Strong and Bitter Vegetables from Traditional Cultivars and Cropping Methods Improve the Health Status of Type 2 Diabetics: A Randomized Control Trial
Abstract
1. Introduction
2. Materials and Methods
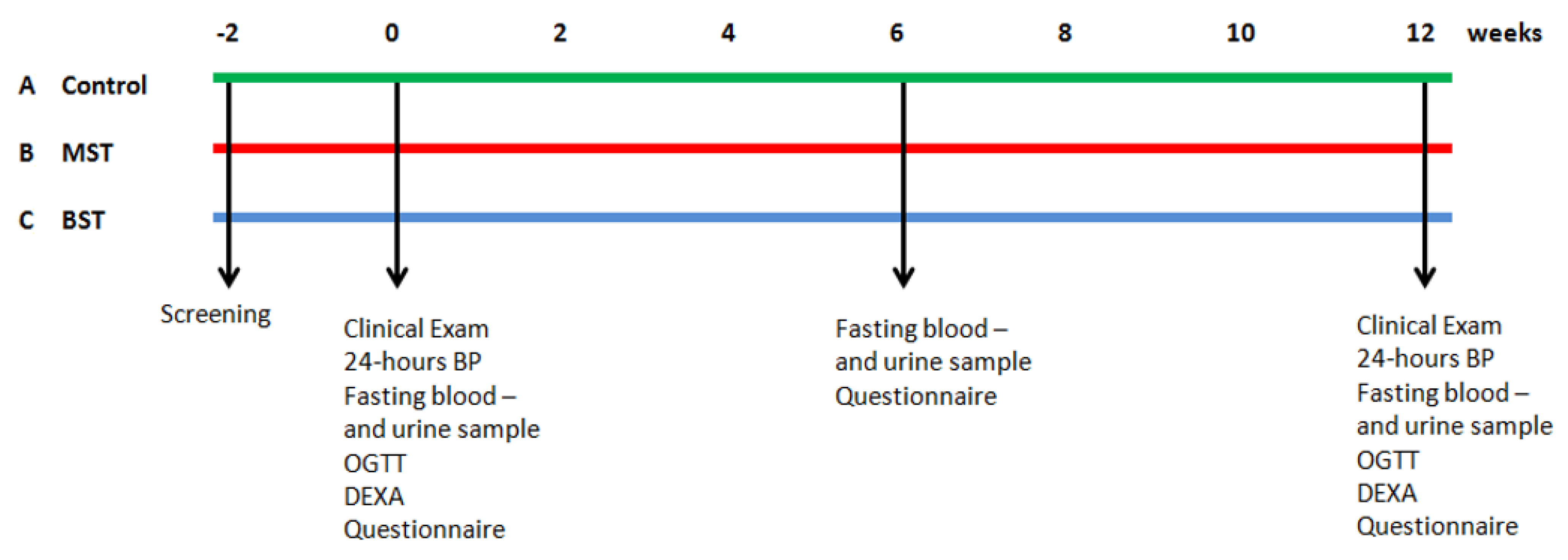
2.1. Study Design
2.2. Ethics Statement
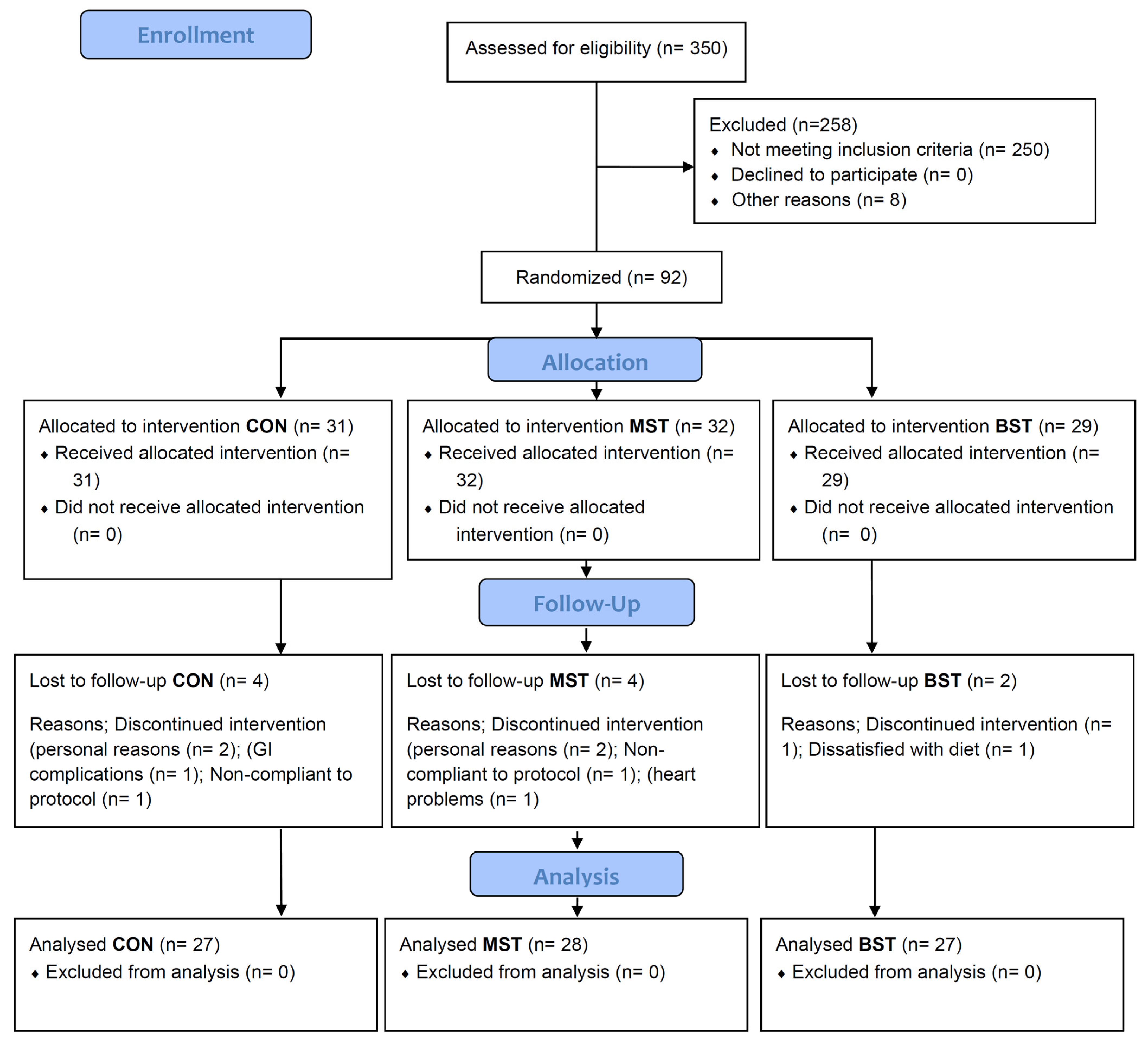
