Deterioration and Protection of Concrete Elements Embedded in Contaminated Soil: A Review
Abstract
:1. Introduction
2. Search Methodology
- i.
- Search protocol: A set of rules and parameters for the search process was used together with logical and relational operators (AND, OR, NOT, <, >, <=, >=, < >, =, etc.).
- ii.
- Analysis: It refers to the consolidation and combination of data according to different criteria, such as most-cited authors, year of publication, and type of journal by creating a database with various articles that meet the search and consolidation criteria.
- iii.
- Data synthesis: It allows to generate conclusions and new knowledge based on the results presented by the different papers analyzed.
- iv.
- Writing: The information was extracted from 76 articles. The results were consolidated through scientific and academic writing.
3. Significance of the Review
4. Results and Discussion
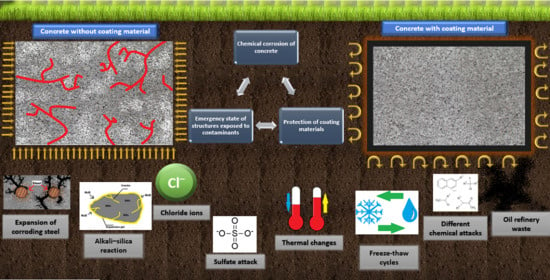
4.1. Chemical Corrosion of Concrete Elements in Contaminated and Noncontaminated Soil
4.2. Characteristics of Reinforced Concrete Elements Embedded in the Ground in Terms of Their Chemical Corrosion
4.3. Characteristics of Emergency State of Structures Caused by Chemical Corrosion of Concrete Elements Embedded in the Ground
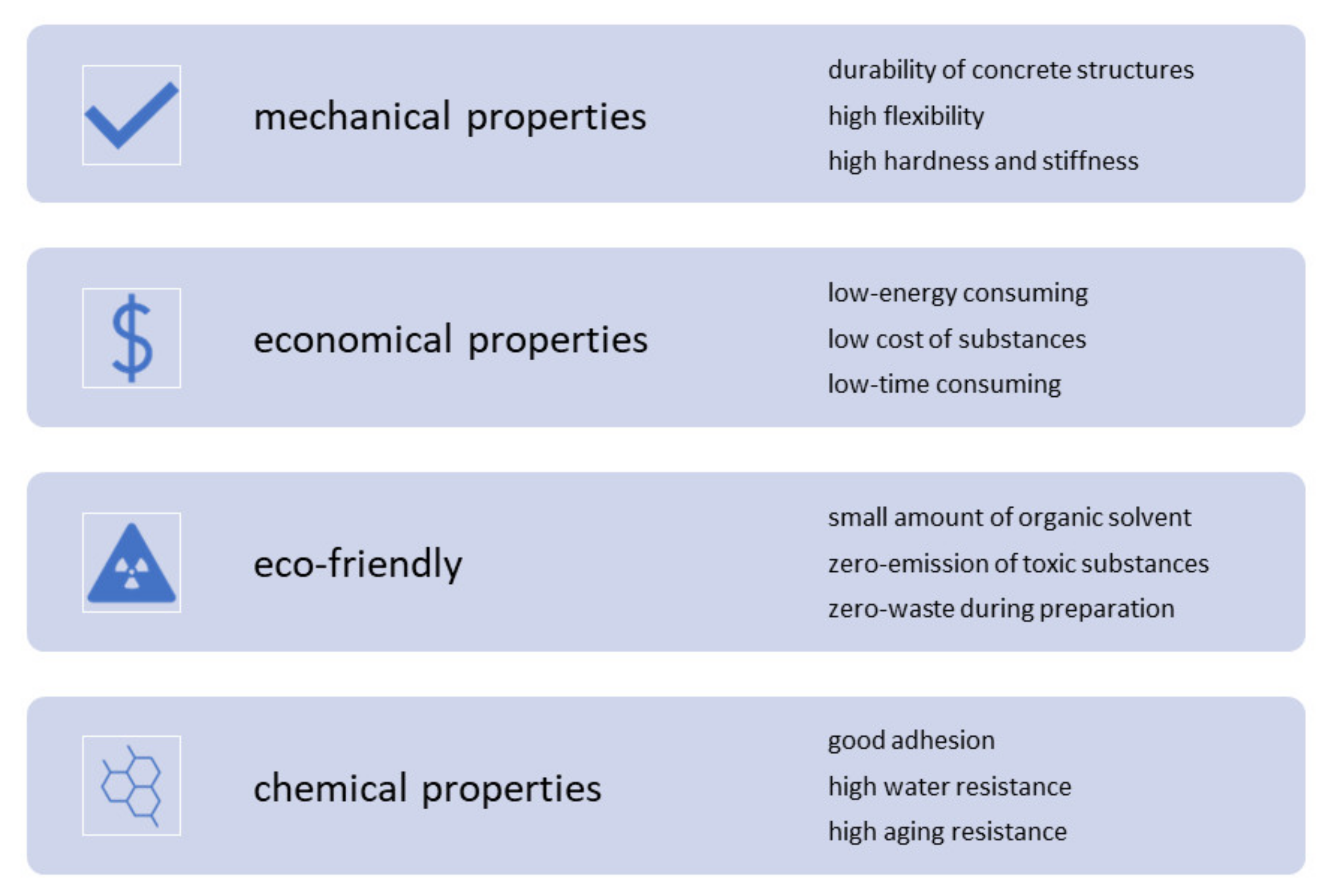
4.4. Coating Materials—Current State, Challenges, and Perspectives
4.4.1. Research Gaps in the Use of Coating Materials
4.4.2. Current Status and Future Challenges
4.4.3. Characteristics of Coating Materials According to Polish–European and American Standards
5. Conclusions
6. Research Limitations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Meaning |
| Cesium 137 | |
| Cesium 134 | |
| ACO | Acetoxy group |
| AISI | American Iron and Steel Institute |
| ASR | Alkali–silica reaction |
| As/PU | Asphalt and polyurethane |
| ASTM | American Society for Testing and Materials |
| BC | Before Christ |
| BS | British standard |
| Calcium carbonate | |
| CEM | Cement |
| CPC | Calcium phosphate cements |
| Tricalcium aluminate | |
| Carbon dioxide | |
| Carbon oxides | |
| DGEBA | Diglycidyl ether of bisphenol A |
| EN | European standards |
| GPa | Gigapascal |
| ISO | International Organization for Standardization |
| ITB | Building Research Institute |
| KOH | Potassium hydroxide |
| LiOH | Lithium hydroxide |
| MgSO4 | Magnesium sulfate |
| Sodium carbonate | |
| Sodium sulfate | |
| NaCl | Sodium chloride |
| NaOH | Sodium hydroxide |
| NCO | Isocyanate chemical group |
| Nitrogen oxide | |
| OH | Hydroxide |
| pH | Potential of hydrogen |
| PN | Polish standards |
| PVC | Polyvinyl chloride |
| RC | Reinforced concrete |
| SEM | Scanning electron microscopy |
| SER | Solid epoxy resins |
| Sulfur oxide | |
| TGA | Thermalgravimetric analysis |
| UNS | Unified number system |
| Uranium oxide | |
| UV | Ultraviolet |
| W/C | Water/cement |
| XRD | X-ray diffraction |
| ZUAT | Recommendations of the Technical Approval Provision |
References
- Popov, B.N. Corrosion of Structural Concrete. Corros. Eng. 2015, 525–556. [Google Scholar] [CrossRef]
- Safiuddin, M. Concrete Damage in Field Conditions and Protective Sealer and Coating Systems. Coatings 2017, 7, 90. [Google Scholar] [CrossRef]
- Vaysburd, A.; Emmons, P. How to make today’s repairs durable for tomorrow—corrosion protection in concrete repair. Constr. Build. Mater. 2000, 14, 189–197. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Owsiak, Z. Testing alkali—Reactivity of selected concrete aggregates. J. Civ. Eng. Manag. 2010, 3730, 8. [Google Scholar] [CrossRef]
