Zinc Biofortification in Food Crops Could Alleviate the Zinc Malnutrition in Human Health
Abstract
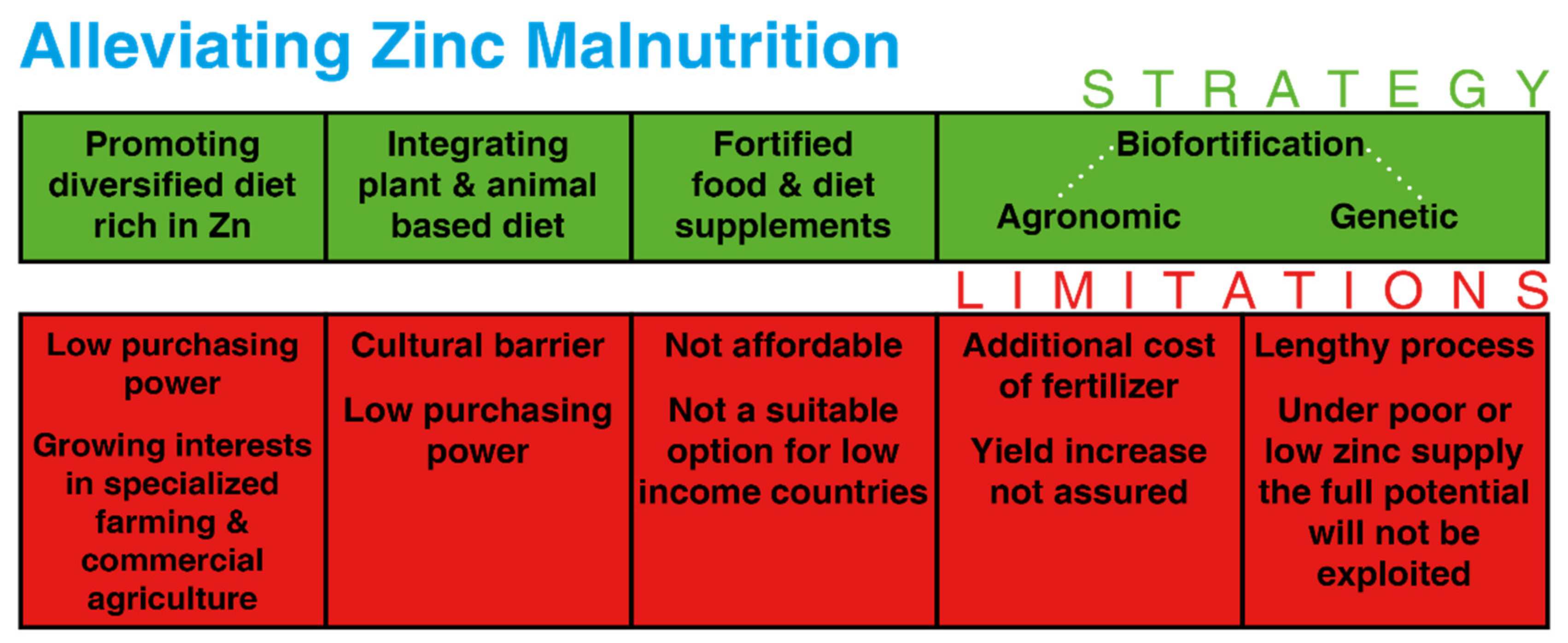
1. Introduction
2. Role of Zn in Human Health
3. Role of Zn in Crop
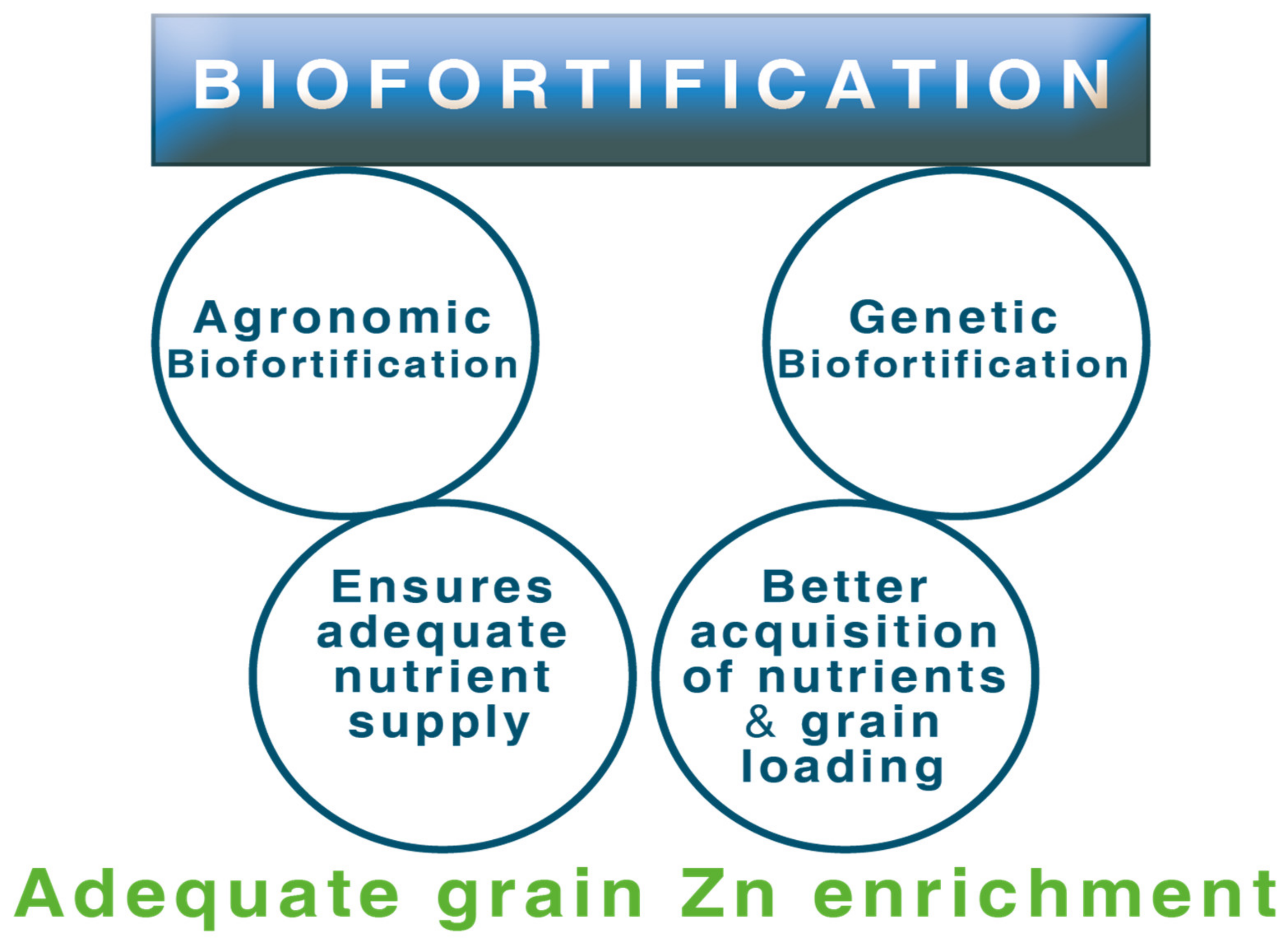
4. Biofortification for Grain Zn Enrichment: The Concept
4.1. Agronomic Biofortification
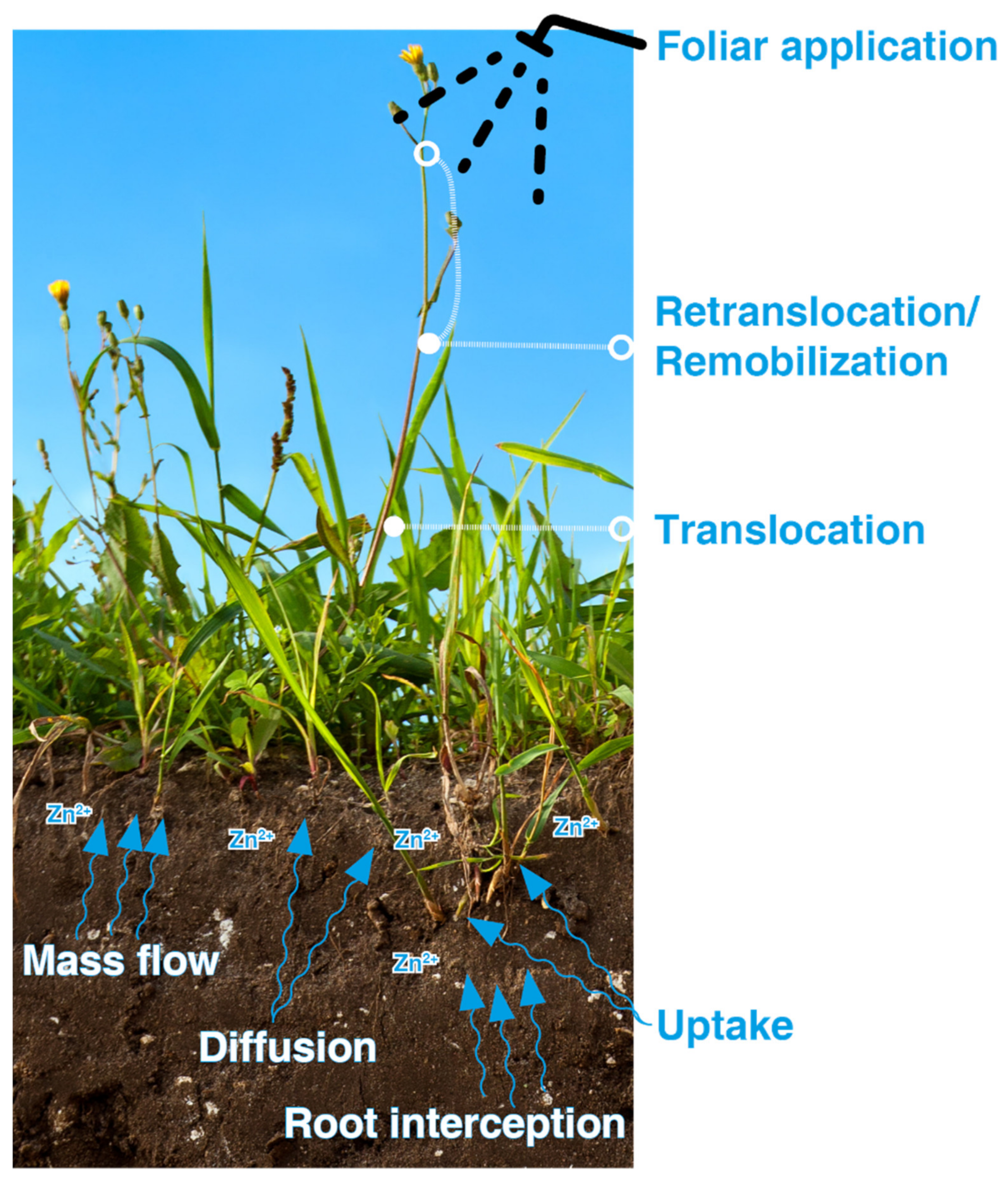
4.1.1. Effect of Different Methods of Zn Application on Grain Zn Enrichment
4.1.2. Soil Application
4.1.3. Foliar Application
4.1.4. Seed Application
4.1.5. Combination of Application Methods
4.1.6. Other Agronomic Practices to Improve Zn Uptake
4.1.7. Additional Benefits of Zn Fertilization
4.2. Genetic Biofortification
4.2.1. Strategies for Genetic Biofortification
4.2.2. Limitations and Constraints of Genetic Biofortification
5. Combining Agronomic and Genetic Biofortification
6. Economic Points of View for Zn Biofortification
7. Future Scopes
- A comprehensive 4R (right place, right time, right source, and right dose) approach of Zn application can be developed for different crops at the regional level and the best combination can be found for achieving high grain Zn concentration.
