Effects of Fatigue Damage on the Microscopic Modulus of Cortical Bone Using Nanoindentation
Abstract
1. Introduction
2. Materials and Methods
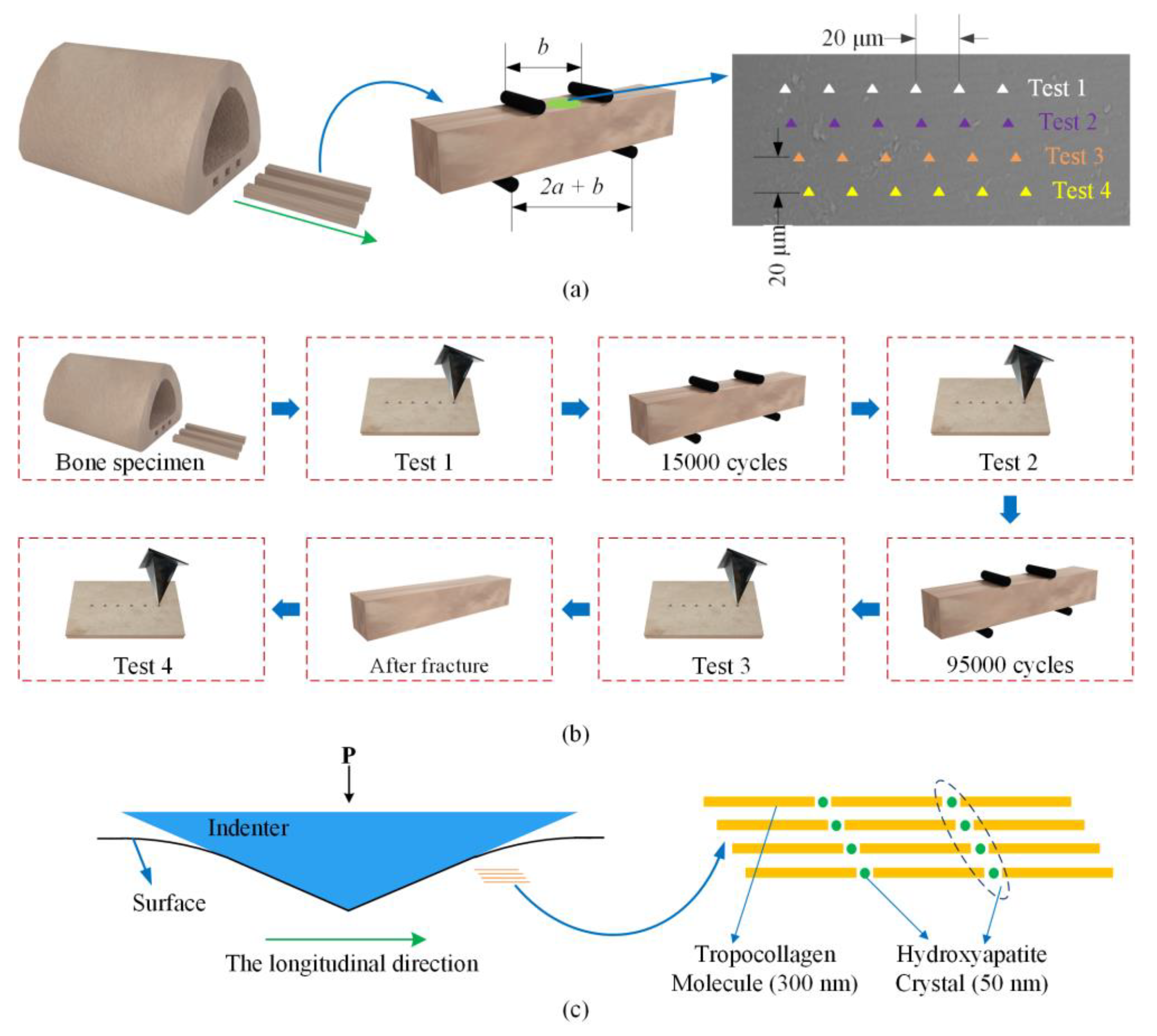
2.1. Specimen Preparation and Fatigue Experiment
2.2. Nanoindentation Test
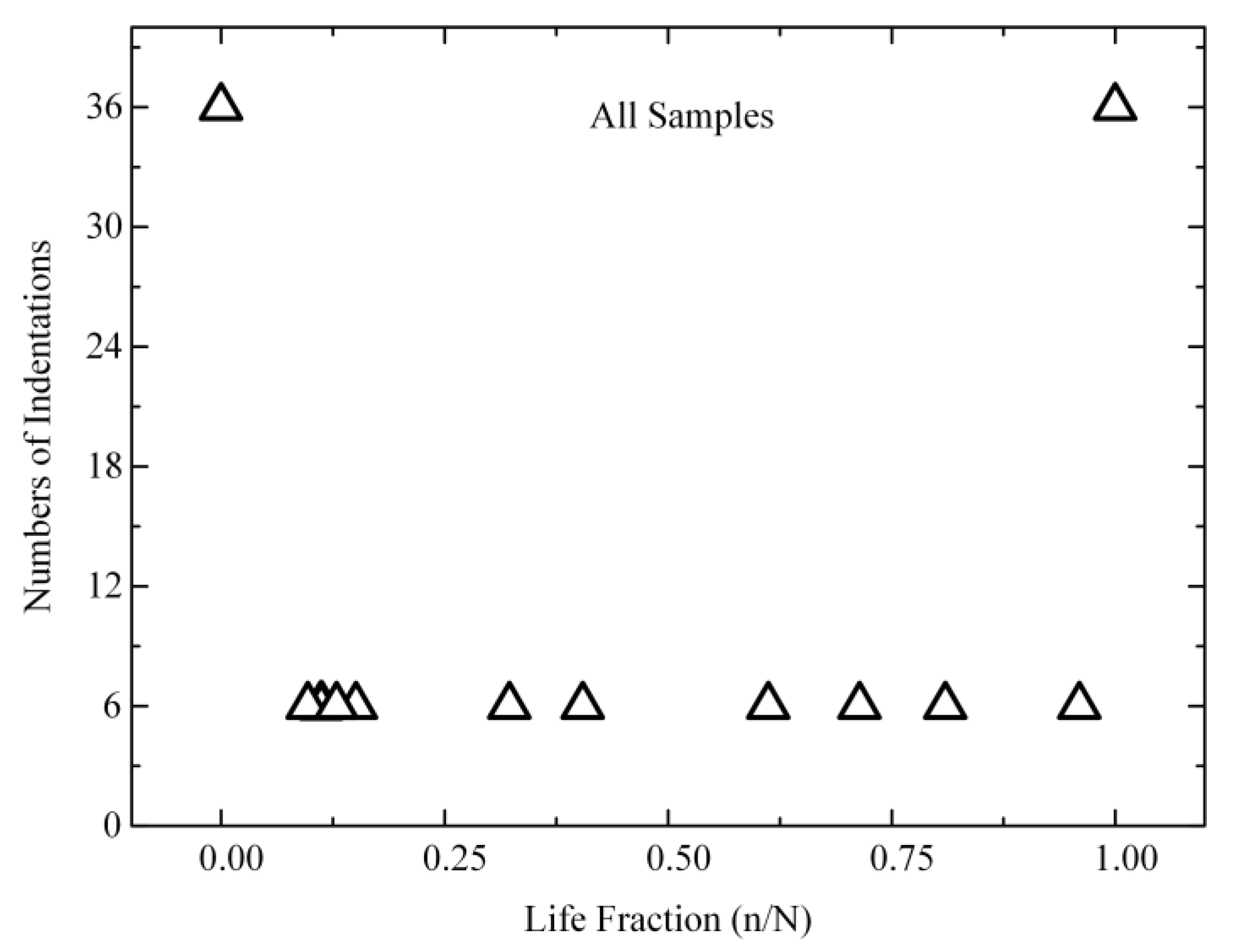
2.3. Statistics
3. Results
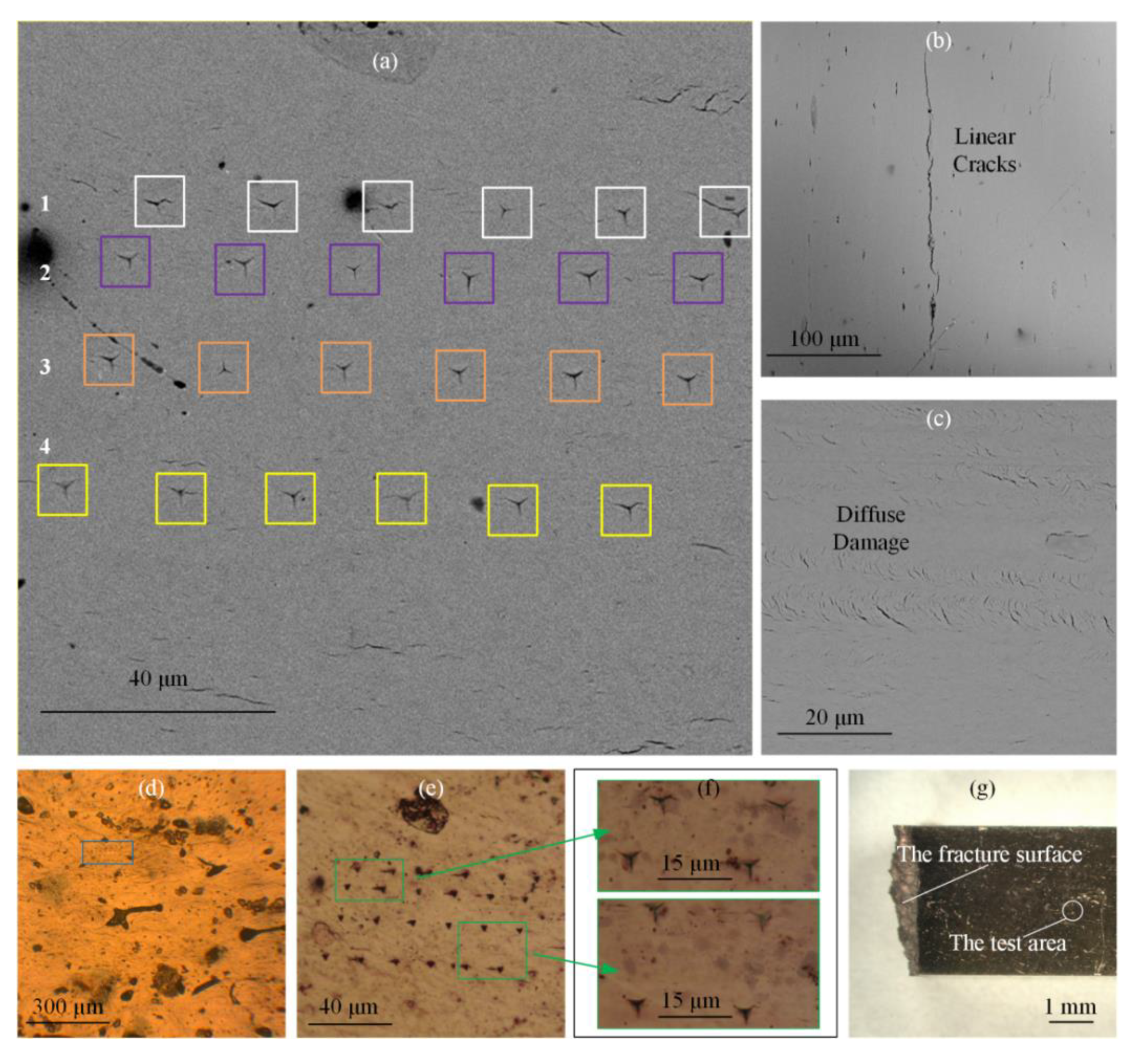
3.1. Optical Microscope and Scanning Electron Microscope (SEM) Analysis
3.2. Evolution of Reduced Modulus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodríguez-Gómez, I.; Mañas, A.; Losa-Reyna, J.; Rodríguez-Mañas, L.; Chastin, S.F.M.; Alegre, L.M.; García-García, F.J.; Ara, I. Compositional Influence of Movement Behaviors on Bone Health during Aging. Med. Sci. Sports Exerc. 2019, 51, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gómez, I.; Mañas, A.; Losa-Reyna, J.; Rodríguez-Mañas, L.; Chastin, S.F.; Alegre, L.M.; García-García, F.J.; Ara, I. Prospective Changes in the Distribution of Movement Behaviors Are Associated With Bone Health in the Elderly According to Variations in their Frailty Levels. J. Bone Miner. Res. 2020, 35, 1236–1245. [Google Scholar] [CrossRef]
- Burge, R.; Dawson-Hughes, B.; Solomon, D.H.; Wong, J.B.; King, A.; Tosteson, A. Incidence and Economic Burden of Osteoporosis-Related Fractures in the United States, 2005–2025. J. Bone Miner. Res. 2006, 22, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.L.; Seeley, D.G.; Lui, L.