1. Introduction
Wound healing is one of the fundamental capabilities of living organisms, which allow them to withstand trauma and disease by regenerating or repairing lost tissue [
1]. Unfortunately, several circumstances or conditions, such as wound extension or disease, can reduce the regenerative potential of tissues; supportive therapies are therefore needed to help tissues achieve their pristine continuity, aesthetic and function [
2]. Dentistry and periodontics are examples of clinical fields where such therapies are sorely needed, as impaired healing may heavily impact oral function and patients’ well-being [
3]. Most regenerative approaches are based on the use of a scaffold or matrix, which fills the gaps within the tissue, and offers a docking point and growth substrate to cell precursors [
4]. Biomimetic approaches are of particular interest because they leverage the self-healing potential of tissues by relying on mechanisms that are already present in the organism [
5]. Accordingly, scaffolds made of molecules that mimic the extracellular matrix are preferable, because they are somewhat already optimized for the task they have to accomplish [
6]. One of such materials is hyaluronic acid, a biocompatible complex proteoglycan that is commonly found in human tissues, where it is responsible for maintaining tissue structure, volume and regulating the interactions of cells and bioactive cues in the extracellular matrix [
7]. Hyaluronic acid has been long known and studied as a scaffold [
8,
9], because of its good tolerability and it ease of use [
10], in different fields, including dentistry [
11]. In the present work we combined hyaluronic acid with polynucleotides (PN), a mixture of double and single stranded deoxyribonucleotides of animal origin. PN are a natural polymer that binds water molecules, thus forming a 3-D gel. Clinically, when infiltrated, PN can moisturize and acquire pronounced viscoelastic properties that can be advantageously used at a tissue level. PN are then enzymatically cleaved and slowly release smaller oligonucleotides and water [
12]. PN has been clinically used for almost 20 years in different class III medical devices because of its wound healing properties [
13]. PNs have been shown to be effective in vitro in several cell models, ranging from dermal fibroblasts to chondrocytes and osteoblasts, and in vivo in many pre-clinical and clinical models, including skin grafts, surgical incision and ulcers [
14]. PN have been shown to act in cells according to multiple pathways, including a salvage pathway, whereby cells are supplemented with an extra nucleotide supply that sustains an increase in cell growth [
9,
15]. Supplementing cells with polynucleotides actually recapitulate some of the molecular events that occur upon wound healing, as ATP and nucleotide strands are released from ruptured cells together with several other cellular components [
16]. Such compounds have evolved to evoke appropriate tissue responses to the wound, with the goal of facilitating healing. A PN and HA-based hydrogel has yielded promising results in soft tissue [
17] and intra-articular [
18] applications. In the present paper we investigated the effects of a PN/Hyaluronic acid hydrogel on primary fibroblasts of gingival origin and assessed cell responses using assays that are relevant to wound healing.
2. Materials and Methods
2.1. Materials
Polynucleotides (PN, Mastelli s.r.l., Sanremo, Italy) are a DNA fraction of different lengths. Salmon trout gonads are the source used to extract PN through a processing that guarantees high DNA yields and chemical purity. All products used for the present study are commercially available as Class III medical devices: PN (7.5 mg/mL) and PN + HA (10 mg/mL PN and 10 mg/mL HA fixed combination with mannitol).
2.2. Cell Culture
Our study used HGF (Human primary fibroblasts; Normal, Human, Adult) of gingival origin from a commercial vendor (ATCC, LGC Standards S.R.L., Milan, Italy). Cells were routinely grown in complete Dulbecco modified MEM (DMEM, LifeTechnologies, Carlsbad, CA, USA) enriched with 10% Fetal Bovine Serum (FBS, LifeTechnologies, Carlsbad, CA, USA), 4 mM L-glutamine (Merck KGaA, Darmstadt, Germany), 100 IU/mL penicillin and 100 μg/mL streptomycin (PenStrep, Merck KGaA, Darmstadt, Germany), in a humidified atmosphere at 37 °C and 5% CO2. Cells were maintained in complete DMEM and monitored with a Nikon TMS inverted optical microscope (Nikon, Tokyo, Japan) in phase contrast. The microscope was equipped with a Nikon Digital Sight DS-2Mv acquisition system (Nikon, Tokyo, Japan) and NIS Elements F software (Nikon, Tokyo, Japan) for image analysis.
Once confluent, cells were detached using trypsin–EDTA (Merck KGaA, Darmstadt, Germany), counted and passaged onto new plates. This in vitro study comprised 3 experimental groups, namely the Control, PN and PN + HA groups. To perform the assays, cells were plated on plasticware and stimulated with either culture medium (Control) or 100 µg/mL PN or 100 µg/mL PN + HA 24 h after seeding.
2.3. Cell Count
To assess cell number, cells in the Control, PN and PN + HA groups were assayed after 96 h and 1 week from treatment. Briefly, cells were seeded in a 24-well plate at a density of 2 × 104 cells/well, stimulated after 24 h and detached with trypsin-EDTA at the desired time point. Cells were resuspended in cold saline and counted with a Coulter Counter (Coulter Electronics Limited, Luton, UK). Counts were carried out in triplicate and supernatants were collected for ELISA assays.