- Physiological constraints of grain Zn accumulation must be identified for different crops under different conditions and agronomic and genetic approaches for ameliorating these constraints may be found to further improve the grain Zn density.
- Biofortification options must be studied under stressed environments and their effects must be evaluated under such conditions. As climate change is expected to bring more weather anomalies, a stress-proof biofortification approach must be developed.
- The bioavailability of Zn obtained through foliar application can be compared with other application methods. Agronomic management that improves grain Zn bioavailability should be studied.
- The environmental implications of continuous Zn application should be studied. Continuous application of Zn over a long period may cause Zn toxicity and therefore should be regularly monitored.
- The performance of Zn-efficient genotypes under different soil Zn availability should be evaluated. The beneficial effects of combining the agronomic and genetic biofortification approach should be explored.
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gundersen, C.; Ziliak, J.P. Food Insecurity and Health Outcomes. Health Aff. 2015, 34, 1830–1839. [Google Scholar] [CrossRef]
- Onyango, A.W. Dietary diversity, child nutrition and health in contemporary African communities. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 136, 61–69. [Google Scholar] [CrossRef]
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World 2020: Transforming Food Systems for Affordable Healthy Diets; FAO: Rome, Italy, 2020. [Google Scholar]
- Cakmak, I.; Pfeiffer, W.H.; McClafferty, B. REVIEW: Biofortification of Durum Wheat with Zinc and Iron. Cereal Chem. J. 2010, 87, 10–20. [Google Scholar] [CrossRef]
- Cakmak, I.; Graham, R.; Welch, R.M. Agricultural and molecular genetic approaches to improving nutrition and preventing micronutrient malnutrition globally. Encycl. Life Support Syst. 2002, 1, 1075–1099. [Google Scholar]
- Velu, G.; Ortiz-Monasterio, I.; Cakmak, I.; Hao, Y.; Singh, R. Biofortification strategies to increase grain zinc and iron concentrations in wheat. J. Cereal Sci. 2014, 59, 365–372. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Aslam, Z.; Yaseen, M.; Ihsan, M.Z.; Khaliq, A.; Fahad, S.; Bashir, S.; Ramzani, P.M.A.; Naeem, M. Zinc bioforti-fication in rice: Leveraging agriculture to moderate hidden hunger in developing countries. Arch. Agron. Soil Sci. 2018, 64, 147–161. [Google Scholar] [CrossRef]
- Ruel, M.T.; Alderman, H.; Maternal and Child Nutrition Study Group. Nutrition-sensitive interventions and programmes: How can they help to accelerate progress in improving maternal and child nutrition? Lancet 2013, 382, 536–551. [Google Scholar] [CrossRef]
- De Valença, A.; Bake, A.; Brouwer, I.; Giller, K. Agronomic biofortification of crops to fight hidden hunger in sub-Saharan Africa. Glob. Food Secur. 2017, 12, 8–14. [Google Scholar] [CrossRef]
- Aciksoz, S.B.; Yazici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification? Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Das, S.; Chaki, A.K.; Hossain, A. Breeding and agronomic approaches for the biofortification of zinc in wheat (Triticum aestivum L.) to combat zinc deficiency in millions of a population: A Bangladesh perspective. Acta Agrobot. 2019, 72. [Google Scholar] [CrossRef]
- Maqbool, M.A.; Beshir, A. Zinc biofortification of maize (Zea mays L.): Status and challenges. Plant Breed. 2019, 138, 1–28. [Google Scholar] [CrossRef]
- Bouis, H.E.; Welch, R.M. Biofortification-A Sustainable Agricultural Strategy for Reducing Micronutrient Malnutrition in the Global South. Crop Sci. 2010, 50, S-20–S-32. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Welch, R.M.; Graham, R.D. Breeding for micronutrients in staple food crops from a human nutrition perspective. J. Exp. Bot. 2004, 55, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, M.K.; Hussain, S. Zinc-biofortified wheat required only a medium rate of soil zinc application to attain the targets of zinc biofortification. Arch. Agron. Soil Sci. 2021, 67, 551–562. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Agronomic Biofortification of Cereal Grains with Iron and Zinc. Adv. Agron. 2014, 125, 55–91. [Google Scholar] [CrossRef]
- Wessells, K.R.; Brown, K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE 2012, 7, e50568. [Google Scholar] [CrossRef]
- Gibson, R.S. Zinc deficiency and human health: Etiology, health consequences, and future solutions. Plant Soil 2012, 361, 291–299. [Google Scholar] [CrossRef]
- Hotz, C.; Brown, K.H. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25, S91–S204. [Google Scholar]
- Prasad, R. Crop Nutrition—Principles and Practices; New Vishal Publications: Delhi, India, 2007; p. 272. [Google Scholar]