-Y.; Cauley, J.A.; Ensrud, K.; Browner, W.S.; Nevitt, M.C.; Cummings, S.R. BMD at Multiple Sites and Risk of Fracture of Multiple Types: Long-Term Results From the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2003, 18, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Oden, A.; Johnell, O.; Johansson, H.; De Laet, C.; Brown, J.; Burckhardt, P.; Cooper, C.; Christiansen, C.; Cummings, S.; et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos. Int. 2007, 18, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.; Hayes, W. Compact bone fatigue damage—I. Residual strength and stiffness. J. Biomech. 1977, 10, 325–337. [Google Scholar] [CrossRef]
- Dominguez, V.M.; Agnew, A.M. Microdamage as a Bone Quality Component: Practical Guidelines for the Two-Dimensional Analysis of Linear Microcracks in Human Cortical Bone. JBMR Plus 2019, 3, e10203. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, S.; Luong, D.H. Stress fractures of the ankle malleoli diagnosed by ultrasound: A report of 6 cases. Skelet. Radiol. 2014, 43, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Diab, T.; Condon, K.W.; Burr, D.B.; Vashishth, D. Age-related change in the damage morphology of human cortical bone and its role in bone fragility. Bone 2006, 38, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Diab, T.; Vashishth, D. Effects of damage morphology on cortical bone fragility. Bone 2005, 37, 96–102. [Google Scholar] [CrossRef]
- Pattin, C.A.; Caler, W.E.; Carter, D.R. Cyclic mechanical property degradation during fatigue loading of cortical bone. J. Biomech. 1996, 29, 69–79. [Google Scholar] [CrossRef]
- Loundagin, L.; Edwards, W. Stressed volume around vascular canals explains compressive fatigue life variation of secondary osteonal bone but not plexiform bone. J. Mech. Behav. Biomed. Mater. 2020, 111, 104002. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. Some effects of ageing in human Haversian systems. J. Anat. 1964, 98, 69–75. [Google Scholar] [PubMed]