2.4. Cell Viability
Cell viability was evaluated by MTT (Roche Applied Science, Monza, Italy) and CellTiter-Glo (Promega, Madison, WI, USA) assays 96 h and 1 week after stimulation. For each experiment, cells were seeded in a 96-well plate at a density of 8 × 103 cells/well and stimulated 24 h after seeding.
The MTT assay was used according to the manufacturer’s indications. Briefly, cells were incubated for three hours with 0.5 mg/mL MTT labeling reagent and assayed by solving the formazane with a solubilization solution and then reading the sample’s absorbance at 570 nm by a microplate reader (Infinite F200 TECAN, Männedorf, Switzerland). For each sample, eight wells were assayed.
The CellTiter-Glo® assay was carried out following the manufacturer’s recommendations. Briefly, assay lysis buffer was added to the wells and mixed for 2 min, thus ensuring the release of intracellular ATP. The signal was stabilized by a 10 min RT incubation, and the samples were then read with a microplate reader (Infinite F200 TECAN, Männedorf, Switzerland). Control wells containing medium without cells were used to measure background luminescence. Four wells were assayed for each sample.
2.5. Clonogenic Efficiency Assay
Cells were seeded in a 6-well plate at final concentration of 150 cells/well. Twenty-four hours after seeding, cells were stimulated and incubated in a CO2-incubator at the saturated humidity conditions at 37 °C in 5% CO2 atmosphere for 2 weeks. Culture medium was renewed every three days. Thereafter, the culture plate with the pre-formed colonies was washed with PBS and fixed. Briefly, an equal volume of methanol/PBS for 2 min was added and then replaced with pure methanol for 10 more minutes. Alcohol residuals were then removed, and the colonies were stained with Giemsa (Merck KGaA, Darmstadt, Germany) for 2 min and observed with an inverted optical microscope with phase contrast to evaluate wound closure. The plates were thoroughly washed of excessive stain and dried at room temperature. All samples were observed with an inverted optical microscope (Nikon, Tokyo, Japan) as described above.
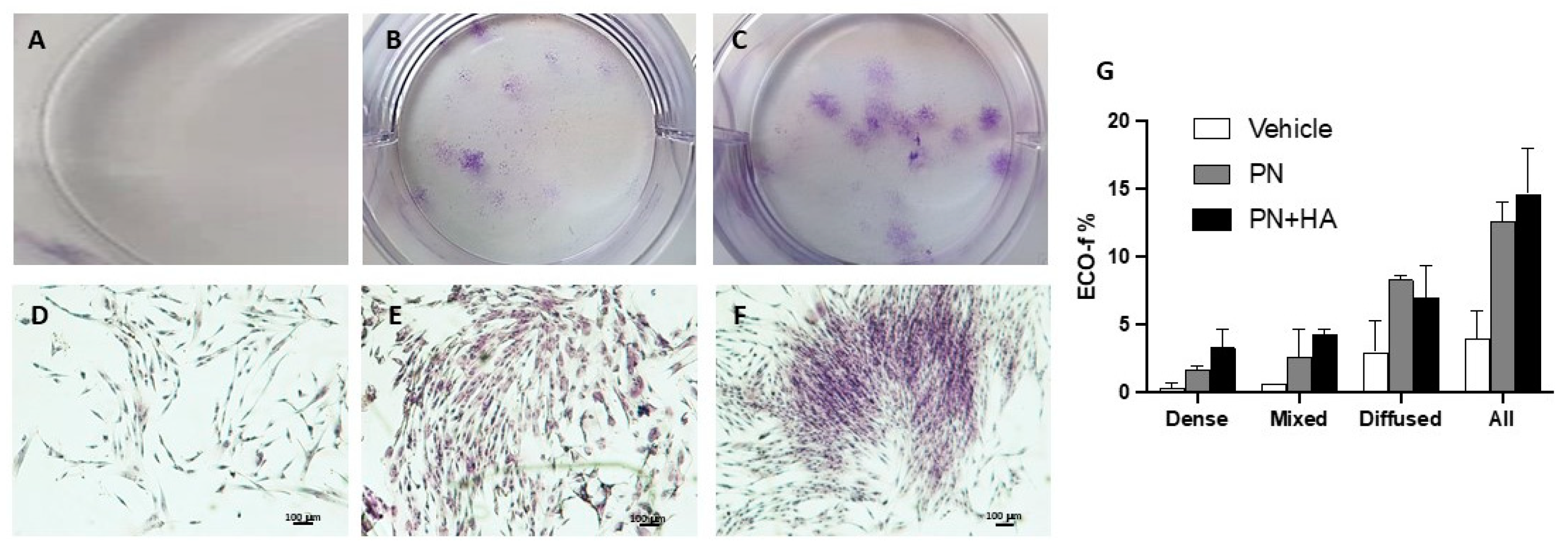
Only colonies that included more than 20 cells were included in the analysis and were ranked in three groups: dense, diffuse and mixed colonies, according to how densely packed cells appeared [
19]. Fibroblast colony forming efficiency (ECO-f) was determined according to Fridenshtein’s equation for stromal progenitors: ECO-f = number of pre-formed colonies/number of seeded cells × 100%.
