Fabrication and Characterization of Gelatin/Zein Nanofiber Films Loading Perillaldehyde for the Preservation of Chilled Chicken
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Electrospinning Solutions
2.3. Properties of Electrospinning Solution
2.4. Preparation of G/Z and G/Z/P Nanofiber Films
2.5. Evaluation of Composite Nanofiber Films Characterization
2.5.1. Morphology of Nanofiber Films
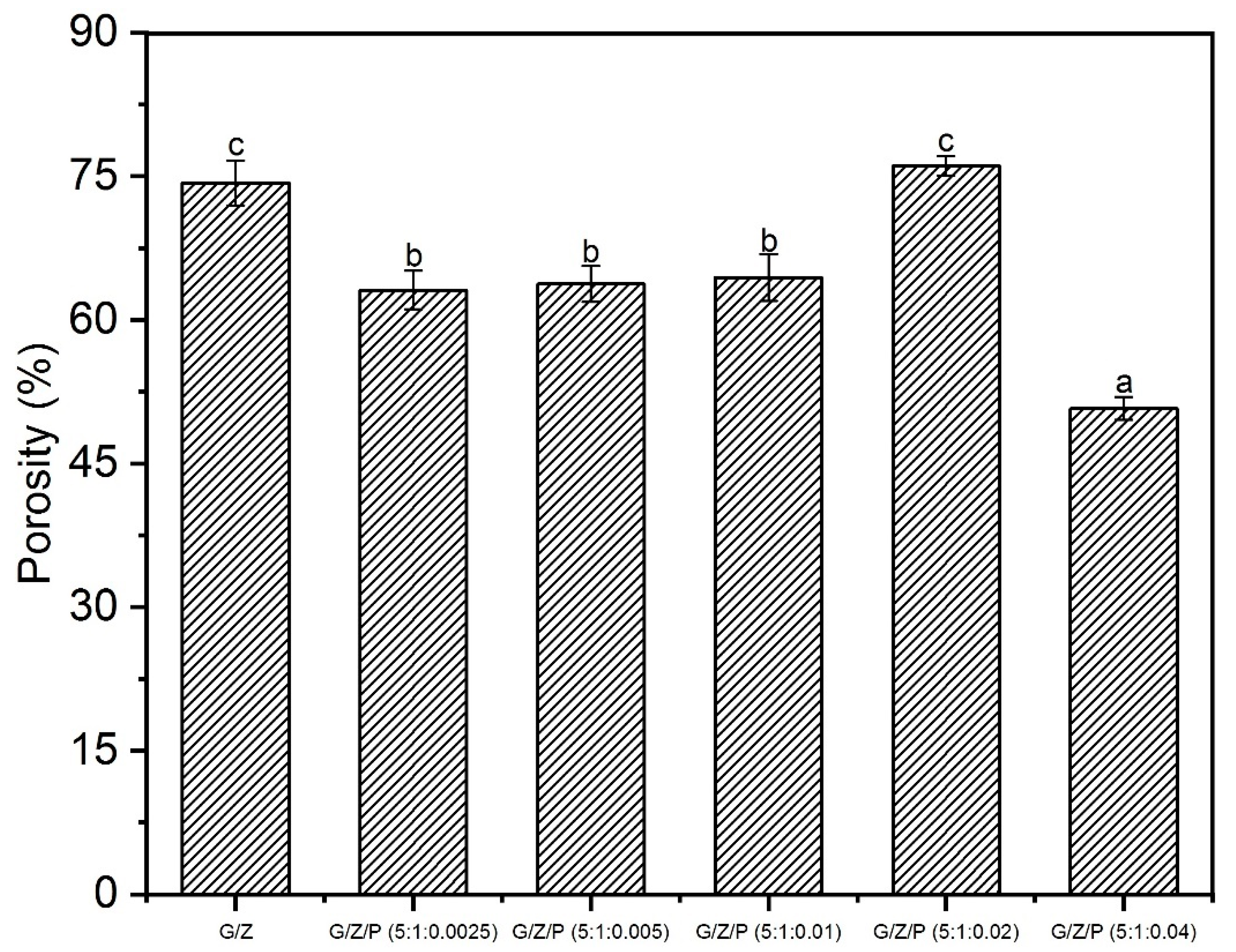
2.5.2. Porosity
2.5.3. FTIR Analysis
2.5.4. X-ray Diffraction (XRD)
2.5.5. Thermal Analysis
2.5.6. Water Contact Angle
2.6. Antibacterial Activities of the Nanofiber Films
2.6.1. Inactivation of Bacterial Cells
2.6.2. Antibacterial Activity of Nanofiber Film on Chicken Breasts
2.7. Statistical Analysis
3. Results
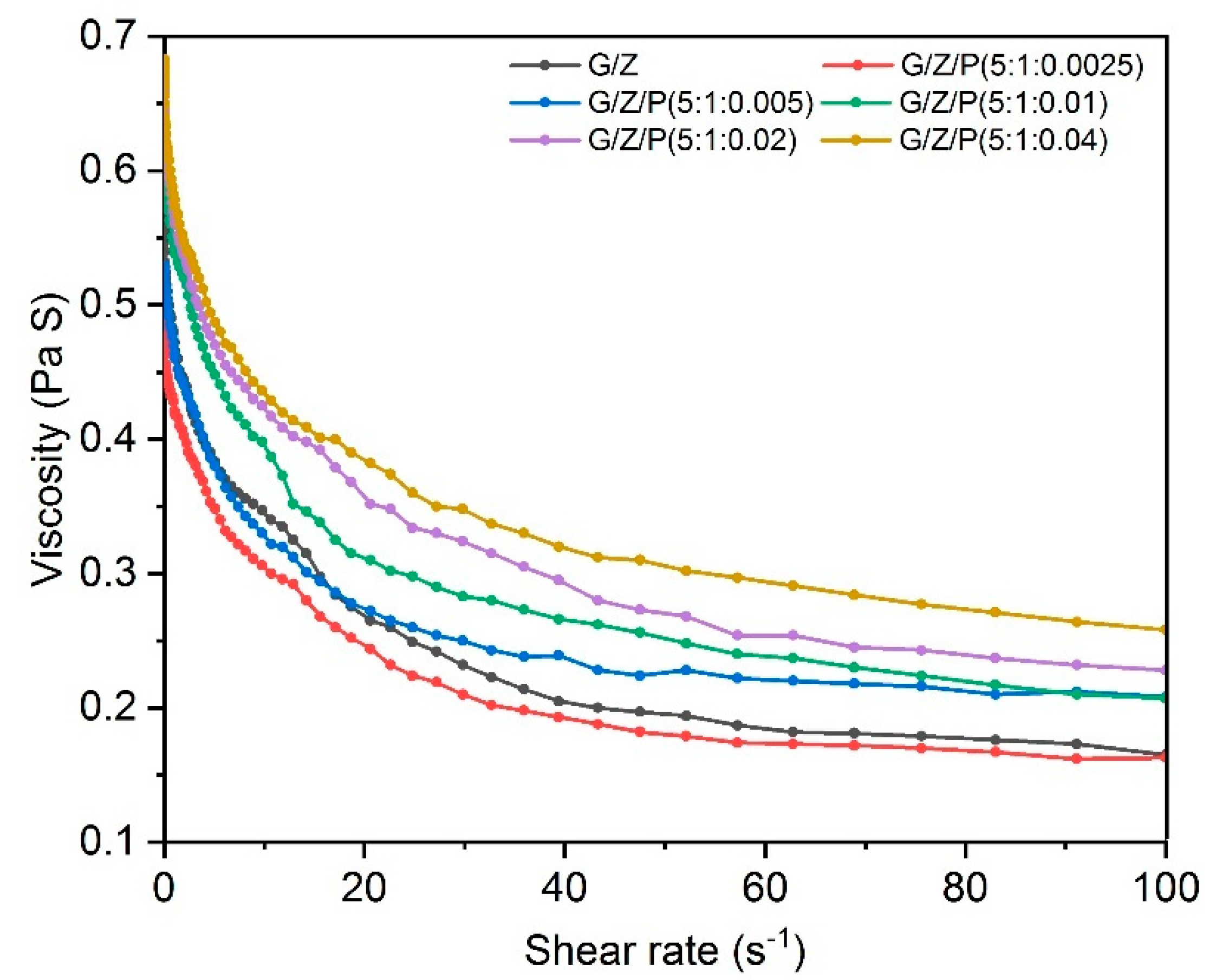
3.1. Characterization of Electrospinning Solutions
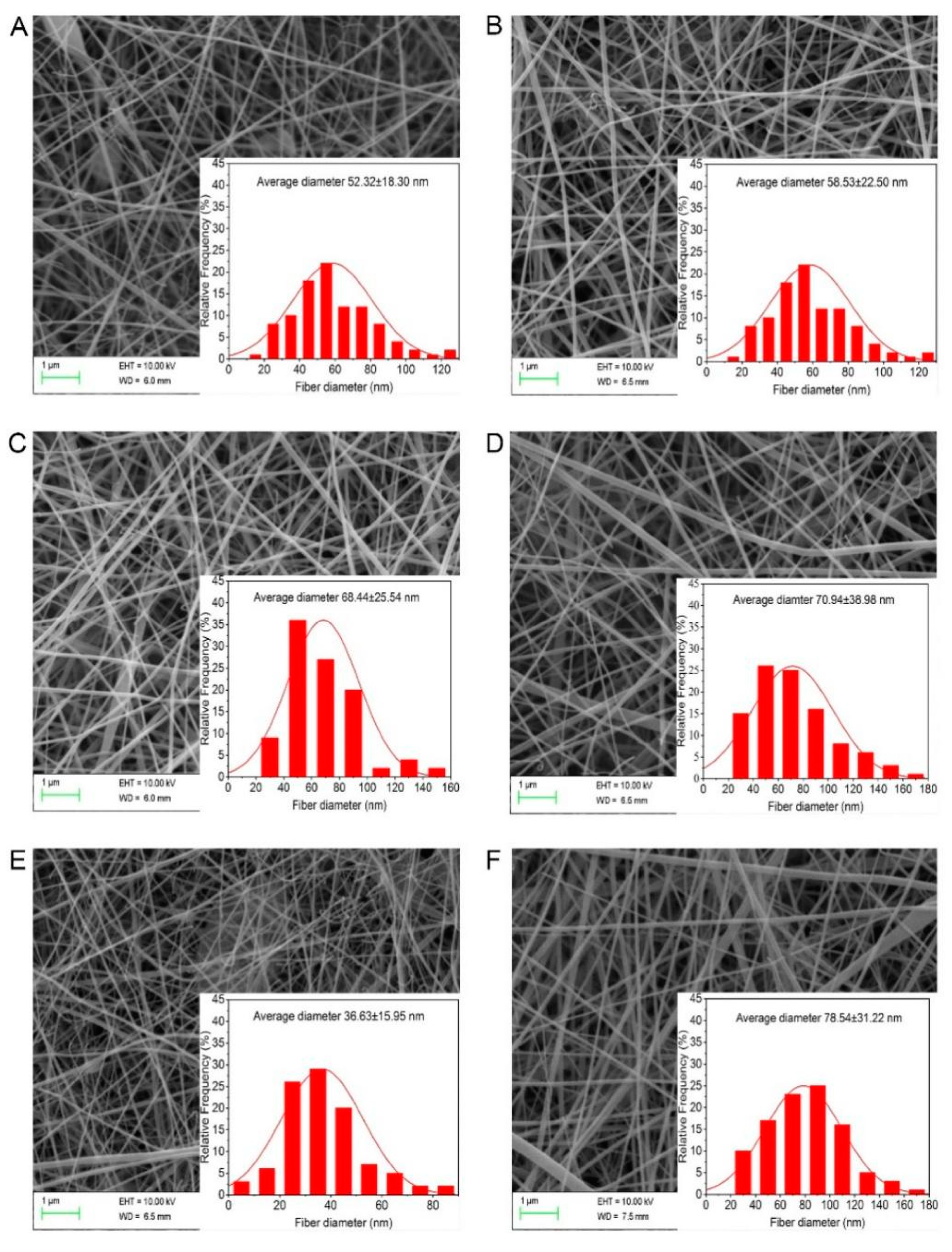
3.2. Morphology and Diameter of the Nanofiber Membrane
3.3. FTIR Analysis
3.4. XRD Analysis
3.5. Thermal Stability
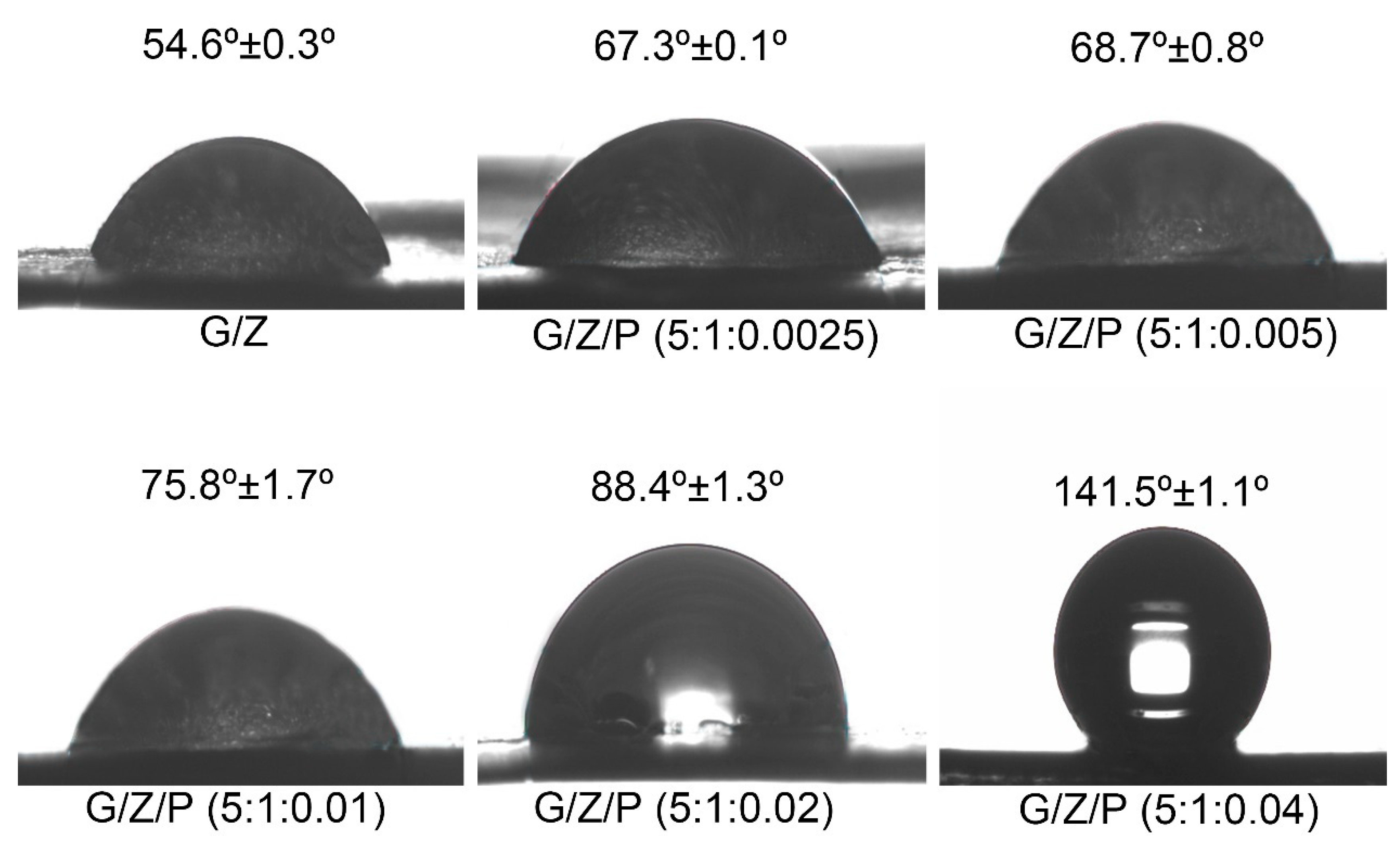
3.6. Water Contact Angle Analysis
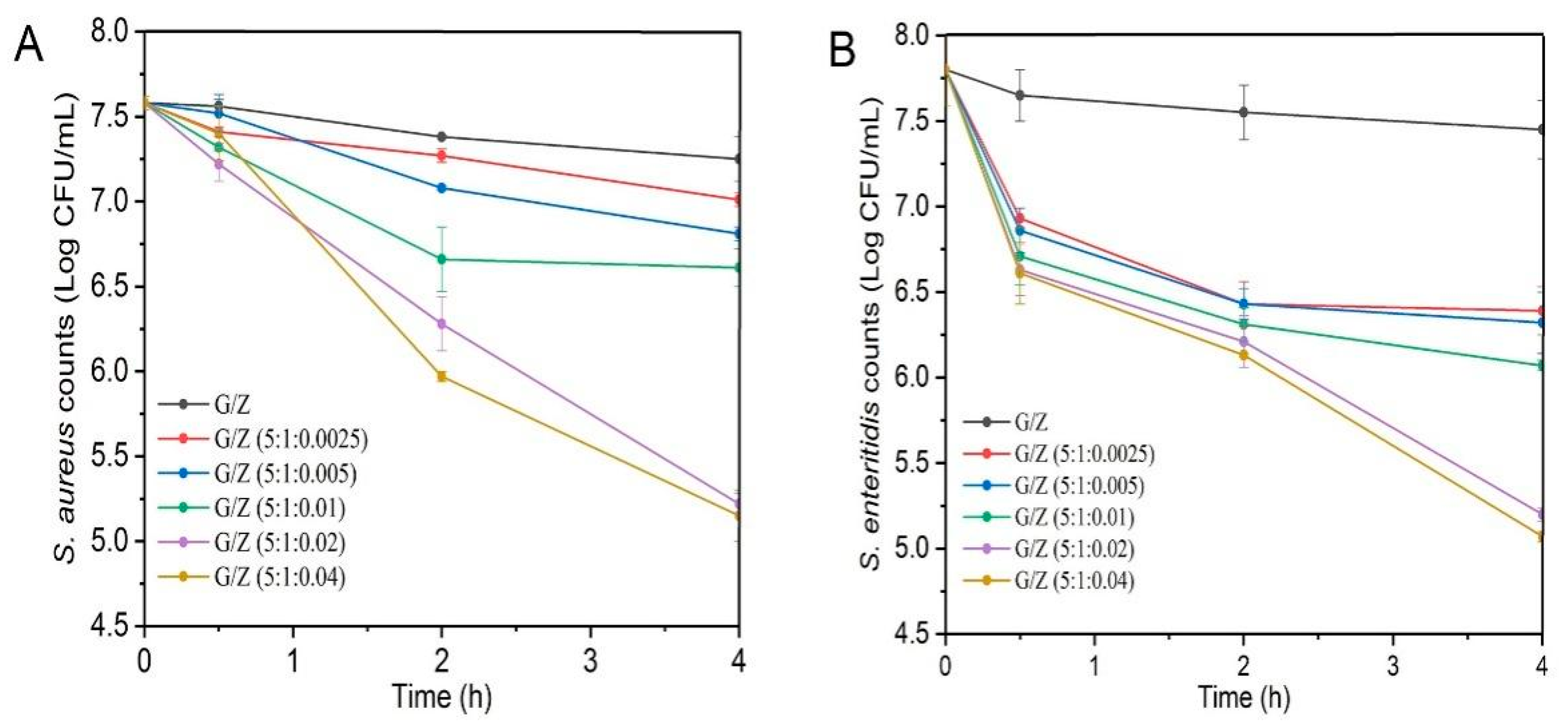
3.7. Antibacterial Activity
