Abstract
Pathological conditions of the tracheal epithelium, such as postoperative injuries and chronic conditions, often compromise the functionality of the respiratory epithelium. Although replacement of the respiratory epithelium using various types of tracheal transplantation has been attempted, there is no predictable and dependable replacement method that holds for safe and practicable long-term use. Therefore, we used a tissue engineering approach for ex vivo regeneration of the respiratory epithelium (RE) construct. Collagen type I was isolated from sheep tendon and it was fabricated in a three-dimensional (3D) scaffold format. Isolated human respiratory epithelial cells (RECs) and fibroblasts from nasal turbinate were co-cultured on the 3D scaffold for 48 h, and epithelium maturation was allowed for another 14 days in an air–liquid interface culture system. The scanning electron microscope results revealed a fabricated porous-structure 3D collagen scaffold. The scaffold was found to be biocompatible with RECs and fibroblasts and allows cells attachment, proliferation, and migration. Immunohistochemical analysis showed that the seeded RECs and fibroblasts were positive for expression of cytokeratin 14 and collagen type I markers, respectively, indicating that the scaffold supports the native phenotype of seeded cells over a period of 14 days. Although a longer maturation period is needed for ciliogenesis to occur in RECs, the findings suggest that the tissue-engineered RE construct is a potential candidate for direct use in tracheal epithelium replacement or tracheal tube reengineering.
1. Introduction
The tracheal epithelial layer is a well-known barrier that prevents underlying tissues from external irritants such as allergens, airborne particulates, infectious agents, and noxious gases, and their potential penetration. As a part of the immune system, it facilitates the communication and activities of cells in the innate and adaptive immune system. Therefore, structural or functional alternations occurring in the respiratory epithelium can severely compromise its effectiveness. Diseases that involve the tracheal epithelium include trauma, post-intubation injuries, infection, and inflammation, and chronic diseases such as asthma [1], rhinitis [2], and obstructive pulmonary disease. In asthma, which is known as a common chronic inflammatory disease of the airway, or in the cases of inhaling harmful chemicals or vapors [3], the injuries in the epithelium cause structural changes to the normal architecture of the nasal epithelium, increasing its susceptibility to irritants and decreasing its efficiency in response to respiratory viruses [4]. The consequence of the injuries introduced into the epithelium can even cause the loss of surface epithelium integrity and partial or complete shedding of the epithelium [5]. Therefore, these conditions necessitate epithelium repair or replacement. Treatments using foreign materials [6], nonviable tissues, autologous tissues, and various types of tracheal epithelial transplantation have been tried; however, no breakthrough had been achieved. It is thought that tissue engineering strategies can offer alternative solutions by developing tissue-engineered epithelium to be used for epithelial replacement or reconstruction. Ex-vivo-engineered respiratory epithelium has been integral to understanding the pathologies and underlying mechanisms of many respiratory-related diseases. Hence, it can potentially be used for studying drug interactions with the reparatory epithelium and developing regenerative therapies for respiratory epithelium diseases [7].
A tissue-engineered respiratory epithelium construct composed of autologous respiratory epithelium cells, fibroblasts, and plasma from sheep’s blood was previously reported in a series of studies, and it was used to replace tracheal mucosal defects in sheep. The post-operative analysis showed encouraging results: immature cilia were found to be present on the surface of epithelial cells. These results indicated that the engineered respiratory epithelium was able to function as a normal tissue, and the presence of autologous fibrin found in blood plasma provided a favorable environment for respiratory epithelial cells adaptation and proliferation [8,9,10].
Aoki et al. investigated de-epithelialized porcine tracheal tissue that was re-populated with human bronchial epithelial cells and its potential application in tracheal tissue engineering. Their findings showed the feasibility of the de-epithelialization of the porcine trachea using sodium dodecyl sulfate, without compromising the integrity of the underlying cartilage and the viability of residing chondrocytes. They reported that the remaining porcine cartilage supports the growth and proliferation of seeded bronchial epithelial cells over 7 days [11], but the tracheal epithelium being partially removed and the presence of residing viable chondrocytes raise concerns about the immunogenicity and clinical application of such grafts [12]. Cellulose-based material from either plant or bacterial sources is another category of materials being extensively studied for their applicability in tissue engineering in general and tracheal tissue engineering in particular. Cellulose, which is a linear polymer comprising a glucose unit [13], shows favorable properties in supporting the attachment, growth, and proliferation of epithelial cells [14] and chondrocytes [15], and allows infiltration and ingrowth of seeded cells [16]. Despite the appealing features of cellulose-based material and their mild immunogenicity in vivo [17], the non-degradability of such materials in the human body [18] make cellulose an unfavorable material for clinical application. The application of denuded amniotic membrane, which is composed of non-fibrillar meshwork containing collagen type III, was reported previously as cell support for the transferring cell layer [19]. In 2018, crosslinking of decellularized amniotic membrane with 0.5% genipin was reported to significantly enhance the biostability and resistance of the amniotic membrane when exposed to enzymatic degradation. It was also shown that the genipin-crosslinked amniotic membrane expresses better biocompatibility with nasal fibroblasts compared to the native one, making it a suitable scaffold for respiratory tissue engineering applications [20]. Another support material that has the potential to be used in respiratory tissue engineering is collagen type I, being the most abundant collagen in the human body. Collagen type I is the major component of the extracellular matrix in most of the tissues and organs, and it is known to support cellular properties in vivo [21]. In vitro studies showed that increasing the concentration of collagen type I promotes the stratification of respiratory epithelial cells (RECs) and affects the morphology of the cells [22]. The commercially available bovine collagen cross-linked with glycosaminoglycan (Integra®) already has the Food and Drug Administration (FDA) and EU approval and is currently used in clinical practice for skin treatment [23]. Collagen fibers also possess some unique structural properties important for tissue engineering. The physical and mechanical properties of bovine collagen type I were extensively studied elsewhere [24]. It was reported that genipin cross-linked bovine collagen exhibits significantly improved mechanical features (stiffness, shrinkage, and swelling properties) and it is suitable for tissue engineering application in vivo. Bovine collage exhibits a higher Young’s modulus/stiffness (~500 MPa) compared to human native trachea (16.92 MPa). Additionally, collagen has high water affinity, low antigenicity, very good cell compatibility, and the ability to promote tissue regeneration [25]. These factors combined make collagen one of the most ideal biopolymers available for tissue engineering applications. However, due to bovine collagen’s fast degradation rate, it is a suitable material for tracheal tissue engineering application [26].