- Roohani, N.; Hurrell, R.; Kelishadi, R.; Schulin, R. Zinc and its importance for human health: An integrative review. J. Res. Med. Sci. 2013, 18, 144–157. [Google Scholar] [PubMed]
- Tapiero, H.; Tew, K.D. Trace elements in human physiology and pathology: Zinc and metallothioneins. Biomed. Pharmacother. 2003, 57, 399–411. [Google Scholar] [CrossRef]
- Zastrow, M.L.; Pecoraro, V.L. Designing Hydrolytic Zinc Metalloenzymes. Biochemistry 2014, 53, 957–978. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc Biochemistry: From a Single Zinc Enzyme to a Key Element of Life. Adv. Nutr. 2013, 4, 82–91. [Google Scholar] [CrossRef]
- Hambidge, K.M. Zinc deficiency in young children. Am. J. Clin. Nutr. 1997, 65, 160–161. [Google Scholar] [CrossRef]
- Veenemans, J.; Milligan, P.; Prentice, A.M.; Schouten, L.R.; Inja, N.; Van Der Heijden, A.C.; De Boer, L.C.; Jansen, E.J.; Koopmans, A.E.; Enthoven, W.T.; et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: A randomised trial. PLoS Med. 2011, 8, e1001125. [Google Scholar] [CrossRef]
- Bhutta, Z.; Black, R.; Brown, K.; Gardner, J.; Gore, S.; Hidayat, A.; Khatun, F.; Martorell, R.; Ninh, N.; Penny, M.; et al. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: Pooled analysis of randomized controlled trials. J. Pediatr. 1999, 135, 689–697. [Google Scholar] [CrossRef]
- Moretti, D.; Biebinger, R.; Bruins, M.J.; Hoeft, B.; Kraemer, K. Bioavailability of iron, zinc, folic acid, and vitamin A from fortified maize. Ann. N. Y. Acad. Sci. 2013, 1312, 54–65. [Google Scholar] [CrossRef]
- Udechukwu, M.C.; Collins, S.A.; Udenigwe, C.C. Prospects of enhancing dietary zinc bioavailability with food-derived zinc-chelating peptides. Food Funct. 2016, 7, 4137–4144. [Google Scholar] [CrossRef]
- Saha, S.; Chakraborty, M.; Padhan, D.; Saha, B.; Murmu, S.; Batabyal, K.; Seth, A.; Hazra, G.C.; Mandal, B.; Bell, R.W. Agro-nomic biofortification of zinc in rice: Influence of cultivars and zinc application methods on grain yield and zinc bioavailability. Field Crops Res. 2017, 210, 52–60. [Google Scholar] [CrossRef]
- Sharma, A.; Patni, B.; Shankhdhar, D.; Shankhdhar, S.C. Zinc—An indispensable micronutrient. Physiol. Mol. Biol. Plants 2013, 19, 11–20. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef]
- Chasapis, C.; Loutsidou, A.C.; Spiliopoulou, C.A.; Stefanidou, M.E. Zinc and human health: An update. Arch. Toxicol. 2012, 86, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Shokrzadeh, M.; Ghaemian, A.; Salehifar, E.; Aliakbari, S.; Saravi, S.S.S.; Ebrahimi, P. Serum Zinc and Copper Levels in Ischemic Cardiomyopathy. Biol. Trace Elem. Res. 2008, 127, 116–123. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kinoshita, M.; Shimada, S.; Kawamura, T. Zinc and Skin Disorders. Nutrients 2018, 10, 199. [Google Scholar] [CrossRef]
- Lansdown, A.B.G.; Mirastschijski, U.; Stubbs, N.; Scanlon, E.; Ågren, M.S. Zinc in wound healing: Theoretical, experimental, and clinical aspects. Wound Repair Regen. 2007, 15, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.R.; Marsh, R.G.; Draelos, Z.D. Zinc and Skin Health: Overview of Physiology and Pharmacology. Dermatol. Surg. 2006, 31, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Rostan, E.F.; DeBuys, H.V.; Madey, D.L.; Pinnell, S.R. Evidence supporting zinc as an important antioxidant for skin. Int. J. Dermatol. 2002, 41, 606–611. [Google Scholar] [CrossRef]
- Favier, A.E. The role of zinc in reproduction. Hormonal mechanism. Biol. Trace Elem. Res. 1992, 32, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Role of Zinc in Male Infertility: Review of Literature. Indian J. Obstet. Gynecol. Res. 2016, 3, 167. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. Review: The role of zinc in the endocrine system. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef]
- Gower-Winter, S.D.; Levenson, C.W. Zinc in the central nervous system: From molecules to behavior. BioFactors 2012, 38, 186–193. [Google Scholar] [CrossRef]
- Brown, K.H.; Rivera, J.A.; Bhutta, Z.; Gibson, R.S.; King, J.C.; Lönnerdal, B.; Ruel, M.T.; Sandtröm, B.; Wasantwisut, E.; Hotz, C. International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food Nutr. Bull. 2004, 25 (Suppl. 2), S99–S203. [Google Scholar] [PubMed]
- Barnett, J.B.; Hamer, D.H.; Meydani, S.N. Low zinc status: A new risk factor for pneumonia in the elderly? Nutr. Rev. 2010, 68, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and Function of Zinc Plants. In Zinc in Soils and Plants; Springer: Dordrecht, The Netherlands, 1993; pp. 93–106. [Google Scholar]
- Tobin, A.J. Carbonic Anhydrase from Parsley Leaves. J. Biol. Chem. 1970, 245, 2656–2666. [Google Scholar] [CrossRef]