3.8. Antibacterial Activity of Nanofiber Film on Chicken Breasts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qu, P.; Zhang, M.; Fan, K.; Guo, Z. Microporous modified atmosphere packaging to extend shelf life of fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2020, 16, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Bhandari, B.; Adhikari, B. Application of electronic tongue for fresh foods quality evaluation: A review. Food Rev. Int. 2018, 34, 746–769. [Google Scholar] [CrossRef]
- More, A.S.; Ranadheera, C.S.; Fang, Z.; Warner, R.; Ajlouni, S. Biomarkers associated with quality and safety of fresh-cut produce. Food Biosci. 2020, 34, 100524. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, H.; Mujumdar, A.S.; Tang, J.; Miao, S.; Wang, Y. Recent developments in high-quality drying of vegetables, fruits, and aquatic products. Crit. Rev. Food Sci. Nutr. 2017, 57, 1239–1255. [Google Scholar] [CrossRef]
- Meng, R.; Wu, Z.Z.; Xie, Q.T.; Cheng, J.S.; Zhang, B. Preparation and characterization of zein/carboxymethyl dextrin nanoparticles to encapsulate curcumin: Physicochemical stability, antioxidant activity and controlled release properties. Food Chem. 2020, 340, 127893. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Otoni, C.G.; Correa, D.S.; Assis, O.B.G.; de Moura, M.R.; Mattoso, L.H.C. Nanostructured antimicrobials in food packaging—Recent advances. Biotechnol. J. 2019, 14, 1900068. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R. Antimicrobial food packaging based on sustainable bio-based materials for reducing foodborne pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Wu, M.; Ma, J.; Lu, P. Bio-based antimicrobial packaging from sugarcane bagasse nanocellulose/nisin hybrid films. Int. J. Biol. Macromol. 2020, 161, 627–635. [Google Scholar] [CrossRef]
- Karim, M.; Fathi, M.; Soleimanian-Zad, S. Nanoencapsulation of cinnamic aldehyde using zein nanofibers by novel needle-less electrospinning: Production, characterization and their application to reduce nitrite in sausages. J. Food Eng. 2020, 288, 110140. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Shi, X.; Zhan, Y.; Tu, H.; Du, Y.; Deng, H.; Jiang, L. Production of thick uniform-coating films containing rectorite on nanofibers through the use of an automated coating machine. Colloids Surf. B Biointerfaces 2017, 149, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Fabra, M.J.; López-Rubio, A.; Lagaron, J.M. Use of the electrohydrodynamic process to develop active/bioactive bilayer films for food packaging applications. Food Hydrocoll. 