2.6. Protein Content
To quantify protein content, cells were seeded in triplicate in a 24-well plate at a density of 2 × 104 cells/well for each experimental condition. Samples were stimulated 24 h after seeding and evaluated 96 h and 1 week after treatment. Briefly, samples were rinsed with PBS, dried and stirred for 1 h in 5% NaOH DOC (Sigma-Aldrich, St. Louis, MO, USA) for solubilization. Samples were stirred for 10 more minutes after addition of Lowry Reagent and then transferred to a sonicator. Folin and Ciocalteu’s Phenol Reagent Working Solution (Sigma-Aldrich, St. Louis, MO, USA) was added to the samples, which were mixed on orbital shaker for 1 h. Protein content was measured by a microplate reader (Infinite F200 TECAN, Männedorf, Switzerland) at 750 nm and a standard curve was used to compute the absolute amount of proteins in samples.
2.7. Extracellular Matrix Deposition
To assess extracellular deposition, cells were seeded in a 6-well plate at a density of 20 × 104 cells/well and stimulated after 24 h. Samples were fixed 21 days after stimulation and stained with Sirius-red F3B200 (Mobay Chemical Co., Pittsburgh, PA, USA) and Alcian Blue 8GS (Bio-Optica Milano s.p.a., Milano, Italy) to evaluate collagen and proteoglycan deposition, respectively. Briefly, samples were washed with PBS and Bouin’s fixative was added. The plate was incubated for 1 h at room temperature, Bouin’s solution was removed, and samples were rinsed with water and allowed to dry. For Collagen staining, after adding 0.1% Sirius red, the plate was incubated for 1 h and then washed with 0.1 N HCl and left to dry. For proteoglycan deposition, 1% Alcian Blue was added, the plate was incubated over-night at room temperature and washed with distilled water and left to dry. All samples were observed with an optical microscope Nikon Eclipse 80i (Nikon, Tokyo, Japan), equipped with a Nikon Digital Sight DS-2Mv camera and imaged with NIS Elements F control software (Nikon, Tokyo, Japan).
To quantify Collagen expression, the supernatants collected in the Control, PN and PN + HA groups (as described above) were assayed using Human Pro-Collagen I alpha 1SimpleStep ELISA Kit (Abcam, Cambridge, UK) and Human Collagen III Alpha (COL3A1) ELISA Kit (Abbkine, Wuhan, China), following the manufacturer’s recommendations and the experiments were performed in triplicate.
For Collagen I detection, after centrifugation, diluted supernatants, standard samples and the antibody cocktail were pipetted into the plate, which was incubated for 1 h at room temperature on an orbital shaker and then washed with 1× Wash Buffer PT. After adding TMB Development Solution, samples were incubated in the dark and a stop solution was then added. The Optical Density (O.D.) was recorded at 450 nm by a microplate reader, as described above.
For Collagen III detection, standard samples and diluted and centrifuged supernatants were added to testing wells, and then incubated at 37 °C for 45 min. The HRP-conjugated detection antibody was added to each well after a washing step. The plate was incubated for 30 min at 37 °C, samples were washed again, and chromogen solution A and chromogen solution B were then added. After gently mixing, the plate was incubated for 15 min at 37 °C, and the Stop Solution was added. The O.D. was read at 450 nm by a microplate reader, as described above.
2.8. In Vitro Scratch Assay
The effect of PN on cell spreading and migration was investigated by a scratch assay. Briefly, 20 × 104 cells were seeded into each well of a 12-well plate and, upon confluence, the cell monolayer was scratched with a 200 μL plastic pipette tip across the center of the well. Cells were rinsed with PBS and culture medium in the absence (control group) or in the presence of PN or PN + HA was added. Scratch closure was monitored with an inverted optical microscope in phase contrast and microphotographs were taken with a digital camera 48 and 96 h after stimuli were added.
Moreover, samples were fixed with methanol after 96 h and then stained with Giemsa solution for 2 min. The samples were then rinsed with tap water, dried and then observed with an inverted optical microscope as described above.
2.9. Statistical Analysis
For the statistical analysis, the collected data were analyzed using Prism X (GraphPad, La Jolla, CA, USA). The results are expressed as means ± standard deviation (mean ± SD) of repeated experiments. The statistical significance of the differences observed between groups was determined using ANOVA test and a p value smaller than 0.05 was considered a statistically significant difference.
4. Discussion
Regeneration is a potent capability of living organisms to recreate parts of tissue that have been lost and thus restore their own integrity [
20]. However, several circumstances, such as defect extension or the presence of disease, may impair this process and call for adjunctive treatments [
21]. Under such conditions, tools may be needed to improve cell growth and healing potential. Biomaterials, one of the mainstays of most regenerative strategies, have proved to be able to provide excellent support to healing tissues, although they often lack the capability to impart specific cues to obtained desired responses from cells [
22]. Recently, biomimetic approaches have become object of in-depth investigation, as they rely on principles of activity that are physiologically found in organisms [
5]. The present study reports on the responses of primary gingival fibroblasts to a hyaluronic acid biopolymer enriched with polynucleotides (PN + HA). PN is a compound containing a mixture of DNA fragments that has accrued a conspicuous body of evidence regarding its capability to enhance cell vitality in several in vitro and in vivo studies and can count on a long clinical experience of use as healing support therapy, especially for wounds or ulcers [
14,
15].
