Beyond the Second Law: Darwinian Evolution as a Tendency for Entropy Production to Increase
Abstract
1. Biologists on Entropy
What is important in the origin of life field is understanding the transitions that led from chemistry to biology. So far, I have not seen that efforts to define life have contributed at all to that understanding.[3]
Entropy The degradation of matter and energy in the universe to an ultimate state of inert uniformity. Entropy can be reached only in a closed system.
According to the Second Law, the universe is unwinding out of a more ordered state into the ultimately disordered state known as the heat death of the universe. What then, are living things? They are things that defy this crumbling into dust, at least for a while, by not being isolated—by taking in from their environment the wherewithal to keep life and limb together…Not just individual organisms, but the whole progress of evolution that creates them, thus, can be seen as fundamental physical phenomena running contrary to the large trend of cosmic time…It is not impossible to oppose the trend of the Second Law, but it is costly.[9], pp 68–69
…to stay alive we need to continually eat so as to combat the inevitable, destructive forces of entropy production. Entropy kills.[10], p. 14–15
It is sometimes claimed that evolution, by producing order, is in conflict with the “law of entropy” of physics, according to which evolutionary change should produce an increase of disorder. Actually, there is no conflict, because the law of entropy is valid only for closed systems, whereas the evolution of a species of organisms takes place in an open system in which organisms can reduce entropy at the expense of the environment….[8]
…Life violates the spirit but not the letter of the second law.[14], p 74
2. What Is the Connection Between Entropy and Biology? Going Beyond the Second Law
The two fundamental laws of thermodynamics are, of course, insufficient to determine the course of events in a physical system. They tell us that certain things cannot happen, but they do not tell us what does happen.[18], p 151
In the jargon of thermodynamics, the formation of patterns in these [complex emergent] systems helps to speed up the dissipation of energy as mandated by the second law.[51], p. 13
3. Paradigm Shift from “We-Eat-Food” to “Food-Has-Produced-Us-to-Eat-It”
…gradients, when steep enough, give rise to far from equilibrium dissipative structures (e.g., galaxies, stars, black holes, hurricanes and life) which emerge spontaneously to hasten the destruction of the gradients which spawned them. This represents a paradigm shift from “we eat food” to “food has produced us to eat it.[54]
Life does not exist despite the second law of thermodynamics; instead, life has evolved to take full advantage of the second law wherever it can.
…life may have a larger role to play than we have yet imagined. Life may succeed against all odds in molding the universe to its own purposes.[74], p. 51
4. Enhancement Compared to What?
5. The Origin of Life: The Emergence and Evolution of Biotic FFEDS from Abiotic FFEDS
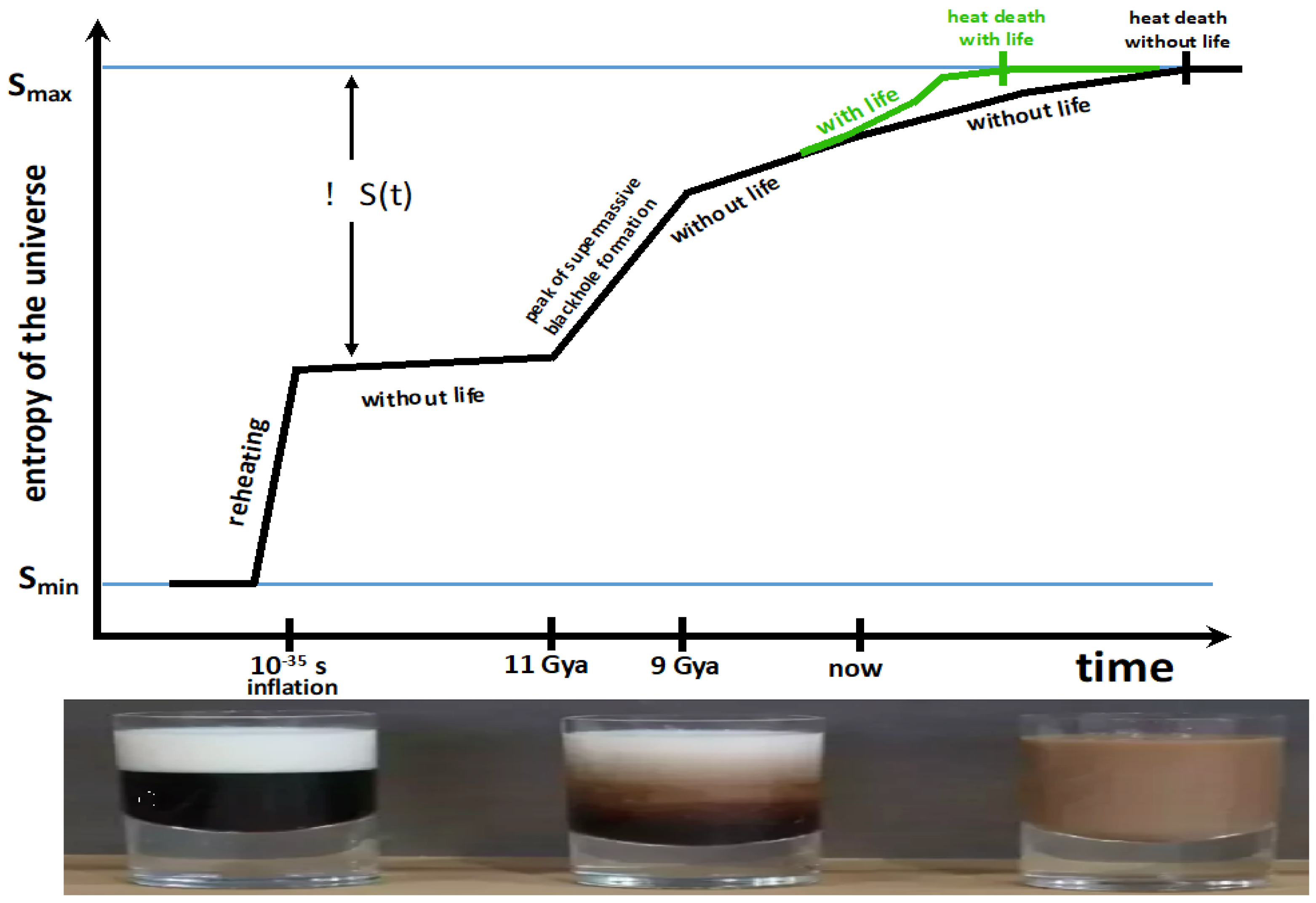
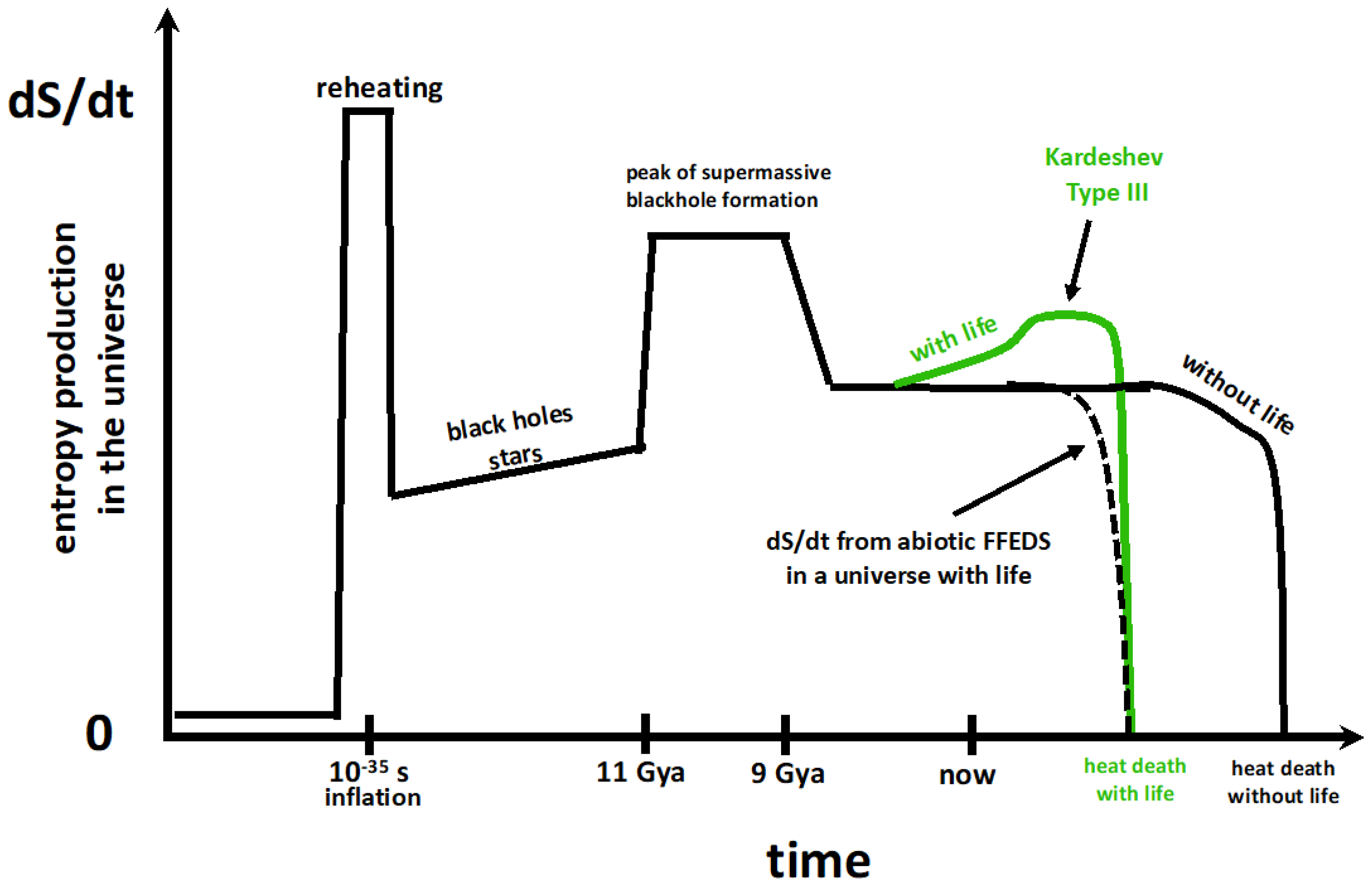
6. A Tendency Towards Increased Entropy Production Can Explain the Apparent Increase in Biological Complexity
Stars are the power plants that drive life’s development toward increasing complexity.[99], p. 159
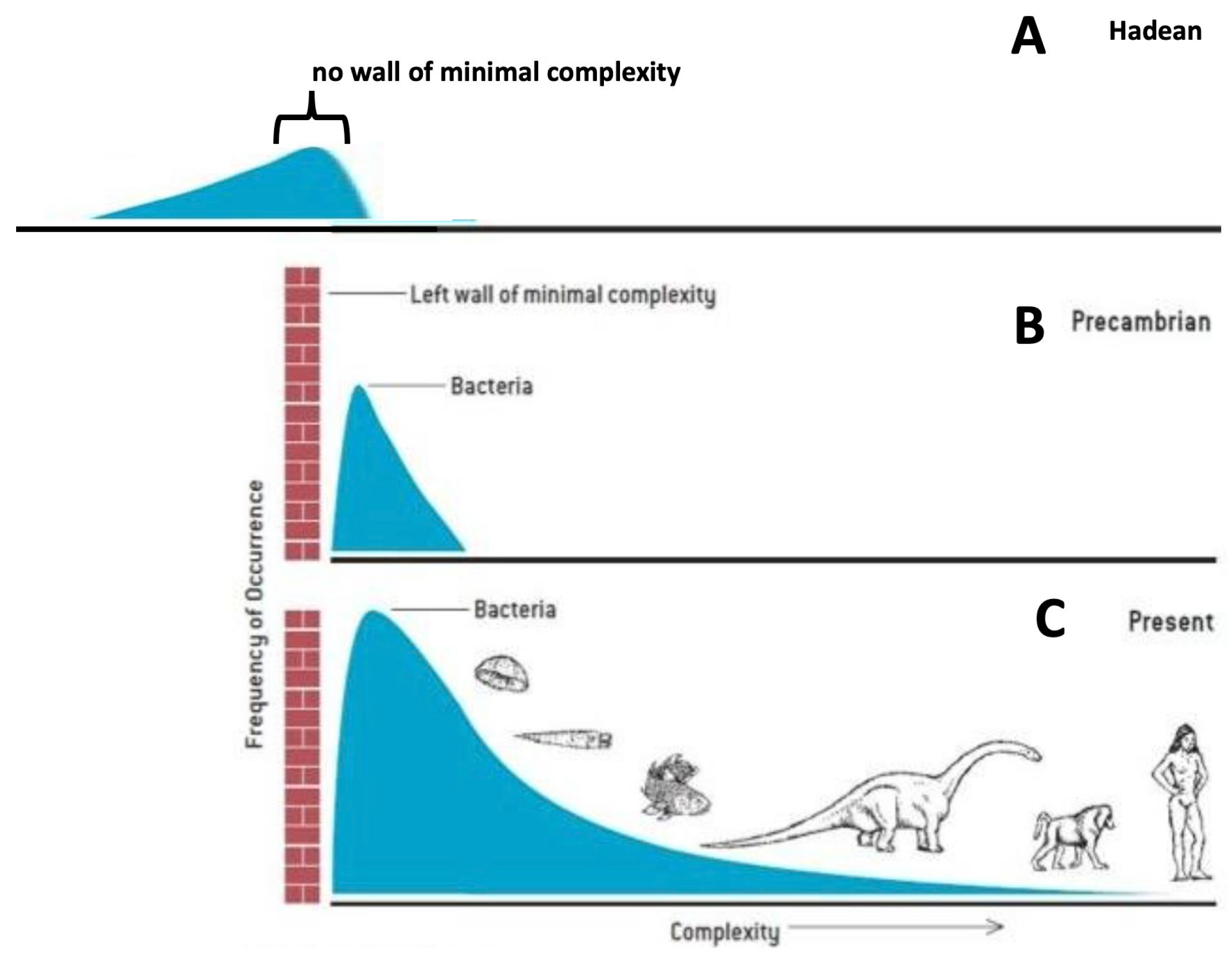
7. Gould’s Wall of Minimal Complexity
For reasons related to the chemistry of life’s origin and the physics of self-organization, the first living things arose at the lower limit of life’s conceivable preservable complexity. Call this lower limit the ‘left wall’ for an architecture of complexity. Since so little space exists between the left wall and life’s initial bacterial mode in the fossil record, only one direction for future increment exists—toward greater complexity at the right.[102]
8. Techno FFEDS