2.3. Participant Recruitment and Screening
2.4. Study Assessments
2.5. Vegetables
2.6. Baseline Characteristics
2.7. Biochemical Analysis
2.8. NMR Spectroscopy
2.9. Analysis of Plasma for β-Carotene (Compliance)
2.10. Statistical Analysis
3. Results
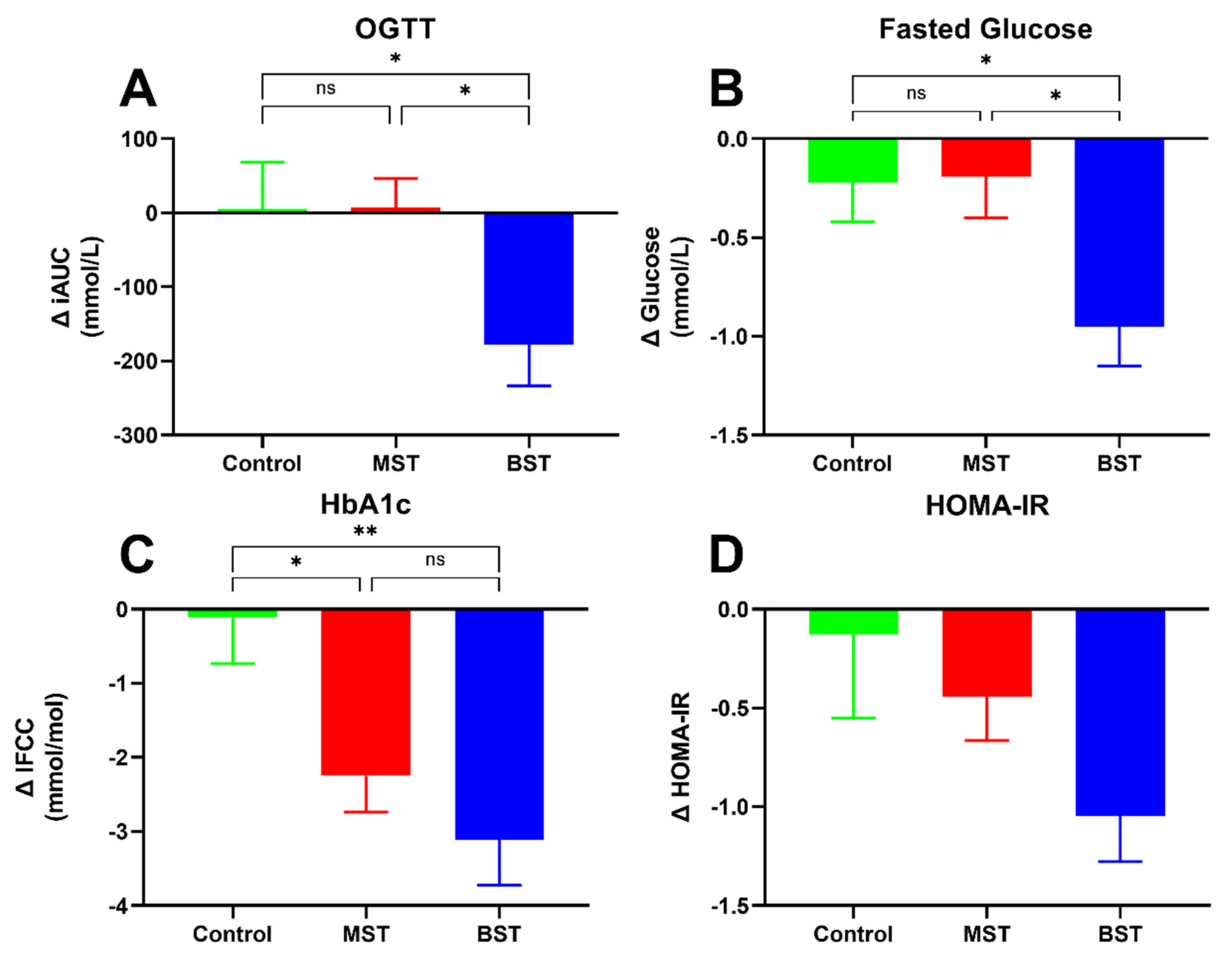
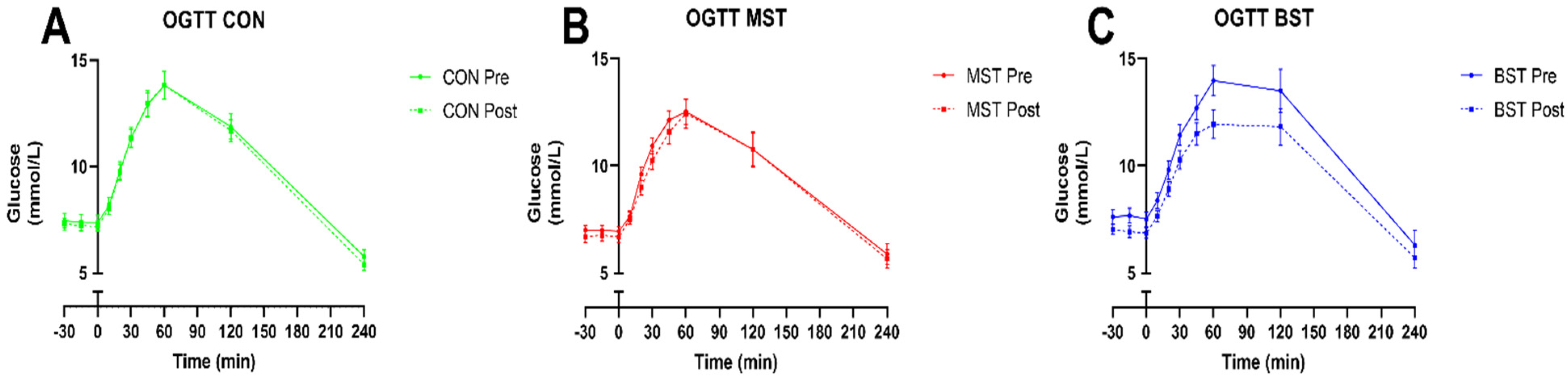