- Du, H.Y.; Liu, D.X.; Liu, G.T.; Liu, H.P.; Kurtenbach, R. Relationship between Polyamines and Anaerobic Respiration of Wheat Seedling Root under Water-Logging Stress. Russ. J. Plant Physiol. 2018, 65, 874–881. [Google Scholar] [CrossRef]
- Miro, B.; Ismail, A.M. Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Front. Plant Sci. 2013, 4, 269. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef]
- Lu, Y.; Hall, D.A.; Last, R.L. A small zinc finger thylakoid protein plays a role in maintenance of photosystem II in Arabidopsis thaliana. Plant Cell 2011, 23, 1861–1875. [Google Scholar] [CrossRef] [PubMed]
- Disante, K.B.; Fuentes, D.; Cortina, J. Response to drought of Zn-stressed Quercus suber L. seedlings. Environ. Exp. Bot. 2011, 70, 96–103. [Google Scholar] [CrossRef]
- Hafeez, B.; Khanif, Y.M.; Saleem, M. Role of Zinc in Plant Nutrition—A Review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Kasim, W.A. Physiological consequences of structural and ultra-structural changes induced by Zn stress in Phaseolus vulgaris. I. Growth and Photosynthetic apparatus. Int. J. Bot. 2007, 3, 15–22. [Google Scholar] [CrossRef]
- Peck, A.W.; McDonald, G.K. Adequate zinc nutrition alleviates the adverse effects of heat stress in bread wheat. Plant Soil 2010, 337, 355–374. [Google Scholar] [CrossRef]
- Tavallali, V.; Rahemi, M.; Eshghi, S.; Kholdebarin, B.; Ramezanian, A. Zinc alleviates salt stress and increases antioxidant enzyme activity in the leaves of pistachio (Pistacia vera L. ‘Badami’) seedlings. Turk. J. Agric. Forest. 2010, 34, 349–359. [Google Scholar]
- Jan, A.; Maruyama, K.; Todaka, D.; Kidokoro, S.; Abo, M.; Yoshimura, E.; Shinozaki, K.; Nakashima, K.; Yamaguchi-Shinozaki, K. OsTZF1, a CCCH-Tandem Zinc Finger Protein, Confers Delayed Senescence and Stress Tolerance in Rice by Regulating Stress-Related Genes. Plant Physiol. 2013, 161, 1202–1216. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wen, F.; Yao, D.; Wang, L.; Guo, J.; Ni, L.; Zhang, A.; Tan, M.; Jiang, M. A novel rice C2H2-type zinc finger protein, ZFP36, is a key player involved in abscisic acid-induced antioxidant defence and oxidative stress tolerance in rice. J. Exp. Bot. 2014, 65, 5795–5809. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Yu, Y.; Wang, D.; Wu, C.; Yang, G.; Huang, J.; Zheng, C. GhZFP1, a novel CCCH-type zinc finger protein from cotton, enhances salt stress tolerance and fungal disease resistance in transgenic tobacco by interacting with GZIRD21A and GZIPR5. New Phytol. 2009, 183, 62–75. [Google Scholar] [CrossRef]
- Li, C.; Lv, J.; Zhao, X.; Ai, X.; Zhu, X.; Wang, M.; Zhao, S.; Xia, G. TaCHP: A Wheat Zinc Finger Protein Gene Down-Regulated by Abscisic Acid and Salinity Stress Plays a Positive Role in Stress Tolerance. Plant Physiol. 2010, 154, 211–221. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C. A Role for Zinc in Plant Defense against Pathogens and Herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Khoshgoftarmanesh, A.H.; Schulin, R.; Chaney, R.L.; Daneshbakhsh, B.; Afyuni, M. Micronutrient-efficient genotypes for crop yield and nutritional quality in sustainable agriculture—A review. Agron. Sustain. Dev. 2010, 30, 83–107. [Google Scholar] [CrossRef]
- McDonald, G.K.; Genc, Y.; Graham, R.D. A simple method to evaluate genetic variation in grain zinc concentration by correcting for differences in grain yield. Plant Soil 2008, 306, 49–55. [Google Scholar] [CrossRef]
- Zou, C.Q.; Zhang, Y.Q.; Rashid, A.; Ram, H.; Savasli, E.; Arisoy, R.Z.; Ortiz-Monasterio, I.; Simunji, S.; Wang, Z.H.; Sohu, V.S.; et al. Biofortification of wheat with zinc through zinc fertilization in seven countries. Plant Soil 2012, 361, 119–130. [Google Scholar] [CrossRef]
- Yilmaz, A.; Ekiz, H.; Torun, B.; Gultekin, I.; Karanlik, S.; Bagci, S.A.; Cakmak, I. Effect of different zinc application methods on grain yield and zinc concentration in wheat cultivars grown on zinc-deficient calcareous soils. J. Plant Nutr. 1997, 20, 461–471. [Google Scholar] [CrossRef]
- Khan, M.U.; Qasim, M.; Subhan, M.; Jamil, M.; Ahmad, R.D. Response of rice to different methods of zinc application in calcareous soil. Pak. J. Appl. Sci. 2003, 3, 524–529. [Google Scholar] [CrossRef]
- Mathpal, B.; Srivastava, P.C.; Shankhdhar, D.; Shankhdhar, S.C. Zinc enrichment in wheat genotypes under various methods of zinc application. Plant Soil Environ. 2015, 61, 171–175. [Google Scholar] [CrossRef]
- Hussain, S.; Maqsood, M.A.; Rengel, Z.; Aziz, T. Biofortification and estimated human bioavailability of zinc in wheat grains as influenced by methods of zinc application. Plant Soil 2012, 361, 279–290. [Google Scholar] [CrossRef]
- Rehman, A.; Farooq, M.; Ahmad, R.; Basra, S. Seed priming with zinc improves the germination and early seedling growth of wheat. Seed Sci. Technol. 2015, 43, 262–268. [Google Scholar] [CrossRef]
