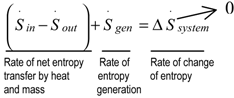
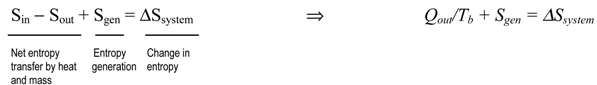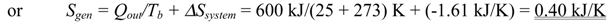Energy, Entropy and Exergy Concepts and Their Roles in Thermal Engineering
Abstract
:Introduction
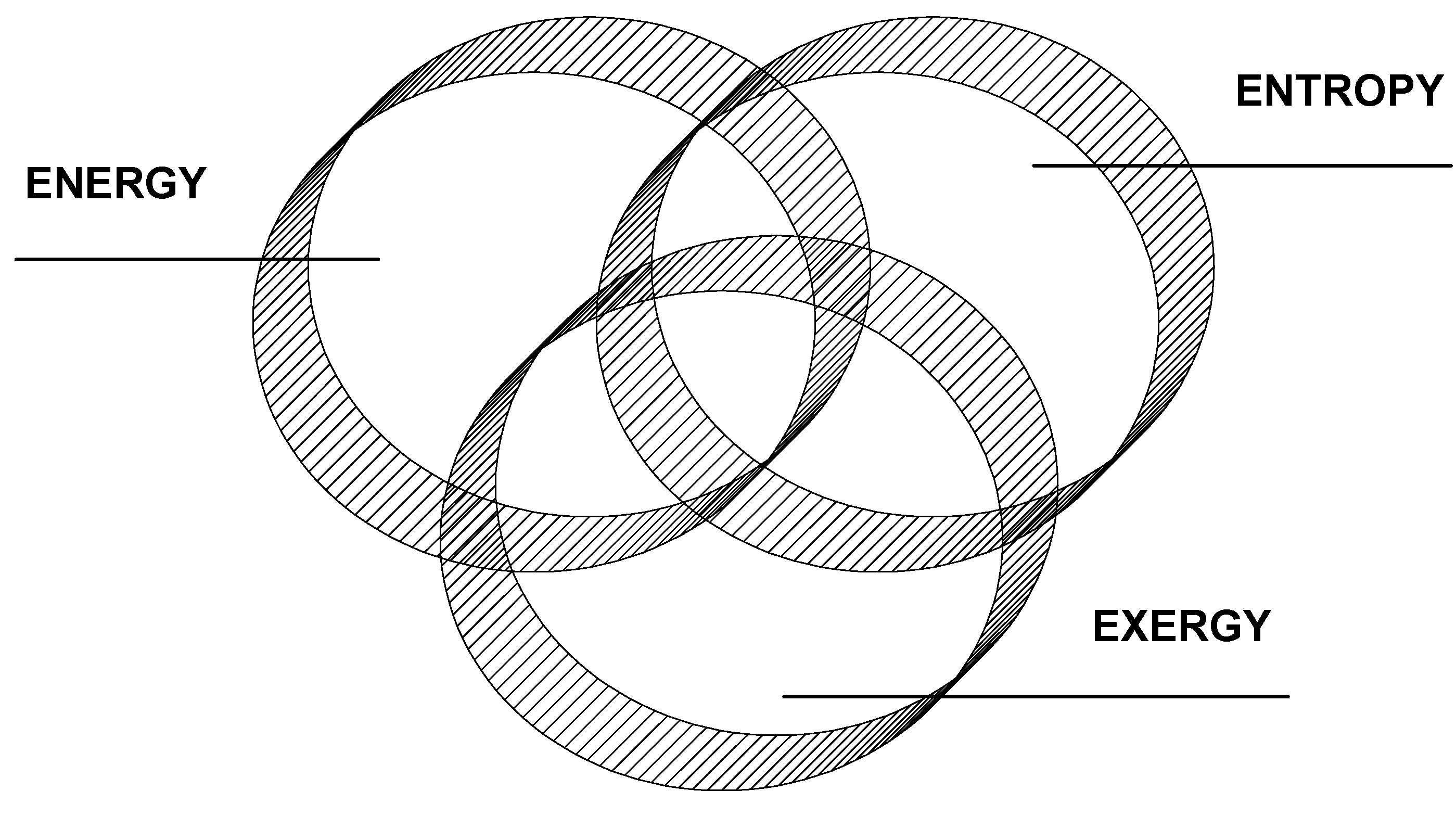
2. Energy
2.1 Introduction
2.2 Concept of Energy
2.3 Forms of Energy
- The macroscopic forms of energy are those where a system possesses as a whole with respect to some outside reference frame such as kinetic and potential energies. For example, the macroscopic energy of an upmoving object changes with velocity and elevation. The macroscopic energy of a system is related to motion and the influence of some external effects such as gravity, magnetism, electricity and surface tension. The energy that a system possesses as a result of its motion relative to some reference frame is called kinetic energy. The energy that a system has as a result of its elevation in a gravitational field is called potential energy. Kinetic energy refers to the energy possessed by the system because of its overall motion, either translational or rotational. The word "overall" is italicized because the kinetic energy to which we refer is the kinetic energy of the entire system, not the kinetic energy of the molecules in the system. If the system is a gas, the kinetic energy is the energy due to the macroscopic flow of the gas, not the motion of individual molecules. The potential energy of a system is the sum of the gravitational, centrifugal, electrical, and magnetic potential energies. To illustrate using gravitational potential energy, a one-kilogram mass, 100 m above the ground, clearly has a greater potential energy than the same kilogram mass on the ground. That potential energy can be converted into other forms of energy, such as kinetic energy, if the mass is allowed to fall freely. Kinetic and potential energy depend on the environment in which the system exists. In particular, the potential energy of a system depends on the choice of a zero level. For example, if the ground level is considered to be at zero potential energy, then the potential energy of the mass at 100 m above the ground will have a positive potential energy equal to the mass (1 kg) multiplied by the gravitational constant (g = 9.807 m/s2 at sea level) and the height above the ground (100 m). Its potential energy will be 980.7 (kgm2)/s2 = 980.7 Newton-meters (Nm), that is, 980.7 J. The datum plane for potential energy can be chosen arbitrarily. If it had been chosen at 100 m above the ground level, the potential energy of the mass would have been zero. Of course, the difference in potential energy between the mass at 100 m and the mass at ground level is the same independent of the datum plane.
