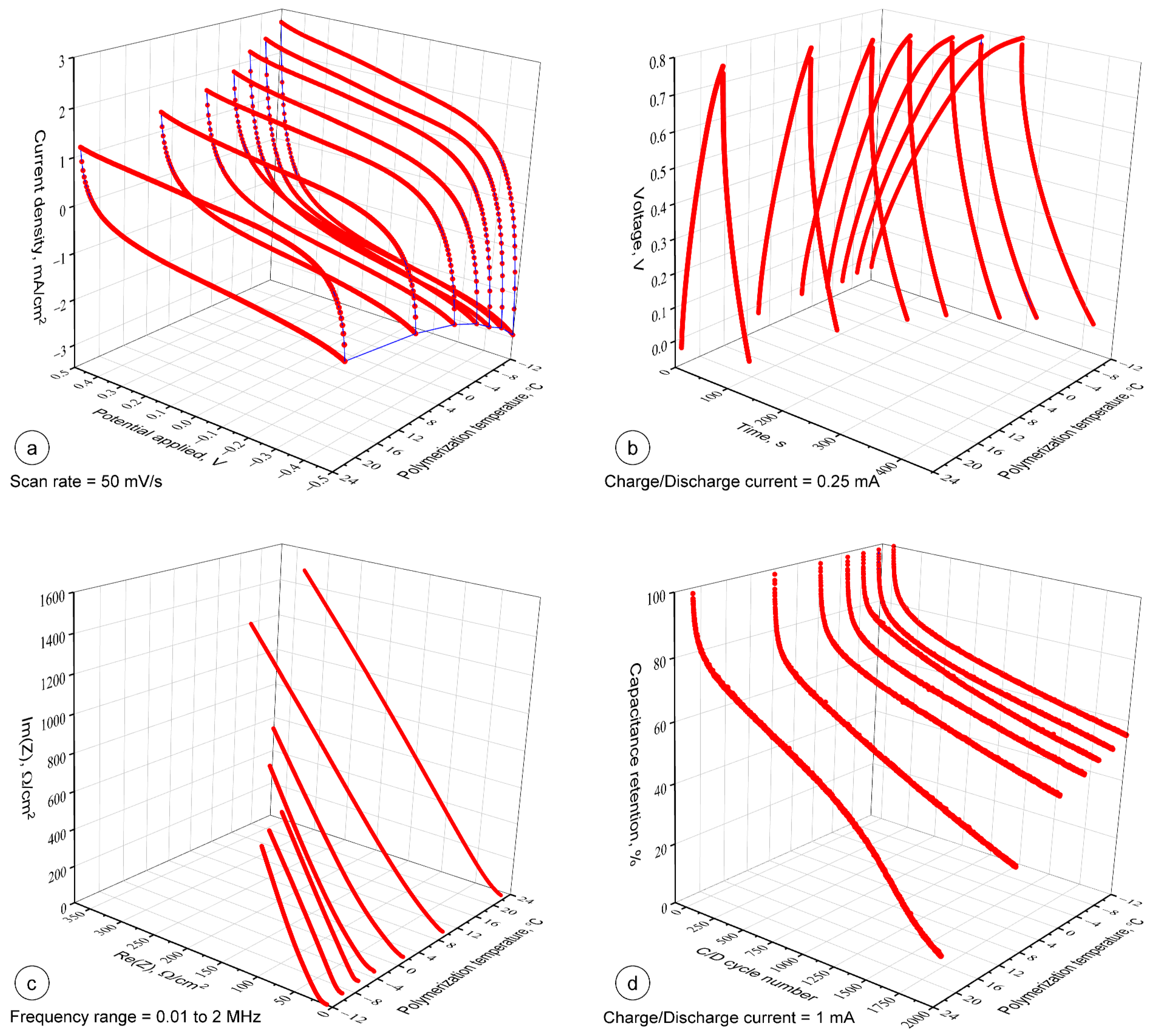
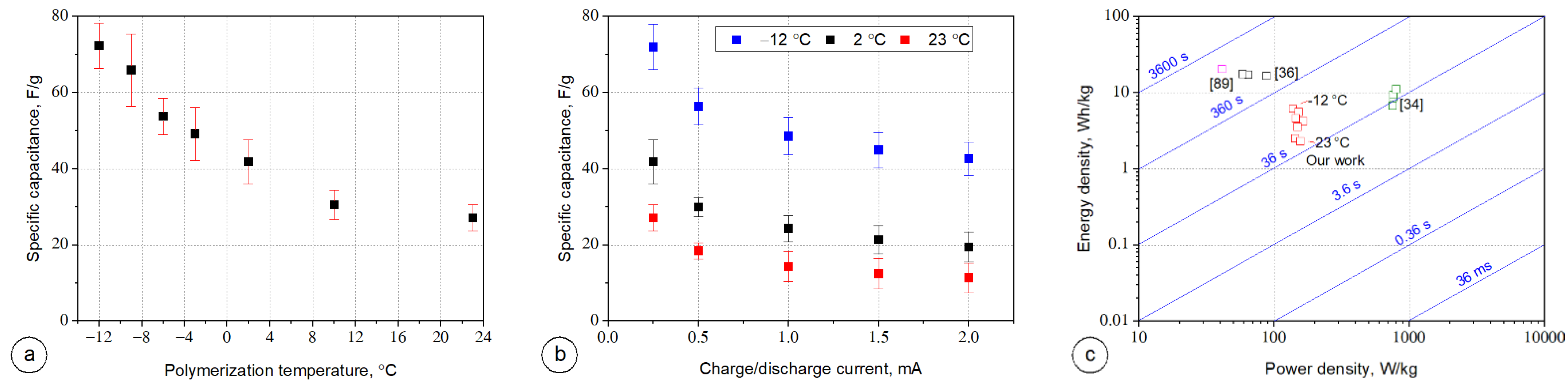
3.5. Electrochemical Performance of PPy Based Textile Solid-State Supercapacitors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, W.-S.; Humphrey, B.D.; MacDiarmid, A.G. Polyaniline, a novel conducting polymer. Morphology and chemistry of its oxidation and reduction in aqueous electrolytes. J. Chem. Soc. Faraday Trans. 1986, 82, 2385–2400. [Google Scholar] [CrossRef]
- Digar, M.L.; Bhattacharyya, S.N.; Mandal, B.M. Conducting polypyrrole particles dispersible in both aqueous and non-aqueous media. J. Chem. Soc. Chem. Commun. 1992, 1, 18–20. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A Polymer with Many Interesting Intrinsic Redox States. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Groenendaal, L.; Jonas, F.; Freitag, D.; Pielartzik, H.; Reynolds, J.R. Poly(3,4-ethylenedioxythiophene) and its derivatives: Past, present, and future. Adv Mater. 2000, 12, 481–494. [Google Scholar] [CrossRef]
- Wang, L.-X.; Li, X.-G.; Yang, Y.-L. Preparation, properties and applications of polypyrroles. React. Funct. Polym. 2001, 47, 125–139. [Google Scholar] [CrossRef]
- Omastová, M.; Trchova, M.; Kovářová, J.; Stejskal, J. Synthesis and structural study of polypyrroles prepared in the presence of surfactants. Synth. Met. 2003, 138, 447–455. [Google Scholar] [CrossRef]
- Xing, S.; Zhao, G. Morphology, structure, and conductivity of polypyrrole prepared in the presence of mixed surfactants in aqueous solutions. J. Appl. Polym. Sci. 2007, 104, 1987–1996. [Google Scholar] [CrossRef]
- Wu, T.-M.; Chang, H.-L.; Lin, Y.-W. Synthesis and characterization of conductive polypyrrole with improved conductivity and processability. Polym. Int. 2009, 58, 1065–1070. [Google Scholar] [CrossRef]
- Sharma, R.K.; Rastogi, A.C.; Desu, S.B. Pulse polymerized polypyrrole electrodes for high energy density electrochemical supercapacitor. Electrochem. Commun. 2008, 10, 268–272. [Google Scholar] [CrossRef]
- Ghouri, A.S.; Aslam, R.; Siddiqui, M.S.; Sami, S.K. Recent Progress in Textile-Based Flexible Supercapacitor. Front. Mater. 2020, 7, 58. [Google Scholar] [CrossRef]
- Dubal, D.P.; Lee, S.H.; Kim, J.G.; Kim, W.B.; Lokhande, C.D. Porous polypyrrole clusters prepared by electropolymerization for a high performance supercapacitor. J. Mater. Chem. 2012, 22, 3044–3052. [Google Scholar] [CrossRef]
- Tang, H.; Wang, J.; Yin, H.; Zhao, H.; Wang, D.; Tang, Z. Growth of polypyrrole ultrathin films on MoS2 monolayers as high-performance supercapacitor electrodes. Adv Mater. 2015, 27, 1117–1123. [Google Scholar] [CrossRef]