- Qin, Q.-H.; Qu, C.; Ye, J. Thermoelectroelastic solutions for surface bone remodeling under axial and transverse loads. Biomaterials 2005, 26, 6798–6810. [Google Scholar] [CrossRef]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 520, 1263–1334. [Google Scholar] [CrossRef]
- Jowsey, J. Studies of Haversian systems in man and some animals. J. Anat. 1966, 100, 857–864. [Google Scholar]
- Qin, Q.H. Mechanics of Cellular Bone Remodeling: Coupled Thermal, Electrical, and Mechanical Field Effects; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2013. [Google Scholar]
- Li, S.; Abdel-Wahab, A.; Silberschmidt, V.V. Analysis of fracture processes in cortical bone tissue. Eng. Fract. Mech. 2013, 110, 448–458. [Google Scholar] [CrossRef]
- Qin, Q.-H.; Ye, J.-Q. Thermoelectroelastic solutions for internal bone remodeling under axial and transverse loads. Int. J. Solids Struct. 2004, 41, 2447–2460. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S. Nanoscale Mechanisms of Bone Deformation and Fracture. In Handbook of Biomineralization; Bäuerlein, E., Ed.; Wiley: Weinheim, Germany, 2008; pp. 397–414. [Google Scholar]
- Carter, D.R.; Caler, W.E.; Spengler, D.M.; Frankel, V.H. Fatigue Behavior of Adult Cortical Bone: The Influence of Mean Strain and Strain Range. Acta Orthop. Scand. 1981, 52, 481–490. [Google Scholar] [CrossRef]
- Evans, F.G. Stress and Strain Bones: Their Relation to Fractures and Osteogenesis; Thomas: Springfield, IL, USA, 1957. [Google Scholar]
- Frost, H.L. Presence of Microscopic Cracks In Vivo In Bone. Henry Ford Hosp. Med. Bull. 1960, 8, 25–35. [Google Scholar]