Despite many recent attempts at the tissue engineering of human respiratory epithelium [27,28], no ideal treatment for respiratory epithelium repair has yet been developed. Proper epithelialization of tissue-engineered airway constructs, poor vascularization, and less-than-favorable mechanical properties are the current challenges. Complex and often non-reproducible differentiation procedures and difficulty in maintaining the mucocilated phenotype of the primary airway epithelial cells are the major complications persisting in the field [29]. Hence, in this study, we introduced a three-dimensional (3D) model of an in vitro respiratory epithelium (RE) construct comprising RECs and fibroblasts from human nasal turbinate, which were incorporated into a freeze-dried ovine collagen scaffold and grown in an air–liquid interface culture condition. The aim was to use the constructed respiratory epithelium for respiratory epithelium regeneration or substitution. To the best of our knowledge, this is the first time that this proposed approach is reported in the tissue engineering of the respiratory epithelium. The significance of the study is the application of FDA-approved collagen from bovine source, which makes the 3D respiratory epithelium construct a promising candidate for future clinical use.
2. Materials and Methods
This study was approved by the University Kebangsaan Malaysia Research Ethic Committee with reference number oUKM1.5.3.5/244/GGPM-2015-035.
2.1. Respiratory Epithelial and Fibroblast Cells Isolation and Culture
Nasal turbinate discarded during turbinectomy was obtained from consenting patients and cleaned of mucus and blood three times using Dulbecco’s phosphate-buffered saline (DPBS; Gibco, Walthan, MA, USA) supplemented with 1% (v/v) antibiotic/antimycotic (Gibco, Walthan, MA, USA). The epithelium layer was minced into 1 mm pieces and digested with 0.6% (w/v) collagenase type I (Worthington, Lakewood, NJ, USA) supplemented with 1% (v/v) antibiotic/antimycotic for 60 min in a shaker incubator at 37 °C. After tissue digestion, it was centrifuged (Hettich Zentaifugen, Westphalia, Germany) for 5 min at 2370× g. Next, the supernatant was discarded and the pellet containing fibroblasts and RECs was washed with DPBS, followed by re-centrifugation for 5 min at 2370× g. Then, the cells were cultured in defined keratinocytes serum-free medium (DKSFM; Gibco, Walthan, MA, USA), F-12, and Dulbecco’s modified Eagle’s medium (DMEM) in a 2:1:1 ratio, supplemented with 5% fetal bovine serum (FBS) (DKSFM:F-12:DMEM + 5%FBS). Cells were incubated at 37 °C in a 5% CO2 incubator (RS Biotech, Irvine, U.K.), and media were changed every 2 days. Once confluent (80–90%), differential trypsinization of fibroblasts was performed using 0.05% trypsin-EDTA (Capricorn Scientific, Ebsdorfergrund, Germany) with 3 min incubation at 37 °C. This step allowed selective detachment of fibroblasts from the culture plate while leaving colonies of RECs in place. The REC colonies in 6-well plates were left to reach confluency. The trypsinized fibroblasts were then seeded back into another 6-well plate and cultured in F-12:DMEM (1:1) + 10% FBS, with media being replaced every 2 days until 80–90% confluency.
2.2. Three-Dimensional (3D) Collagen Scaffold Fabrication
Collagen type I was isolated and purified from sheep tendon as previously described [30,31]. Dialyzed collagen was freeze-dried for 24–48 h and redissolved in 0.35 M acetic acid for a final concentration of 14.25 mg/mL. The collagen solution was neutralized to pH 7.0 using 1 M sodium hydroxide (Sigma, St. Louis, MO, USA) and poured into a 12-well plate as a mold, followed by freezing at −30 °C for 6 h and freeze-drying (Ilshin, Gyeonggido, Korea) for 24–48 h. The collagen scaffold was sterilized using 70% ethanol and incubated with culture medium for 30 min prior to cell seeding.
2.3. Formation of Human Respiratory Epithelium Construct
At day 0, fibroblasts were seeded in a 12-well plate with 2 × 104 cells/well cell seeding density. At day 1, the floating fibroblasts were removed and the pre-soaked fabricated 3D scaffold was placed on top of the fibroblasts in the 12-well plate. RECs (2 × 104) were seeded on the top surface of the collagen scaffold. The cell-seeded scaffold was maintained submerged in co-culture medium (DKSFM:F-12:DMEM + 5% FBS in 2:1:1 ratio) for 48 h, in a 5% CO2 incubator at 37 °C. Afterward, to initiate the air–liquid interface (ALI) culture, the media were completely aspirated from the 12-well plate and a fresh co-culture medium was added into the well, to a level that allowed the upper surface of the collagen scaffold to be exposed to the air and the media to be perfused only from the lower side of the scaffold. The 3D respiratory epithelium (RE) construct was maintained under ALI culture condition for 2 weeks to promote the functional differentiation of the epithelium. The medium was replaced every 2 day; each time, the liquid on the surface of the 3D constructs was gently aspirated to maintain an ALI microenvironment [14]. The experiment was repeated with 4 biological samples.