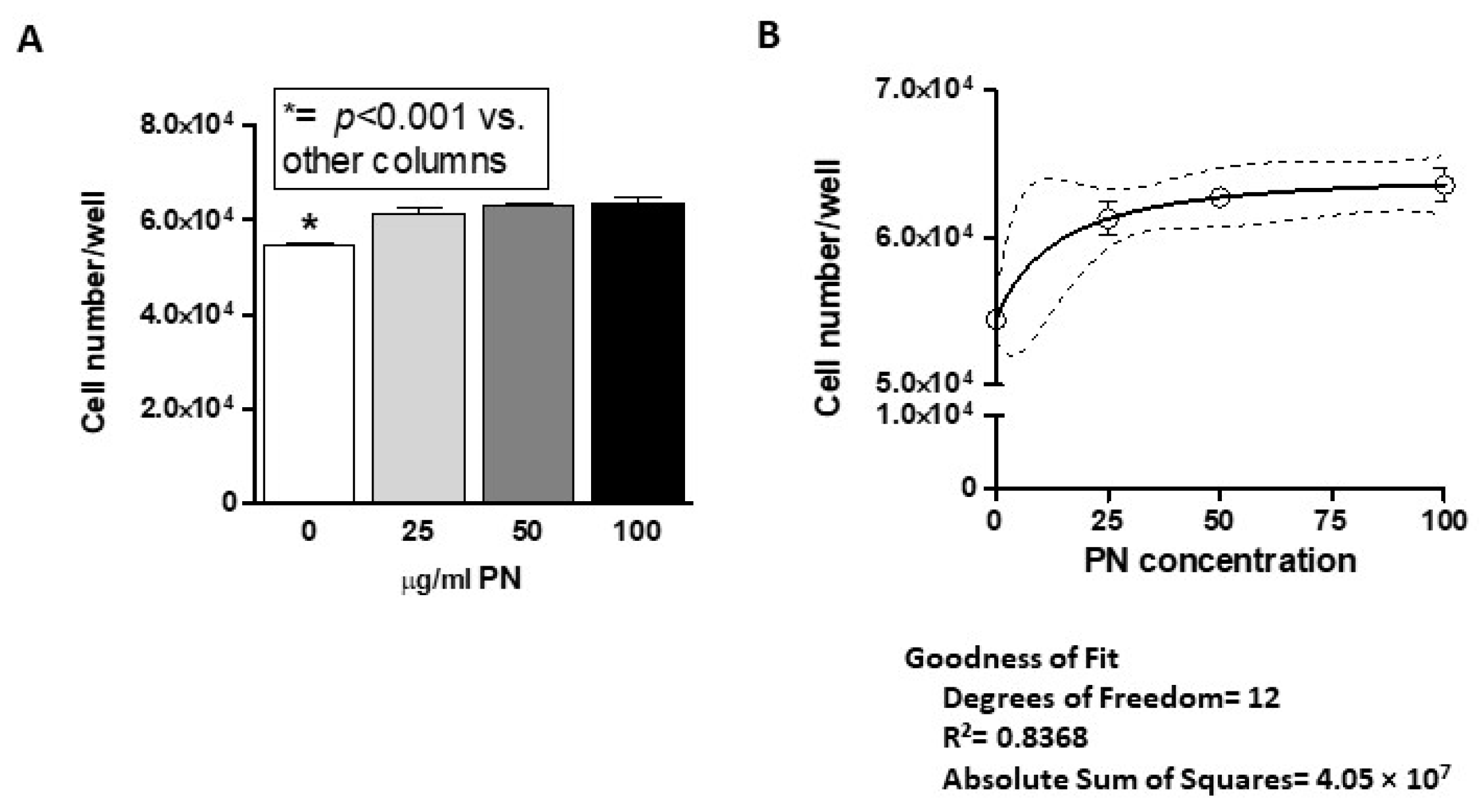
Our data confirmed that PN are effective in promoting cell growth in gingival fibroblasts (
Figure 1), with consistent results across several assays. Addition of HA appeared to exert a marginal effect on growth, which was observed at direct count and, partially, at luminescence, although MTT noticeably failed to pick this up (
Figure 1). Most of the available literature reports that 100 μg/mL is the optimal dose of polynucleotides for in vitro and in vivo applications and our data confirm that gingival fibroblasts too achieve their peak response at that concentration.