- The microscopic forms of energy are those related to the molecular structure of a system and the degree of the molecular activity, and they are independent of outside reference frames. The sum of all the microscopic forms of energy is called the internal energy of a system. The internal energy of a system depends on the inherent qualities, or properties, of the materials in the system, such as composition and physical form, as well as the environmental variables (temperature, pressure, electric field, magnetic field, etc.). Internal energy can have many forms, including, sensible and latent (i.e., thermal), chemical, nuclear, electrical, mechanical, magnetic, and surface energy. For example, a spring that is compressed has a higher internal energy (mechanical energy) than a spring that is not compressed, because the compressed spring can do some work on changing (expanding) to the uncompressed state. As another example, consider two identical vessels, each containing hydrogen and oxygen. In the first, the gases are contained in the elemental form, pure hydrogen and pure oxygen in a ratio of 2:1. In the second, the identical number of atoms is contained, but in the form of water. One can appreciate that the internal energy of the first is different from the second. A spark set off in the first container will result in a violent release of energy. The same will not be true in the second. Clearly, the internal energy present differs in these two situations. Any energy balance will have to take this difference into account.
2.4 The First law of Thermodynamics (FLT)
2.5 FLT vs Energy


3. Entropy
3.1 Introduction
3.2 Entropy Aspects
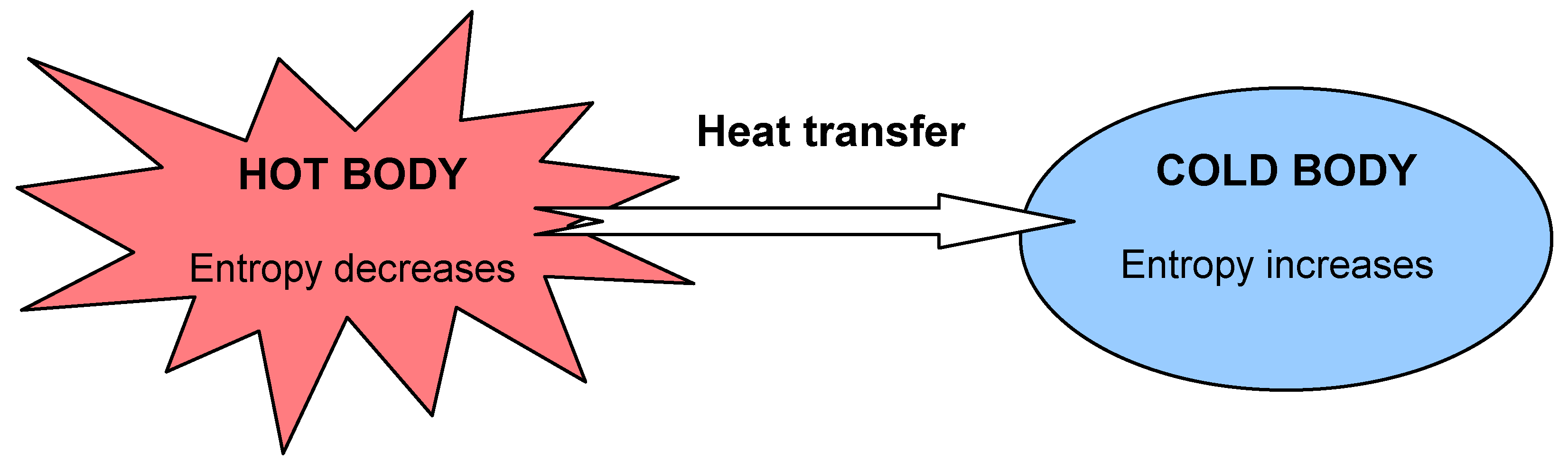
- The entropy of a system is a measure of the amount of molecular disorder within the system.
- A system can only generate, not destroy, entropy.
- The entropy of a system can be increased or decreased by energy transports across the system boundary.
3.3 Significance of Entropy
3.4 Carnot's Contribution
3.5 The Second Law of Thermodynamics (SLT)
3.6 SLT Statements
- The Clausius statement. It is impossible for a system to transfer heat from a lower temperature reservoir to a higher temperature reservoir. Simply, heat transfer can only occur spontaneously in the direction of temperature decrease. For example, we can not construct a refrigerator that operates without any work input.
- The Kelvin-Planck statement. It is impossible for a system to receive a given amount of heat from a high-temperature reservoir and provide an equal amount of work output. While a system converting work to an equivalent energy transfer as heat is possible, a device converting heat to an equivalent energy transfer as work is impossible. For example, we can not build a heat engine that has a thermal efficiency of 100%.
3.7 The Clausius Inequality
- Sgen = ΔStotal = ΔSsys + ΔSsurr = 0 for a reversible process.
- Sgen = ΔStotal = ΔSsys + ΔSsurr > 0 for an irreversible process.
3.8 UsefulRelationships
- δq = du + δw: This is a statement of the FLT and is applicable to any simple compressible closed system.
- δq = du + pdv: This is a statement of the FLT and is restricted to reversible processes of a closed system only.
- Tds = du + δw: This is a combined statement of the FLT and SLT (with Tds = δq).
- Tds = du + pdv: This is a combined statement of the FLT and SLT and is valid for all processes between equilibrium states.
4. Exergy
4.1 Introduction
4.2 Exergy Aspects
- A system in complete equilibrium with its environment does not have any exergy. No difference appears in temperature, pressure, or concentration etc. for running any processes.
- The more a system deviates from the environment, the more exergy it carries. Hot water has a higher content of exergy during the winter than it has on a hot summer day. A block of ice carries hardly any exergy in winter while it does in summer.
- When the energy loses its quality, it results in exergy destroyed. The exergy is the part of the energy which is useful in the society and therefore has an economic value and is worth taking care of.
- Almost all energy, converted in the thin layer on the earth’s surface, where life can be found, derives from the sun. Sunlight, rich in exergy, reaches the earth. A lot of it is reflected but the energy absorbed on the earth is converted and finally leaves the earth as heat radiation with no exergy relative to the earth. The net exergy absorbed by the earth is consequently gradually destroyed but during this destruction it manages to drive the water/wind system and the life on earth. The green plants absorb exergy from the sunlight and convert it via photosynthesis into chemical exergy. The chemical exergy then passes through different food chains in the ecosystems. On every tropical level exergy is consumed and micro-organisms live on the last level in this food chain. There exists no waste.