3.1. Diabetes Status
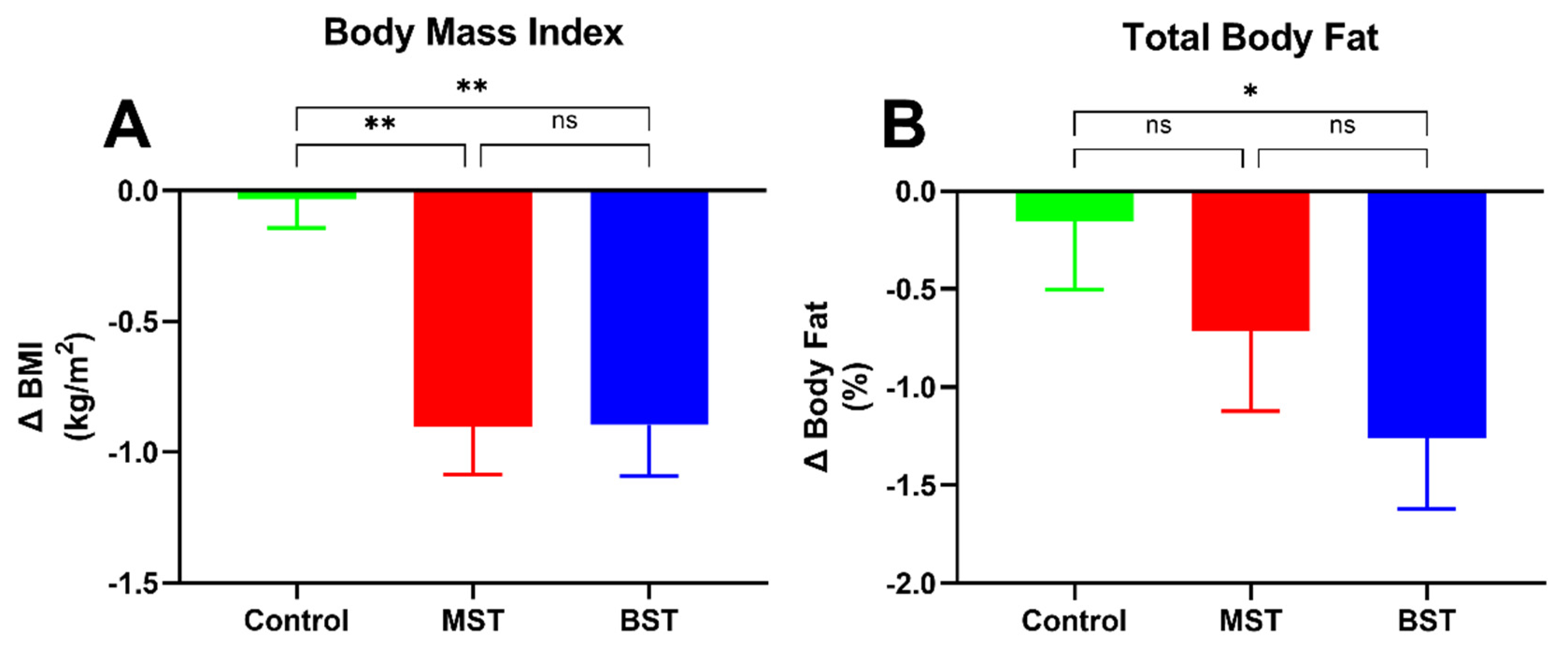
3.2. Body Composition
3.3. Blood Pressure
3.4. Lipids
3.5. PTH and Vitamin D
3.6. NMR Spectroscopy
3.7. Compliance Study
3.8. Sensory Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IDF Diabetes Atlas 2015, 7th ed.; International Diabetes Federation: Brussels, Belgium, 2015.