- Figueira, R.B.; Sousa, R.; Coelho, L.; Azenha, M.; de Almeida, J.M.; Jorge, P.A.S.; Silva, C.J.R. Alkali-silica reaction in concrete: Mechanisms, mitigation and test methods. Constr. Build. Mater. 2019, 222, 903–931. [Google Scholar] [CrossRef]
- Portland Cement Association. Types and Causes of Concrete Deterioration. Portl. Cem. Assoc. Concr. Inf. PCA R.D. Se 2002, 5420 Old Orchard Road, 1–16. Available online: www.cement:docs/default-source/fc_concrete_technology/durability/is536-types-and-causes-of-concrete-deterioration.pdf?sfvrsn=4&sfvrsn=4 (accessed on 28 February 2021).
- Wang, K. Carbonate Aggregate in Concrete. Available online: http://www.lrrb:pdf/201514.pdf (accessed on 28 February 2021).
- Wang, C.; Chen, F. Durability of Polypropylene Fiber Concrete Exposed to Freeze-Thaw Cycles with Deicing Salts; Atlantis Press: Dordrecht, The Netherlands, 2019; Volume 181, pp. 203–206. [Google Scholar] [CrossRef]
- Enshassi, A.; Kochendoerfer, B.; Rizq, E. Evaluación de los impactos medioambientales de los proyectos de construcción. Rev. Ing. Construcción 2014, 29, 234–254. [Google Scholar] [CrossRef] [Green Version]
- Zolfagharian, S.; Nourbakhsh, M.; Irizarry, J.; Ressang, A.; Gheisari, M. Environmental impacts assessment on construction sites. In Proceedings of the Construction Research Congress 2012: Construction Challenges in a Flat World, EE. UU, Indiana, IN, USA, 21–23 May 2012; pp. 1750–1759. [Google Scholar] [CrossRef] [Green Version]
- Bonić, Z.; Ćurčć, G.T.; Davidovič, N.; Savič, J. Damage of concrete and reinforcement of reinforced-concrete foundations caused by environmental effects. Procedia Eng. 2015, 117, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Popov, B.N. High-Temperature Corrosion. Corros. Eng. Elsevier 2015, 481–523. [Google Scholar] [CrossRef]
- Stambaugh, N.D.; Bergman, T.L.; Srubar, W.V. Numerical service-life modeling of chloride-induced corrosion in recycled-aggregate concrete. Constr. Build. Mater. 2018, 161, 236–245. [Google Scholar] [CrossRef]
- Xia, J.; Shen, J.; Li, T.; Jin, W.-L. Corrosion prediction models for steel bars in chloride-contaminated concrete: A review. Mag. Concr. Res. 2021, 1–20. [Google Scholar] [CrossRef]
- Yin, G.J.; Zuo, X.B.; Tang, Y.J.; Ayinde, O.; Wang, J.L. Numerical simulation on time-dependent mechanical behavior of concrete under coupled axial loading and sulfate attack. Ocean Eng. 2017, 142, 115–124. [Google Scholar] [CrossRef]
- Silva, M.A.G.; Cunha, M.P.; Pinho-Ramos, A.; da Fonseca, B.S.; Pinho, F.F.S. Accelerated action of external sulfate and chloride to study corrosion of tensile steel in reinforced concrete. Mater. Constr. 2017. [Google Scholar] [CrossRef] [Green Version]
- Pernicová, R.; Dobiáš, D. Resistance of surface layers of concrete against aggressive environment. Key Eng. Mater. 2017, 722, 44–51. [Google Scholar] [CrossRef]
- Wei, J.; Liu, J.; Bai, Y.; Song, Z.; Feng, Q.; Lu, Y.; Sun, S. Effect on the resistance of concrete acid corrosion in superficial soil layers. Adv. Civ. Eng. 2018. [Google Scholar] [CrossRef]
- Kozubal, J.; Wyjadłowski, M.; Steshenko, D. Probabilistic analysis of a concrete column in an aggressive soil environment. PLoS ONE 2019, 14, e0212902. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Yang, C. Theoretical approach for prediction of service life of RC pipe piles with original incomplete cracks in chloride-contaminated soils, Constr. Build. Mater. 2019, 228, 116717. [Google Scholar] [CrossRef]
- Kim, J.-H.J.; Lim, Y.M.; Won, J.P.; Park, H.G. Fire resistant behavior of newly developed bottom-ash-based cementitious coating applied concrete tunnel lining under RABT fire loading. Constr. Build. Mater. 2010, 24, 1984–1994. [Google Scholar] [CrossRef]