- A concentrated deposit of mineral ‘contrasts’ with the environment and this contrast increases with the concentration of the mineral. The mineral is thus a carrier of exergy. When the mineral is mined the exergy content of the mineral is kept constant, and if it is enriched the exergy content increases. A poor deposit of mineral contains less exergy and can accordingly be utilised only through a larger input of external exergy. Today this substitution of exergy often comes from exergy forms such as coal and oil. When a concentrated mineral is dispersed the exergy content is decreased (item 3).
- An apparent difficulty in the definition of exergy is that it depends on the environment. This difficulty could, however, be solved through conventions, one could define a ‘standard environment’ with a given chemical composition at a certain temperature and pressure. A possible standard environment for global use could, for instance, be a standard atmosphere, a standard sea and a standard bed-rock. One principal problem is, however, that these systems are not in equilibrium with each other. Sometimes one should, in addition to this, use local standards depending on the season (item 2).
- An engineer designing a system is expected to aim for the highest possible technical efficiency at a minimum cost under the prevailing technical, economic and legal conditions, but also with regard to ethical, ecological and social consequences. Exergy is a concept that makes this work a great deal easier. Thus, exergetics offers a unique insight where losses and possible improvements can be determined, and Life Cycle Exergy Analysis (LCEA) is suggested as a method to better meet environmental conditions.
- It is a primary tool in best addressing the impact of energy resource utilization on the environment.
- It is an effective method using the conservation of mass and conservation of energy principles together with the second law of thermodynamics for the design and analysis of energy systems.
- It is a suitable technique for furthering the goal of more efficient energy-resource use, for it enables the locations, types, and true magnitudes of wastes and losses to be determined.
- It is an efficient technique revealing whether or not and by how much it is possible to design more efficient energy systems by reducing the inefficiencies in existing systems.
- It is a key component in obtaining sustainable development.