- Ritchie, R.O. The conflicts between strength and toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.S. Skeletal tissue mechanics. Am. J. Phys. Anthropol. 2000, 112, 435–436. [Google Scholar] [CrossRef]
- Vashishth, D.; Koontz, J.; Qiu, S.; Lundin-Cannon, D.; Yeni, Y.; Schaffler, M.; Fyhrie, D. In vivo diffuse damage in human vertebral trabecular bone. Bone 2000, 26, 147–152. [Google Scholar] [CrossRef]
- Lee, T.C.; O’Brien, F.J.; Taylor, D. The nature of fatigue damage in bone. Int. J. Fatigue 2000, 22, 847–853. [Google Scholar] [CrossRef]
- Zioupos, P.; Currey, J.D. The extent of microcracking and the morphology of microcracks in damaged bone. J. Mater. Sci. 1994, 29, 978–986. [Google Scholar] [CrossRef]
- Burr, D.B.; Turner, C.H.; Naick, P.; Forwood, M.R.; Ambrosius, W.; Hasan, M.S.; Pidaparti, R. Does microdamage accumulation affect the mechanical properties of bone? J. Biomech. 1998, 31, 337–345. [Google Scholar] [CrossRef]
- Zimmermann, E.; Ritchie, R.O. Bone as a Structural Material. Adv. Heal. Mater. 2015, 4, 1287–1304. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.; Gupta, H.S.; Zaslansky, P.; Wagner, H.D.; Fratzl, P. Tough Lessons from Bone: Extreme Mechanical Anisotropy at the Mesoscale. Adv. Funct. Mater. 2008, 18, 1905–1911. [Google Scholar] [CrossRef]
- Koester, K.J.; Ager, J.W., 3rd; Ritchie, R.O. The true toughness of human cortical bone measured with realistically short cracks. Nat. Mater. 2008, 7, 672–677. [Google Scholar] [CrossRef]
- Tertuliano, O.A.; Greer, J.R. The nanocomposite nature of bone drives its strength and damage resistance. Nat. Mater. 2016, 15, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Wagermaier, W.; Klaushofer, K.; Fratzl, P. Fragility of Bone Material Controlled by Internal Interfaces. Calcif. Tissue Int. 2015, 97, 201–212. [Google Scholar] [CrossRef]