- Fattuni, N.I.; Hughes, B.P. Effect of acid attack on concrete with different admixtures or protective coatings. Cem. Concr. Res. 1983, 13, 655–665. [Google Scholar] [CrossRef]
- Taylor, S.R. Coatings for Corrosion Protection: Inorganic. Encycl. Mater. Sci. Technol. 2001, 1263–1269. [Google Scholar] [CrossRef]
- Taylor, S.R. Coatings for Corrosion Protection: Organic. Encycl. Mater. Sci. Technol. Elsevier 2001, 1274–1279. [Google Scholar] [CrossRef]
- Guma, T.N.; Aku, S.Y.; Yawas, D.S.; Dauda, M. Bitumen in Coating Corrosion Protection of Steel-The Position and Prognosis of Nigerian Bitumen. Am. J. Eng. Res. 2015, 4, 101–111. [Google Scholar]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible Coating of Fruits and Vegetables: A Review View Project. 2016. Available online: www.researchgate.net/publication/331298687 (accessed on 7 February 2021).
- Singh, A.K.; Bhadauria, A.S.; Kumar, P.; Bera, H.; Saha, S. Bioactive and drug-delivery potentials of polysaccharides and their derivatives. In Polysaccharide Carriers for Drug Delivery; Woodhead Publishing: Sawston, UK, 2019; pp. 19–48. [Google Scholar] [CrossRef]
- Popov, B.N. Organic Coatings. Corros. Eng. Elsevier 2015, 557–579. [Google Scholar] [CrossRef]
- Pham, H.Q.; Marks, M.J. Epoxy Resins, Assesments of Potential BPA Emissions. In Construction Research Congress 2012: Construction Challenges in a Flat World; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; p. 90. [Google Scholar]
- Chattopadhyay, D.K.; Raju, K.V.S.N. Structural engineering of polyurethane coatings for high performance applications. Prog. Polym. Sci. 2007, 32, 352–418. [Google Scholar] [CrossRef]
- Americus. Coatings update: Chlorinated rubber technology. Pigment Resin Technol. 1978, 7, 10–12. [Google Scholar] [CrossRef]
- Ibrahim, M.; Al-Gahtani, A.S.; Maslehuddin, M.; Dakhil, F.H. Use of surface treatment materials to improve concrete durability. J. Mater. Civ. Eng. 1999, 11, 36–40. [Google Scholar] [CrossRef]
- Satoshi, S. Solution Casting Method. U.S. Patent Application US 2005/0212172 A1, 2005. [Google Scholar]
- Myers, R.R. History of Coatings Science and Technology. J. Macromol. Sci. Part A. Chem. 1981, 15, 1133–1149. [Google Scholar] [CrossRef]
- Edwards, K.N.; Mislang, H.B. History of coatings. Appl. Polym. Sci. 21st Century Elsevier 2000, 439–447. [Google Scholar] [CrossRef]
- Soucek, M.; Johansson, M.K.G. Alkyds for the 21st century. Prog. Org. Coatings 2012, 73, 273. [Google Scholar] [CrossRef]
- Pilcher, G.R.; The ChemQuest Group. Entering the Second Decade of the 21st Century: The State of the U.S. Paint and Coatings Industry. CoatingsTech 2019, 16, 8. [Google Scholar]
- Merachtsaki, D.; Tsardaka, E.-C.; Tsampali, E.; Simeonidis, K.; Anastasiou, E.; Yiannoulakis, H.; Zouboulis, A. Study of Corrosion Protection of Concrete in Sewage Systems with Magnesium Hydroxide Coatings. Environ. Sci. Proc. 2020, 2, 27. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.M.; de Gutiérrez, R.M. Alkali-activated protective coatings for reinforced concrete exposed to chlorides. Constr. Build. Mater. 2021, 268, 121098. [Google Scholar] [CrossRef]
- Sakr, M.R.; Bassuoni, M.T.; Taha, M.R. Effect of coatings on concrete resistance to physical salt attack. ACI Mater. J. 2019, 116, 255–267. [Google Scholar] [CrossRef]
- Ferenhof, H.A.; Fernandes, R.F. Desmistificando a Revisão de Literatura Como Base Para Redação cientíFica: Método SSF, n.d. Available online: www.researchgate.net/publication/325070845 (accessed on 4 May 2021).