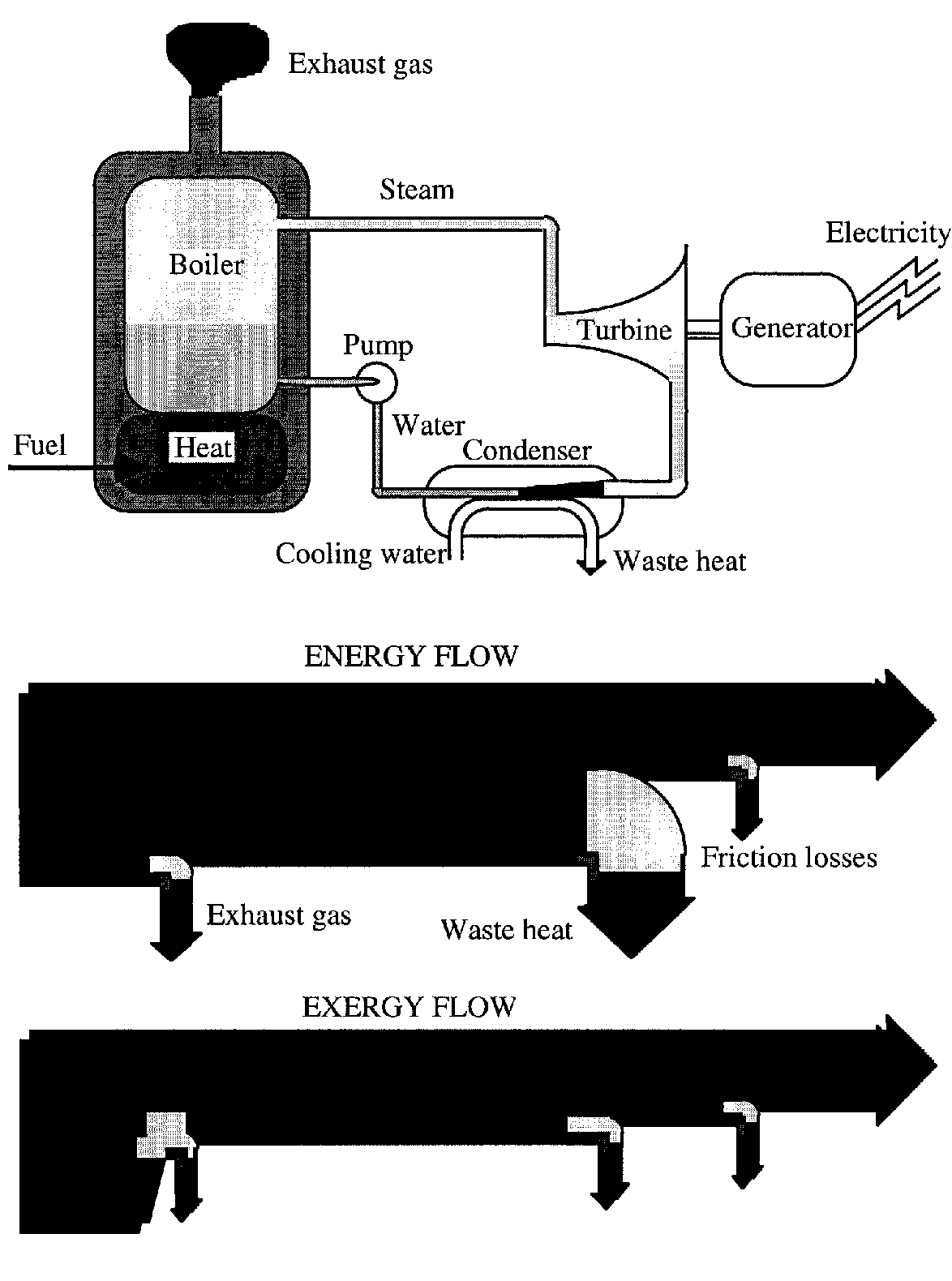
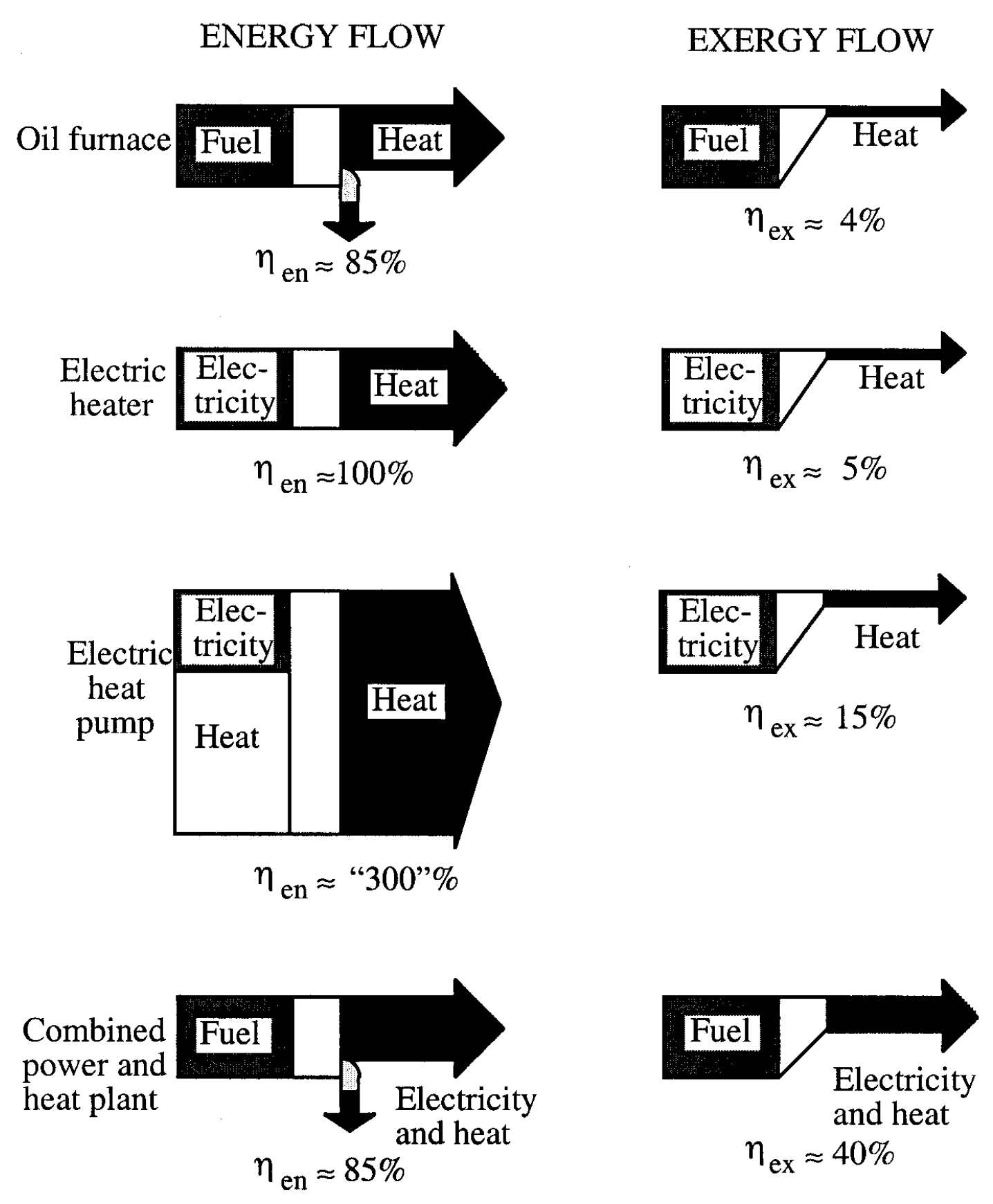
4.3 Exergy vs Energy
| ENERGY | EXERGY |
|---|---|
|
|
Example-I: for Difference Between Energy and Exergy Description
Example-II: Difference Between Energy and Exergy Description
4.4 Interdisciplinary Nature of Exergy
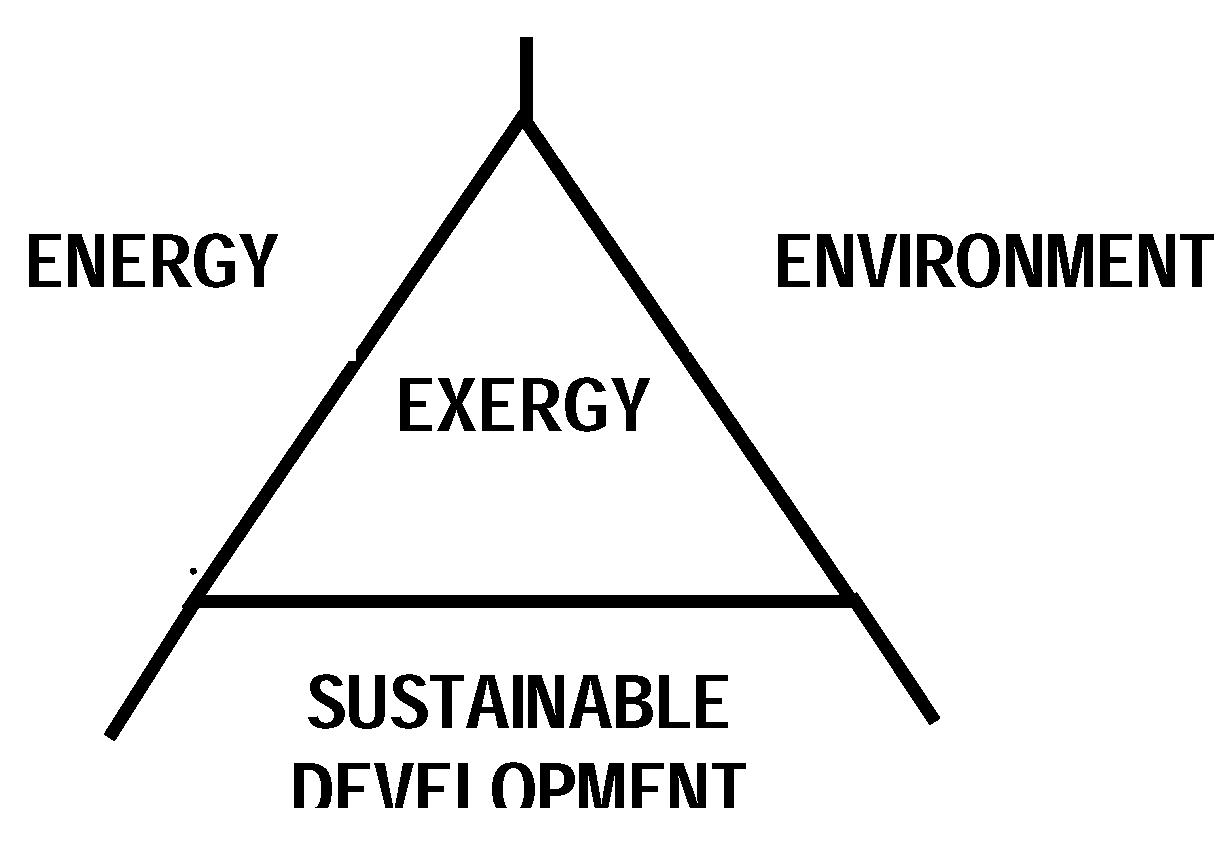
4.5 Exergy Analysis
4.6 Exergy Models
- Ex = T0(St,eq - St)
- Ex = U + P0V – T0S – Σi µi0ni
- Ex = (U – Ueq) + P0(V – Veq ) – T0 (S – Seq ) – Σi µi0(ni – nieq)
- Ex = RT0nln(c/c0)
- Ex = n[µ – µ0 + RT0ln(c/c0)]
4.7 Definition of Environment
- Natural-environment-subsystem models: These models attempt to simulate realistically subsystems of the natural environment. This model consists of saturated moist air and liquid water in phase equilibrium. An extension of that model was proposed to allow sulphur-containing materials to be analyzed. The temperature and pressure of this reference environment (Table 2) are normally taken to be 25°C and 1 atm, respectively, and the chemical composition is taken to consist of air saturated with water vapor, and the following condensed phases at 25°C and 1 atm: water (H2O), gypsum (CaSO4•2H2O), and limestone (CaCO3). The stable configurations of C, O and N respectively are taken to be those of CO2, O2 and N2 as they exist in air saturated with liquid water at T0 and P0 (the temperature and pressure for the reference environment); of hydrogen is taken to be in the liquid phase of water saturated with air at T0 and P0; and of S and Ca respectively are taken to be those of CaSO4•2H2O and CaCO3 at T0 and P0.
- Reference-substance models: With this model, a "reference substance" is selected for every chemical element and assigned zero exergy. This is the model in which the reference substances are selected as the most valueless substances found in abundance in the natural environment. The criterion for selecting such reference substances is consistent with the notion of simulating the natural environment, but is primarily economic in nature, and is vague and arbitrary with respect to the selection of reference substances. Part of this environment is the composition of moist air, including N2, O2, CO2, H2O and the noble gases; gypsum (for sulphur) and limestone (for calcium). This model is not similar to the natural environment. Consequently absolute exergies evaluated with this model do not relate to the natural environment, and can not be used rationally to evaluate efficiencies or environmental impact.