- Huang, Y.; Li, H.; Wang, Z.; Zhu, M.; Pei, Z.; Xue, Q.; Zhi, C. Nanostructured Polypyrrole as a flexible electrode material of supercapacitor. Nano Energy 2016, 22, 422–438. [Google Scholar] [CrossRef]
- Vaz, Á.; De Queiroz, K.; Cavalcanti, G.; Lins, R.; Dos Santos, C.; Nascimento, M.; de Melo, C. Study of the interface effects of a free-grown polypyrrole layer in PPV OLEDs. Synth. Met. 2001, 121, 1727–1728. [Google Scholar] [CrossRef]
- Ma, M.; Guo, L.; Anderson, D.G.; Langer, R. Bio-Inspired Polymer Composite Actuator and Generator Driven by Water Gradients. Science 2013, 339, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhao, F.; Hu, C.; Zhao, Y.; Luo, H.; Dai, L.; Qu, L. Vapor-Activated Power Generation on Conductive Polymer. Adv. Funct. Mater. 2016, 26, 8784–8792. [Google Scholar] [CrossRef]
- Hernandez, S.C.; Chaudhuri, D.; Chen, W.; Myung, N.V.; Mulchandani, A. Single Polypyrrole Nanowire Ammonia Gas Sensor. Electroanalysis 2007, 19, 2125–2130. [Google Scholar] [CrossRef]
- Carquigny, S.; Sanchez, J.-B.; Berger, F.; Lakard, B.; Lallemand, F. Ammonia gas sensor based on electrosynthesized polypyrrole films. Talanta 2009, 78, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Do, J.S.; Wang, S.H. On the sensitivity of conductimetric acetone gas sensor based on polypyrrole and polyaniline conducting polymers. Sens. Actuators B Chem. 2013, 185, 39–46. [Google Scholar] [CrossRef]
- Šetka, M.; Drbohlavová, J.; Hubálek, J. Nanostructured Polypyrrole-Based Ammonia and Volatile Organic Compound Sensors. Sensors 2017, 17, 562. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, L.; Wang, W.; Du, C.; Fuchs, H.; Hu, W.; Chi, L. High-Performance and Stable Organic Transistors and Circuits with Patterned Polypyrrole Electrodes. Adv. Mater. 2012, 24, 2159–2164. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.-H.; Cho, J.; Jang, J.; Jang, H.S.; Park, E.S.; Song, K.; Kim, S.H. Polypyrrole top-contact electrodes patterned by inkjet printing assisted vapor deposition polymerization in flexible organic thin-film transistors. Org. Electron. 2012, 13, 715–720. [Google Scholar] [CrossRef]
- Inzelt, G. Recent advances in the field of conducting polymers. J. Solid State Electrochem. 2017, 21, 1965–1975. [Google Scholar] [CrossRef]
- Le, T.-H.; Kim, Y.; Yoon, H. Electrical and Electrochemical Properties of Conducting Polymers. Polymers 2017, 9, 150. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Kang, T.S.; Lee, S.W.; Joo, J.; Lee, J.Y. Electrically conducting polypyrrole fibers spun by electrospinning. Synth. Met. 2005, 153, 61–64. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Grapenson, S.; Jakob, A. Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties. Polymer 2006, 47, 1597–1603. [Google Scholar] [CrossRef]