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
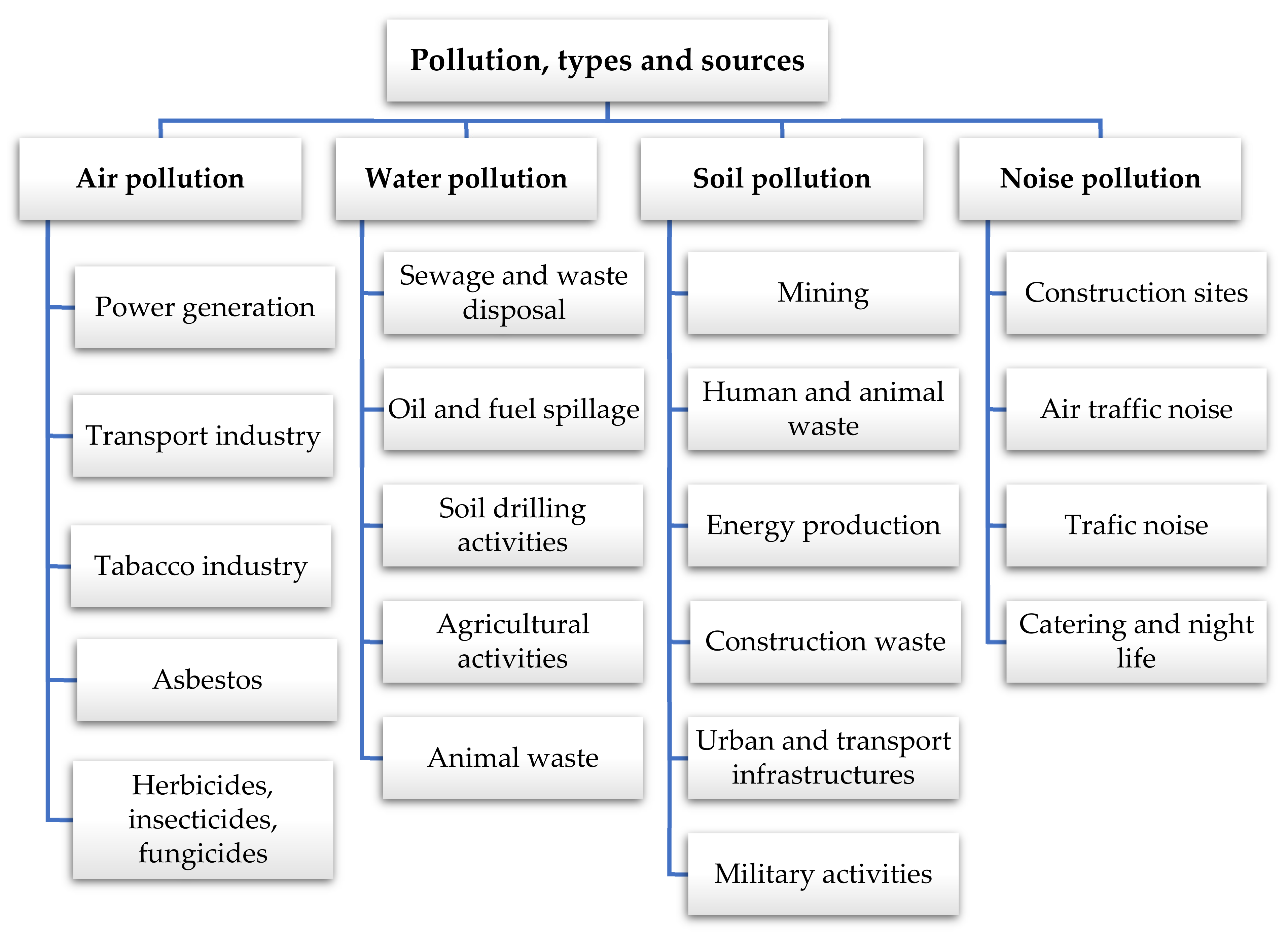
- Saha, J.K.; Selladurai, R.; Kundu, S.; Patra, A.K. Chapter 9 Water, Agriculture, Soil and Environmental. Tech. Instrum. Anal. Chem. 1989, 155–191. [Google Scholar] [CrossRef]
- Mirsal, I.A. Soil Pollution. Origin, Monitoring and Remediation, 2nd ed.; Springer: New York, NY, USA, 2004. [Google Scholar]
- Pallise, J. Impactos ambientales de la producción de electricidad. Asoc. Prod. Energías Renov. 2014, 42. Available online: http://proyectoislarenovable.iter.es/wp-content/uploads/2014/05/17_Estudio_Impactos_MA_mix_electrico_APPA.pdf (accessed on 14 January 2021).
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, N.; McLaughlin, F.M.; Pennock, D. Soil Pollution: A Hidden Reality. 2018. Available online: www.fao.org/ (accessed on 17 February 2021).