9. Entropy Production as a Useful Expression of ‘Complexity’
10. A Tendency to Increase Entropy Production Can Resolve Some Problems with Darwinian Explanations
11. Summary
Funding
Data Availability Statement
Conflicts of Interest
References
- Schrödinger, E. What Is Life? The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1944. [Google Scholar]
- Benner, S.A. Defining life. Astrobiology 2010, 10, 1021–1030. [Google Scholar] [CrossRef]
- Szostak, J.W. Attempts to Define Life Do Not Help to Understand the Origin of Life. J. Biomol. Struct. Dyn. 2012, 29, 599–600. [Google Scholar] [CrossRef]
- Smith, J.M.; Szathmary, E. The Origins of Life: From the Birth of Life to the Origins of Language; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Gould, S.J. The Structure of Evolutionary Theory; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Ruse, M.; Travis, J. Evolution: The First Four Billion Years; Belkap Harvard University Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Lenton, T.; Watson, A. Revolutions that Made the Earth; Oxford University Press: Oxford, UK, 2011. [Google Scholar]
- Mayr, E. What is Evolution? Weidenfeld & Nicolson: London, UK, 2001. [Google Scholar]
- Dennett, D.C. Darwin’s Dangerous Idea: Evolution and the Meanings of Life; Simon & Schuster: New York, NY, USA, 1995. [Google Scholar]
- West, G. Scale: The Universal Laws of Life and Death in Organisms, Cities and Companies; Weidenfeld and Nicolson: London, UK, 2017. [Google Scholar]
- Smith, E.; Morowitz, H.J. The Origin and Nature of Life on Earth: The Emergence of the Fourth Geosphere; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Quaster, R. The Emergence of Biological Organization; Yale University Press: New Haven, CT, USA, 1964. [Google Scholar]
- Brillouin, L. Science and Information Theory, 2nd ed.; Dover Phoenix Editions: Mineola, NY, USA, 2004. [Google Scholar]
- Brown, G. The Energy of Life; Flamingo Harpur Collins: London, UK, 1999. [Google Scholar]
- Schneider, E.D.; Sagan, D. Into the Cool: Energy Flow, Thermodynamics and Life; University of Chicago Press: Chicago, IL, USA, 2005. [Google Scholar]
- Martyushev, L.M. Life Defined in Terms of Entropy Production: 20th Century Physics Meets 21st Century Biology. BioEssays 2020, 42, 2000101. [Google Scholar] [CrossRef]
- Clausius, R. The Mechanical Theory of Heat with its Applications to the Steam-Engine and to the Physical Properties of Bodies; John Van Voorst: London, UK, 1867. [Google Scholar]
- Lotka, A.J. Natural selection as a physical principle. Proc. Natl. Acad. Sci. USA 1922, 8, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Boltzmann, L. Der zweite Hauptsatz der mechanischen Wärmetheorie. p. 21, Vortrag., Popular Schriften, Essay 3, Wein bei Gerold. Address to a formal meeting of the Kaiserlichen Akademie der Wissenschaften. 29 May 1886. [Google Scholar]
- Lotka, A.J. Contribution to the energetics of evolution. Proc. Nat. Acad. Sci. USA 1922, 8, 147–151. [Google Scholar] [CrossRef]
- Lotka, A.J. The Law of Evolution as a Maximal Principle. Hum. Biol. 1945, 17, 167–194. [Google Scholar]
- Prigogine, I.; Stengers, I. Order out of Chaos: Man’s New Dialog with Nature; W.H. Freeman and Co.: New York, NY, USA, 1984. [Google Scholar]
- Brooks, D.R.; Wiley, E.O. Evolution as Entropy, 2nd ed.; University of Chicago Press: Chicago, IL, USA, 1988. [Google Scholar]
- Wicken, J.S. The Generation of Complexity in Evolution: A Thermodynamic and Information-Theoretical Discussion. J. Theor. Biol. 1979, 77, 349–365. [Google Scholar] [CrossRef]
- Wicken, J.S. Evolution, Thermodynamics, and Information: Extending the Darwinian Program; Oxford University Press: New York, NY, USA, 1987. [Google Scholar]
- Ulanowicz, R.E.; Hannon, B.M. Life and the production of entropy. Proc. R. Soc. B 1987, 232, 181–192. [Google Scholar]
- Weber, B.H.; Depew, D.J.; Smith, J.D. Entropy, Information and Evolution; MIT Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Lehninger, A. Principles of Biochemistry, 2nd ed.; Worth Publishers: New York, NY, USA, 1993. [Google Scholar]