- Chougule, M.A.; Pawar, S.G.; Godse, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. Synthesis and Characterization of Polypyrrole (PPy) Thin Films. Soft Nanosci. Lett. 2011, 1, 6–10. [Google Scholar] [CrossRef]
- Sakthivel, S.; Boopathi, A. Synthesis and Characterization of Polypyrrole (PPY) Thin Film by Spin Coating Technique. J. Chem. Chem. Sci. 2014, 4, 150–155. [Google Scholar]
- Weng, B.; Morrin, A.; Shepherd, R.; Crowley, K.; Killard, A.J.; Innis, P.C.; Wallace, G.G. Wholly printed polypyrrole nanoparticle-based biosensors on flexible substrate. J. Mater. Chem. B 2014, 2, 793–799. [Google Scholar] [CrossRef]
- Stempien, Z.; Rybicki, T.; Kozanecki, M.; Szynkowska, M. In-situ deposition of polyaniline and polypyrrole electroconductive layers on textile surfaces by the reactive ink-jet printing technique. Synth. Met. 2015, 202, 49–62. [Google Scholar] [CrossRef]
- Asavapiriyanont, S.; Chandler, G.K.; Gunawardena, G.A.; Pletcher, D. The electrodeposition of polypyrrole films from aqueous solutions. J. Electroanal. Chem. 1984, 177, 229–244. [Google Scholar] [CrossRef]
- Sabouraud, G.; Sadki, S.; Brodie, N. The mechanisms of pyrrole electropolymerization. Chem. Soc. Rev. 2000, 29, 283–293. [Google Scholar] [CrossRef]
- Yue, B.; Wang, C.; Ding, X.; Wallace, G.G. Polypyrrole coated nylon lycra fabric as stretchable electrode for supercapacitor applications. Electrochim. Acta 2012, 68, 18–24. [Google Scholar] [CrossRef]
- Yue, B.; Wang, C.; Ding, X.; Wallace, G. Electrochemically synthesized stretchable polypyrrole/fabric electrodes for supercapacitor. Electrochim. Acta 2013, 113, 17–22. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, L.; Zhong, Y.; Sui, X.; Wang, B.; Chen, Z.; Feng, X.; Xu, H.; Mao, Z. High-performance polypyrrole coated knitted cotton fabric electrodes for wearable energy storage. Org. Electron. 2019, 74, 59–68. [Google Scholar] [CrossRef]
- Xu, J.; Wang, D.; Fan, L.; Yuan, Y.; Wei, W.; Liu, R.; Gu, S.; Xu, W. Fabric electrodes coated with polypyrrole nanorods for flexible supercapacitor application prepared via a reactive self-degraded template. Org. Electron. 2015, 26, 292–299. [Google Scholar] [CrossRef]
- Wang, B.; Song, W.; Gu, P.; Fan, L.; Yin, Y.; Wang, C. A stretchable and hydrophobic polypyrrole/knitted cotton fabric electrode for all-solid-state supercapacitor with excellent strain capacitance. Electrochim. Acta 2019, 297, 794–804. [Google Scholar] [CrossRef]
- Martinez, J.G.; Richter, K.; Persson, N.-K.; Jager, E.W.H. Investigation of electrically conducting yarns for use in textile actuators. Smart Mater. Struct. 2018, 27, 074004. [Google Scholar] [CrossRef]