- Owa, F.D. Water pollution: Sources, effects, control and management. Mediterr. J. Soc. Sci. 2013, 4, 65–68. [Google Scholar] [CrossRef]
- Haufe, J.; Vollpracht, A. Tensile strength of concrete exposed to sulfate attack. Cem. Concr. Res. 2019, 116, 81–88. [Google Scholar] [CrossRef]
- Meng, C.; Li, W.; Cai, L.; Shi, X.; Jiang, C. Experimental research on durability of high-performance synthetic fibers reinforced concrete: Resistance to sulfate attack and freezing-thawing. Constr. Build. Mater. 2020, 262, 120055. [Google Scholar] [CrossRef]
- Osuji, S.; Nwankwo, E. Effect of Crude Oil Contamination on the Compressive Strength of Concrete. Niger. J. Technol. 2015, 34, 259. [Google Scholar] [CrossRef]
- Adewuyi, A.P.; Olaniyi, O.A.; Olafusi, O.S.; Fawumi, A.S. Compressive and Flexural Behaviour of Unstressed Concrete Substructure in Cassava Effluent Contaminated Soils. Open J. Civ. Eng. 2015, 5, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Adewuyi, A. Strength and durability assessment of concrete substructure in organic and hydrocarbon polluted soil. Int. J. Mod. Res. Eng. Technol. 2017, 2, 34–42. [Google Scholar]
- Yu, X.T.; Chen, D.; Feng, J.R.; Zhang, Y.; di Liao, Y. Behavior of mortar exposed to different exposure conditions of sulfate attack. Ocean Eng. 2018, 157, 1–12. [Google Scholar] [CrossRef]
- Jedidi, M.; Benjeddou, O. Chemical causes of concrete degradation. MOJ Civ. Eng. 2018, 4, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-F.; Chen, J.-W. The experimental investigation of concrete carbonation depth. Cem. Concr. Res. 2006, 36, 1760–1767. [Google Scholar] [CrossRef]
- Baltazar-Zamora, M.A.; Mendoza-Rangel, J.M.; Croche, R.; Gaona-Tiburcio, C.; Hernández, C.; López, L.; Olguín, F.; Almeraya-Calderón, F. Corrosion Behavior of Galvanized Steel Embedded in Concrete Exposed to Soil Type MH Contaminated With Chlorides. Front. Mater. 2019, 6, 1–12. [Google Scholar] [CrossRef]
- Melchers, R.E.; Li, C.Q. Reinforcement corrosion initiation and activation times in concrete structures exposed to severe marine environments. Cem. Concr. Res. 2009, 39, 1068–1076. [Google Scholar] [CrossRef]
- Ramli, M.; Kwan, W.H.; Abas, N.F. Strength and durability of coconut-fiber-reinforced concrete in aggressive environments. Constr. Build. Mater. 2013, 38, 554–566. [Google Scholar] [CrossRef]
- Zhong, R.; Wille, K. Deterioration of residential concrete foundations: The role of pyrrhotite-bearing aggregate. Cem. Concr. Compos. 2018, 94, 53–61. [Google Scholar] [CrossRef]
- Oliveira, I.; Cavalaro, S.H.P.; Aguado, A. Evolution of pyrrhotite oxidation in aggregates for concrete. Mater. Construcción 2014, 64, e038. [Google Scholar] [CrossRef] [Green Version]
- Tagnit-Hamou, A.; Saric-Coric, M.; Rivard, P. Internal deterioration of concrete by the oxidation of pyrrhotitic aggregates. Cem. Concr. Res. 2005, 35, 99–107. [Google Scholar] [CrossRef]
- Yoshida, N. Sulfate attack on residential concrete foundations in Japan. J. Sustain. Cem. Mater. 2019, 8, 327–336. [Google Scholar] [CrossRef]
- Tulliani, J.M.; Montanaro, L.; Negro, A.; Collepardi, M. Sulfate attack of concrete building foundations induced by sewage waters. Cem. Concr. Res. 2002, 32, 843–849. [Google Scholar] [CrossRef]
- Santiago-Hurtado, G. Electrochemical evaluation of reinforcement concrete exposed to soil type SP contaminated with sulphates. Int. J. Electrochem. Sci. 2016, 11, 4850–4864. [Google Scholar] [CrossRef]
- Baltazar-Zamora, M.A.; Bastidas, D.M.; Santiago-Hurtado, G.; Mendoza-Rangel, J.M.; Gaona-Tiburcio, C.; Bastidas, J.M.; Almeraya-Calderón, F. Effect of Silica Fume and Fly Ash Admixtures on the Corrosion Behavior of AISI 304 Embedded in Concrete Exposed in 3.5% NaCl Solution. Materials 2019, 12, 4007. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; He, Y.; Long, Z.; Zhan, Y. Synergistic effect of functional carbon nanotubes and graphene oxide on the anti-corrosion performance of epoxy coating. Polym. Adv. Technol. 2017, 28, 754–762. [Google Scholar] [CrossRef]
- Patil, R. Waterproofing: Types, Advantages & Disadvantages. 2016. Available online: constructionor.com/waterproofing/ (accessed on 22 February 2021).
- Rabiot, D.; Morizur, M.F. Polymer-modified bitumen emulsions an advantage for the various road applications. Eurasphalt Eurobitume Congr. 1996, 6, 161. [Google Scholar]
- Paliukaite, M.; Vorobjovas, V.; Bulevičius, M.; Andrejevas, V. Evaluation of Different Test Methods for Bitumen Adhesion Properties. Transp. Res. Procedia 2016, 14, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.N.L.; Do, T.V.; Nguyen, T.V.; Dao, P.H.; Trinh, V.T.; Mac, V.P.; Nguyen, A.H.; Dinh, D.A.; Nguyen, T.A.; Vo, T.K.A.; et al. Antimicrobial activity of acrylic polyurethane/Fe3O4-Ag nanocomposite coating. Prog. Org. Coatings 2019, 132, 15–20. [Google Scholar] [CrossRef]
- Alessandrini, G.; Aglietto, M.; Castelvetro, V.; Ciardelli, F.; Peruzzi, R.; Toniolo, L. Comparative evaluation of fluorinated and unfluorinated acrylic copolymers as water-repellent coating materials for stone. J. Appl. Polym. Sci. 2000, 76, 962–977. [Google Scholar] [CrossRef]
- Samimi, A.; Zarinabadi, S. Application Polyurethane as Coating in Oil and Gas Pipelines Calculation of Corrosion in Oil and Gas Refinery with EOR Method View project Application Polyurethane as Coating in Oil and Gas Pipelines. Int. J. Sci. Eng. Investig. 2012, 1, 43–45. [Google Scholar]
- Alchimica, Advantages and Disadvantages of Polyurethane Waterproofing Materials. 2019. Available online: alchimica.com.ua/en/2019/05/13/advantages-and-disadvantages-of-polyurethane-waterproofing-materials/ (accessed on 23 February 2021).