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 37, S81–S90. [Google Scholar] [CrossRef]
- Cooper, A.J.; Sharp, S.J.; Lentjes, M.; Luben, R.N.; Khaw, K.-T.; Wareham, N.J.; Forouhi, N.G. A Prospective Study of the Association Between Quantity and Variety of Fruit and Vegetable Intake and Incident Type 2 Diabetes. Diabetes Care 2012, 35, 1293–1300. [Google Scholar] [CrossRef]
- Cooper, A.J.; Forouhi, N.G.; Ye, Z.; Buijsse, B.; Arriola, L.; Balkau, B.; Barricarte, A.; Beulens, J.W.; Boeing, H.; Büchner, F.L.; et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur. J. Clin. Nutr. 2012, 66, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef]
- Carter, P.; Gray, L.J.; Troughton, J.; Khunti, K.; Davies, M.J. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: Systematic review and meta-analysis. BMJ 2010, 341, c4229. [Google Scholar] [CrossRef]
- Roswall, N.; Olsen, A.; Boll, K.; Christensen, J.; Halkjær, J.; Sørensen, T.I.; Dahm, C.C.; Overvad, K.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; et al. Consumption of predefined ‘Nordic’ dietary items in ten European countries—An investigation in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Public Health Nutr. 2014, 17, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Mithril, C.; Dragsted, L.O.; Meyer, C.; Tetens, I.; Biltoft-Jensen, A.; Astrup, A. Dietary composition and nutrient content of the New Nordic Diet. Public Health Nutr. 2013, 16, 777–785. [Google Scholar] [CrossRef]
- Lippmann, D.; Lehmann, C.; Florian, S.; Barknowitz, G.; Haack, M.; Mewis, I.; Wiesner, M.; Schreiner, M.; Glatt, H.; Brigelius-Flohé, R.; et al. Glucosinolates from pak choi and broccoli induce enzymes and inhibit inflammation and colon cancer differently. Food Funct. 2014, 5, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.E.; Terschluesen, A.M.; Rimbach, G. Health Promoting Effects of Brassica-Derived Phytochemicals: From Chemopreventive and Anti-Inflammatory Activities to Epigenetic Regulation. Oxidative Med. Cell. Longev. 2013, 2013, 964539. [Google Scholar] [CrossRef]
- Wu, Q.J.; Yang, Y.; Vogtmann, E.; Wang, J.; Han, L.H.; Li, H.L.; Xiang, Y.B. Cruciferous vegetables intake and the risk of colorectal cancer: A meta-analysis of observational studies. Ann. Oncol. 2013, 24, 1079–1087. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, X.-O.; Xiang, Y.-B.; Yang, G.; Li, H.; Gao, J.; Cai, H.; Gao, Y.-T.; Zheng, W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am. J. Clin. Nutr. 2011, 94, 240–246. [Google Scholar] [CrossRef]
- Cheng, D.M.; Pogrebnyak, N.; Kuhn, P.; Krueger, C.G.; Johnson, W.; Raskin, I. Development and Phytochemical Characterization of High Polyphenol Red Lettuce with Anti-Diabetic Properties. PLoS ONE 2014, 9, e91571. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H. Potential Synergy of Phytochemicals in Cancer Prevention: Mechanism of Action. J. Nutr. 2004, 134, 3479S–3485S. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef]
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef]
- Kreutzmann, S.; Christensen, L.P.; Edelenbos, M. Investigation of bitterness in carrots (Daucus carota L.) based on quantitative chemical and sensory analyses. LWT Food Sci. Technol. 2008, 41, 193–205. [Google Scholar] [CrossRef]
- Cox, D.N.; Melo, L.; Zabaras, D.; Delahunty, C.M. Acceptance of health-promoting Brassica vegetables: The influence of taste perception, information and attitudes. Public Health Nutr. 2012, 15, 1474–1482. [Google Scholar] [CrossRef]
- Groenbaek, M.; Jensen, S.; Neugart, S.; Schreiner, M.; Kidmose, U.; Kristensen, H.L. Influence of Cultivar and Fertilizer Approach on Curly Kale (Brassica oleracea L. var. sabellica). 1. Genetic Diversity Reflected in Agronomic Characteristics and Phytochemical Concentration. J. Agric. Food Chem. 2014, 62, 11393–11402. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. General Guidance for the Selection, Training and Monitoring of Assessors; ISO 8586-1; ISO: Geneva, Switzerland, 1993. [Google Scholar]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes—2012. Diabetes Care 2012, 35, S11–S63. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes—2015: Summary of Revisions. Diabetes Care 2014, 38, S4. [Google Scholar] [CrossRef]
- Howarth, N.C.; Saltzman, E.; Roberts, S.B. Dietary Fiber and Weight Regulation. Nutr. Rev. 2001, 59, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Ray, T.K.; Mansell, K.M.; Knight, L.C.; Malmud, L.S.; Owen, O.E.; Boden, G. Long-term effects of dietary fiber on glucose tolerance and gastric emptying in noninsulin-dependent diabetic patients. Am. J. Clin. Nutr. 1983, 37, 376–381. [Google Scholar] [CrossRef]
- Diabetes Prevention Program Research Group. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 2009, 374, 1677–1686. [Google Scholar] [CrossRef]
- Gregg, E.W.; Chen, H.; Wagenknecht, L.E.; Clark, J.M.; Delahanty, L.M.; Bantle, J.; Pownall, H.; Johnson, K.C.; Safford, M.M.; Kitabchi, A.E.; et al. Association of an Intensive Lifestyle Intervention with Remission of Type 2 Diabetes. JAMA 2012, 308, 2489–2496. [Google Scholar] [CrossRef]