2.4. Histological Analysis of the 3D Construct
At harvest, half of the 3D human RE construct was used for histological analysis. The RE construct was fixed in 10% neutral-buffered formalin in PBS and was dehydrated in a series of increasing concentrations of ethanol. The construct was cleared in xylene and was embedded in paraffin. The paraffin blocks were sectioned (5 μm thickness) and hematoxylin and eosin (H&E) staining was subsequently performed on slices.
2.5. Immunohistochemical Analysis of the 3D Construct
For immunohistochemical staining of 3D human RE construct, 5 μm slices were heated at 95 °C for 15 min in citrate buffer (pH 6) as the antigen-retrieval solution. Next, the permeabilization was performed using 0.1% triton-X (Sigma-Aldrich, St. Louis, MO, USA) in PBS for 5 min. To eliminate non-specific binding, the slices were blocked with 10% goat serum for 1 h at 37 °C. Afterward, tissues were incubated with mouse anti-cytokeratin 14 antibody (ab7800, Abcam, Cambridge, UK) in a 1:200 dilution ratio and mouse-anti collagen type I antibody (ab6308, Abcam, Cambridge, UK) with dilution ratio of 1:300 at 4 °C overnight, followed by staining with secondary antibody (Alexa Fluor Invitrogen, Waltham, MA, USA) for 2 h at 37 °C. We used 4′,6-diamidino-2-phenylindole (DAPI) for nuclei counter staining. The stained tissues were visualized under a confocal microscope.
2.6. Scanning Electron Microscopy Analysis
For scanning electron microscope analysis, the 3D human RE construct was fixed overnight in a neutral aqueous buffer containing 2.5% glutaraldehyde and 0.1 M sodium cacodylate. The fixed 3D human RE constructs was post-fixed in 1% osmium tetroxide followed by dehydration to 100% ethanol. To preserve the surface structure, the 3D construct was critical-point dried under CO2, mounted, and sputter-coated for viewing in a scanning electron microscope machine (QUANTA 650F, FEI, Hillsboro, OR, USA).
3. Results
The primary culture of human RECs and fibroblast were successfully established from nasal turbinate samples (Figure 1). In our previous studies, we successfully characterized the RECs isolated from nasal turbinate via gene expression (CK18 and 14, MUC5B, and Ki67) [32] and immunocytochemical analysis (acetylated β-tubulin, CK14, MUC5AC, and Ki67) [32,33].
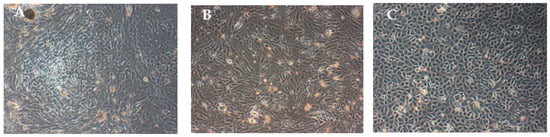
Figure 1.
Monolayer cells cultured in 6-well plate: (A) Co-culture of respiratory epithelial cells (RECs), and fibroblasts, (B) fibroblasts, and (C) RECs. The co-cultured cells became confluent in a week’s time and fibroblasts were separated by differential trypsinization before seeding into a new flask.
The overall appearance of the collagen scaffold was spongy and white in color, and the irregular-sized pores on the scaffold were observable with the naked eye (Figure 2A). The SEM analysis showed that the scaffold had interconnected pores with size range of 100 to 200 µm (Figure 2B,C).
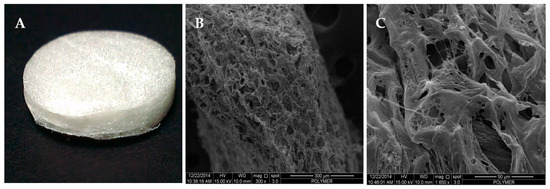
Figure 2.
The freeze-dried collagen type I (Col I) scaffold at −80 °C: (A) overall view, (B) view under scanning electron microscope (SEM) at 300×, and (C) view under SEM at 1650×.
The hematoxylin and eosin (H&E) staining of the 3D human RE construct cross-section showed that the collagen scaffold contained interconnected pores and the cells (RECs and fibroblasts) were able to migrate into the collagen scaffold upon 4 weeks of culture (Figure 3). However, the cell attachment and migration were not uniform, as some parts of the 3D human RE construct showed patchy cell organization while there were no cells present on other parts.
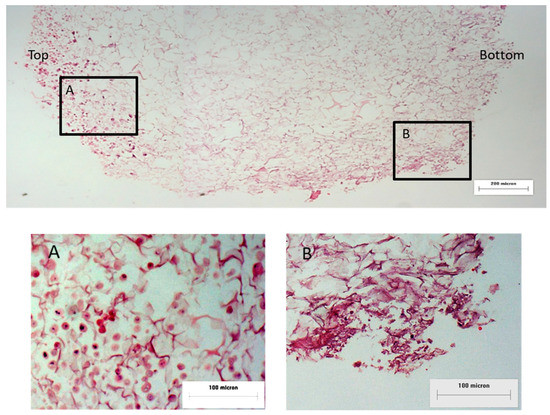
Figure 3.
Cross-section view of the hematoxylin and eosin (H&E)-stained 3D human RE construct: (A) top layer of collagen construct with RECs and (B) bottom layer of collagen scaffold with fibroblasts. The cells were found to enter and migrate into the collagen scaffold at 4 weeks post cell seeding.
The presence of distinct RECs and fibroblasts layers on the collagen scaffold was confirmed by the immunostaining analysis. As the anti-collagen type I antibody bound to the collagen scaffold as well as the seeded fibroblasts, it was difficult to identify the presence of fibroblasts based on collagen type I expression. So, the cells with a nucleus stained with DAPI and negative expression of CK 14 were assumed to be the seeded fibroblasts on the collagen scaffold. We found that the RECs and fibroblast co-existed in the same area and the RECs had not yet migrated to form a separate layer (Figure 4).