- Kongahage, D.; Foroughi, J. Actuator materials: Review on recent advances and future outlook for smart textiles. Fibers 2019, 7, 21. [Google Scholar] [CrossRef]
- Kaynak, A.; Håkansson, E. Generating heat from conducting polypyrrole-coated PET fabrics. Adv. Polym. Technol. 2005, 24, 194–207. [Google Scholar] [CrossRef]
- Sparavigna, A.C.; Florio, L.; Avloni, J.; Henn, A. Polypyrrole Coated PET Fabrics for Thermal Applications. Mater. Sci. Appl. 2010, 1, 253–259. [Google Scholar] [CrossRef]
- Maity, S.; Chatterjee, A.; Singh, B.; Singh, A.P. Polypyrrole based electro-conductive textiles for heat generation. J. Text. Inst. 2014, 105, 887–893. [Google Scholar] [CrossRef]
- Kincal, D.; Kumar, A.; Child, A.D.; Reynolds, J.R. Conductivity switching in polypyrrole-coated textile fabrics as gas sensors. Synth. Met. 1998, 92, 53–56. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Leung, M.; Tsang, J.; Tao, X.; Yuen, M. A flexible strain sensor from polypyrrole-coated fabrics. Synth. Met. 2005, 155, 89–94. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Sawan, M.; Ghafar-Zadeh, E.; Savadogo, O.; Chodavarapu, V.P. A Polypyrrole-based Strain Sensor Dedicated to Measure Bladder Volume in Patients with Urinary Dysfunction. Sensors 2008, 8, 5081–5095. [Google Scholar] [CrossRef]
- Tjahyono, A.P.; Aw, K.C.; Devaraj, H.; Surendra, W.; Haemmerle, E.; Travas-Sejdic, J. A five-fingered hand exoskeleton driven by pneumatic artificial muscles with novel polypyrrole sensors. Ind. Robot. Int. J. 2013, 40, 251–260. [Google Scholar] [CrossRef]
- Liang, W.; Lei, J.; Martin, C.R. Effect of synthesis temperature on the structure, doping level and charge-transport properties of polypyrrole. Synth. Met. 1992, 52, 227–239. [Google Scholar] [CrossRef]
- Kassim, A.; Basar, Z.B.; Mahmud, H.N.M.E. Effects of preparation temperature on the conductivity of polypyrrole conducting polymer. J. Chem. Sci. 2002, 114, 155–162. [Google Scholar] [CrossRef]
- Qi, G.; Huang, L.; Wang, H. Highly conductive free standing polypyrrole films prepared by freezing interfacial polymerization. Chem. Commun. 2012, 48, 8246–8248. [Google Scholar] [CrossRef] [PubMed]
- Tekin, E.; Smith, P.J.; Schubert, U.S. Inkjet Printing as a Deposition and Patterning Tool for Polymers and Inorganic Particles. Soft Matter 2008, 4, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Shepherd, R.L.; Crowley, K.; Killard, A.J.; Wallace, G. Printing conducting polymers. Analyst 2010, 135, 2779–2789. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Medarametla, S.P.; Li, H.; Zhou, C.; Lin, D. 3D Printing of Graphene Aerogels. Small 2016, 12, 1702–1708. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, X.; Li, B.; Chen, Z.; Mi, S.; Lao, C. Fabrication and Characterization of 3D-Printed Highly-Porous 3D LiFePO4 Electrodes by Low Temperature Direct Writing Process. Materials 2017, 10, 934. [Google Scholar] [CrossRef]