- Alberti, K.G.M.; Zimmet, P.; Shaw, J. The metabolic syndrome—A new worldwide definition. Lancet 2005, 366, 1059–1062. [Google Scholar] [CrossRef]
- Laskey, M. Dual-energy X-ray absorptiometry and body composition. Nutrients 1996, 12, 45–51. [Google Scholar] [CrossRef]
- Svendsen, O.L.; Haarbo, J.; Hassager, C.; Christiansen, C. Accuracy of measurements of body composition by dual-energy x-ray absorptiometry in vivo. Am. J. Clin. Nutr. 1993, 57, 605–608. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care in Diabetes-2019 Abridged for Primary Care Providers. Clin. Diabetes. 2019, 37, 11–34. [Google Scholar] [CrossRef] [PubMed]
- Kishore, P.; Kim, S.H.; Crandall, J.P. Glycemic Control and Cardiovascular Disease: What’s a Doctor to Do? Curr. Diabetes Rep. 2012, 12, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Rydén, L.; Grant, P.J.; Anker, S.D.; Berne, C.; Cosentino, F.; Danchin, N.; Deaton, C.; Escaned, J.; Hammes, H.P.; Huikuri, H.; et al. Pre-Diabetes Task Force on The Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD—Summary. Diabetes Vasc. Dis. Res. 2014, 11, 133–173. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Ž.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Hearth J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef]
- Miselli, M.-A.; Nora, E.D.; Passaro, A.; Tomasi, F.; Zuliani, G. Plasma triglycerides predict ten-years all-cause mortality in outpatients with type 2 diabetes mellitus: A longitudinal observational study. Cardiovasc. Diabetol. 2014, 13, 135. [Google Scholar] [CrossRef]
- Tenenbaum, A.; Klempfner, R.; Fisman, E.Z. Hypertriglyceridemia: A too long unfairly neglected major cardiovascular risk factor. Cardiovasc. Diabetol. 2014, 13, 1–10. [Google Scholar] [CrossRef]
- Parati, G.; Bilo, G.; Mancia, G. Blood pressure measurement in research and in clinical practice: Recent evidence. Curr. Opin. Nephrol. Hypertens. 2004, 13, 343–357. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A Clinical Trial of the Effects of Dietary Patterns on Blood Pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Hikmat, F.; Appel, L.J. Effects of the DASH diet on blood pressure in patients with and without metabolic syndrome: Results from the DASH trial. J. Hum. Hypertens. 2014, 28, 170–175. [Google Scholar] [CrossRef]
- Poulsen, S.K.; Due, A.; Jordy, A.B.; Kiens, B.; Stark, K.; Stender, S.; Holst, C.; Astrup, A.; Larsen, T.M. Health effect of the New Nordic Diet in adults with increased waist circumference: A 6-mo randomized controlled trial. Am. J. Clin. Nutr. 2013, 99, 35–45. [Google Scholar] [CrossRef]
- Collins, R.; Peto, R.; MacMahon, S.; Godwin, J.; Qizilbash, N.; Hebert, P.; Eberlein, K.; Taylor, J.; Hennekens, C.; Fiebach, N. Blood pressure, stroke, and coronary heart disease. Lancet 1990, 335, 827–838. [Google Scholar] [CrossRef]
- Cook, N.R.; Cohen, J.; Hebert, P.R.; Taylor, J.O.; Hennekens, C.H. Implications of Small Reductions in Diastolic Blood Pressure for Primary Prevention. Arch. Intern. Med. 1995, 155, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Lidder, S.; Webb, A.J. Vascular Effects of Dietary Nitrate (as Found in Green Leafy Vegetables and Beetroot) via the Nitrate-Nitrite-Nitric Oxide Pathway. Br. J. Clin. Pharmacol. 2013, 75, 677–696. [Google Scholar] [CrossRef]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute Blood Pressure Lowering, Vasoprotective, and Antiplatelet Properties of Dietary Nitrate via Bioconversion to Nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. NO Generation from Nitrite and Its Role in Vascular Control. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, D.A.; Kaffa, N.; George, T.W.; Methven, L.; Lovegrove, J.A. Blood pressure-lowering effects of beetroot juice and novel beetroot-enriched bread products in normotensive male subjects. Br. J. Nutr. 2012, 108, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Crawford, S.O.; Hoogeveen, R.C.; Brancati, F.L.; Astor, B.C.; Ballantyne, C.M.; Schmidt, M.I.; Young, J.H. Association of blood lactate with type 2 diabetes: The Atherosclerosis Risk in Communities Carotid MRI Study. Int. J. Epidemiol. 2010, 39, 1647–1655. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A Branched-Chain Amino Acid-Related Metabolic Signature that Differentiates Obese and Lean Humans and Contributes to Insulin Resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Baldrick, F.R.; Woodside, J.V.; Elborn, J.S.; Young, I.S.; McKinley, M.C. Biomarkers of Fruit and Vegetable Intake in Human Intervention Studies: A Systematic Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 795–815. [Google Scholar] [CrossRef]
- Brown, E.D.; Micozzi, M.S.; Craft, N.E.; Bieri, J.G.; Beecher, G.; Edwards, B.K.; Rose, A.; Taylor, P.R.; Smith, J.J.C. Plasma carotenoids in normal men after a single ingestion of vegetables or purified beta-carotene. Am. J. Clin. Nutr. 1989, 49, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Promoting Fruit and Vegetable Consumption around the World. World Health Organization. Available online: http://www.who.int/dietphysicalactivity/fruit/en/ (accessed on 18 September 2018).