| T0 = 25°C = 298.15 K | |||
| P0 = 1 aim | |||
| Composition: | (i) Atmospheric air saturated with H2O at T0 and P0 (having the following composition): | ||
| Air Constituents | Mole Fraction | ||
| N2 | 0.7567 | ||
| O2 | 0.2035 | ||
| H2O | 0.0303 | ||
| Ar | 0.0091 | ||
| CO2 | 0.0003 | ||
| H2 | 0.0001 | ||
| (ii) The following condensed phases at T0 and P0: | |||
| Water (H2O) | |||
| Limestone (CaCO3) | |||
| Gypsum (CaSO4•2H2O) | |||
- Equilibrium and constrained-equilibrium models: It is the model in which all the materials present in the atmposphere, oceans and a layer of the crust of the earth are pooled together and an equilibrium composition is calculated for a given temperature. The selection of the thickness of crust considered is subjective and is intended to include all materials accessible to technical processes. Thicknesses varying from 1 m to 1000 m, and a temperature of 25°C were considered. For all thicknesses, the model differed significantly from the natural environment. Exergy values obtained using these environments are significantly dependent on the thickness of crust considered, and represent the absolute maximum amount of work obtainable from a material. Since there is no technical process available which can obtain this work from materials, this equilibrium model does not give meaningful exergy values when applied to the analysis of real processes. Its modified version of the equilibrium environment was proposed later in which the calculation of an equilibrium composition excludes the possibility of the formation of nitric acid (HNO3) and its compounds. That is, all chemical reactions in which these substances are formed are in constrained equilibrium, and all other reactions are in unconstrained equilibrium. When a thickness of crust of 1 m and temperature of 25°C are used, the model is similar to the natural environment.
- Process-dependent models: This is the model which contains only components that participate in the process being examined in a stable equilibrium composition at the temperature and pressure of the natural environment. This model is dependent on the process examined, and is not general. Exergies evaluated for a specific process-dependent model are relevant only to the process. They can not rationally be compared with exergies evaluated for other process-dependent models or used in environmental assessments.