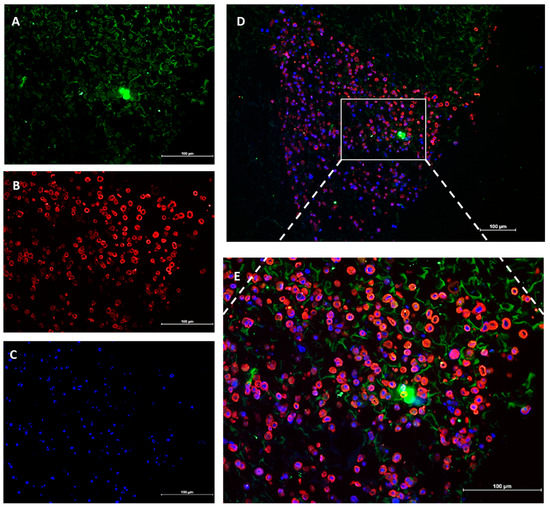
Figure 4.
The top view of the cell-seeded collagen scaffold immunostained with (A) anti-Col type I antibody (green) as the fibroblast marker and (B) anti-cytokeratin 14 antibody (red) as the marker of RECs. (C). The cell nuclei were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). (D) Merged image 10× and (E) 20×. (A). The anti-Col type I antibody was found to bind to the scaffold in addition to the fibroblasts. The intense green color located almost centrally (A) formed due to the accumulation of green fluorescence in unknown debris.
The ultrastructural study of the 3D human RE construct revealed that 4 weeks post cell seeding, the seeded cells had formed a confluent layer on top of the scaffold and were actively secreting extracellular matrix (Figure 5). The SEM results showed collagen degradation, which indicated the biodegradability of the scaffold. However, no cilia were found on the cells, which showed that the RE layer was not yet functional.
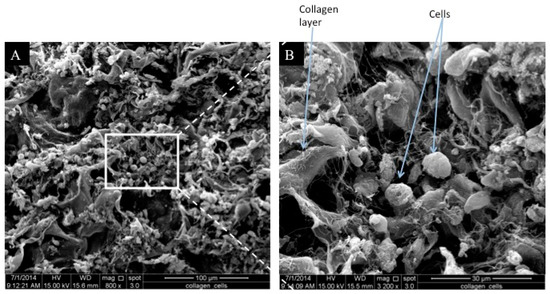
Figure 5.
The SEM observation (top view) of cell-seeded 3D human RE construct: (A) 300× and (B) 1600×. The cells were found to be confluent and were covering the entire scaffold 4 weeks post cell seeding. Collagen fibrils were also observed, which indicated collagen degradation. No cilia were detected on the seeded RECs.
4. Discussion
Tissue engineering of respiratory epithelium refers to the organization of airway cells (RECs and fibroblasts) into a controlled and specific arrangement that resembles the native respiratory epithelium, both structurally and functionally [34]. For the development of functional 3D epithelial tissue, incorporation of these three factors is essential: (1) a basal lamina equivalent, consisting of collagen fibers for cell–cell interactions and cell polarization; (2) extracellular factors of mesenchymal fibroblasts; and (3) an air–liquid interface culture system for the proliferation and mucocillary differentiation of the epithelial cells [35,36]. Homeostasis in any tissue is achieved via complex interactions between different types of cells and their growth bed in the form of the extracellular matrix. It is widely thought that maintaining the in vivo homeostasis in respiratory tissues mostly relies on cellular and molecular signaling between the RECs and fibroblasts; therefore, co-culturing of fibroblasts and RECs is crucial for the successful engineering of 3D respiratory epithelial constructs [37]. The presence of fibroblast markedly speeds up the growth of epithelial cells as the growth factors secreted by fibroblasts stimulate epithelial cell migration, proliferation, and differentiation [38,39].
It was previously reported that the growth and relative level of differentiation in human RECs are much better on porous compared to the solid structures [40]. Optimal cell infiltration and adhesion depend on the scaffold pore size [41] and pore density. Notably, for successful engineering of 3D human RE constructs, a sophisticated pore size range and a balanced porosity are essential. Cells can span the adjacent cells and the pores by elongation; therefore, to promote multi-layered epithelial tissue formation with an architecture similar to the native one, the pores must be small enough to prevent tracheal epithelial cells migration and ingrowth into the scaffold [42]. Endothelial cells infiltration into the scaffold is necessary as well for revascularization and the successful engraftment of tissue construct in vivo. It was previously shown that pore sizes within a range of 40 to 600 μm are favorable for capillary ingrowth [43,44]. Respiratory epithelial cells cultured on high pore density (HPD) inserts (1 × 108 pores/cm2) formed columnar, pseudostratified epithelium with a well-defined single layer of basal cells and a higher number of ciliated cells, which more resembled the native epithelium. In contrast, epithelial cells grown on low density (LPD) inserts (2 × 106 pores/cm2) tend to form stratified squamous epithelium with a lower number of ciliated cells; hence, they failed to replicate the native epithelium morphology. Application of HPD inserts resulted in the formation of significantly thicker epithelium compared to that formed with LPD inserts [45]. Our findings showed the formation of a confluent layer of co-cultured RECs and fibroblasts on the surface of the porous freeze-dried collagen type I scaffold, which indicated the presence of favorable porosity and pore size for RECs and fibroblasts to form a multilayer structure. The introduced tissue-engineered RE construct, which has a pore size ranging from 100 to 200 μm, is suitable for capillary ingrowth and vascularization, demonstrating the potential in vivo application suitability of the 3D construct.