- Ministry of Food, Agriculture and Fisheries. Version Current 15 January 2015. Available online: http://altomkost.dk/raad-og-anbefalinger/de-officielle-kostraad/spis-frugt-og-mange-groensager/01/15/2015 (accessed on 15 May 2018).
- Davis, D.R.; Epp, M.D.; Riordan, H.D. Changes in USDA Food Composition Data for 43 Garden Crops, 1950 to 1999. J. Am. Coll. Nutr. 2004, 23, 669–682. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Li, T.Y.; Joshipura, K.J.; Hu, F.B. Intake of Fruit, Vegetables, and Fruit Juices and Risk of Diabetes in Women. Diabetes Care 2008, 31, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Kamada, C.; Yoshimura, H.; Okumura, R.; Iimuro, S.; Ohashi, Y.; Araki, A.; Umegaki, H.; Sakurai, T.; Yoshimura, Y.; et al. Effects of total and green vegetable intakes on glycated hemoglobin A1c and triglycerides in elderly patients with type 2 diabetes mellitus: The Japanese Elderly Intervention Trial. Geriatr. Gerontol. Int. 2012, 12, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Welch, A.A.; Luben, R.; Bingham, S.A.; Day, N.E. Measurement of Fruit and Vegetable Consumption with Diet Questionnaires and Implications for Analyses and Interpretation. Am. J. Epidemiol. 2005, 161, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Manchali, S.; Murthy, K.N.C.; Patil, B.S. Crucial facts about health benefits of popular cruciferous vegetables. J. Funct. Foods 2012, 4, 94–106. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Baatrup, G.; KhataeiNotabi, M.; El-Houri, R.B.; Pipó-Ollé, E.; Arnspang, E.C.; Christensen, E.A. Dietary Polyacetylenic Oxylipins Falcarinol and Falcarindiol Prevent Inflammation and Colorectal Neoplastic Transformation: A Mechanistic and Dose-Response Study in A Rat Model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef]
- Christensen, L.P. Bioactive C17 and C18 Acetylenic Oxylipins from Terrestrial Plants as Potential Lead Compounds for Anticancer Drug Development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef]
- El-Houri, R.B.; Kotowska, D.; Christensen, K.B.; Bhattacharya, S.; Oksbjerg, N.; Wolber, G.; Kristiansen, K.; Christensen, L.P. Polyacetylenes from carrots (Daucus carota) improve glucose uptake in vitro in adipocytes and myotubes. Food Funct. 2015, 6, 2135–2144. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Talalay, P. Direct and indirect antioxidant properties of inducers of cytoprotective proteins. Mol. Nutr. Food Res. 2008, 52, S128–S138. [Google Scholar] [CrossRef]
- Birringer, M. Hormetics: Dietary Triggers of an Adaptive Stress Response. Pharm. Res. 2011, 28, 2680–2694. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, P.; Wang, J.; Gregg, E.W.; Yang, W.; Gong, Q.; Li, H.; Li, H.; Jiang, Y.; An, Y.; et al. The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: A 20-year follow-up study. Lancet 2008, 371, 1783–1789. [Google Scholar] [CrossRef]
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar] [CrossRef]
- Ballali, S.; Lanciai, F. Functional food and diabetes: A natural way in diabetes prevention? Int. J. Food Sci. Nutr. 2011, 63, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P. Functional foods-based diet as a novel dietary approach for management of type 2 diabetes and its complications: A review. World J. Diabetes 2014, 5, 267–281. [Google Scholar] [CrossRef] [PubMed]
| Strategy | Bitter and Strong (BST) | Mild and Sweet (MST) |
|---|---|---|