4.8 Exergy Efficiencies
5. Illustrative Examples
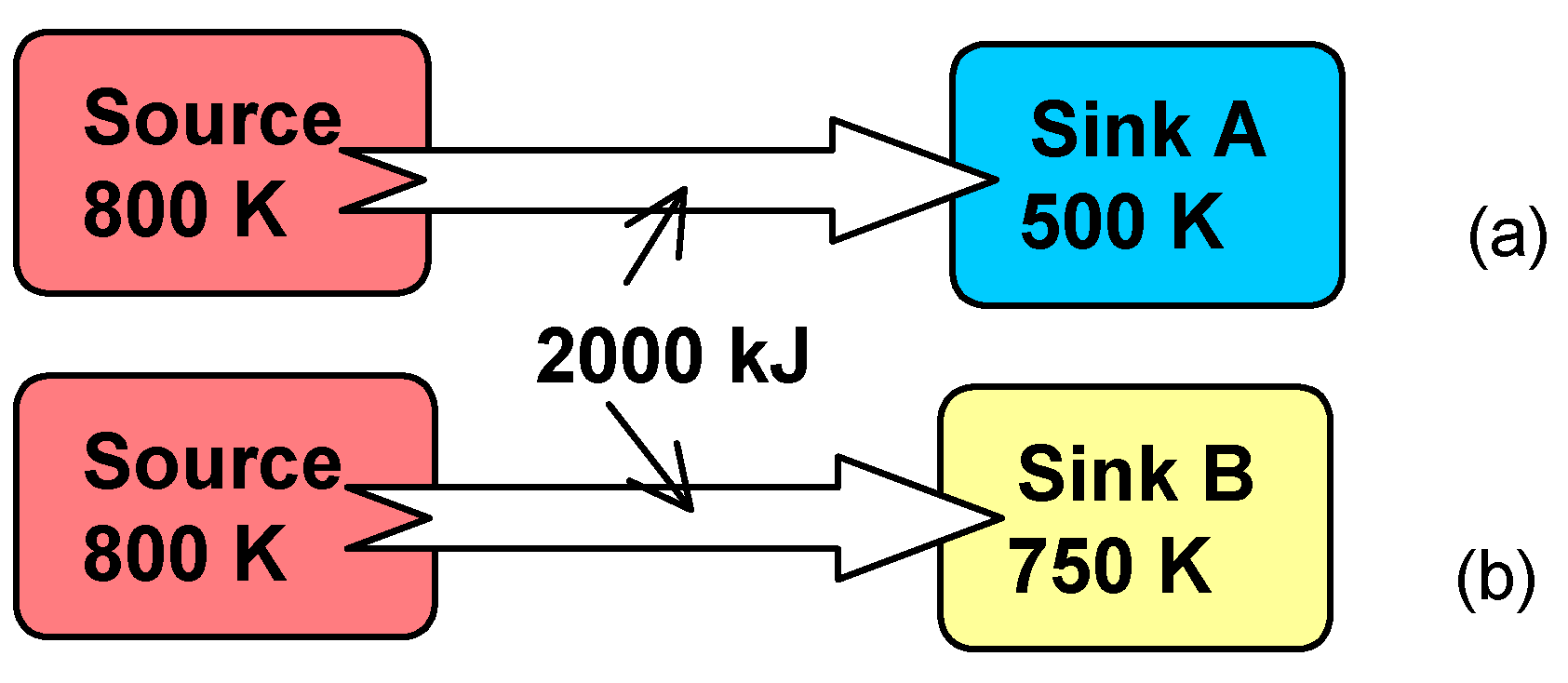
5.1 Illustrative Example 1
- (a)
- For the heat transfer process to a sink at 500 K:
- (b)
- Repeating the calculations in part (a) for a sink temperature of 750 K, we obtain
5.2 Illustrative Example 2
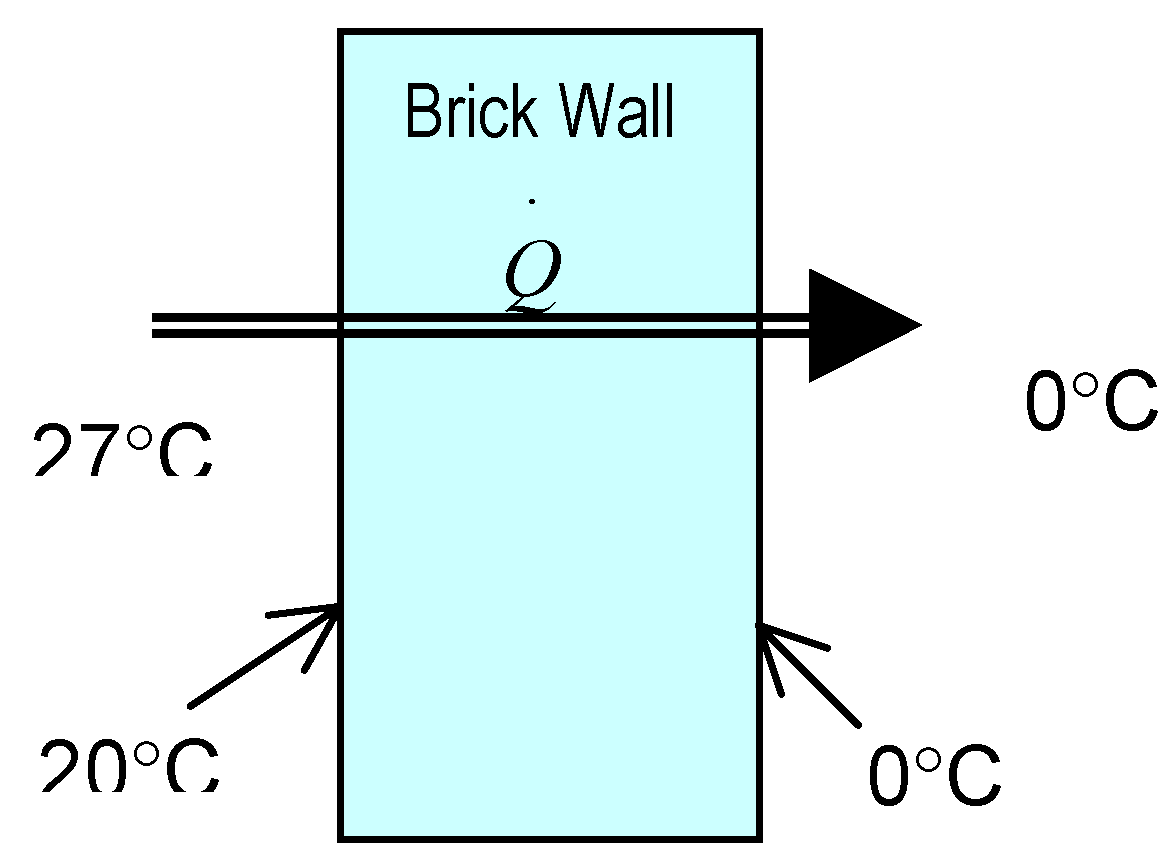
5.3 Illustrative Example 3
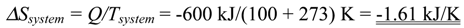
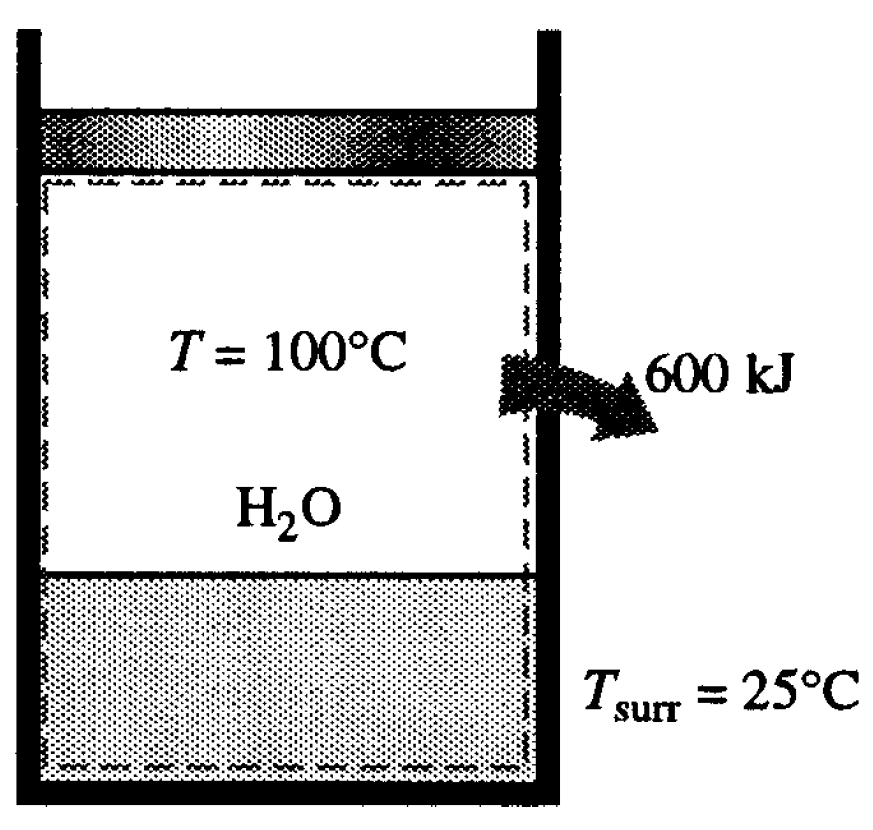
- (b)
- To determine the total entropy generation during this process, we consider the extended system, which includes the water, the piston-cylinder device, and the region immediately outside the system that experiences a temperature change so that the entire boundary of the extended system is at the surrounding temperature of 25°C. The entropy balance for this extended system (system immediate surroundings) yields
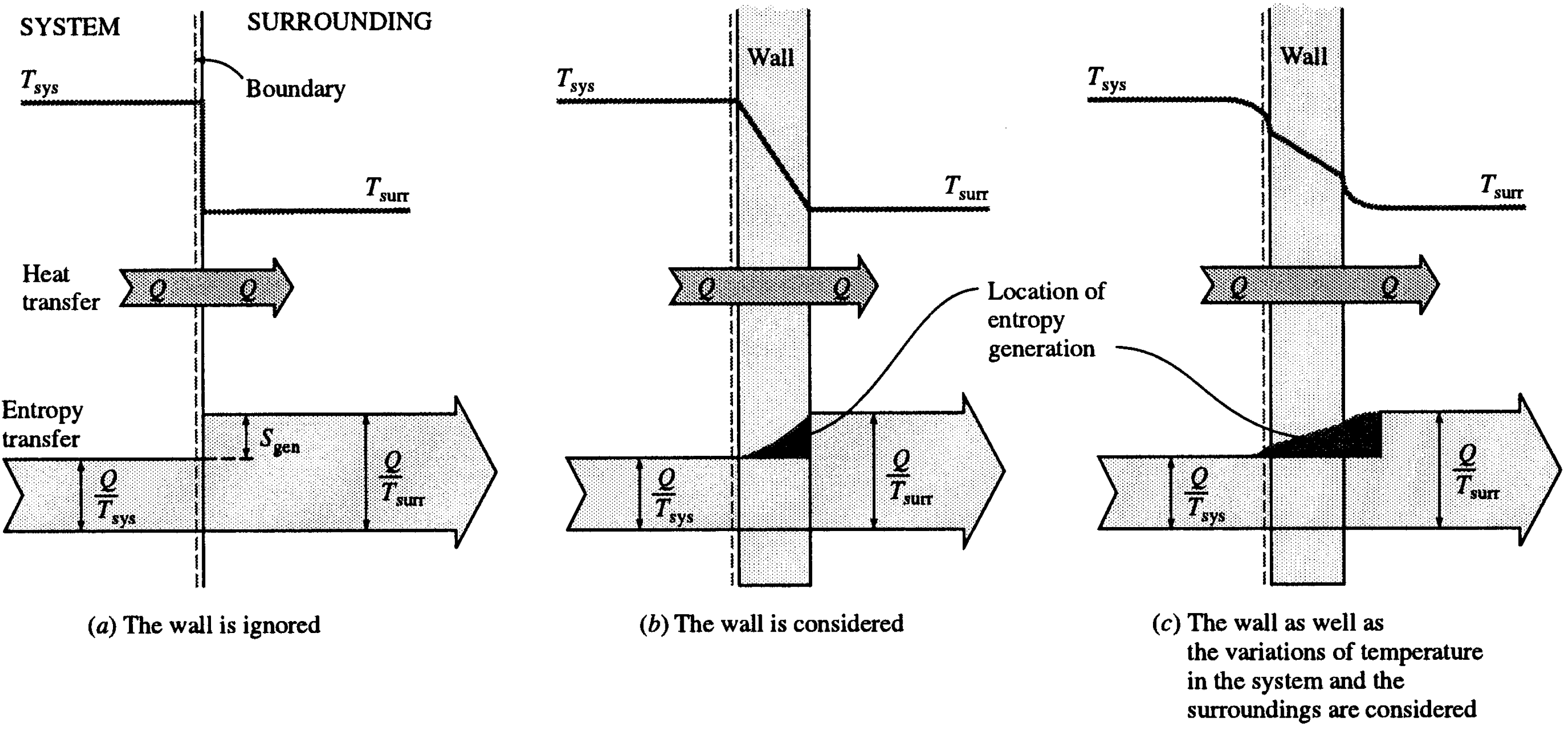
5.4 Illustrative Example 4
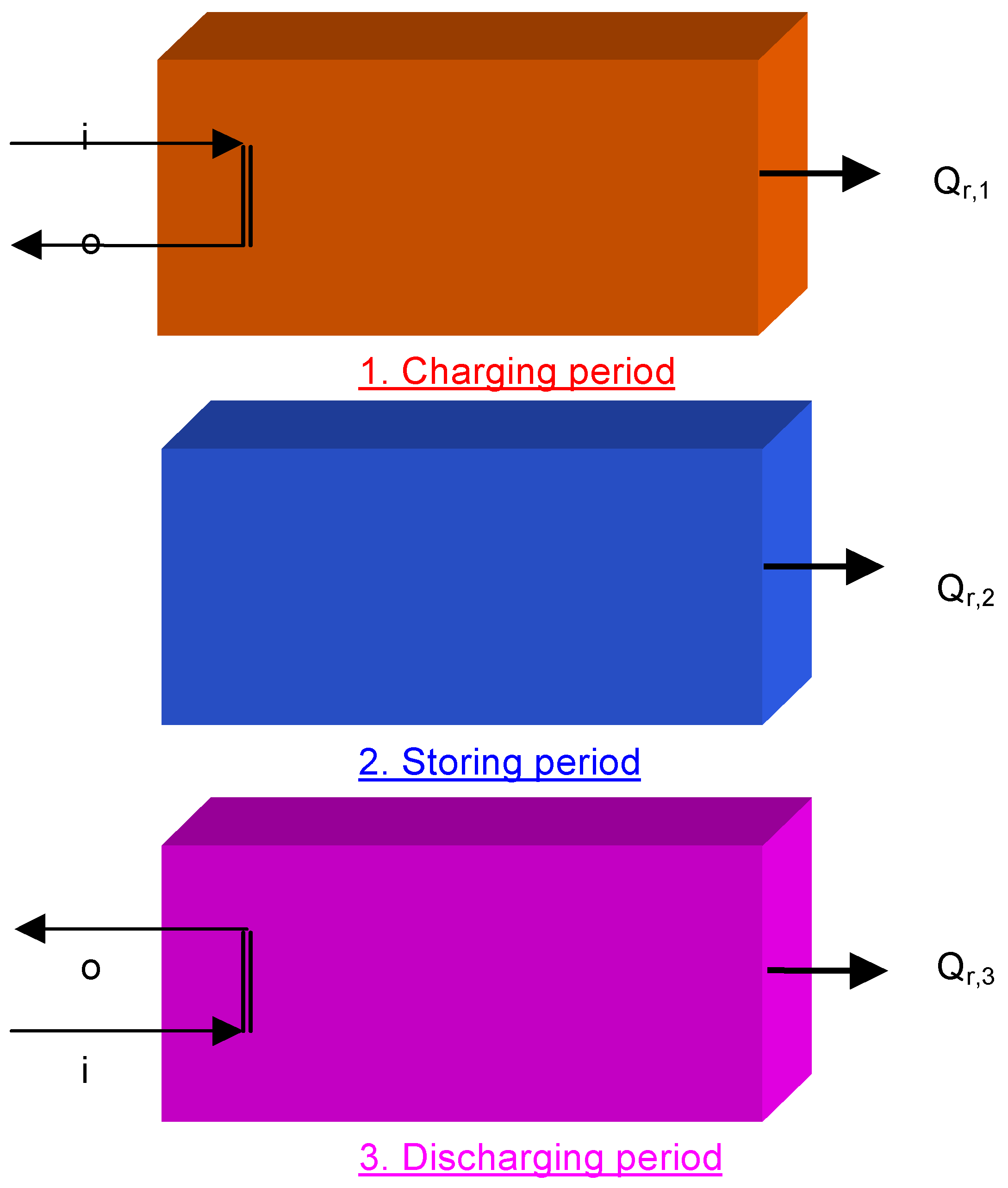
- It provides more proper accounting of the loss of availability of heat in SES system using the conservation of mass and energy principles together with the second law of thermodynamics for the goals of design and analysis.
- It gives more meaningful and useful information than energy analysis regarding the efficiency, losses and performance of SES systems.