- Burr, D.B.; Martin, R.; Schaffler, M.B.; Radin, E.L. Bone remodeling in response to in vivo fatigue microdamage. J. Biomech. 1985, 18, 189–200. [Google Scholar] [CrossRef]
- Bentolila, V.; Boyce, T.; Fyhrie, D.; Drumb, R.; Skerry, T.; Schaffler, M. Intracortical remodeling in adult rat long bones after fatigue loading. Bone 1998, 23, 275–281. [Google Scholar] [CrossRef]
- Qu, C.; Qin, Q.-H.; Kang, Y. A hypothetical mechanism of bone remodeling and modeling under electromagnetic loads. Biomaterials 2006, 27, 4050–4057. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- McCalden, R.W.; A McGeough, J.; Barker, M.B.; Court-Brown, C.M. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J. Bone Jt. Surg. Am. Vol. 1993, 75, 1193–1205. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Hodgson, P.; Wen, C. Elastic modulus and hardness of cortical and trabecular bovine bone measured by nanoindentation. Trans. Nonferrous Met. Soc. China 2006, 16, s744–s748. [Google Scholar] [CrossRef]
- Zebaze, R.M.; Jones, A.C.; Pandy, M.G.; Knackstedt, M.A.; Seeman, E. Differences in the degree of bone tissue mineralization account for little of the differences in tissue elastic properties. Bone 2011, 48, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Zysset, P.K.; Guo, X.E.; Hoffler, C.E.; E Moore, K.; A Goldstein, S. Elastic modulus and hardness of cortical and trabecular bone lamellae measured by nanoindentation in the human femur. J. Biomech. 1999, 32, 1005–1012. [Google Scholar] [CrossRef]