- Schneider, E.D.; Kay, J.J. Life as a Manifestation of the Second Law of Thermodynamics. Mathl. Comput. Model. 1994, 19, 25–84. [Google Scholar] [CrossRef]
- Aoki, I. Entropy production in living systems: From organisms to ecosystems. Thermochim. Acta 1995, 250, 359–370. [Google Scholar] [CrossRef]
- Annila, A.; Annila, E. Why did life emerge? Int. J. Astrobiol. 2008, 7, 293–300. [Google Scholar] [CrossRef]
- Gusev, A.O.; Martyushev, L.M. An Evolution Based on Various Energy Strategies. Entropy 2021, 23, 317. [Google Scholar] [CrossRef]
- Cushman, S.A. Entropy, Ecology and Evolution: Toward a Unified Philosophy of Biology. Entropy 2023, 25, 405. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, H.; Shimokawa, S.; Sakuma, H. Thermodynamics of fluid turbulence: A unified approach to the maximum transport properties. Phys. Rev. E 2001, 64, 026303. [Google Scholar] [CrossRef]
- Ozawa, H.; Ohmura, A.; Lorenz, R.D.; Pujol, T. The second law of thermodynamics and the global climate systems: A review of the maximum entropy production principle. Rev. Geophys. 2003, 41, 1018–1041. [Google Scholar] [CrossRef]
- Kleidon, A.; Lorenz, R.D. (Eds.) Non-Equilibrium Thermodynamics and the Production of Entropy: Life, Earth and Beyond; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Dewar, R.C. Maximum entropy production and the fluctuation theorem. J. Phys. A 2005, 38, 371–381. [Google Scholar] [CrossRef]
- Dewar, R.C. Maximum entropy production as an algorithm that translates physical assumptions into macroscopic predictions: Don’t shoot the messenger. Entropy 2009, 11, 931–944. [Google Scholar] [CrossRef]
- Martyushev, L.M.; Seleznev, V.D. Maximum entropy production principle in physics, chemistry and biology. Phys. Rep. 2006, 426, 1–45. [Google Scholar] [CrossRef]
- Bruers, S. Classification and discussion of macroscopic entropy production principles. arXiv 2007, arXiv:cond-mat/0604482. [Google Scholar]
- Bruers, S. Energy and Ecology: On Entropy Production and the Analogy Between Fluid, Climate and Ecosystems. Ph.D. Thesis, Katholieke Universiteit Leuven, Instituut voor Theoretische Fysica, Leuven, Belgium, 2007. [Google Scholar]
- Bruers, S. A discussion on maximum entropy production and information theory. J. Phys. A Math. Theor. 2007, 40, 7441–7450. [Google Scholar] [CrossRef]
- Niven, R.K. Steady State of a Dissipative Flow-Controlled System and the Maximum Entropy Production Principle. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2009, 80, 021113. [Google Scholar] [CrossRef]
- Kleidon, A. A basic introduction to the thermodynamics of the Earth system far from equilibrium and maximum entropy production. Phil. Trans. R. Soc. B 2010, 365, 1303–1315. [Google Scholar] [CrossRef]
- Kleidon, A. Life, hierarchy, and the thermodynamic machinery of planet Earth. Phys. Life Rev. 2010, 7, 424. [Google Scholar] [CrossRef]
- Volk, T.; Pauluis, O. It is not the entropy you produce, rather, how you produce it. Phil. Trans. Biol. Sci. 2010, 365, 1545. [Google Scholar] [CrossRef]
- Herrmann-Pillatha, C.; Salthe, S.N. Triadic conceptual structure of the maximum entropy approach to evolution. Biosystems 2011, 103, 315–330. [Google Scholar] [CrossRef]
- Dewar, R.C.; Lineweaver, C.H.; Niven, R.K.; Regenauer-Lieb, K. (Eds.) Beyond the Second Law: Entropy Production and Non-equilibrium Systems; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Dewar, R.C.; Lineweaver, C.H.; Niven, R.K.; Regenauer-Lieb, K. Beyond the Second Law: An Overview. In Beyond the Second Law: Entropy Production and Non-equilibrium Systems; Springer: Berlin/Heidelberg, Germany, 2014; Chapter 1; pp. 3–30. [Google Scholar]
- Endres, R.G. Entropy Production selects nonequilibrium states in multistable systems. Sci. Rep. 2017, 7, 14437. [Google Scholar] [CrossRef]
- Hazen, R.M. Genesis: The Scientific Quest for Life’s Origin; Joseph Henry Press: Washington, DC, USA, 2005. [Google Scholar]