2016, 55, 11–18. [Google Scholar] [CrossRef]
- Chong, E.J.; Phan, T.T.; Lim, I.J.; Zhang, Y.Z.; Bay, B.H.; Ramakrishna, S.; Lim, C.T. Evaluation of electrospun pcl/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomater. 2007, 3, 321–3302. [Google Scholar] [CrossRef]
- Mendes, A.C.; Stephansen, K.; Chronakis, I.S. Electrospinning of food proteins and polysaccharides. Food Hydrocoll. 2017, 68, 53–68. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Xin, S.L.; Xiao, L.; Dong, X.P.; Li, X.; Wang, Y.; Hu, X.X.; Sameen, D.; Qin, W.; Zhu, B. Preparation of chitosan/curcumin nanoparticles based zein and potato starch composite films for schizothorax prenati fillet preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef]
- Fathi, M.; Donsi, F.; McClements, D.J. Protein-Based Delivery Systems for the Nanoencapsulation of Food Ingredients. Compr. Rev. Food Sci. Food Saf. 2018, 17, 920–936. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, L. Fabrication and characterization of novel assembled prolamin protein nanofabrics with improved stability, mechanical property and release profiles. J. Mater. Chem. 2012, 22, 21592–21601. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Sun, Z.; Wang, D.; Wu, H.; Du, L.; Wang, D. Preparation and antibacterial properties of ε-polylysine-containing gelatin/chitosan nanofiber films. Int. J. Biol. Macromol. 2020, 164, 3376–3387. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, X.; Li, Y.; Que, F.; Kang, X.F.; Liu, Y.; Feng, F.; Zhang, H. Characterization of gelatin/zein nanofibers by hybrid electrospinning. Food Hydrocoll. 2018, 75, 72–78. [Google Scholar] [CrossRef]
- Liu, T.; Liu, L. Fabrication and characterization of chitosan nanoemulsions loading thymol or thyme essential oil for the preservation of refrigerated pork. Int. J. Biol. Macromol. 2020, 162, 1509–1515. [Google Scholar] [CrossRef]
- Catto, C.; de Vincenti, L.; Borgonovo, G.; Bassoli, A.; Marai, S.; Villa, F.; Cappitelli, F.; Saracchi, M. Sub-lethal concentrations of perilla frutescens essential oils affect phytopathogenic fungal biofilms. J. Environ. Manag. 