| Traditional cultivars 1 | Curly kale Brassica oleracea L. var. sabellica L. ‘Halvhøj Kruset Konserva’ White cabbage B. oleracea L. var. capitata ‘Dural’ Celeriac Apium graveolens var. rapaceum ‘Balder Maskot’, ‘Balder Baro’, ‘Balder Bonnet’ Carrot Daucus carota var. sativus ‘Valoria’ | Curly kale ‘Høj Amager Toftø’ White cabbage ‘Amager Høj Grøn Kalida’, ‘Amager Lav Capo’ |
| Modern cultivars 1 | Carrot ‘Mellow Yellow’ Beetroot Beta vulgaris var. vulgaris ‘Chioggio’, ‘Burbees’ | Celeriac ‘Rowena’ Carrot ‘Bolero’, ‘White satin’ Beetroot ‘Pablo’, ‘Taunus’ |
| Nitrogen and sulfur fertilization 2 | High (standard) nitrogen and sulfur fertilization of curly kale and pointed cabbage 3 | Low nitrogen (half) and sulfur (zero) fertilization of curly kale and pointed cabbage 3 |
| Storage conditions | Two weeks in ethylene atmosphere of carrot ‘Bolero’ | No storage of carrot ‘Bolero’ |
| Experimental Diets | ||||
|---|---|---|---|---|
| Control a | Mild and Sweet a | Bitter and Strong a | p-Value b | |
| Participants (n) | 27 | 28 | 27 | - |
| Age (years) | 63.6 ± 1.3 | 62.6 ± 1.4 | 61.6 ± 1.1 | 0.58 |
| Diagnosis (years) | 3.3 ± 0.7 | 2.8 ± 0.7 | 3.5 ± 1.3 | 0.43 |
| Daily vegetable intake (g) | 222 ± 23 | 157 ± 20 | 183 ± 25 | NS |
| β-carotene (μg/mL plasma) * | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | NS |
| Weight (kg) | 96 ± 3.8 | 94 ± 3.3 | 91.3 ± 3.2 | 0.75 |
| Waist circumference (cm) | 109.2 ± 3.8 | 104.1 ± 2.6 | 102.5 ± 2.2 | 0.26 |
| BMI (kg/m2) | 33.1 ± 1.3 | 32.9 ± 1.5 | 31.8 ± 1 | 0.73 |
| Total body fat (%) | 39.3 ± 1.8 | 39 ± 2 | 37.9 ± 1.6 | 0.85 |
| Plasma glucose (mmol/L) | 7.3 ± 0.3 | 6.7 ± 0.3 | 7.6 ± 0.3 | 0.06 |
| HbA1c mmol/mol | 48.2 ± 1.0 | 47.8 ± 1.4 | 50.4 ± 1.7 | 0.37 |
| iAUC glucose (mmol/L) | 755 ± 59 | 706.9 ± 62.1 | 911.1 ± 100.3 | 0.15 |
| Plasma insulin (pmol/L) | 68.8 ± 8.3 | 67.2 ± 9.6 | 73.5 ± 7.0 | 0.87 |
| Plasma glucagon (pg/mL) | 64 ± 3.94 | 64.7 ± 2.96 | 72.1 ± 3.09 | 0.17 |
| HOMA-IR | 4.1 ± 0.6 | 3.3 ± 0.5 | 4.25 ± 0.4 | 0.39 |
| Plasma cholesterol (mmol/L) | 4.4 ± 0.2 | 4.4 ± 0.2 | 4.7 ± 0.2 | 0.58 |
| Plasma LDL–cholesterol (mmol/L) | 2.2 ± 0.2 | 2.4 ± 0.2 | 2.4 ± 0.2 | 0.74 |
| Plasma HDL–cholesterol (mmol/L) | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 | 0.93 |
| Plasma triglycerides (mmol/L) | 2.0 ± 0.3 | 1.6 ± 0.2 | 1.6 ± 0.2 | 0.37 |
| Free fatty acids (mmol/L) | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.5 ± 0.3 | 0.56 |
| Experimental Diets | |||||||
|---|---|---|---|---|---|---|---|
| Control | Mild and Sweet | Bitter and Strong | between Groups | ||||
| Changes a | p-Value b | Changes a | p-Value b | Changes a | p-Value b | p-Value c | |
| Fasting insulin (pmol/L) | −1.99 ± 4.1 | 0.63 | −7.77 ± 2.8 | <0.01 | −9.0 ± 3.33 | <0.05 | 0.29 |
| Fasting glucagon (pg/mL) | −2.25 ± 2.19 | 0.32 | −2.23 ± 1.73 | 0.21 | −2.17 ± 1.78 | 0.28 | 0.99 |
| AUC insulin240 min, (pmol/L ∗ 240 min) † | −632.7 ± 3355 | 0.85 | −2795 ± 2777 | 0.32 | −7199 ± 3476 | < 0.05 | 0.21 |
| AUC glucagon240 min (pg/mL ∗ 240 min) | 142.7 ± 561.3 | 0.80 | −159.5 ± 416.2 | 0.71 | −13.6 ± 524.3 | 0.98 | 0.92 |
| HOMA-IR † | −0.13 ± 0.42 | 0.76 | −0.46 ± 0.23 | 0.06 | −1.05 ± 0.23 | <0.0001 | 0.057 |
| Experimental Diets | |||||||
|---|---|---|---|---|---|---|---|
| Control | Mild and Sweet | Bitter and Strong | Between Groups | ||||
| Changes a | p-Value b | Changes a | p-Value b | Changes a | p-Value b | p-Value c | |
| Weight (kg) | −0.004 ± 0.39 | 0.99 | −2.54± 0.51 | <0.0001 | −2.4 ± 0.54 | <0.0001 | <0.01 d,e |
| Waist circumference (cm) | −3.82 ± 2.29 | 0.11 | −4.00 ± 0.63 | <0.0001 | −4.45 ± 0.86 | <0.0001 | 0.91 |