- Prigogine, I. Dissipative Structures, Dynamics and Entropy. Int. J. Quantum Chem. 1975, 9, 443–456. [Google Scholar] [CrossRef]
- Hoelzer, G.A.; Smith, E.; Pepper, J.W. On the logical relationship between natural selection and self-organization. J. Evol. Biol. 2006, 19, 1785–1794. [Google Scholar] [CrossRef]
- Lineweaver, C.H.; Egan, C. Life, gravity and the second law of thermodynamics. Phys. Life Rev. 2008, 5, 225–242. [Google Scholar] [CrossRef]
- Wang, G.M.; Sevick, E.M.; Mittag, E.; Searles, D.J.; Evans, D.J. Experimental Demonstration of Violations of the Second Law of Thermodynamics for Small Systems and Short Time Scales. Phys. Rev. Lett. 2002, 89, 050601. [Google Scholar] [CrossRef] [PubMed]
- Onsager, L. Reciprocal relations in irreversible processes I. Phys Rev. 1931, 37, 405–426. [Google Scholar] [CrossRef]
- Onsager, L. Reciprocal relations in irreversible processes II. Phys Rev. 1931, 38, 2265–2279. [Google Scholar] [CrossRef]
- Kondepudi, D.; Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures; Wiley & Sons: New York, NY, USA, 2006. [Google Scholar]
- Županović, P.; Kuić, D.; Juretić, D.; Dobovišek, A. On the Problem of Formulating Principles in Nonequilibrium Thermodynamics. Entropy 2010, 12, 926–931. [Google Scholar] [CrossRef]
- Ben-Naim, A. Entropy and Information Theory: Uses and Misuses. Entropy 2019, 21, 1170. [Google Scholar] [CrossRef]
- Sharma, V.; Annila, A. Natural process—Natural selection. Biophys. Chem. 2007, 127, 123–128. [Google Scholar] [CrossRef] [PubMed]
- De Martino, A.; De Martino, D. An introduction to the maximum entropy approach and its application to inference problems in biology. Heliyon 2018, 4, e00596. [Google Scholar] [CrossRef] [PubMed]
- Virgo, N. From maximum entropy to maximum entropy production: A new approach. Entropy 2010, 12, 107–126. [Google Scholar] [CrossRef]
- Kleidon, A. Beyond Gaia: Thermodynamics of life and Earth system functioning. Clim. Change 2004, 66, 271–319. [Google Scholar] [CrossRef]
- Toniazzo, T.; Lenton, T.M.; Cox, P.M.; Gregory, J. Entropy and Gaia: Is there a link between MEP and self-regulation in the climate system? In Non-Equilibrium Thermodynamics and the Production of Entropy: Life, Earth, and Beyond; Kleidon, A., Lorenz, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 223–242. [Google Scholar]
- Kleidon, A.; Malhi, Y.; Cox, P.M. Maximum entropy production in environmental and ecological systems. Phil. Trans. Roy. Soc. B 2010, 365, 1297–1302. [Google Scholar] [CrossRef]
- Arthur, R.; Nicholson, A. Selection principles for Gaia. J. Theor. Biol. 2021, 533, 110940. [Google Scholar] [CrossRef]
- Penrose, R. The Road to Reality: A Complete Guide to the Laws of the Universe; Vintage Books: London, UK, 2005; pp. 699–707. [Google Scholar]
- Lineweaver, C.H. The Entropy of the Universe and the Maximum Entropy Production Principle. In Beyond the Second Law: Entropy Production and Non-Equilibrium Systems; Dewar, R.C., Lineweaver, C.H., Niven, R.K., Regenauer-Lieb, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Chapter 22; pp. 415–427. [Google Scholar]
- Lineweaver, C.H. A simple treatment of complexity: cosmological entropic boundary conditions on increasing complexity. In Complexity and the Arrow of Time; Cambridge University Press: Cambridge, UK, 2013; pp. 42–67. [Google Scholar]
- Vallino, J.J.; Algar, C.K.; González, N.F.; Huber, J.A. Use of Receding Horizon Optimal Control to Solve MaxEP-Based Biogeochemistry Problems. In Beyond the Second Law: Entropy Production and Non-Equilibrium Systems; Springer: Berlin/Heidelberg, Germany, 2014; Chapter 18; pp. 337–359. [Google Scholar]
- Lovelock, J.E.; Margulis, L. Biological Modulation of the Earth’s Atmosphere. Icarus 1974, 21, 471–489. [Google Scholar]
- Hoffman, P.M. Life’s Ratchet: How Molecular Machines Extract Order from Chaos; Basic Books: New York, NY, USA, 2012. [Google Scholar]
- Dyson, F. Energy in the Universe. Sci. Am. 1971, 225, 50–58. [Google Scholar] [CrossRef]