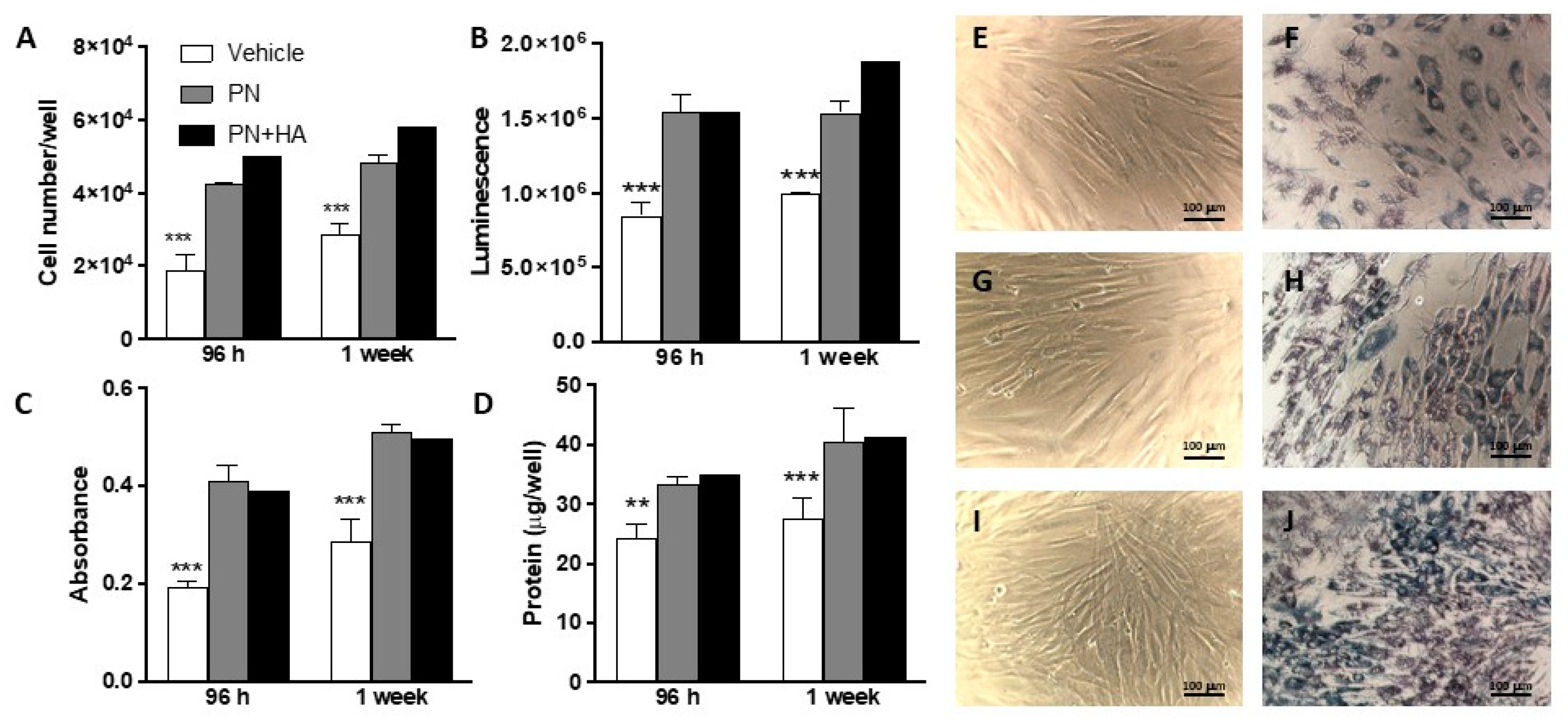
Microscopic observations confirmed the impression of a higher cellular density in the presence of PN + HA hydrogel than in the control group or with PN alone, consistently with direct count data. Microphotographs may also offer a clue as to why metabolic viability assays like MTT failed to observe a further increase in cell number in the PN + HA group.
MTT is a very common and well established cellular assay that is based on the conversion of MTT reagent to a blue compound formazan, which can then be quantified, and thus relies on the cellular metabolic activity [
23]. Microphotographs show that cells in the PN + HA group tended to form nodules and to stack up to form multilayers. Although our cell model was a commercially available primary cell model that tended to grow in monolayers, it can be hypothesized that the presence of HA may have facilitated the formation of nodules, thanks to a more abundant biopolymer matrix, and thus of cell-rich areas that did not provide a linear MTT response.
Indeed, our clonogenic assay showed that PN and PN + HA significantly improved colony number as compared to vehicle, which can be easily explained by their effects on cell growth (
Figure 2), and which possibly became even more visible in a challenging situation for cells in CFU-assay. In a clonogenic assays, cells are seeded at very low density [
19], where paracrine stimulation from neighboring cells is very poor and PN may have thus provided just enough stimulation to allow the formation of 3–4 times the number of colonies as in the control group. Moreover, though the overall number of colonies in the PN + HA group was not higher than in the PN alone group, the colony patter was distinctively different. Most colonies in the PN alone group were diffused, i.e., were formed by sparse cells, scattered quite far apart. On the contrary, PN + HA appeared to induce the formation of a higher number of dense or mixed colonies that control or PN alone, confirming that PN + HA, while possessing a pro-growth effect on cells, is also endowed with the capability to affect cell arrangement and alignment (
Figure 2F,G).