- Kanimozhi, K.; Prabunathan, P.; Selvaraj, V.; Alagar, M. Bio-based silica-reinforced caprolactam-toughened epoxy nanocomposites. High Perform. Polym. 2016, 28, 189–197. [Google Scholar] [CrossRef]

| Causes of Deterioration | Deterioration Type |
|---|---|
| Caused by concrete components |
|
| Caused by external agents |
|
| Year/Period | Event Description |
|---|---|
| Pre-History | |
| Before 4000 B.C | Varnishes and paints were used during the stone age art |
| Before 6000 BC | Development of organic pigments (gum Arabic, egg white, gelatin, and beeswax) |
| Since 5000 BC | Use of protective coatings by Egyptians to seal ships |
| Ancient Age | |
| 1300 B.C-1400 B.C | Use of oleo-resinous varnishes by Egyptians |
| Since 1122 BC | Introduction of polymers as the main component in coatings |
| 350 B.C | First written record of uses of varnishes |
| Middle Age | |
| 476 B.C-1453 | Use of different organic paints and varnishes for the protection of exposed wood surfaces |
| Modern Age | |
| 1550–1750 | Researches about coatings for protection of musical instruments made in wood |
| 1575 | The first use of yellow amber resin as a primary component in coatings |
| Since 1760 | Significant emergence of coating materials as a high technology industry, the development of synthetic resins in solutions, emulsion, latexes, and waterborne polymers |
| 1763 | First varnish patent |
| Contemporary Age | |
| 1815 | Start industrial varnish production |
| 1839 | The first production of styrene monomer used as a modifier in polymer coatings |
| 1910 | Casein powder paints |
| 1912 | Patented acrylic resin |
| 1939–1945 | Development of alkyds, urethane, and epoxy resins |
| 1948 | Incorporation of latex resins in the coating industry |
| 1961–1965 | Development of coil coatings, electrodeposition curtain coating, computer color control electrostatic powder spray, fluorocarbon resins |
| 1970 | Use of emulsion resin to control penetration in substrates |
| 1966–1970 | Development of radiation curable coatings |
| 1970–1975 | Development of aqueous industrial enamels electron beam curing, and ultraviolet curing |
| 1976–1980 | Development of high solid epoxy and polyurethane coatings resins |
| 1981–1985 | Development of high-performance pigments, polyurea resins, and high solids alkyd paints |
| 1986–1999 | Waterborne epoxy coatings and waterborne polyurethanes |
| 21st Century | New systems based on alkyd technology, synthetic polymer-based coating resins, e.g., PVC-plastisol, acrylate dispersion, melamine/polyester, 2K urethanes, and inclusion of new drier systems for alkyds by replacing the cobalt driers |
| Year | Total of Publications Used |
|---|---|
| 2021 | 4 |
| 2020 | 3 |
| 2019 | 13 |
| 2018 | 6 |
| 2017 | 7 |
| 2016 | 6 |
| 2015 | 9 |
| 2014 | 3 |
| 2013 | 2 |
| 2012 | 4 |
| 2010 | 3 |
| 2007 | 2 |
| 2005 | 3 |
| 2004 | 1 |
| 2002 | 2 |
| 2001 | 2 |
| 2000 | 4 |
| 1989 | 3 |
| 1983 | 1 |
| 1981 | 1 |
| 1978 | 1 |
| Country | Total of Publications Used |
|---|---|
| USA | 20 |
| China | 13 |
| India | 7 |
| Germany | 6 |
| Nigeria | 4 |
| United Kingdom | 4 |
| Mexico | 3 |
| Poland | 3 |
| Canada | 2 |
| Spain | 2 |
| Italy | 2 |
| Portugal | 2 |
| Saudi Arabia | 2 |
| Australia | 1 |
| Brazil | 1 |
| Colombia | 1 |
| Czech Republic | 1 |
| Greece | 1 |
| Japan | 1 |
| Lithuania | 1 |
| Russia | 1 |
| Serbia | 1 |
| Material | Laboratory Type | Number of Articles |
|---|---|---|
| Concrete | Compressive strength | 19 |
| Flexural strength | 4 | |
| Loss of concrete weight | 5 | |
| Slump test | 6 | |
| Permeability | 4 | |
| Expansion behavior | 4 | |
| Carbonation depth | 14 | |
| Steel | Corrosion potential | 5 |
| Corrosion kinetics | 5 | |
| Aggregates | Moisture | 3 |
| Bulk density and gravity | 3 |
| Exposition Time (Days) | Number of Articles |
|---|---|
| <100 | 4 |
| >100 and <200 | 7 |
| >200 | 13 |
| Shape | Sample Size (mm) | Number of Articles |
|---|---|---|
| Prismatic | 120 × 150 × 70 | 5 |
| 150 × 150 × 150 | 12 | |
| 150 × 100 × 1000 | 4 | |
| Cylindric | 150 × 300 | 3 |
| 50 × 100 | 4 |
| Aim of Research | Materials | Laboratories | Results | Ref. |
|---|---|---|---|---|
| Determination of the compressive and flexural strength behavior of unstressed concrete samples embedded in polluted soil | ➢ Ordinary Portland cement grade 42.5 ➢ Dimension of cubic samples: 15cm × 15cm × 15cm ➢ Dimension of beams: 15cm × 15cm × 100cm ➢ Concrete mix 1:1.5:3 | ➢ Compressive and flexural test at curing ages of 28 up to 196 days ➢ Compressive strength of concrete samples exposed to progressive heat in five cycles ➢ Consistency, gravity, soundness, and compressive strength of cement ➢ Determination of moisture, bulk density, and the gravity of aggregates | ➢ Reduction in the compressive strength up to 9.47% during the first 28 days ➢ Reduction in the flexural strength up to 34.50% during the first 28 days | [53] |