2019, 245, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, S.; Zhao, Z.; Gao, W.; Ma, Y.; Wang, X.; Yan, S.; Luo, D. Ultrasound pre-treatment combined with microwave-assisted hydrodistillation of essential oils from Perilla frutescens (L.) Britt. leaves and its chemical composition and biological activity. Ind. Crops Prod. 2019, 143, 111908. [Google Scholar] [CrossRef]
- Zhou, Q.; Hu, Z.; Du, L.; Liu, F.; Yuan, K. Inhibition of enterococcus faecalis growth and cell membrane integrity by perilla frutescens essential oil. Foodborne Pathog. Dis. 2020, 17, 547–554. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Lu, Z.; Sun, C.; Zhang, M.; Zhu, A.; Peng, X. Perillaldehyde, a promising antifungal agent used in food preservation, triggers apoptosis through a metacaspase-dependent pathway in Aspergillus flavus. J. Agric. Food Chem. 2016, 64, 7404. [Google Scholar] [CrossRef]
- Laha, A.; Yadav, S.; Majumdar, S.; Sharma, C.S. In-vitro release study of hydrophobic drug using electrospun cross-linked gelatin nanofibers. Biochem. Eng. J. 2016, 105, 481–488. [Google Scholar] [CrossRef]
- GB 5009.228-2016, China Food Safety Standard. Determination of Volatile Basic Nitrogen in Food. Available online: https://max.book118.com/html/2017/0719/122941754.shtm. (accessed on 19 July 2017).