- Martin, W.; Russell, M.J. On the origins of cells: A hypothesis for the evolutionary transitions from abiotic geochemistry to chemoautotrophic prokaryotes, and from prokaryotes to nucleated cells. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2003, 358, 59–85. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, G. From volcanic origins of chemoautotrophic life to Bacteria, Archaea and Eukarya. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1787–1808. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M.C.; Sousa, F.L.; Mrnjavac, N.; Neukirchen, S.; Roettger, M.; Nelson-Sathi, S.; Martin, W. The physiology and habitat of the last universal common ancestor. Nat. Microbiol. 2016, 1, 16116. [Google Scholar] [CrossRef]
- Harrison, S.A.; Rammu, H.; Liu, F.; Halpern, A.; Nunes Palmeira, R.; Lane, N. Life as a guide to its own origins. Annu. Rev. Ecol. Evol. Syst. 2023, 54, 327–350. [Google Scholar] [CrossRef]
- Branscomb, E.; Russell, M.J. Turnstiles and bifurcators: The disequilibrium converting engines that put metabolism on the road. Biochim. Biophys. Acta 2013, 1827, 62–78. [Google Scholar] [CrossRef]
- Bejan, A. The Constructal Law of Configuration Generation. In Advanced Engineering Thermodynamics, 3rd ed.; Wiley: Hoboken, NJ, USA, 2006; Chapter 13; pp. 705–841. [Google Scholar]
- Bejan, A.; Lorente, S. The constructal law and the evolution of design in nature. Phys. Life. Rev. 2011, 8, 209–240. [Google Scholar] [CrossRef]
- Russell, M.J.; Nitschke, W.; Branscomb, E. The inevitable journey to being. Philos. Trans. Roy. Soc. B 2013, 368, 20120254. [Google Scholar] [CrossRef]
- McDonald, M.D.; Owusu-Ansah, C.; Ellenbogen, J.B.; Malone, Z.D.; Ricketts, M.P.; Frolking, S.E.; Ernakovich, J.G.; Ibba, M.; Bagby, S.C.; Weissman, J.L. What is microbial dormancy? Trends Microbiol. 2023, 32, 142–150. [Google Scholar] [CrossRef]
- Smith, E. Thermodynamics of natural selection III: Landauer’s principle in computation and chemistry. J. Theor. Biol. 2008, 252, 213–220. [Google Scholar] [CrossRef]
- Lineweaver, C.H. We have not detected extraterrestrial life, or have we? In Life as We Know It; Seckbach, J., Ed.; Springer Science & Business Media: London, UK, 2006; Volume 10, pp. 445–457. [Google Scholar]
- Pascal, R.; Pross, A.; Sutherland, J.D. Towards an evolutionary theory of the origin of life based on kinetics and thermodynamics. Open Biol. 2013, 3, 130156. [Google Scholar] [CrossRef]
- Lifson, S. On the crucial stages in the origin of animate matter. J. Mol. Evol. 1997, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dobovišek, A.; Županović, P.; Brumen, M.; Juretić, D. Maximum Entropy Production and Maximum Shannon Entropy as Germane Principles for the Evolution of Enzyme Kinetics. In Beyond the Second Law: Entropy Production and Non-equilibrium Systems; Springer: Berlin/Heidelberg, Germany, 2014; Chapter 19. [Google Scholar]
- Weng, J.-K. The evolutionary paths towards complexity: A metabolic perspective. New Phytol. 2013, 201, 1141–1149. [Google Scholar] [CrossRef]
- Juretić, D. Exploring the Evolution-Coupling Hypothesis: Do Enzymes’ Performance Gains Correlate with Increased Dissipation? Entropy 2025, 27, 365. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Fenchel, T.; Delong, E.F. The Microbial Engines That Drive Earth’s Biogeochemical Cycles. Science 2008, 320, 1034–1039. [Google Scholar] [CrossRef]
- Mäkelä, T.; Annila, A. Natural patterns of energy dispersal. Phys. Life Rev. 2010, 7, 477–498. [Google Scholar] [CrossRef]
- Vallino, J.J.; Algar, C.K. The Thermodynamics of Marine Biogeochemical Cycles: Lotka Revisited. Annu. Rev. Mar. Sci. 2016, 8, 333. [Google Scholar] [CrossRef]
- Doolittle, W.F.; Inkpen, S.A. Processes and patterns of interaction as units of selection: An introduction to ITSNTS thinking. Proc. Natl. Acad. Sci. USA 2018, 115, 4006–4014. [Google Scholar] [CrossRef]
- Lineweaver, C.H.; Chopra, A. Other Earths, Gaian Bottlenecks and Darwinized Gaias. Phil. Trans. R. Soc. B. 2025, 380, 20240438. Available online: https://royalsocietypublishing.org/doi/10.1098/rstb.2024.0438 (accessed on 4 August 2025). [CrossRef] [PubMed]