Mechanical stabilization and the ability to withstand physiological pressure changes imposed by the process of respiration [46], are other factors that are essential in airway epithelium engineering and its eventual transplantation, since the ultimate goal of any tissue-engineered construct is their application in the clinical context. In an in vivo application, collagen-based scaffold would degrade completely, enabling the integration of the implanted tissue to the native tissue. The application of a scaffold fabricated from non-resorbable materials, such as titanium mesh, often results in unfavorable outcomes such as chronic inflammatory response with extrusion and granulation tissue [47,48]. In contrast, the resorbable property of the collagen scaffold and its similar properties to tracheal cartilage makes it an ideal material for use in airway epithelium tissue engineering. Davenport and Nettesheim reported that besides collagen improving the adhesion of cells to the surface of the scaffold, it enhances and accelerates ciliogenesis in RECs. Most probably, the favorable influence of collagen type I in tracheal epithelial differentiation occurs via modulating cell morphology, modulating the nutrition access to the cells, and the regulation of cilia-specific gene expression by cell–ECM interactions [49]. Our fabricated 3D collagen scaffold showed favorable characteristics in terms of stability and biodegradability and in supporting the attachment of seeded RECs and fibroblasts, as apparent in the histological and immunohistochemical staining results. The biodegradability of our 3D collagen scaffold demonstrated in the SEM results indicated that it is an ideal option and allows integration of 3D human RE construct into the native tissue in in vivo applications.
Tracheal ciliated epithelial cells are located only at the luminal surface of the tracheal epithelium and they are specialized cells that are responsible for transporting secretions across the airway [50] and maintaining the airway’s immunoprotection [29]. Whether achieved via transdifferentiation of primary cells or through ciliogenesis in undifferentiated mesenchymal stem cells, having epithelial cells with intact cilia is a critical factor for the successful engineering of tracheal epithelial tissue. Even though human primary respiratory epithelium is the best choice for studying airway epithelium [51], in the case of using primary epithelial cells, there is a high possibility of cilia de-differentiation [52] and impairment or loss during cells cryopreservation [50]. However, culturing cells at the ALI is a well-established technique [53,54] in cell culture, which stimulates cilia regeneration after the native cilia have been lost [50]. In a recent study, nasal epithelial cells grown in ALI conditions were shown to well-represent their in vivo counterparts in terms of transcriptome and gene expression profile [55]. In another study, culturing nasal epithelial cells on a porous support using the ALI cell culture model stimulated the ciliary differentiation of nasal epithelial cells. However, in the classical submerged single-layer culture model, ciliary differentiation of epithelial cells did not occur [56]. The ALI cell culture model enabled the cells to undergo mucociliary differentiation [57]; this is of more importance regarding therapeutic application to enable the stem cells or progenitor cells to differentiate into the correct phenotype. The co-orientation of differentiated cells into a functional assembly of tissues is another vital prerequisite for successful and functional engineered tissue [58]. In our study, ciliogenesis in seeded RECs did not occur fully over the period of 4 weeks in the ALI culture system, which suggested that an extended ALI culture period is needed.
Many studies have indicated the beneficial effect of retinoic acid (RA) in promoting ciliogenesis, maintaining the mucociliary status of tracheal epithelial cells, and enhancing the morphological and functional aspects of regenerating cilia [59]. Therefore, supplementing culture media with retinoid as a ciliogenic regulatory element [60] can improve the ciliation of seeded RECs on the collagen scaffold. Moreover, Luengen et al. showed in 2020 that a balanced combination of RA and vascular endothelial growth factor, epidermal growth factor, and fibroblast growth factor β in culture medium plays a critical role in the differentiation status of respiratory epithelium; it needs to be considered in future studies [29].
5. Conclusions
We successfully isolated and purified collagen type I from sheep tendon and freeze-dried it into collagen sponge with suitable pore size and biostability. Both the human RECs and nasal fibroblasts were found to attach, proliferate, and form a confluent layer on the scaffold. Even though the RECs did not show the presence of cilia, which implied that this tissue was not yet functional, this can be overcome by extending the period of ALI culturing and RA supplementation. Bovine collagen was proved to be clinically safe [61], and since autologous cells (REC and fibroblasts) can be used for the production of tissue-engineered epithelium, there would be no risk of immunogenicity and graft rejection by the recipient. Therefore, the proposed in vitro respiratory epithelium 3D construct is a potential candidate for use as tissue-engineered epithelium in respiratory epithelium reconstructive surgeries in the future and can serve as an in vitro model for drug testing.
Author Contributions
Conceptualization, Y.L., R.B.H.I., A.B.S., and M.H.M.Y.; methodology, Y.L., M.B.F., and R.C.M.; software, Y.L.; validation Y.L. and M.H.M.Y.; formal analysis, Y.L.; investigation, Y.L.; resources, R.B.H.I., A.B.S., and M.H.M.Y.; data curation, Y.L.; writing—original draft preparation, Y.L. and Z.R.; writing—review and editing, Z.R. and M.H.M.Y.; visualization, Y.L.; supervision, M.H.M.Y.; project administration, M.H.M.Y. and R.B.H.I.; funding acquisition, M.H.M.Y., R.B.H.I., and A.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Kebangsaan Malaysia under research university grants GUP-2013-021 and GGPM-2015-035.
Institutional Review Board Statement
The study was conducted according to and approved by the Institutional Ethics Committee at the University of Kebangsaan Malaysia with approval reference number UKM1.5.3.5/244/GGPM-2015-035.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, which their nasal turbinate was collected.