- Xie, J.H.; Tang, W.; Jin, M.L.; Li, J.E.; Xie, M.Y. Recent advances in bioactive polysaccharides from Lycium barbarum L., Zizyphus jujuba Mill, Plantago spp., and Morus spp.: Structures and functionalities. Food Hydrocoll. 2016, 60, 148–160. [Google Scholar] [CrossRef]
- Nie, S.P.; Xie, M.Y. A review on the isolation and structure of tea polysaccharides and their bioactivities. Food Hydrocoll. 2011, 25, 144–149. [Google Scholar] [CrossRef]
- Feng, X.; Fu, C.; Yang, H. Gelatin addition improves the nutrient retention, texture and mass transfer of fish balls without altering their nanostructure during boiling. LWT 2017, 77, 142–151. [Google Scholar] [CrossRef]
- Feng, X.; Ng, V.K.; Marta, M.K.; Yang, H. Effects of fish gelatin and tea polyphenol coating on the spoilage and degradation of myofibril in fish fillet during cold storage. Food Bioproc. Technol 2017, 10, 89–102. [Google Scholar]
- Shao, P.; Niu, B.; Chen, H.; Sun, P. Fabrication and characterization of tea polyphenols loaded pullulan-CMC electrospun nanofiber for fruit preservation. Int. J. Biol. Macromol. 2017, 107, 1908–1914. [Google Scholar] [CrossRef]
- Oraby, M.A.; Waley, A.I.; El-Dewany, A.I.; Saad, E.A.; Abd El-Hady, B.M. Electrospun gelatin nanofibers: Effect of gelatin concentration on morphology and fiber diameters. J. Appl. Sci. Res. 2013, 9, 534–540. [Google Scholar]
- Jridi, M.; Hajji, S.; Ayed, H.B.; Lassoued, I.; Mbarek, A.; Kammoun, M.; Souissi, N.; Nasri, M. Physical, structural, antioxidant and antimicrobial properties of gelatin-chitosan composite edible films. Int. J. Biol. Macromol. 2014, 67, 373–379. [Google Scholar] [CrossRef]
- Zhan, F.; Yan, X.; Sheng, F.; Li, B. Facile in situ synthesis of silver nanoparticles on tannic acid/zein electrospun membranes and their antibacterial, catalytic and antioxidant activities. Food Chem. 2020, 330, 127172. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, L.; Wu, P.; Liu, Y.; Nie, F.; Huang, F. Fabrication of RDX, HMX and CL-20 based microcapsules via in situ polymerization of melamine-formaldehyde resins with reduced sensitivity. Chem. Eng. J. 2015, 268, 60–66. [Google Scholar] [CrossRef]
- Chrayteh, M.; Huet, T.R.; Drean, P. Gas-phase hydration of perillaldehyde investigated by microwave spectroscopy assisted by computational chemistry. J. Phys Chem. A 2020, 124, 6511–6520. [Google Scholar] [CrossRef] [PubMed]
- Topuz, F.; Uyar, T. Electrospinning of gelatin with tunable fiber morphology from round to flat/ribbon. Mater. Sci. Eng. C 2017, 80, 371–378. [Google Scholar] [CrossRef]
- Ki, C.S.; Baek, D.H.; Gang, K.D.; Lee, K.H.; Um, I.C.; Park, Y.H. Characterization of gelatin nanofiber prepared from gelatineformic acid solution. Polymer 2005, 46, 5094–5102. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Sun, B.; Zhang, Y.; Zhang, D.; Tang, Z.; Chen, X.; Wang, C. Electrospun chitosan/sericin composite nanofibers with antibacterial property as potential wound dressings. Int. J. Biol. Macromol. 2014, 68, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Venugopal, J.; Huang, Z.M.; Lim, C.; Ramakrishna, S. Crosslinking of the electrospun gelatin nanofibers. Polymer 2006, 47, 2911–2917. [Google Scholar] [CrossRef]