- Hohmann-Marriott, M.F.; Blankenship, R.E. Evolution of Photosynthesis. Annu. Rev. Plant Biol. 2011, 62, 515–548. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, C.H.; Davies, P.C.W.; Ruse, M. Complexity and the Arrow of Time; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Lineweaver, C.H.; Davies, P.C.W.; Ruse, M. What is complexity? Is it increasing? In Complexity and the Arrow of Time; Cambridge University Press: Cambridge, UK, 2013; pp. 3–16. [Google Scholar]
- Adams, F.C. Origins of Existence: How Life Emerged in the Universe; The Free Press: New York, NY, USA, 2002. [Google Scholar]
- Conway Morris, S. Life: The final frontier for complexity. In Complexity and the Arrow of Time; Cambridge University Press: Cambridge, UK, 2013; Chapter 7. [Google Scholar]
- Ruse, M. Wrestling with biological complexity: From darwin to dawkins. In Complexity and the Arrow of Time; Cambridge University Press: Cambridge, UK, 2013; Chapter 12; pp. 279–307. [Google Scholar]
- Gould, S.J. The Evolution of Life on Earth. Sci. Am. 1994, 271, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Dollo, L. Les lois de l’évolution. Bull. Soc. Belge Geol. Pal. Hydr. 1893, 7, 164–166. [Google Scholar]
- Gould, S.J. Dollo on Dollo’s law: Irreversibility and the status of evolutionary laws. J. Hist. Biol. 1970, 3, 189–212. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J. The Mismeasure of Man; Penguin: London, UK, 1981. [Google Scholar]
- Hug, L.A.; Baker, B.J.; Anantharaman, K.; Brown, C.T.; Probst, A.J.; Castelle, C.J.; Butterfield, C.N.; Hernsdorf, A.W.; Amano, Y.; Ise, K.; et al. A new view of the tree of life. Nat. Microbiol. 2016, 1, 16048. [Google Scholar] [CrossRef]
- Castelle, C.J.; Banfield, J.F. Major new microbial groups expand diversity and alter our understanding of the tree of life. Cell 2018, 172, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, C.H. What Do the DPANN Archaea and the CPR Bacteria Tell Us About the Last Universal Common Ancestors? In Extremophiles as Astrobiological Models; Seckbach, J., Stan-Lotter, H., Eds.; Scrivener Publishing: Beverly, MA, USA, 2020; Chapter 17; pp. 359–368. [Google Scholar]
- Al-Shayeb, B.; Sachdeva, R.; Chen, L.-X.; Ward, F.; Munk, P.; Devoto, A.; Castelle, C.J.; Olm, M.R.; Bouma-Gregson, K.; Amano, Y.; et al. Clades of huge phages from across Earth’s ecosystems. Nature 2020, 578, 425–431. [Google Scholar] [CrossRef]
- Hou, X.; He, Y.; Fang, P.; Mei, S.Q.; Xu, Z.; Wu, W.C.; Tian, J.H.; Zhang, S.; Zeng, Z.Y.; Gou, Q.Y.; et al. Using artificial intelligence to document the hidden RNA virosphere. Cell 2024, 187, 6929–6942. [Google Scholar] [CrossRef]
- Dupré, J.; O’Malley, M.A. Varieties of Living Things: Life at the Intersection of Lineage and Metabolism. Philos. Theor. Biol. 2009, 1, e003. [Google Scholar] [CrossRef]
- Nasir, A.; Caetano-Anolles, G. A phylogenomic data-driven exploration of viral origins and evolution. Sci. Adv. 2015, 1, e1500527.24. [Google Scholar] [CrossRef]
- Diener, T.O. Potato spindle tuber “virus”. IV. A replicating, low molecular weight RNA. Virology 1971, 45, 411–428. [Google Scholar] [CrossRef]
- Keller, E.F. A Feeling for the Organism: The Life and Work of Barbara McClintock; Freeman: New York, NY, USA, 1983. [Google Scholar]
- Gilbert, W. The RNA World. Nature 1986, 319, 618. [Google Scholar] [CrossRef]
- Neveu, M.; Kim, H.J.; Benner, S.A. The “strong” RNA world hypothesis: Fifty years old. Astrobiology 2013, 13, 391–403. [Google Scholar] [CrossRef]
- Cech, T. The RNA Worlds in Context. Cold Spring Harb. Perspect. Biol. 2012, 4, a006742. [Google Scholar] [CrossRef]
- Cech, T. The Catalyst: RNA and the Quest to Unlock Life’s Deepest Secret; W.W. Norton: New York, NY, USA, 2024. [Google Scholar]
- Koonin, E.V.; Dolja, V.V. Virus world as an evolutionary network of viruses and capsidless selfish elements. Microbiol. Mol. Biol. Rev. 2014, 78, 278–303. [Google Scholar] [CrossRef] [PubMed]