- It is more correct reflecting the thermodynamic and economic values of the operation of SES systems.
- It is an efficient technique revealing whether or not and by how much it is possible to design more efficient SES systems by reducing the inefficiencies in the existing units.
6. Conclusions
References
- Wall, G. (1977); Exergy—A Useful Concept within Resource Accounting; Institute of Theoretical Physics: Göteborg, Report No. 77-42. [Google Scholar]
- Wall, G. (1983); Exergy Flows in Industrial Processes; Physical Resource Theory Group, Chalmers University of Technology and University of Göteborg. [Google Scholar]
- Wall, G. Exergy–A Useful Concept. Ph.D. Thesis, Chalmers University of Technology, S-412 96 Göteborg, Sweden, (1983). [Google Scholar]
- Dincer, I.; Rosen, M. (1999); The Intimate Connection between Exergy and the Environment. In Thermodynamic Optimization of Complex Energy Systems (Edited by A. Bejan and E. Mamut); Kluwer Academic: the Netherlands; pp. 221–230. [Google Scholar]
- Rosen, M.A.; Dincer, I. (1999); Thermal storage and exergy analysis: the impact of stratification. In Transactions of the CSME; Volume 23(1B), pp. 173–186. [Google Scholar]
- Rosen, M.A.; Morris, D.R.; Steward, F.R. (1988); Exergy Analysis of Thermal, Chemical, and Metallurgical Processes; Hemisphere; p. 332. New York. [Google Scholar]