| Analyze the influence of organic abattoir waste and disposal hydrocarbon contamination on the durability of concrete | ➢ Ordinary Portland cement grade 42.5 ➢ Dimension of cubic samples: 15cm × 15cm × 15cm ➢ Dimension of beams: 15cm × 15cm × 90cm ➢ Steel for beams Ø10mm and Ø8mm | ➢ Compressive strengths of samples (every seven days until the 84th day in cubes) ➢ Flexural strength at the age of 84 days ➢ Density of concrete ➢ Physical and chemical properties of contaminated and not contaminated soil | ➢ The physical and mechanical properties of the concrete were affected by the presence of soil contaminants ➢ Hydrocarbon contamination had a more significant effect on the load-carrying capacity of concrete | [54] |
| Determination of the influence of crude oil on the compressive strength of concrete | ➢ Dimension of samples: 15cm × 15cm × 15cm ➢ Concrete mix with 0%, 1%, 2%, 3%, 4%, and 5% of contaminated aggregates | ➢ Characterization of physical properties of aggregates used to manufacture the concrete. ➢ Concrete Slump Test ➢ Compressive strength at 7, 14, 28, and 56 days | ➢ The presence of crude oil in concrete samples significantly decreased the mechanical properties ➢ Increase in percentages of crude oil in the fine aggregate cause higher workability of concrete | [52] |
| Analyze the mechanical and physical properties behavior of concrete samples | ➢ Dimension of samples: Ø50 mm × 100 mm ➢ Dimension of samples: prismatic: 25 mm × 25 mm × 285 mm ➢ solution | ➢ Compressive strength ➢ Measurement of elastic modulus ➢ Permeability ➢ Expansion behavior ➢ Carbonation depth | ➢ Increments in the expansion material of about 0.5% higher than the limit expansion stated in the standards ➢ Compressive strength shows a reduction in the resistance of about 30% | [55] |
| Comparison of the concentration and intensity distribution of in concrete samples | ➢ Dimension of samples: Ø150 mm × 300 mm ➢ Type I ordinary Portland cement ➢ Phenolphthalein indicator | ➢ Carbonation depth ➢ Thermalgravimetric analysis (TGA) method ➢ X-ray diffraction analysis tests ➢ Fourier transformation infrared spectroscopy (FTIR) test ➢ pH measurement ➢ Compressive strength | ➢ TGA, FTIR, and XRDA test show very similar results in the carbonation depth of about 35 mm up to 16 weeks ➢ Carbonation depth measured by the phenolphthalein test shows a value of 17 mm in the same frame of time | [57] |
| Analyze the behavior of corrosion in reinforced concrete embedded in soil contaminated with chlorides and sulfates | ➢ Dimension of samples: 120 mm × 70 mm × 180 mm ➢ Soil type MH ➢ Portland cement, CPC 30R RS, and CPC 30R ➢ Steel bars of AISI 1018 Carbon Steel and Galvanized Steel, Ø 3/8 and steel bars of UNS S31600 | ➢ Characterization of concrete mixtures in a fresh state ➢ Initial compressive strength ➢ Measurement of corrosion potential ➢ Physical description of the soil | ➢ Concrete samples exposed to soil contamination with NaCl content higher to 2% present the highest probability to suffer from premature corrosion in the steel bars during the first 103 days ➢ Lower Icorr magnitudes in samples made with Portland type V | [58] |
| Evaluation of the electromechanical behavior of concrete samples embedded in contaminated soil with different percentages of magnesium sulfate | ➢ Dimension of samples: 120 mm × 70 mm × 180 mm ➢ Soil type SP ➢ Portland cement, CPC 30R RS, and CPC 30R ➢ Steel bars of AISI 1018 carbon steel and galvanized steel Ø 3/8” and bars of UNS S31600 | ➢ Measurement of corrosion potential ➢ Measurement of corrosion kinetics | ➢ In concentrations between 1% and 2% of the corrosion resistance varies according to the Portland cement and steels bars type, being higher in concrete made with CPC 30R RS and reinforced with galvanized bars ➢ All concrete samples present a high and moderate level of corrosion during the first 130 days in soils, with 3% of content | [66] |
| Evaluation of the corrosion behavior of carbon and stainless steel bars using different concrete mixtures, including the addition of silica fumes and fly ash | ➢ Dimension of samples: Ø150mm× 300mm and 120mm × 70mm × 150mm ➢ AISI 1018 carbon steel and AISI 304 stainless steel with Ø 0.95 mm ➢ Concrete mixtures, 100% CPC, 80% CPC, and 20% silica fume, and 80% CPC and 20% fly ash | ➢ Measurement of corrosion potential ➢ Characterization of concrete aggregates ➢ Physical and mechanical characterization of fresh and hardened concrete mixtures ➢ Initial compressive strength | ➢ Severe corrosion in all concrete samples during the 365 days of exposure ➢ Samples with 20% of fly ash and silica fume addition showed a reduction of around 70% in the kinetic corrosion in comparison with the specimens without mineral additions | [67] |