- Feng, L.; Chittenden, M.; Schirer, J.; Dickinson, M.; Jasiuk, I. Mechanical properties of porcine femoral cortical bone measured by nanoindentation. J. Biomech. 2012, 45, 1775–1782. [Google Scholar] [CrossRef]
- Poundarik, A.A.; Vashishth, D. Multiscale imaging of bone microdamage. Connect. Tissue Res. 2015, 56, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Tai, K.; Dao, M.; Suresh, S.; Palazoglu, A.; Ortiz, C. Nanoscale heterogeneity promotes energy dissipation in bone. Nat. Mater. 2007, 6, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Burket, J.C.; Brooks, D.J.; MacLeay, J.M.; Baker, S.P.; Boskey, A.L.; van der Meulen, M.C. Variations in nanomechanical properties and tissue composition within trabeculae from an ovine model of osteoporosis and treatment. Bone 2013, 52, 326–336. [Google Scholar] [CrossRef]
- Subit, D.; Dios, E.d.P.d.; Valazquez-Ameijide, J.; Arregui-Dalmases, C.; Crandall, J. Tensile material properties of human rib cortical bone under quasi-static and dynamic failure loading and influence of the bone microstucture on failure characteristics. arXiv 2011, arXiv:1108.0390. [Google Scholar]
- Draper, E.R.; E Goodship, A. A novel technique for four-point bending of small bone samples with semi-automatic analysis. J. Biomech. 2003, 36, 1497–1502. [Google Scholar] [CrossRef]
- Donnelly, E.; Baker, S.P.; Boskey, A.L.; van der Meulen, M.C.H. Effects of surface roughness and maximum load on the mechanical properties of cancellous bone measured by nanoindentation. J. Biomed. Mater. Res. 2006, 77, 426–435. [Google Scholar] [CrossRef] [PubMed]
- IBM SPSS Statistics 26, R26.0.0.0, edition; IBM: Armonk, NY, USA, 2019.
- Origin 9.0, 9.0.0 x64 b45 64-bit, edition; Originlab: Northampton, MA, USA, 2012.