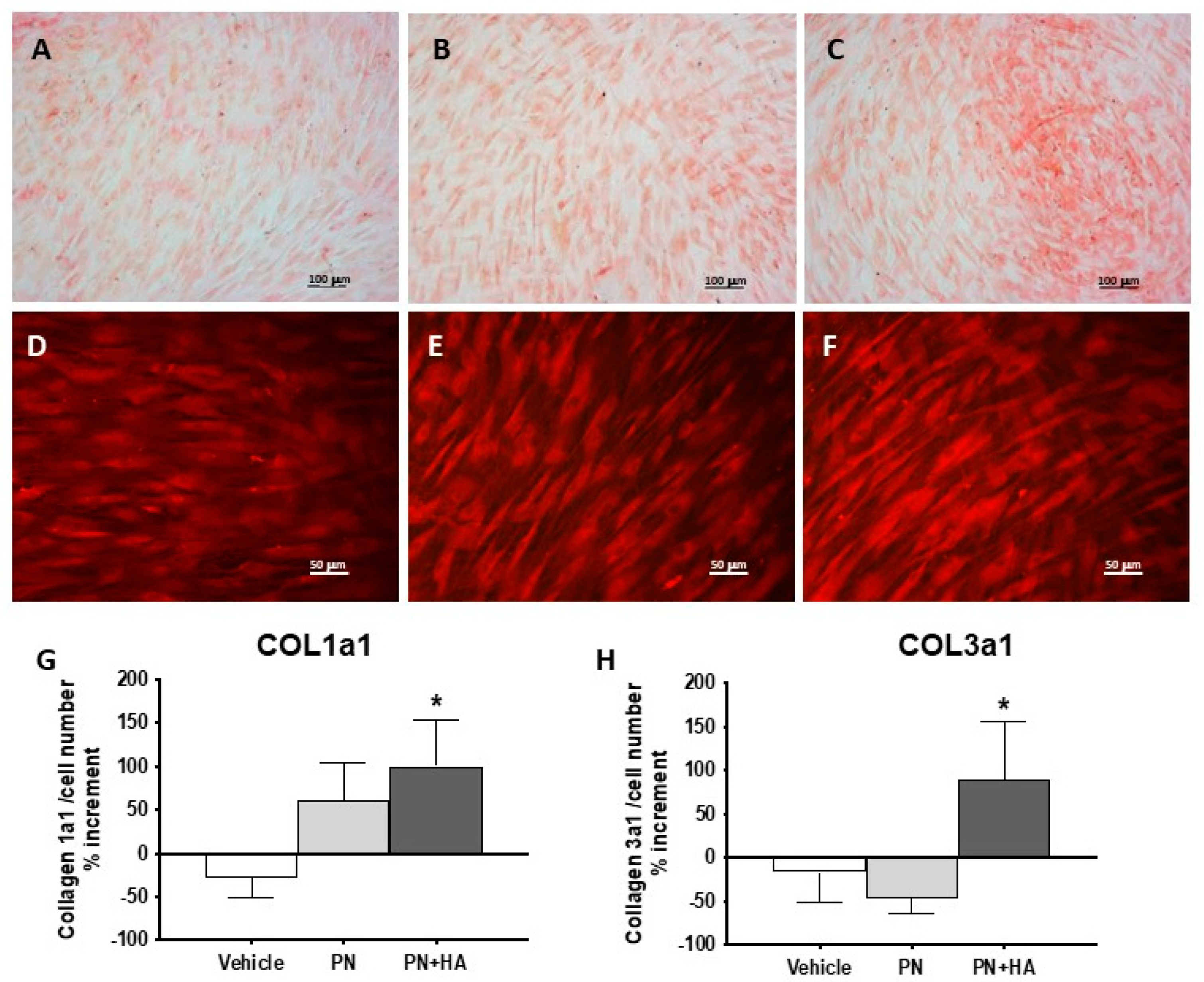
A further confirmation was obtained by Sirius red staining [
24], which visualized collagen deposits (
Figure 3A–C) and revealed that PN and PN + HA were able to progressively increase collagen deposition. PN + HA furthermore facilitated the formation of more numerous collagen-positive nodules with high cells density and multilayer cell stacking, which becomes visible at fluorescence microscopy (
Figure 3F). It appears that, as expected, these high-density nodules were also centers of higher matrix formation. Though this may be in part due to the mere presence of more cells, we collected evidence that points at an increased production of collagen (
Figure 3G,H). Even when adjusting by cell number, we observed an increased in collagen 1a1 expression by cells stimulated with PN and PN + HA, which in this latter case was also significantly higher than the control group, where no change in expression was measured. Moreover, if the expression of Collagen 3a1 did not appear to change in the control group or event tended to decrease in the PN group, there was a significant increased, adjusted by cell number, after stimulation with PN + HA (
Figure 3H). This finding is of particular interest because Collagen 3a1 is an early product of wound healing and is known to play a role in orchestrating matrix assembly during regeneration [
25]. It has actually been shown that an increased Collagen 1a1/3a1 ratio is conductive to healing impairment and keloid formation [
26]. A sustained Collagen 3a1 production is therefore possibly predictive of reduced risk of abnormal wound healing.