- Kam, D.; Chasnitsky, M.; Nowogrodski, C.; Braslavsky, I.; Abitbol, T.; Magdassi, S.; Shoseyov, O. Direct Cryo Writing of Aerogels Via 3D Printing of Aligned Cellulose Nanocrystals Inspired by the Plant Cell Wall. Colloids Interfaces 2019, 3, 46. [Google Scholar] [CrossRef]
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-Graphene Core-Sheath Microfibers for All-Solid-State, Stretchable Fibriform Supercapacitors and Wearable Electronic Textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Yan, C.; Li, K.; Zheng, Z. Wearable energy-dense and power-dense supercapacitor yarns enabled by scalable graphene-metallic textile composite electrodes. Nat. Commun. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Liu, W.-W.; Yan, X.-B.; Lang, J.-W.; Peng, C.; Xue, Q.-J. Flexible and conductive nanocomposite electrode based on graphene sheets and cotton cloth for supercapacitor. J. Mater. Chem. 2012, 22, 17245. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Li, S.-M.; Hsiao, S.-T.; Liao, W.-H.; Chen, P.-H.; Yang, S.-Y.; Tien, H.-W.; Ma, C.-C.M.; Hu, C.-C. Integration of tailored reduced graphene oxide nanosheets and electrospun polyamide-66 nanofabrics for a flexible supercapacitor with high-volume- and high-area-specific capacitance. Carbon N. Y. 2014, 73, 87–98. [Google Scholar] [CrossRef]
- Xu, L.L.; Guo, M.X.; Liu, S.; Bian, S.W. Graphene/cotton composite fabrics as flexible electrode materials for electrochemical capacitors. RSC Adv. 2015, 5, 25244–25249. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Karim, N.; Vallés, C.; Afroj, S.; Novoselov, K.S.; Yeates, S.G. Ultraflexible and robust graphene supercapacitors printed on textiles for wearable electronics applications. 2D Mater. 2017, 4, 035016. [Google Scholar] [CrossRef]
- Stempien, Z.; Khalid, M.; Kozicki, M.; Kozanecki, M.; Varela, H.; Filipczak, P.; Pawlak, R.; Korzeniewska, E.; Sąsiadek, E. In-situ deposition of reduced graphene oxide layers on textile surfaces by the reactive inkjet printing technique and their use in supercapacitor applications. Synth. Met. 2019, 256. [Google Scholar] [CrossRef]
- Li, X.; Liu, R.; Xu, C.; Bai, Y.; Zhou, X.; Wang, Y.; Yuan, G. High-Performance Polypyrrole/Graphene/SnCl2 Modified Polyester Textile Electrodes and Yarn Electrodes for Wearable Energy Storage. Adv. Funct. Mater. 2018, 28, 1800064. [Google Scholar] [CrossRef]