- Moody, E.R.R.; Alvarez-Carretero, S.; Mahendrarajah, T.A.; Clark, J.W.; Beets, H.C.; Dombrowski, N.; Szantho, L.L.; Boyle, R.A.; Daines, S.; Chen, X.; et al. The nature of the last universal common ancestor and its impact on the early Earth system. Nat. Ecol. Evol. 2024, 8, 1654–1666. [Google Scholar] [CrossRef]
- Segré, D.; Lancet, D. A Statistical chemistry approach to the origin of life, Chemtrasts. Biochem. Mol. Biol. 1999, 12, 382–397. [Google Scholar]
- Dyson, F. Origins of Life, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- McShea, D.W.; Brandon, R.N. Biology’s First Law: The Tendency for Diversity and Complexity to Increase in Evolutionary Systems; University of Chicago Press: Chicago, IL, USA, 2010. [Google Scholar]
- Chaisson, E.J. Cosmic Evolution: The Rise of Complexity in Nature; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Chaisson, E.J. Energy rate density. II. Probing further a new complexity metric. Complexity 2011, 17, 44–63. [Google Scholar] [CrossRef]
- Wrangham, R. Catching Fire: How Cooking Made Us Human; Basic Books: New York, NY, USA, 2009. [Google Scholar]
- Haff, P.K. Maximum Entropy Production by Technology. In Beyond the Second Law: Entropy Production and Non-Equilibrium Systems; Springer: Berlin/Heidelberg, Germany, 2014; Chapter 21; pp. 397–414. [Google Scholar]
- Bromm, V. Formation of the first stars. Rep. Prog. Phys. 2013, 76, 112901. [Google Scholar] [CrossRef] [PubMed]
- Penrose, R.; Floyd, R.M. Extraction of Rotational Energy from a Black Hole. Nat. Phys. Sci. 1971, 229, 177–179. [Google Scholar] [CrossRef]
- Kardashev, N.S. Transmission of information by extraterrestrial civilizations. Sov. Astron. 1964, 8, 217. [Google Scholar]
- Kardashev, N.S. Cosmology and Civilizations. Astrophys. Space Sci. 1997, 252, 25–40. [Google Scholar] [CrossRef]
- Holmlid, L.; Zeiner-Gundersen, S. Future interstellar rockets may use laser-induced annihilation reactions for relativistic drive. Acta Astronaut. 2020, 175, 32–36. [Google Scholar] [CrossRef]
- Dyson, F. Search for Artificial Stellar Sources of Infrared Radiation. Science 1960, 131, 1667–1668. [Google Scholar] [CrossRef]
- Guth, A.H. Can a man-made universe be created by quantum tunneling without an initial singularity? Phys. Scr. 1991, T36, 237. [Google Scholar] [CrossRef]
- Sommeria, J. Entropy production in turbulent mixing. In Non-Equilibrium Thermodynamics and the Production of Entropy: Life, Earth, and Beyond; Kleidon, A., Lorenz, R.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 79–91. [Google Scholar] [CrossRef]
- Ozawa, H.; Murayama-Ogino, S.; Kleidon, A. Thermodynamics of Morphogenesis: Beading and Branching Pattern Formation in Diffusion-Driven Salt Finger Plumes. Entropy 2025, 27, 106. [Google Scholar] [CrossRef]
- Aaronson, S.; Carroll, S.; Ouellett, L. Quantifying the rise and fall of complexity in closed systems: The coffee automaton. arXiv 2014, arXiv:1405.6903. [Google Scholar] [CrossRef]
- Carroll, S. The Big Picture: On the Origin of Life, Meaning, and the Universe Itself; Penguin: London, UK, 2017. [Google Scholar]
- Adami, C. What is complexity? BioEssays 2002, 24, 1085–1094. [Google Scholar] [CrossRef]
- Lloyd, S. Measures of Complexity a non-exhaustive list. IEEE Control. Syst. Mag. 2001, 21, 7–8. [Google Scholar] [CrossRef]
- Gell-Mann, M. What is Complexity? In Complexity and Industrial Clusters. Contributions to Economics; Curzio, A.Q., Fortis, M., Eds.; Physica-Verlag HD: Berlin/Heidelberg, Germany, 2002. [Google Scholar] [CrossRef]
- Kolmogorov, A.N. Three approaches to the quantitative definition of information. Probl. Inf. Transm. 1968, 2, 157–168. [Google Scholar] [CrossRef]
- Albert, D. Time and Chance; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Ainsworth, P.M. Cosmic Inflation and the past hypothesis. Synthese 2008, 162, 157–165. [Google Scholar] [CrossRef]