- Kotas, T.J. (1995); The Exergy Method of Thermal Plant Analysis, Reprint ed; Krieger: Malabar, Florida. [Google Scholar]
- Moran, M.J. (1982); Availability Analysis: A Guide to Efficient Energy Use; p. 263. Prentice-Hall: New Jersey. [Google Scholar]
- Bejan, A. (1997); Advanced Engineering Thermodynamics; John Wiley & Sons: New York. [Google Scholar]
- Cengel, Y.A.; Boles, M.A. (2001); Thermodynamics: An Engineering Approach, 4th edition; p. 1056. McGraw-Hill: New York. [Google Scholar]
© 2001 by MDPI. All rights reserved.
Share and Cite
Dincer, I.; Cengel, Y.A. Energy, Entropy and Exergy Concepts and Their Roles in Thermal Engineering. Entropy 2001, 3, 116-149. https://doi.org/10.3390/e3030116
Dincer I, Cengel YA. Energy, Entropy and Exergy Concepts and Their Roles in Thermal Engineering. Entropy. 2001; 3(3):116-149. https://doi.org/10.3390/e3030116
Chicago/Turabian StyleDincer, Ibrahim, and Yunus A. Cengel. 2001. "Energy, Entropy and Exergy Concepts and Their Roles in Thermal Engineering" Entropy 3, no. 3: 116-149. https://doi.org/10.3390/e3030116
APA StyleDincer, I., & Cengel, Y. A. (2001). Energy, Entropy and Exergy Concepts and Their Roles in Thermal Engineering. Entropy, 3(3), 116-149. https://doi.org/10.3390/e3030116