- Zang, X.; Li, X.; Zhu, M.; Li, X.; Zhen, Z.; He, Y.; Wang, K.; Wei, J.; Kang, F.; Zhu, H. Graphene/polyaniline woven fabric composite films as flexible supercapacitor electrodes. Nanoscale 2015, 7, 7318–7322. [Google Scholar] [CrossRef]
- Song, P.; He, X.; Xie, M.; Tao, J.; Shen, X.; Ji, Z.; Yan, Z.; Zhai, L.; Yuan, A. Polyaniline wrapped graphene functionalized textile with ultrahigh areal capacitance and energy density for high-performance all-solid-state supercapacitors for wearable electronics. Compos. Sci. Technol. 2020, 198, 108305. [Google Scholar] [CrossRef]
- Cheng, H.; Dong, Z.; Hu, C.; Zhao, Y.; Hu, Y.; Qu, L.; Chen, N.; Dai, L. Textile electrodes woven by carbon nanotube-graphene hybrid fibers for flexible electrochemical capacitors. Nanoscale 2013, 5, 3428–3434. [Google Scholar] [CrossRef] [PubMed]
- Shakir, I.; Ali, Z.; Bae, J.; Park, J.; Kang, D.J. Layer by layer assembly of ultrathin V2O5 anchored MWCNTs and graphene on textile fabrics for fabrication of high energy density flexible supercapacitor electrodes. Nanoscale 2014, 6, 4125. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Hu, L.; Vosgueritchian, M.; Wang, H.; Xie, X.; McDonough, J.R.; Cui, X.; Cui, Y.; Bao, Z. Solution-processed graphene/MnO2 nanostructured textiles for high-performance electrochemical capacitors. Nano Lett. 2011, 11, 2905–2911. [Google Scholar] [CrossRef]
- Guo, M.X.; Bian, S.W.; Shao, F.; Liu, S.; Peng, Y.H. Hydrothermal synthesis and electrochemical performance of MnO2/graphene/polyester composite electrode materials for flexible supercapacitors. Electrochim. Acta 2016, 209, 486–497. [Google Scholar] [CrossRef]
- Jin, L.N.; Liu, P.; Jin, C.; Zhang, J.N.; Bian, S.W. Porous WO3/graphene/polyester textile electrode materials with enhanced electrochemical performance for flexible solid-state supercapacitors. J. Colloid Interface Sci. 2018, 510, 1–11. [Google Scholar] [CrossRef]
- Zhao, C.; Shu, K.; Wang, C.; Gambhir, S.; Wallace, G.G. Reduced graphene oxide and polypyrrole/reduced graphene oxide composite coated stretchable fabric electrodes for supercapacitor application. Electrochim. Acta 2015, 172, 12–19. [Google Scholar] [CrossRef]
- Borisova, M.; Kamalov, A.; Jayasinghe, B. Electrical and electret properties of non-woven “spunbond” polypropylene. In 2018 IEEE 59th Annual International Scientific Conference on Power and Electrical Engineering of Riga Technical University, RTUCON 2018—Proceedings; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Electrical resistivity of fabrics, AATCC Test Method 76-2005. In Technical Manual of the American Association of Textile Chemists and Colorists; American Association of Textile Chemists and Colorists: Research Triangle Park, NC, USA, 2005.