Conflicts of Interest
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yuksel, H.; Turkeli, A. Airway epithelial barrier dysfunction in the pathogenesis and prognosis of respiratory tract diseases in childhood and adulthood. Tissue Barriers 2017, 5, e1367458. [Google Scholar] [CrossRef] [PubMed]
- Toppila-Salmi, S.; van Drunen, C.M.; Fokkens, W.J.; Golebski, K.; Mattila, P.; Joenvaara, S.; Renkonen, J.; Renkonen, R. Molecular mechanisms of nasal epithelium in rhinitis and rhinosinusitis. Curr. Allergy Asthma Rep. 2015, 15, 495. [Google Scholar] [CrossRef] [PubMed]
- Saeed, O.; Boyer, N.L.; Pamplin, J.C.; Driscoll, I.R.; Dellavolpe, J.; Cannon, J.; Cancio, L.C. Inhalation injury and toxic industrial chemical exposure. Mil. Med. 2018, 183, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Puddicombe, S.M.; Field, S.; Haywood, J.; Broughton-Head, V.; Puxeddu, I.; Haitchi, H.M.; Vernon-Wilson, E.; Sammut, D.; Bedke, N.; et al. Defective epithelial barrier function in asthma. J. Allergy Clin. Immunol. 2011, 128. [Google Scholar] [CrossRef]
- Puchelle, E.; Zahm, J.-M.; Tournier, J.-M.; Coraux, C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006, 3, 726–733. [Google Scholar] [CrossRef]
- Jungebluth, P.; Go, T.; Asnaghi, M.A.; Bellini, S.; Martorell, J.; Calore, C.; Urbani, L.; Ostertag, H.; Mantero, S.; Conconi, M.T.; et al. Structural and morphologic evaluation of a novel detergent–enzymatic tissue-engineered tracheal tubular matrix. J. Thorac. Cardiovasc. Surg. 2009, 138, 586–593. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue engineering approaches in the design of healthy and pathological in vitro tissue models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Heikal, M.Y.; Aminuddin, B.S.; Jeevanan, J.; Chen, H.C.; Sharifah, S.; Ruszymah, B.H. A scanning electron microscopic study of in vivi tissue engineered respiratory epithelium in sheep. Med. J. Malays. 2008, 63, 34. [Google Scholar]
- Yunus, M.H.M.; Aminuddin, B.; Jeevanan, J.; Chen, H.C.; Sharifah, S.; Ruszymah, B. Autologous implantation of bilayered tissue-engineered respiratory epithelium for tracheal mucosal regenesis in a sheep model. Cells Tissues Organs 2010, 192, 292–302. [Google Scholar] [CrossRef]
- Heikal, M.; Roy Chowdhury, S.; Busra, M.; Aminuddin, B.; Ruszymah, B. Quality evaluation of human tissue engineered respiratory epithelium constructs. Regen. Res. 2012, 1, 61. [Google Scholar]
- Aoki, F.G.; Varma, R.; Marin-Araujo, A.E.; Lee, H.; Soleas, J.P.; Li, A.H.; Soon, K.; Romero, D.; Moriya, H.T.; Haykal, S.; et al. De-epithelialization of porcine tracheal allografts as an approach for tracheal tissue engineering. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Wong, M.; Griffiths, L. Immunogenicity in xenogeneic scaffold generation: Antigen removal versus decellularization. Acta Biomater. 2014, 10, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.; Guedes, G.; Sousa, F.L.; Freire, C.S.R.; Santos, H.A. Latest advances on bacterial cellulose-based materials for wound healing, delivery systems, and tissue engineering. Biotechnol. J. 2019, 14, e1900059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cao, J.; Zhao, S.; Luo, H.; Yang, Z.; Gama, M.; Zhang, Q.; Su, D.; Wan, Y. Biocompatibility evaluation of bacterial cellulose as a scaffold material for tissue-engineered corneal stroma. Cellulose 2020, 27, 2775–2784. [Google Scholar] [CrossRef]
- Kumbhar, J.V.; Jadhav, S.H.; Bodas, D.S.; Barhanpurkar-Naik, A.; Wani, M.R.; Paknikar, K.M.; Rajwade, J.M. In vitro and in vivo studies of a novel bacterial cellulose-based acellular bilayer nanocomposite scaffold for the repair of osteochondral defects. Int. J. Nanomed. 2017, 12, 6437–6459. [Google Scholar] [CrossRef]
- Akaraonye, E.; Filip, J.; Safarikova, M.; Salih, V.; Keshavarz, T.; Knowles, J.C.; Roy, I. Composite scaffolds for cartilage tissue engineering based on natural polymers of bacterial origin; thermoplastic Poly(3- hydroxybutyrate) and micro-fibrillated bacterial cellulose. Polym. Int. 2016, 65, 780–791. [Google Scholar] [CrossRef]
- Miyamoto, T.; Takahashi, S.I.; Ito, H.; Inagaki, H.; Noishiki, Y. Tissue biocompatibility of cellulose and its derivatives. J. Biomed. Mater. Res. 1989, 23, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Märtson, M.; Viljanto, J.; Hurme, T.; Laippala, P.; Saukko, P. Is cellulose sponge degradable or stable as implantation material? An in vivo subcutaneous study in the rat. Biomaterials 1999, 20, 1989–1995. [Google Scholar] [CrossRef]
- Niknejad, H.; Peirovi, H.; Jorjani, M.; Ahmadiani, A.; Ghanavi, J.; Seifalian, A.M. Properties of the amniotic membrane for potential use in tissue engineering. Eur. Cells Mater. 2008, 15, 88–99. [Google Scholar] [CrossRef]
- Gobinathan, S.; Zainol, S.S.; Azizi, S.F.; Iman, N.M.; Muniandy, R.; Hasmad, H.N.; Bin Yusof, M.R.; Husain, S.; Aziz, H.A.; Lokanathan, Y. Decellularization and genipin crosslinking of amniotic membrane suitable for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 2051–2067. [Google Scholar] [CrossRef] [PubMed]