| Type of Coating | Advantages | Disadvantages |
|---|---|---|
| Epoxy resin | ✓ Excellent adhesion properties on different substrates | ✗ Poor impact resistance |
| ✓ High chemical and solvent resistance | ✗ Low-temperature resistance | |
| ✓ Control concrete carbonation | ✗ Inherent brittleness | |
| ✓ Fluidity in the application due to its low viscosity properties | ✗ Inferior weathering resistance | |
| ✓ Good electrical properties | ✗ Complex removal procedure | |
| ✓ Excellent anticorrosion performance | ✗ Costly maintenance | |
| ✗ Strong toxic fumes | ||
| Bitumen | ✓ Good penetration into the surface due to its fluidity | ✗ Its protectiveness can be affected by polymer grade |
| ✓ When used in pavements, it improves the sticking between different layers and increases the resistance to deformation | ✗ It is affected by the temperature in the summer season by making the coating soft | |
| ✓ High water resistance | ✗ Difficult to apply to plastic surfaces | |
| ✓ High resistance to mechanical damage | ✗ Overheat buildings when it is used to the roof | |
| ✓ High resistance to UV radiation | ||
| Acrylics | ✓ Highly resistant to variations in temperature | ✗ Complex removal procedure |
| ✓ High impact resistance | ✗ Fast drying | |
| ✓ High chemical resistance | ✗ Poor water repellent | |
| ✓ User friendly, easy to apply | ✗ Low UV radiation resistance | |
| ✓ High fungus resistance | ||
| ✓ Lower cost applications | ||
| ✓ Good adhesion properties | ||
| Polyurethane resin | ✓ High performance in its mechanical properties such as flexibility, strength, hardness, and stiffness | ✗ It is sensitive to humidity |
| ✓ Control concrete carbonation | ✗ Delays the natural breathing capability of concrete | |
| ✓ Long service life | ✗ Low weathering resistance | |
| ✓ High resistance to UV radiation | ✗ Strong toxic fumes | |
| ✓ Economic maintenance | ✗ Less alkali-resistant than epoxy coating | |
| ✓ High hardness and impact resistance | ✗ High cost |
| Standard Reference | Standard Title | Parts |
|---|---|---|
| PN-EN ISO 2811 | Density determination, paints, and varnishes | Part 1: Pycnometric method (2016) |
| Part 2: Immersed body (plummet) method (2011) | ||
| Part 3: Oscillation method (2011) | ||
| Part 4: Pressure cup method (2011) | ||
| PN-EN ISO 2884:2007 | Viscosity determination, paints, and varnishes | Part 1: High shear cone-plate viscometer |
| Part 2: Viscometer with disc or ball, fixed speed | ||
| PN-EN ISO 2431:2019 | Part 1: Determination of flow time by use of flow-cups | |
| PN-EN ISO 2808: 2020 | Measurement of coating thickness, paint, and varnishes | Part 1: Determination of the coating thickness |
| PN-EN ISO 2178: 2016 | Part 1: Non-magnetic coatings on a magnetic substrate—magnetic method | |
| PN-EN ISO 2360: 2017 | Part 1: Amplitude-sensitive eddy-current method | |
| PN-EN ISO 4624: 2016 | Adhesion of the coating to the substrate, paints, and varnishes | Part 1: Pull of test |
| PN-EN ISO 2409: 2013 | Part 1: Cross-cut test | |
| PN-EN 14891:2017 | Ceramic tiling bonded with adhesives - requirements, test methods, and liquid applied water-impermeable products. | |
| Standard Reference Used | Type of Laboratory Performed | Number of Articles |
|---|---|---|
| PN-EN ISO 62:2008 | Water absorption | 1 |
| ASTM C642-97 | 2 | |
| PN-EN ISO-527-1,3 | Tensile stress | 2 |
| PN-EN 14891:2012/17 | 3 | |
| ZUAT-15/IV.13/2002 | Adhesion | 1 |
| ASTM D4541-17 | 2 | |
| ZUAT-15/IV.13/2002 | Resistance to freeze/thaw cycles | 1 |
| PN-EN 1504-2:2006 | 2 | |
| PN-EN 14891:2017 | 2 | |
| ASTM D562-10 | Viscosity | 1 |
| PN-EN 1504-2:2006 | Ability to cover cracks | 2 |
| EN ISO 9117-1:2009 | Curing time | 1 |
| ASTM D1640 | 2 | |
| ASTM C642-97 | Water absorption | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millán Ramírez, G.P.; Byliński, H.; Niedostatkiewicz, M. Deterioration and Protection of Concrete Elements Embedded in Contaminated Soil: A Review. Materials 2021, 14, 3253. https://doi.org/10.3390/ma14123253
Millán Ramírez GP, Byliński H, Niedostatkiewicz M. Deterioration and Protection of Concrete Elements Embedded in Contaminated Soil: A Review. Materials. 2021; 14(12):3253. https://doi.org/10.3390/ma14123253
Chicago/Turabian StyleMillán Ramírez, Ginneth Patricia, Hubert Byliński, and Maciej Niedostatkiewicz. 2021. "Deterioration and Protection of Concrete Elements Embedded in Contaminated Soil: A Review" Materials 14, no. 12: 3253. https://doi.org/10.3390/ma14123253
APA StyleMillán Ramírez, G. P., Byliński, H., & Niedostatkiewicz, M. (2021). Deterioration and Protection of Concrete Elements Embedded in Contaminated Soil: A Review. Materials, 14(12), 3253. https://doi.org/10.3390/ma14123253