- Lindsay, W.L.; Mortvedt, J.J. Inorganic Equilibria Affecting Micronutrients in Soils. Micronutr. Agric. 2018, 4, 89–112. [Google Scholar] [CrossRef]
- Alloway, B.J. Soil factors associated with zinc deficiency in crops and humans. Environ. Geochem. Health 2009, 31, 537–548. [Google Scholar] [CrossRef]
- Sparks, D.L. Kinetics of Soil Chemical Phenomena: Future Directions. Future Prospect. Soil Chem. 2015, 55, 81–101. [Google Scholar] [CrossRef]
- Tye, A.; Young, S.; Crout, N.; Zhang, H.; Preston, S.; Barbosa-Jefferson, V.; Davison, W.; McGrath, S.; Paton, G.; Kilham, K.; et al. Predicting the activity of Cd2+ and Zn2+ in soil pore water from the radio-labile metal fraction. Geochim. Cosmochim. Acta 2003, 67, 375–385. [Google Scholar] [CrossRef]
- Wilkinson, H.F.; Loneragan, J.F.; Quirk, J.P. The Movement of Zinc to Plant Roots1. Soil Sci. Soc. Am. J. 1968, 32, 831–833. [Google Scholar] [CrossRef]
- Rengel, Z. Availability of Mn, Zn and Fe in the rhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 397–409. [Google Scholar] [CrossRef]
- Cakmak, I.; Yilmaz, A.; Ekiz, H.; Torun, B.; Erenoglu, B.; Braun, H.J. Zinc deficiency as a critical nutritional problem in wheat production in Central Anatolia. Plant Soil 1996, 180, 165–172. [Google Scholar] [CrossRef]
- Graham, R.D.; Ascher, J.S.; Hynes, S.C. Selecting zinc-efficient cereal genotypes for soils of low zinc status. Plant Soil 1992, 146, 241–250. [Google Scholar] [CrossRef]
- Erenoglu, E.B.; Kutman, U.B.; Ceylan, Y.; Yildiz, B.; Cakmak, I. Improved nitrogen nutrition enhances root uptake, root-to-shoot translocation and remobilization of zinc ( 65 Zn) in wheat. New Phytol. 2011, 189, 438–448. [Google Scholar] [CrossRef]
- Kutman, U.B.; Yildiz, B.; Ozturk, L.; Cakmak, I. Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chem. 2010, 87, 1–9. [Google Scholar] [CrossRef]
- Mousavi, S.R. Zinc in crop production and interaction with phosphorus. Aust. J. Basic Appl. Sci. 2011, 5, 1503–1509. [Google Scholar]
- Prasad, R.; Shivay, Y.S.; Kumar, D. Interactions of zinc with other nutrients in soils and plants—A Review. Indian J. Fertil. 2016, 12, 16–26. [Google Scholar]
- Gao, X.; Hoffland, E.; Stomph, T.; Grant, C.A.; Zou, C.; Zhang, F. Improving zinc bioavailability in transition from flooded to aerobic rice. A review. Agron. Sustain. Dev. 2012, 32, 465–478. [Google Scholar] [CrossRef]
- Aeron, A.; Kumar, S.; Pandey, P.; Maheshwari, D.K. Emerging role of plant growth promoting rhizobacteria in agrobiology. In Bacteria in Agrobiology: Crop Ecosystems; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–36. [Google Scholar]
- Cakmakçi, R.; Dönmez, F.; Aydın, A.; Şahin, F. Growth promotion of plants by plant growth-promoting rhizobacteria under greenhouse and two different field soil conditions. Soil Biol. Biochem. 2006, 38, 1482–1487. [Google Scholar] [CrossRef]
- Vejan, P.; Abdullah, R.; Khadiran, T.; Ismail, S.; Nasrulhaq Boyce, A. Role of plant growth promoting rhizobacteria in agricultural sustainability—A review. Molecules 2016, 21, 573. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, T.R. The role of arbuscular mycorrhizas in improving plant zinc nutrition under low soil zinc concentrations: A review. Plant Soil 2008, 304, 315–325. [Google Scholar] [CrossRef]
- Pearson, J.; Rengel, Z. Distribution and remobilization of Zn and Mn during grain development in wheat. J. Exp. Bot. 1994, 45, 1829–1835. [Google Scholar] [CrossRef]
- Ozturk, L.; Yazici, M.A.; Yucel, C.; Torun, A.; Cekic, C.; Bagci, A.; Ozkan, H.; Braun, H.J.; Sayers, Z.; Cakmak, I. Concentration and localization of zinc during seed development and germination in wheat. Physiol. Plant. 2006, 128, 144–152. [Google Scholar] [CrossRef]
- Malesh, A.A.; Mengistu, D.K.; Aberra, D.A. 2016. Linking agriculture with health through genetic and agronomic biofortification. Agric. Sci. 2016, 7, 295–307. [Google Scholar]
- Haslett, B.S.; Reid, R.J.; Rengel, Z. Zinc Mobility in Wheat: Uptake and Distribution of Zinc Applied to Leaves or Roots. Ann. Bot. 2001, 87, 379–386. [Google Scholar] [CrossRef]
- Karim, R.; Zhang, Y.-Q.; Zhao, R.-R.; Chen, X.-P.; Zhang, F.-S.; Zou, C.-Q. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Cakmak, I. Tansley Review No. 111: Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Ekiz, H.; Gültekin, I.; Torun, B.; Barut, H.; Karanlik, S.; Cakmak, I. Effect of seed zinc content on grain yield and zinc concentration of wheat grown in zinc-deficient calcareous soils. J. Plant Nutr. 1998, 21, 2257–2264. [Google Scholar] [CrossRef]
- Harris, D.; Rashid, A.; Miraj, G.; Arif, M.; Yunas, M. ‘On-farm’ seed priming with zinc in chickpea and wheat in Pakistan. Plant Soil 2008, 306, 3–10. [Google Scholar] [CrossRef]