- Busra, F.M.; Lokanathan, Y.; Nadzir, M.M.; Saim, A.; Idrus, R.H.; Chowdhury, S.R. Attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen scaffolds: A comparative study. Malays. J. Med. Sci. 2017, 24, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Pageau, S.C.; Sazonova, O.V.; Wong, J.Y.; Soto, A.M.; Sonnenschein, C. The effect of stromal components on the modulation of the phenotype of human bronchial epithelial cells in 3D culture. Biomaterials 2011, 32, 7169–7180. [Google Scholar] [CrossRef]
- Moiemen, N.; Yarrow, J.; Hodgson, E.; Constantinides, J.; Chipp, E.; Oakley, H.; Shale, E.; Freeth, M. Long-term clinical and histological analysis of integra dermal regeneration template. Plast. Reconstr. Surg. 2011, 127, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Mh Busra, F.; Rajab, N.F.; Tabata, Y.; Saim, A.B.; BHIdrus, R.; Chowdhury, S.R. Rapid treatment of full-thickness skin loss using ovine tendon collagen type I scaffold with skin cells. J. Tissue Eng. Regen. Med. 2019, 13, 874–891. [Google Scholar] [CrossRef] [PubMed]
- Koláčná, L.; Bakešová, J.; Varga, F.; Košťáková Kuželova, E.; Plánka, L.; Nečas, A.; Lukáš, D.; Amler, E.; Pelouch, V. Biochemical and biopgysical aspects of collagen nanostructure in the extracellular matrix. Physiol. Res. 2007, 56, 51–60. [Google Scholar]
- Safshekan, F.; Tafazzoli-Shadpour, M.; Abdouss, M.; Shadmehr, M.B. Mechanical characterization and constitutive modeling of human trachea: Age and gender dependency. Materials 2016, 9, 456. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Machino, R.; Taniguchi, D.; Tetsuo, T.; Takeoka, Y.; Takeoka, Y.; Oyama, S.; Moriyama, M.; Taura, Y.; Yamasaki, N.; et al. Tracheal replacement using a bio-3D printed scaffold-free engineered tissue based trachea. Am. J. Respir. Crit. Care Med. 2018, 197, A7722. [Google Scholar]
- Park, J.-H.; Yoon, J.-K.; Lee, J.B.; Shin, Y.M.; Lee, K.-W.; Bae, S.-W.; Lee, J.; Yu, J.; Jung, C.-R.; Youn, Y.-N.; et al. Experimental tracheal replacement using 3-dimensional bioprinted artificial trachea with autologous epithelial cells and chondrocytes. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luengen, A.E.; Kniebs, C.; Buhl, E.M.; Cornelissen, C.G.; Schmitz-Rode, T.; Jockenhoevel, S.; Thiebes, A.L. Choosing the right differentiation medium to develop Mucociliary phenotype of primary nasal epithelial cells in vitro. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.; Ruszymah, B.; Chowdhury, S. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C 2016, 68, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Yunus, M.H.M.; Shuid, A.N.; Busra, M.F.; Chua, K.H.; Ghafar, N.A.; Rani, R.A. The effect of stichopus chloronotus aqueous extract on human osteoarthritis articular chondrocytes in three-dimensional collagen Type I hydrogel in vitro. Sains Malays. 2019, 48, 1671–1683. [Google Scholar] [CrossRef]
- Man, R.C.; Lokanathan, Y.; Razali, R.A.; Chowdury, S.R.; Bin Saim, A.; Idrus, R.B.H. Nasal fibroblast conditioned medium promotes cell attachment and migration of human respiratory epithelium. Sains Malays. 2020, 49, 429–437. [Google Scholar] [CrossRef]
- Rabiatul, A.R.; Lokanathan, Y.; Rohaina, C.M.; Chowdhury, S.R.; Aminuddin, B.S.; Ruszymah, B.H. Surface modification of electrospun poly(methyl methacrylate) (PMMA) nanofibers for the development of in vitro respiratory epithelium model. J. Biomater. Sci. Polym. Ed. 2015, 26, 1297–1311. [Google Scholar] [CrossRef] [PubMed]
- Soleas, J.P.; Paz, A.; Marcus, P.; McGuigan, A.; Waddell, T.K. Engineering airway epithelium. J. Biomed. Biotechnol. 2012, 2012, 982971. [Google Scholar] [CrossRef] [PubMed]
- Roomans, G.M. Tissue engineering and the use of stem/progenitor cells for airway epithelium repair. Eur. Cell Mater. 2010, 19, 284–299. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Zhao, Y.; Chang, M. Growth and differentiation of conducting airway epithelial cells in culture. Eur. Respir. J. 1997, 10, 2398–2403. [Google Scholar] [CrossRef] [PubMed]
- Walimbe, T.; Panitch, A.; Sivasankar, M.P. An in vitro scaffold-free epithelial-fibroblast coculture model for the larynx. Laryngoscope 2017, 127, E185–E192. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Nomoto, Y.; Suzuki, T.; Tada, Y.; Miyake, M.; Hazama, A.; Kanemaru, S.; Nakamura, T.; Omori, K. Effect of fibroblasts on tracheal epithelial regeneration in vitro. Tissue Eng. 2006, 12, 2619–2628. [Google Scholar] [CrossRef]
- Kobayashi, K.; Suzuki, T.; Nomoto, Y.; Tada, Y.; Miyake, M.; Hazama, A.; Wada, I.; Nakamura, T.; Omori, K. A tissue-engineered trachea derived from a framed collagen scaffold, gingival fibroblasts and adipose-derived stem cells. Biomaterials 2010, 31, 4855–4863. [Google Scholar] [CrossRef]
- Widdicombe, J.; Sachs, L.; Finkbeiner, W. Effects of growth surface on differentiation of cultures of human tracheal epithelium. In Vitro Cell. Dev. Biol. Anim. 2003, 39, 51–55. [Google Scholar] [CrossRef]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials 2004, 25, 1077–1086. [Google Scholar] [CrossRef]