- Skrypnychuk, V.; Boulanger, N.; Nordenström, A.; Talyzin, A.V. Aqueous Activated Graphene Dispersions for Deposition of High-Surface Area Supercapacitor Electrodes. J. Phys. Chem. Lett. 2020, 11, 3032–3038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Li, D.; Kaner, R.B. How nucleation affects the aggregation of nanoparticles. J. Mater. Chem. 2007, 17, 2279–2282. [Google Scholar] [CrossRef]
- Wilson, P.; Arthur, J.; Haymet, A. Ice Premelting during Differential Scanning Calorimetry. Biophys. J. 1999, 77, 2850–2855. [Google Scholar] [CrossRef]
- Rahman, M.S. State Diagram of Date Flesh Using Differential Scanning Calorimetry (DSC). Int. J. Food Prop. 2004, 7, 407–428. [Google Scholar] [CrossRef]
- Van Durme, K.; Van Assche, G.; Nies, E.; Van Mele, B. Phase Transformations in Aqueous Low Molar Mass Poly(vinyl methyl ether) Solutions: Theoretical Prediction and Experimental Validation of the Peculiar Solvent Melting Line, Bimodal LCST, and (Adjacent) UCST Miscibility Gaps. J. Phys. Chem. B 2007, 111, 1288–1295. [Google Scholar] [CrossRef]
- Czaderna-Lekka, A.; Kozanecki, M.; Matusiak, M.; Kadlubowski, S. Phase transitions of poly(oligo(ethylene glycol) methyl ether methacrylate)-water systems. Polymer 2021, 212, 123247. [Google Scholar] [CrossRef]
- Pastorczak, M.; Dominguez-Espinosa, G.; Okrasa, L.; Pyda, M.; Kozanecki, M.; Kadlubowski, S.; Rosiak, J.M.; Ulanski, J. Poly(vinyl methyl ether) hydrogels at temperatures below the freezing point of water—molecular interactions and states of water. Colloid Polym. Sci. 2014, 292, 1775–1784. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Somorjai, G.A. Surface premelting of ice. J. Phys. Chem. C 2007, 111, 9631–9637. [Google Scholar] [CrossRef]
- Slater, B.; Michaelides, A. Surface premelting of water ice. Nat. Rev. Chem. 2019, 3, 172–188. [Google Scholar] [CrossRef]
- Bae, W.J.; Kim, K.H.; Jo, W.H.; Park, Y.H. A water-soluble and self-doped conducting polypyrrole graft copolymer. Macromolecules 2005, 38, 1044–1047. [Google Scholar] [CrossRef]
- Park, D.P.; Sung, J.H.; Lim, S.T.; Choi, H.J.; Jhon, M.S. Synthesis and characterization of soluble polypyrrole and polypyrrole/organoclay nanocomposites. J. Mater. Sci. Lett. 2003, 22, 1299–1302. [Google Scholar] [CrossRef]
- Shi, Y.; Pan, L.; Liu, B.; Wang, Y.; Cui, Y.; Bao, Z.; Yu, G. Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 6086–6091. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, L.; Chen, L.Y.; Liu, P.; Hirata, A.; Chen, M.W. Raman characterization of pseudocapacitive behavior of polypyrrole on nanoporous gold. Phys. Chem. Chem. Phys. 2014, 16, 3523–3528. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.P.; Rastogi, A.C. Graphene-based all-solid-state supercapacitor with ionic liquid gel polymer electrolyte. In Materials Research Society Symposium Proceedings; Cambridge University Press: Cambridge, UK, 2012; Volume 1440, pp. 7–12. [Google Scholar]