- Cakmak, I. Zinc Fertilizer Strategy for Improving Yield. Fluid J. 2012, 20, 4–7. [Google Scholar]
- Gao, X.; Zou, C.; Fan, X.; Zhang, F.; Hoffland, E. From Flooded to Aerobic Conditions in Rice Cultivation: Consequences for Zinc Uptake. Plant Soil 2006, 280, 41–47. [Google Scholar] [CrossRef]
- Kutman, U.B.; Yildiz, B.; Cakmak, I. Effect of nitrogen on uptake, remobilization and partitioning of zinc and iron throughout the development of durum wheat. Plant Soil 2011, 342, 149–164. [Google Scholar] [CrossRef]
- Manzeke, M.G.; Mtambanengwe, F.; Watts, M.J.; Broadley, M.R.; Murray, L.R.; Mapfumo, P. Nitrogen effect on zinc bio-fortification of maize and cowpea in Zimbabwean smallholder farms. Agron. J. 2020, 112, 2256–2274. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, S.; Hu, A. Zinc nutrition and metabolism of plants as influenced by supply of phosphorus and zinc. Pedosphere 1999, 9, 265–274. [Google Scholar]
- Joshi, A.K.; Crossa, J.; Arun, B.; Chand, R.; Trethowan, R.; Vargas, M.; Ortiz-Monasterio, I. Genotype × environment interaction for zinc and iron concentration of wheat grain in eastern Gangetic plains of India. Field Crops Res. 2010, 116, 268–277. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Galavi, M.; Rezaei, M. The interaction of zinc with other elements in plants: A review. Int. J. Agric. Crop Sci. 2012, 4, 1881–1884. [Google Scholar]
- Ghasemi-Fasaei, R.; Ronaghi, A. Interaction of Iron with Copper, Zinc, and Manganese in Wheat as Affected by Iron and Manganese in a Calcareous Soil. J. Plant Nutr. 2008, 31, 839–848. [Google Scholar] [CrossRef]
- Rajaie, M.; Ejraie, A.K.; Owliaie, H.R.; Tavakoli, A.R. Effect of zinc and boron interaction on growth and mineral composition of lemon seedlings in a calcareous soil. Int. J. Plant Prod. 2012, 3, 39–50. [Google Scholar]
- Malewar, G.U.; Kate, S.D.; Waikar, S.L.; Ismail, S. Interaction effects of zinc and boron on yield, nutrient uptake and quality of mustard (Brassica juncea L.) on a typic haplustert. J. Indian Soc. Soil Sci. 2001, 49, 763–765. [Google Scholar]
- Kurdi, F.; Doner, H.E. Zinc and copper sorption and interaction in soils. Soil Sci. Soc. Am. J. 1983, 47, 873–876. [Google Scholar] [CrossRef]
- Buerkert, A.; Haake, C.; Ruckwied, M.; Marschner, H. Phosphorus application affects the nutritional quality of millet grain in the Sahel. Field Crops Res. 1998, 57, 223–235. [Google Scholar] [CrossRef]
- Egli, I.; Davidsson, L.; Zeder, C.; Walczyk, T.; Hurrell, R. Dephytinization of a Complementary Food Based on Wheat and Soy Increases Zinc, but Not Copper, Apparent Absorption in Adults. J. Nutr. 2004, 134, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Hotz, C.; Gibson, R.S. Traditional food-processing and preparation practices to enhance the bioavailability of micronutrients in plant-based diets. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Zinc: The missing link in combating micronutrient malnutrition in developing countries. Proc. Nutr. Soc. 2006, 65, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.M. Importance of seed mineral nutrient reserves in crop growth and development. In Mineral Nutrition of Crops: Fundamental Mechanisms and Implications; Food Products Press: New York, NY, USA, 1999; pp. 205–226. [Google Scholar]
- Braun, H.J. Prospects of turkey’s wheat industry, Breeding and Biotechnology. In Hububat Sempozyum; Ekiz, H., Ed.; International Winter Cereal Research Center: Konya, Turkey, 1999; pp. 1–744. [Google Scholar]
- White, P.J.; Broadley, M. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef] [PubMed]
- Davis, D.R. Declining Fruit and Vegetable Nutrient Composition: What Is the Evidence? HortScience 2009, 44, 15–19. [Google Scholar] [CrossRef]
- Garvin, D.F.; Welch, R.M.; Finley, J.W. Historical shifts in the seed mineral micronutrient concentration of US hard red winter wheat germplasm. J. Sci. Food Agric. 2006, 86, 2213–2220. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M. Physiological Limits to Zinc Biofortification of Edible Crops. Front. Plant Sci. 2011, 2, 80. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.D.; Welch, R.M.; Bouis, H. Addressing micronutrient malnutrition through enhancing the nutritional quality of staple foods: Principles, perspectives and knowledge gaps. Adv. Agron. 2001, 70, 77–142. [Google Scholar]
- Urbano, G.; Lopez-Jurado, M.; Aranda, P.; Vidal-Valverde, C.; Tenorio, E.; Porres, J. The role of phytic acid in legumes: An-tinutrient or beneficial function? J. Physiol. Biochem. 2000, 56, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Gemede, H.F.; Ratta, N. Antinutritional factors in plant foods: Potential health benefits and adverse effects. Int. J. Nutr. Food Sci. 2014, 3, 284–289. [Google Scholar] [CrossRef]