- Serjeant, S.; Bertoldi, F.; Blain, A.W.; Clements, D.L.; Cooray, A.; Danese, L.; Dunlop, J.; Eales, S.; Falder, J.; Hatziminaoglou, E.; et al. Herschel ATLAS: The cosmic star formation history of quasar host galaxies. Astron. Astrophys. 2010, 518, L7. [Google Scholar] [CrossRef]
- Egan, C.; Lineweaver, C.H. A larger estimate of the entropy of the universe. Astrophys. J. 2010, 710, 1825–1834. [Google Scholar] [CrossRef]
- Planavsky, N.J.; Fakhraee, M.; Bolton, E.W.; Reinhard, C.T.; Isson, T.T.; Zhang, S.; Mills, B.J.W. On Carbon Burial and Net Primary Production Through Earth’s History. Am. J. Sci. 2022, 322, 413–460. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 3rd ed.; Worth Publishers: New York, NY, USA, 2000. [Google Scholar]
- Benner, S.A. Enzyme kinetics and molecular evolution. Chem. Rev. 1989, 89, 789–806. [Google Scholar] [CrossRef]
- Tolman, R.C.; Fine, P.C. On the irreversible production of entropy. Rev. Mod. Phys. 1948, 20, 51. [Google Scholar] [CrossRef]
- Woese, C.R. On the evolution of cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747. [Google Scholar] [CrossRef]
- Godfrey-Smith, P. Darwinian Populations and Natural Selection; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Wilson, D.S. This View of Life: Contemplating the Darwinian Revolution; Pantheon Books: New York, NY, USA, 2019. [Google Scholar]
- Dawkins, R. The Selfish Gene; Oxford University Press: Oxford, UK, 1976. [Google Scholar]
- Bouchard, F. Darwinism without populations: A more inclusive understanding of the ‘survival of the fittest’. Stud. Hist. Philos. Biol. Biomed. Sci. 2011, 42, 106–114. [Google Scholar] [CrossRef]
- Bouchard, F. Ecosystem Evolution is About Variation and Persistence, not Populations and Reproduction. Biol. Theory 2014, 9, 382–391. [Google Scholar] [CrossRef]
- Okasha, S. Evolution and the Levels of Selection; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Doolittle, W.F. Darwinizing Gaia; MIT Press: Cambridge, MA, USA, 2025. [Google Scholar]
- Malthus, T. An Essay on the Principle of Population: Or, a View of Its Past and Present Effects on Human Happiness; Reeves & Turner: London, UK, 1798. [Google Scholar]
- Kauffman, S.A. The Origins of Order: Self-Organization and Selection in Evolution; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Goodwin, B.C. How the Leopard Changed Its Spots: The Evolution of Complexity; Simon & Schuster: New York, NY, USA, 1994. [Google Scholar]
- Arango-Restrepo, A.; Barragán, D.; Rubi, J.M. Self-assembling outside equilibrium: Emergence of structures mediated by dissipation. Phys. Chem. Chem. Phys. 2019, 21, 17475–17493. [Google Scholar] [CrossRef]
- Arango-Restrepo, A.; Barragan, D.; Rubi, J.M. A criterion for the formation of nonequilibrium self-assembled structures. J. Phys. Chem. B 2021, 125, 1838–1845. [Google Scholar] [CrossRef] [PubMed]
- MacLean, R.C. Adaptive radiation in microbial microcosms. J. Evol. Biol. 2005, 18, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Lenski, R.E. Convergence and Divergence in a Long-Term Experiment with Bacteria. Am. Nat. 2017, 190, S57–S68. [Google Scholar] [CrossRef] [PubMed]
- Brunet, T.D.P.; Doolittle, W.F. The generality of Constructive Neutral Evolution. Biol. Philos. 2018, 33, 2. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lineweaver, C.H. Beyond the Second Law: Darwinian Evolution as a Tendency for Entropy Production to Increase. Entropy 2025, 27, 850. https://doi.org/10.3390/e27080850
Lineweaver CH. Beyond the Second Law: Darwinian Evolution as a Tendency for Entropy Production to Increase. Entropy. 2025; 27(8):850. https://doi.org/10.3390/e27080850
Chicago/Turabian StyleLineweaver, Charles H. 2025. "Beyond the Second Law: Darwinian Evolution as a Tendency for Entropy Production to Increase" Entropy 27, no. 8: 850. https://doi.org/10.3390/e27080850
APA StyleLineweaver, C. H. (2025). Beyond the Second Law: Darwinian Evolution as a Tendency for Entropy Production to Increase. Entropy, 27(8), 850. https://doi.org/10.3390/e27080850