| DEXA | |||||||
| Lean mass (kg) | 0.40 ± 0.4 | 0.25 | −0.68 ± 0.3 | <0.05 | −0.04 ± 0.3 | 0.90 | 0.07 |
| 24 h Diastolic BP (mmHg) | 1.76 ± 1.06 | 0.11 | −1.15 ± 0.93 | 0.22 | −2.12 ± 1.28 | <0.05 | <0.05 f |
| 24 h Systolic BP (mmHg) | 3.16 ± 2.04 | 0.14 | −1.22 ± 1.5 | 0.42 | −3.23 ± 2.23 | 0.16 | 0.07 |
| Experimental Diets | |||||||
|---|---|---|---|---|---|---|---|
| Control | Mild and Sweet | Bitter and Strong | between Groups | ||||
| Changes a | p-Value b | Changes a | p-Value b | Changes a | p-Value b | p-Value d | |
| TC (mmol/L) | −0.03 ± 0.11 | 0.79 | −0.19 ± 0.11 | 0.11 | −0.30 ± 0.12 | <0.05 | 0.26 |
| Trig (mmol/L) † | −0.14 ± 0.11 | 0.23 | −0.11 ± 0.09 | 0.19 | −0.15 ± 0.08 | 0.07 | 0.42 |
| LDL (mmol/L) † | −0.01 ± 0.8 | 0.95 | −0.09 ± 0.8 | 0.24 | −0.14 ± 0.8 | 0.08 | 0.23 |
| FFA (mmol/L) | 0.05 ± 0.04 | 0.10 | 0.04 ± 0.02 | 0.30 | −0.05 ± 0.16 | 0.13 | 0.07 |
| HDL (mmol/L) | −0.01 ± 0.03 | 0.74 | −0.04 ± 0.04 | 0.28 | −0.01 ± 0.5 | 0.86 | 0.8 |
| PTH (mmol/l) | −0.37 ± 0.24 | 0.14 | 0.05 ± 0.2 | −0.56 | 0.02 ± 0.2 | 0.45 | 0.23 |
| Vitamin D2 + D3 (ng/mL) | −11.18 ± 4.1 | 0.11 | −13.96 ± 2.26 | <0.0001 | −17.04 ± 3.74 | <0.0001 | 0.49 |
| β-carotene (%) c | 1.26 ± 3.4 | NS | 2.58± 3.6 | NS | 9.44 ± 5.1 | NS | NS |
| Lactate (%) | −7.7 ± 4.3 | NS | −8.8 ± 5.3 | <0.05 | −13.6 | <0.01 | NS |
| Isoleucine (%) | −1.3 ± 5.9 | NS | 0.74 ± 5.8 | NS | −11.6 ± 4.5 | <0.05 |
| Year 1 | Year 2 | ||||
|---|---|---|---|---|---|
| Date | 27.9 | 15.11 | 25.09 | 13.11 | |
| Vegetable | Groups | p-Value | p-Value | p-Value | p-Value |
| Carrot | Sweet vs. bitter | <0.0001 | <0.0001 | <0.001 | <0.0001 |
| Celeriac | Sweet vs. bitter | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Kale | Sweet vs. bitter | <0.0001 | <0.001 | <0.0001 | NS |
| Pointed cabbage | Sweet vs. bitter | <0.0001 | NS | 0.0015 | <0.01 |
| White cabbage | Sweet vs. bitter | NI a | <0.0001 | NI a | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorup, A.C.; Kristensen, H.L.; Kidmose, U.; Lambert, M.N.T.; Christensen, L.P.; Fretté, X.; Clausen, M.R.; Hansen, S.M.; Jeppesen, P.B. Strong and Bitter Vegetables from Traditional Cultivars and Cropping Methods Improve the Health Status of Type 2 Diabetics: A Randomized Control Trial. Nutrients 2021, 13, 1813. https://doi.org/10.3390/nu13061813
Thorup AC, Kristensen HL, Kidmose U, Lambert MNT, Christensen LP, Fretté X, Clausen MR, Hansen SM, Jeppesen PB. Strong and Bitter Vegetables from Traditional Cultivars and Cropping Methods Improve the Health Status of Type 2 Diabetics: A Randomized Control Trial. Nutrients. 2021; 13(6):1813. https://doi.org/10.3390/nu13061813
Chicago/Turabian StyleThorup, Anne Cathrine, Hanne Lakkenborg Kristensen, Ulla Kidmose, Max Norman Tandrup Lambert, Lars Porskjær Christensen, Xavier Fretté, Morten Rahr Clausen, Steen Møller Hansen, and Per Bendix Jeppesen. 2021. "Strong and Bitter Vegetables from Traditional Cultivars and Cropping Methods Improve the Health Status of Type 2 Diabetics: A Randomized Control Trial" Nutrients 13, no. 6: 1813. https://doi.org/10.3390/nu13061813
APA StyleThorup, A. C., Kristensen, H. L., Kidmose, U., Lambert, M. N. T., Christensen, L. P., Fretté, X., Clausen, M. R., Hansen, S. M., & Jeppesen, P. B. (2021). Strong and Bitter Vegetables from Traditional Cultivars and Cropping Methods Improve the Health Status of Type 2 Diabetics: A Randomized Control Trial. Nutrients, 13(6), 1813. https://doi.org/10.3390/nu13061813