- Yin, L.; Venkatesan, S.; Webb, D.; Kalyanasundaram, S.; Qin, Q.-H. Effect of cryo-induced microcracks on microindentation of hydrated cortical bone tissue. Mater. Charact. 2009, 60, 783–791. [Google Scholar] [CrossRef]
- Zioupos, P.; Wang, X.T.; Currey, J.D. Experimental and theoretical quantification of the development of damage in fatigue tests of bone and antler. J. Biomech. 1996, 29, 989–1002. [Google Scholar] [CrossRef]
- Novitskaya, E.; Chen, P.-Y.; Lee, S.; Castro-Ceseña, A.; Hirata, G.; Lubarda, V.A.; McKittrick, J. Anisotropy in the compressive mechanical properties of bovine cortical bone and the mineral and protein constituents. Acta Biomater. 2011, 7, 3170–3177. [Google Scholar] [CrossRef]
- Bonfield, W.; Grynpas, M.D. Anisotropy of the Young’s modulus of bone. Nature 1977, 270, 453–454. [Google Scholar] [CrossRef]
- Fan, Z.; Swadener, J.G.; Rho, J.Y.; Roy, M.; Pharr, G.M. Anisotropic properties of human tibial cortical bone as measured by nanoindentation. J. Orthop. Res. 2002, 20, 806–810. [Google Scholar] [CrossRef]
- Isaksson, H.; Nagao, S.; MaŁkiewicz, M.; Julkunen, P.; Nowak, R.; Jurvelin, J.S. Precision of nanoindentation protocols for measurement of viscoelasticity in cortical and trabecular bone. J. Biomech. 2010, 43, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.; Shapter, J.G.; Short, K.; Smith, L.J.; Fazzalari, N.L. Micromechanical properties of human trabecular bone: A hierarchical investigation using nanoindentation. J. Biomed. Mater. Res. Part A 2008, 87, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Currey, J.D. Stress Concentrations in Bone. J. Cell Sci. 1962, 3, 111–133. [Google Scholar] [CrossRef]