- Zhu, L.; Wu, L.; Sun, Y.; Li, M.; Xu, J.; Bai, Z.; Liang, G.; Liu, L.; Fang, D.; Xu, W. Cotton fabrics coated with lignosulfonate-doped polypyrrole for flexible supercapacitor electrodes. RSC Adv. 2014, 4, 6261–6266. [Google Scholar] [CrossRef]
- Christen, T.; Carlen, M.W. Theory of ragone plots. J. Power Sources 2000, 91, 210–216. [Google Scholar] [CrossRef]



| Textile Substrate | Electrode Material | PPy Deposition Method | Capacitance, F/g | Capacitance, mF/cm2 | Reference |
|---|---|---|---|---|---|
| Knitted fabric (80% Nylon and 20% Lycra) | PPy doped with Na2NDS | Dip-coating | 108.5 (1 A/g) | not given | [34] |
| Knitted cotton fabric | PPy doped with NaSSA | Dip-coating | not given | 1167.9 (1 mA/cm2) | [36] |
| Cotton fabric | PPy nanorods | Dip-coating | 325 (0.6 mA/cm2) | not given | [37] |
| Knitted cotton fabric | PPy doped with 5-sulfosalicylic acid | Dip-coating | not given | 450 (1 mA/cm2) | [38] |
| PP non-woven fabric | PPy | Reactive inkjet printing in sub-zero temperature | 72.3 (0.6 A/g) | 30.5 (0.25 mA/cm2) | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stempien, Z.; Khalid, M.; Kozanecki, M.; Filipczak, P.; Wrzesińska, A.; Korzeniewska, E.; Sąsiadek, E. Inkjet Printing of Polypyrrole Electroconductive Layers Based on Direct Inks Freezing and Their Use in Textile Solid-State Supercapacitors. Materials 2021, 14, 3577. https://doi.org/10.3390/ma14133577
Stempien Z, Khalid M, Kozanecki M, Filipczak P, Wrzesińska A, Korzeniewska E, Sąsiadek E. Inkjet Printing of Polypyrrole Electroconductive Layers Based on Direct Inks Freezing and Their Use in Textile Solid-State Supercapacitors. Materials. 2021; 14(13):3577. https://doi.org/10.3390/ma14133577
Chicago/Turabian StyleStempien, Zbigniew, Mohmmad Khalid, Marcin Kozanecki, Paulina Filipczak, Angelika Wrzesińska, Ewa Korzeniewska, and Elżbieta Sąsiadek. 2021. "Inkjet Printing of Polypyrrole Electroconductive Layers Based on Direct Inks Freezing and Their Use in Textile Solid-State Supercapacitors" Materials 14, no. 13: 3577. https://doi.org/10.3390/ma14133577
APA StyleStempien, Z., Khalid, M., Kozanecki, M., Filipczak, P., Wrzesińska, A., Korzeniewska, E., & Sąsiadek, E. (2021). Inkjet Printing of Polypyrrole Electroconductive Layers Based on Direct Inks Freezing and Their Use in Textile Solid-State Supercapacitors. Materials, 14(13), 3577. https://doi.org/10.3390/ma14133577