- Xue, W.; Krishna, B.V.; Bandyopadhyay, A.; Bose, S. Processing and biocompatibility evaluation of laser processed porous titanium. Acta Biomater. 2007, 3, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Crowley, C.; Klanrit, P.; Butler, C.R.; Varanou, A.; Platé, M.; Hynds, R.E.; Chambers, R.C.; Seifalian, A.M.; Birchall, M.A.; Janes, S.M. Surface modification of a POSS-nanocomposite material to enhance cellular integration of a synthetic bioscaffold. Biomaterials 2016, 83, 283–293. [Google Scholar] [CrossRef] [PubMed]
- De Mel, A.; Punshon, G.; Ramesh, B.; Sarkar, S.; Darbyshire, A.; Hamilton, G.; Seifalian, A.M. In situ endothelialisation potential of a biofunctionalised nanocomposite biomaterial-based small diameter bypass graft. Biomed. Mater. Eng. 2009, 19, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Cozens, D.; Grahame, E.; Sutherland, E.; Taylor, G.; Berry, C.C.; Davies, R.L. Development and optimization of a differentiated airway epithelial cell model of the bovine respiratory tract. Sci. Rep. 2018, 8, 853. [Google Scholar] [CrossRef] [PubMed]
- Maughan, E.F.; Hynds, R.E.; Proctor, T.J.; Janes, S.M.; Elliott, M.; Birchall, M.A.; Lowdell, M.W.; De Coppi, P. Autologous cell seeding in tracheal tissue engineering. Curr. Stem Cell Rep. 2017, 3, 279–289. [Google Scholar] [CrossRef]
- Daneshi, A.; Mohammadi, S.; Hassannia, F. Delayed laryngotracheal reconstruction with titanium plate: Report of 10 cases. J. Voice 2010, 24, 755–757. [Google Scholar] [CrossRef]
- Yener, M.; Acar, G.O.; Cansiz, H.; Oz, B.; Cigerciogullari, E.; Seymen, O. Use of titanium mesh in laryngotracheal reconstruction: An experimental study on rabbits. Eur. Arch. Oto-Rhino-Laryngol. 2010, 267, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Davenport, E.A.; Nettesheim, P. Regulation of mucociliary differentiation of rat tracheal epithelial cells by type I collagen gel substratum. Am. J. Respir. Cell Mol. Biol. 1996, 14, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wang, Y.; Yuan, W.; Wong, L.B. Ciliogenesis in cryopreserved mammalian tracheal epithelial cells cultured at the air–liquid interface. Cryobiology 2009, 59, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Papazian, D.; Würtzen, P.A.; Hansen, S. Polarized airway epithelial models for immunological co-culture studies. Int. Arch. Allergy Immunol. 2016, 170, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Butler, C.R.; Hynds, R.E.; Gowers, K.H.C.; Lee, D.D.H.; Brown, J.M.; Crowley, C.; Teixeira, V.H.; Smith, C.M.; Urbani, L.; Hamilton, N.J.; et al. Rapid expansion of human epithelial stem cells suitable for airway tissue engineering. Am. J. Respir. Crit. Care Med. 2016, 194, 156–168. [Google Scholar] [CrossRef] [PubMed]
- De Jong, P.M.; Van Sterkenburg, M.A.; Hesseling, S.C.; Kempenaar, J.A.; Mulder, A.A.; Mommaas, A.M.; Dijkman, J.H.; Ponec, M. Ciliogenesis in human bronchial epithelial cells cultured at the air-liquid interface. Am. J. Respir. Cell. Mol. Biol. 1994, 10, 271–277. [Google Scholar] [CrossRef]
- Antunes, M.B.; Woodworth, B.A.; Bhargave, G.; Xiong, G.; Aguilar, J.L.; Ratner, A.J.; Kreindler, J.L.; Rubenstein, R.C.; Cohen, N.A. Murine nasal septa for respiratory epithelial air-liquid interface cultures. Biotechniques 2007, 43, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Park, B.; Bhowmik, D.; Nishida, K.; Lauver, M.; Putcha, N.; Gao, P.; Ramanathan, M., Jr.; Hansel, N.N.; Biswal, S.; et al. Strong correlation between air-liquid interface cultures and in vivo transcriptomics of nasal brush biopsy. Am. J. Physiol. Cell. Mol. Physiol. 2020, 318, L1056–L1062. [Google Scholar] [CrossRef]
- Yeh, T.-H.; Tsai, C.-H.; Chen, Y.-S.; Hsu, W.-C.; Cheng, C.-H.; Hsu, C.-J.; Lee, S.-Y. Increased communication among nasal epithelial cells in air-liquid interface culture. Laryngoscope 2007, 117, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Even-Tzur, N.; Jaffa, A.; Gordon, Z.; Gottlieb, R.; Kloog, Y.; Einav, S.; Wolf, M.; Elad, D. Air–liquid interface culture of nasal epithelial cells on denuded amniotic membranes. Cell. Mol. Bioeng. 2010, 3, 307–318. [Google Scholar] [CrossRef]
- Cortiella, J.; Nichols, J.E.; Kojima, K.; Bonassar, L.J.; Dargon, P.; Roy, A.K.; Vacant, M.P.; Niles, J.A.; Vacanti, C.A. Tissue-engineered lung: An in vivo and in vitro comparison of polyglycolic acid and pluronic F-127 hydrogel/somatic lung progenitor cell constructs to support tissue growth. Tissue Eng. 2006, 12, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Erickson, V.R.; Antunes, M.; Chen, B.; Cohen, N.A.; Hwang, P.H. The effects of retinoic acid on ciliary function of regenerated sinus mucosa. Am. J. Rhinol. 2008, 22, 334–336. [Google Scholar] [CrossRef]
- Chowdhury, P.; Powell, R.T.; Stephan, C.; Uray, I.P.; Talley, T.; Karki, M.; Tripathi, D.N.; Park, Y.S.; Mancini, M.A.; Davies, P.; et al. Bexarotene—A novel modulator of AURKA and the primary cilium in VHL-deficient cells. J. Cell Sci. 2018, 131, jcs219923. [Google Scholar] [CrossRef]
- Shieh, H.F.; Graham, C.D.; Brazzo, J.A., III; Zurakowski, D.; Fauza, D.O. Comparisons of human amniotic mesenchymal stem cell viability in FDA-approvedcollagen-based scaffolds: Implications for engineered diaphragmatic replacement. J. Pediatr. Surg. 2017, 52, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).