- Mazur, K.; Singh, R.; Friedrich, R.P.; Gen, H.; Cicha, I. The effect of antibacterial particle incorporation on the mechanical properties, biodegradability, and biocompatibility of PLA and PHBV composites. Macromol. Mater. Eng. 2020, 305, 2000244. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gimenez, E.; Lagaron, J.M. Characterization of the morphology and thermal properties of zein prolamine nanostructures obtained by electrospinning. Food Hydrocoll. 2008, 22, 601–614. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.; Meniqueti, A.; Martins, A.; Rubira, A.; Muniz, E. Recent advances in foodpacking, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, M.; Tang, W.W.; Wang, Y. Formation and mechanism of superhydrophobic/hydrophobic surfaces made from amphiphiles through droplet-mediated evaporation-induced self-assembly. J. Phys. Chem. B 2015, 119, 5321–5327. [Google Scholar] [CrossRef]
- Park, M.H.; Seol, N.G.; Chang, P.S.; Yoon, S.H.; Lee, J.H. Effects of roasting conditions on the physicochemical properties and volatile distribution in perilla oils (Perilla frutescens var. japonica). J. Food Sci. 2011, 76, C808–C816. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, G.; Ye, K.; Wang, S.; Xu, X.; Li, C. Microbial changes in vacuum-packed chilled pork during storage. Meat Sci. 2015, 100, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Chotimarkorn, C. Quality changes of anchovy (stolephorus heterolobus) under refrigerated storage of different practical industrial methods in thailand. J. Food Sci. Technol. 2014, 51, 285–293. [Google Scholar] [CrossRef]
- Yu, D.; Xu, Y.; Regenstein, J.M.; Xia, W.; Yang, F.; Jiang, Q.; Wang, B. The effects of edible chitosan-based coatings on flavor quality of raw grass carp (Ctenopharyngodon idellus) fillets during refrigerated storage. Food Chem. 2018, 242, 412–420. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Li, X.; Zhong, K.; Li, Y.; Li, J. Effects of different freezing treatments on physicochemical responses and microbial characteristics of Japanese sea bass (Lateolabrax japonicas) fillets during refrigerated storage. LWT 2014, 59, 122–129. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Liu, Y.; Sun, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Fabrication and Characterization of Gelatin/Zein Nanofiber Films Loading Perillaldehyde for the Preservation of Chilled Chicken. Foods 2021, 10, 1277. https://doi.org/10.3390/foods10061277
Wang D, Liu Y, Sun J, Sun Z, Liu F, Du L, Wang D. Fabrication and Characterization of Gelatin/Zein Nanofiber Films Loading Perillaldehyde for the Preservation of Chilled Chicken. Foods. 2021; 10(6):1277. https://doi.org/10.3390/foods10061277
Chicago/Turabian StyleWang, Debao, Yini Liu, Jinyue Sun, Zhilan Sun, Fang Liu, Lihui Du, and Daoying Wang. 2021. "Fabrication and Characterization of Gelatin/Zein Nanofiber Films Loading Perillaldehyde for the Preservation of Chilled Chicken" Foods 10, no. 6: 1277. https://doi.org/10.3390/foods10061277
APA StyleWang, D., Liu, Y., Sun, J., Sun, Z., Liu, F., Du, L., & Wang, D. (2021). Fabrication and Characterization of Gelatin/Zein Nanofiber Films Loading Perillaldehyde for the Preservation of Chilled Chicken. Foods, 10(6), 1277. https://doi.org/10.3390/foods10061277