- Burr, D.B. Stress concentrations and bone microdamage: John Currey’s contributions to understanding the initiation and arrest of cracks in bone. Bone 2019, 127, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Reilly, G.C.; Currey, J.D. The development of microcracking and failure in bone depends on the loading mode to which it is adapted. J. Exp. Biol. 1999, 202, 543–552. [Google Scholar] [CrossRef]

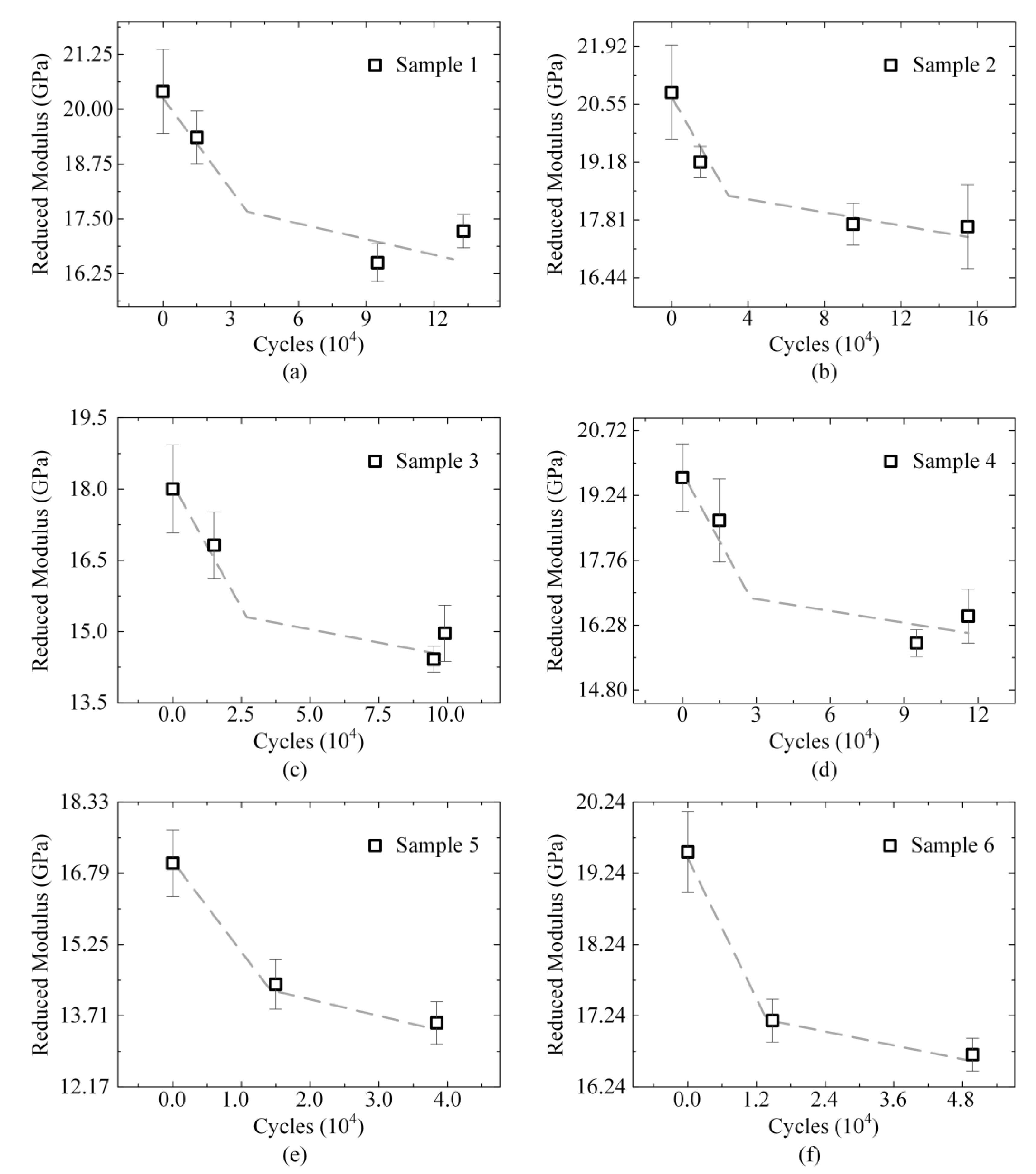
| Samples | Cycles (104) | Modulus (0) | Modulus (1.5) | Modulus (9.5) | Modulus (Failure) | CVmax |
|---|---|---|---|---|---|---|
| 1 | 13.3 | 20.41 ± 0.94 | 19.36 ± 0.60 | 16.70 ± 0.43 | 17.22 ± 0.38 | 4.61% |
| 2 | 15.5 | 20.83 ± 1.12 | 19.18 ± 0.37 | 17.71 ± 0.48 | 17.62 ± 1.02 | 5.79% |
| 3 | 9.9 | 18.04 ± 1.23 | 17.05 ± 0.70 | 14.66 ± 0.28 | 14.84 ± 0.59 | 6.82% |
| 4 | 11.6 | 19.64 ± 0.77 | 18.80 ± 0.95 | 15.93 ± 0.30 | 16.49 ± 0.62 | 5.05% |
| 5 | 3.8 | 16.95 ± 0.59 | 14.11 ± 0.55 | - | 13.66 ± 0.52 | 3.81% |
| 6 | 4.9 | 19.57 ± 0.85 | 17.53 ± 0.48 | - | 16.74 ± 0.33 | 4.34% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Qu, C.; Fu, D.; Qu, C. Effects of Fatigue Damage on the Microscopic Modulus of Cortical Bone Using Nanoindentation. Materials 2021, 14, 3252. https://doi.org/10.3390/ma14123252
Meng X, Qu C, Fu D, Qu C. Effects of Fatigue Damage on the Microscopic Modulus of Cortical Bone Using Nanoindentation. Materials. 2021; 14(12):3252. https://doi.org/10.3390/ma14123252
Chicago/Turabian StyleMeng, Xianjia, Chuanyong Qu, Donghui Fu, and Chuan Qu. 2021. "Effects of Fatigue Damage on the Microscopic Modulus of Cortical Bone Using Nanoindentation" Materials 14, no. 12: 3252. https://doi.org/10.3390/ma14123252
APA StyleMeng, X., Qu, C., Fu, D., & Qu, C. (2021). Effects of Fatigue Damage on the Microscopic Modulus of Cortical Bone Using Nanoindentation. Materials, 14(12), 3252. https://doi.org/10.3390/ma14123252