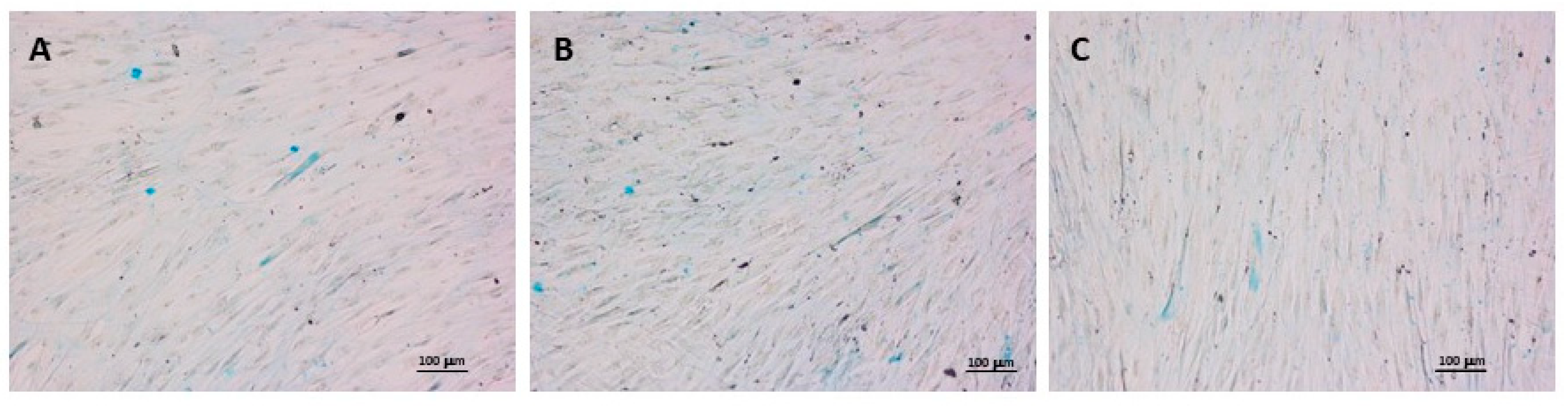
Furthermore, despite the presence of HA in the formulation, Alcian Blue staining did not show any suppression of proteoglycan production, as similar levels can be detected in control, PN and PN + HA groups (
Figure 4). It cannot be excluded that some of the visible staining was actually obtained from exogenous HA from the hydrogel, but the net effect appears to be the presence of comparable levels of proteoglycans in the matrix.
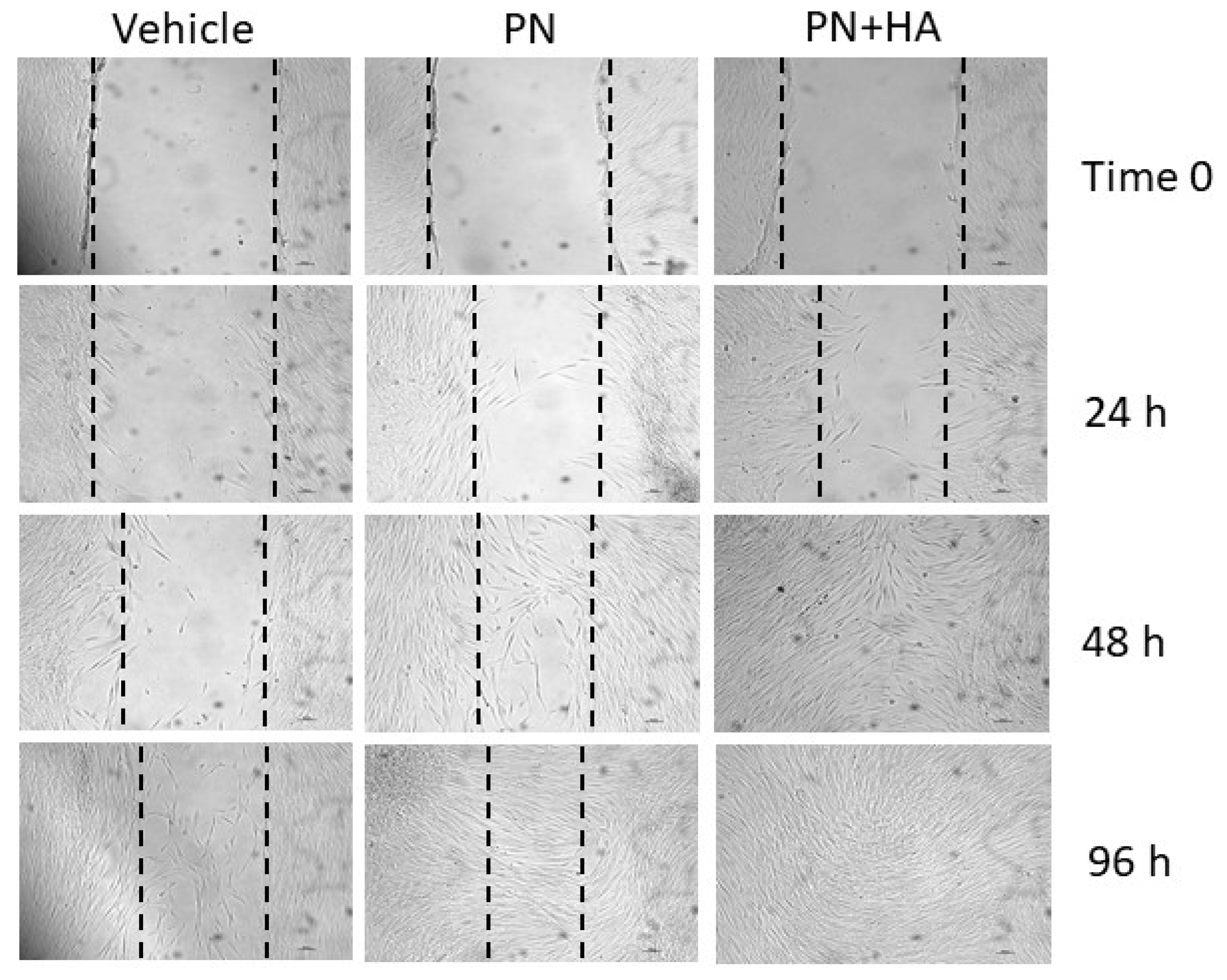
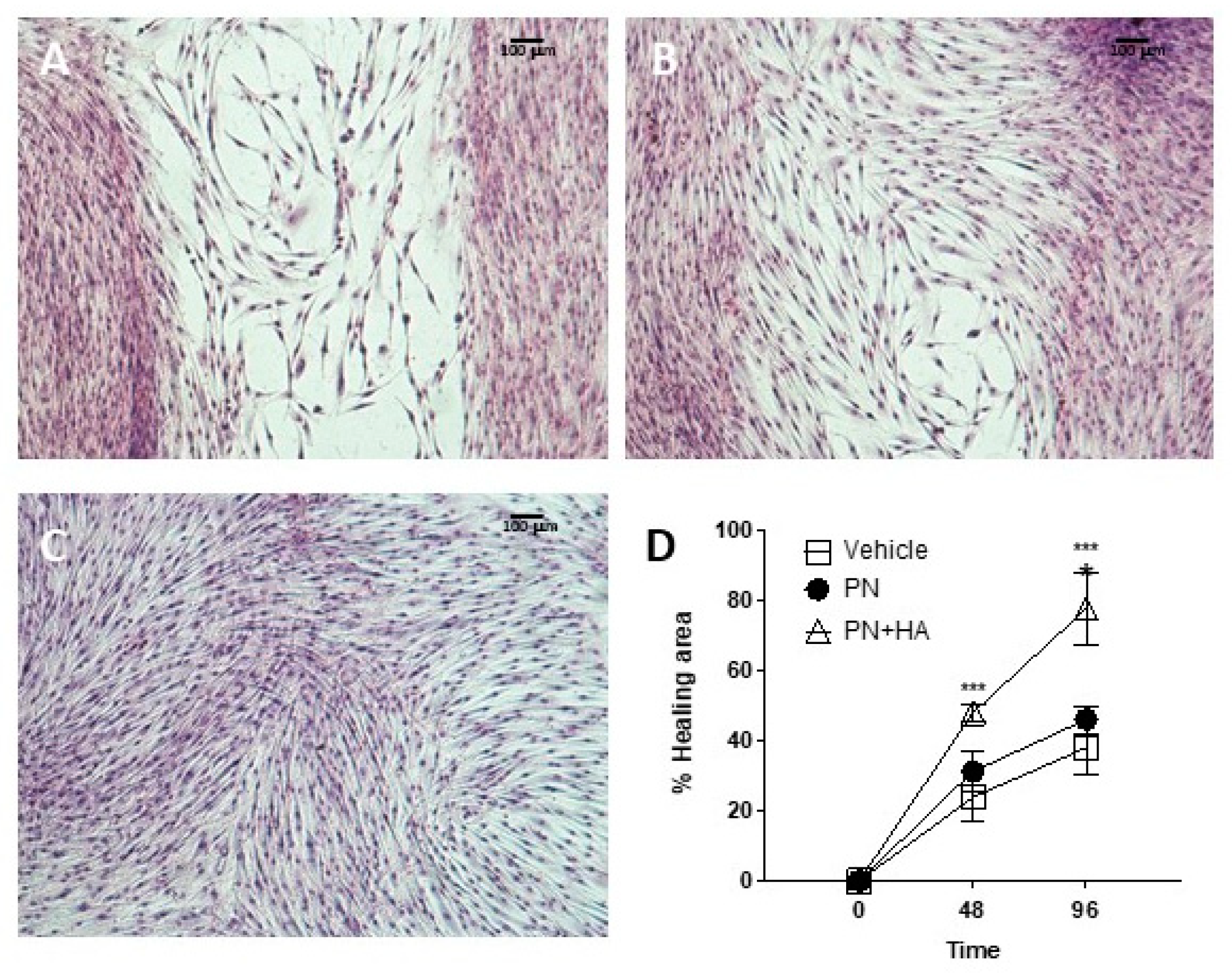
The effects of PN + HA biopolymer on wound healing can be gauged from the scratch assay (
Figure 5), which is a well-established, simple test to assess the capability of an in vitro model to bridge an artificially induced gap in the cell layer [
27]. An optimal wound closure requires a combination of a coordinated array of signals and cell activities, including cell growth and migration. Cells at the borders may well duplicate but will also need to migrate into the defect to adequately and quickly fill it. In an in vivo setting, a wound will cause the release of several mediated from the injured area, including molecules from damaged and ruptured cells [
28]. Such compounds include ATPs and DNA from dying cells and these have been shown to act as important cues and stimulate wound repair. PN may be in part replicating this signaling, while also providing building blocks for duplicating cells, through a well-characterized salvage pathway. This alone appeared to improve scratch healing to a certain, albeit limited, extent, but the combination of PN and HA turned out to be critical to ensure a faster gap closure already after 48 h (
Figure A2).
This suggests that cell migration may also be facilitated by PN + HA, possibly by the presence of HA, itself a normal component of granulation tissue in wounds, which provides a provisional matrix over the gap that fibroblast can more easily cross. Further studies will have to better characterize the growth vs. migration effects of this compound, to tune them according to actual clinical needs.