- Nissar, J.; Ahad, T.; Naik, H.R.; Hussain, S.Z. A review phytic acid: As antinutrient or nutraceutical. J. Pharmacogn. Phytochem. 2017, 6, 1554–1560. [Google Scholar]
- Guttieri, M.J.; Peterson, K.M.; Souza, E.J. Agronomic Performance of Low Phytic Acid Wheat. Crop Sci. 2006, 46, 2623–2629. [Google Scholar] [CrossRef]
- Oltmans, S.E.; Fehr, W.R.; Welke, G.A.; Raboy, V.; Peterson, K.L. Agronomic and Seed Traits of Soybean Lines with Low-Phytate Phosphorus. Crop Sci. 2005, 45, 593–598. [Google Scholar] [CrossRef]
- Schachtman, D.; Barker, S.J. Molecular approaches for increasing the micronutrient density in edible portions of food crops. Field Crops Res. 1999, 60, 81–92. [Google Scholar] [CrossRef]
- Eide, D.J. Zinc transporters and the cellular trafficking of zinc. Biochim. Biophys. Acta Bioenerg. 2006, 1763, 711–722. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.A.; Choimes, S.; Schachtman, D. Over-expression of an Arabidopsis zinc transporter in Hordeum vulgare increases short term zinc uptake after zinc deprivation and seed zinc content. Plant Mol. Biol. 2004, 54, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Zinc in Soils and Crop Nutrition; IZA Publications; International Zinc Association: Brussels, Belgium, 2004; pp. 1–116. [Google Scholar]
- White, J.G.; Zasoski, R.J. Mapping soil micronutrients. Field Crops Res. 1999, 60, 11–26. [Google Scholar] [CrossRef]
- Darnton-Hill, I.; Webb, P.; Harvey, P.W.; Hunt, J.M.; Dalmiya, N.; Chopra, M.; Ball, M.J.; Bloem, M.W.; De Benoist, B. Micronutrient deficiencies and gender: Social and economic costs. Am. J. Clin. Nutr. 2005, 81, 1198S–1205S. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Zou, C.-Q.; Mirza, Z.; Li, H.; Zhang, Z.-Z.; Li, D.-P.; Xu, C.-L.; Zhou, X.-B.; Shi, X.-J.; Xie, D.-T.; et al. Cost of agronomic biofortification of wheat with zinc in China. Agron. Sustain. Dev. 2016, 36, 44. [Google Scholar] [CrossRef]
| Organ Systems | Role of Zn | References |
|---|---|---|
| Cardiovascular | A cardioprotective role, reduces risk of heart failure, and an important role in cardiovascular health | [35,36] |
| Integumentary system | Skin health, wound healing, protection against UV radiation, and acts as antioxidant | [37,38,39,40] |
| Reproductive system | An important role in formation and maturation of spermatozoa, for ovulation and fertilization; functioning of the male and female reproductive system | [41,42,43] |
| Nervous system | The modulator of neuronal excitability, an important role in neuronal metabolism, and the modulator of synaptic activity and neuronal plasticity | [44,45] |
| Respiratory system | Reduces the incidence of acute lower respiratory infection; low Zn may increase the risk of pneumonia in elderly | [46,47] |
| Endocrine system | Thyroid hormone metabolism, structure, and activity of insulin. | [43] |
| Application Methods | Advantages | Limitations |
|---|---|---|
| Soil Application | Minimizes soil Zn deficiency The residual effect may benefit subsequent crops | High fertilizer requirement Availability to plant may decrease due to adverse soil properties |
| Foliar application | Lower fertilizer requirement Not affected by adverse soil characteristics | Crop requirement in the early seedling stage is not met Very high dose of nutrient cannot be applied using the foliar method |
| Seed priming | Lower fertilizer requirement Suitable for stressed environments | A higher amount of nutrient cannot be applied using this method as a high concentration of priming solution may negatively affect germination |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Praharaj, S.; Skalicky, M.; Maitra, S.; Bhadra, P.; Shankar, T.; Brestic, M.; Hejnak, V.; Vachova, P.; Hossain, A. Zinc Biofortification in Food Crops Could Alleviate the Zinc Malnutrition in Human Health. Molecules 2021, 26, 3509. https://doi.org/10.3390/molecules26123509
Praharaj S, Skalicky M, Maitra S, Bhadra P, Shankar T, Brestic M, Hejnak V, Vachova P, Hossain A. Zinc Biofortification in Food Crops Could Alleviate the Zinc Malnutrition in Human Health. Molecules. 2021; 26(12):3509. https://doi.org/10.3390/molecules26123509
Chicago/Turabian StylePraharaj, Subhashisa, Milan Skalicky, Sagar Maitra, Preetha Bhadra, Tanmoy Shankar, Marian Brestic, Vaclav Hejnak, Pavla Vachova, and Akbar Hossain. 2021. "Zinc Biofortification in Food Crops Could Alleviate the Zinc Malnutrition in Human Health" Molecules 26, no. 12: 3509. https://doi.org/10.3390/molecules26123509
APA StylePraharaj, S., Skalicky, M., Maitra, S., Bhadra, P., Shankar, T., Brestic, M., Hejnak, V., Vachova, P., & Hossain, A. (2021). Zinc Biofortification in Food Crops Could Alleviate the Zinc Malnutrition in Human Health. Molecules, 26(12), 3509. https://doi.org/10.3390/molecules26123509










