The Criticality of Consciousness: Excitatory–Inhibitory Balance and Dual Memory Systems in Active Inference
Abstract
1. Introduction and Overview
1.1. Subjective Experience as Evidence for a Theory of Consciousness
1.2. The Privileged Subjectivity of Qualia
1.3. The Feeling of Consciousness
1.4. Computing Experience
1.5. Regulating Synaptic Connectivity, and Consciousness, with Our Feelings
1.6. Unconscious Self-Organization in Sleep
1.7. Affective Control of Criticality
2. Key Terms
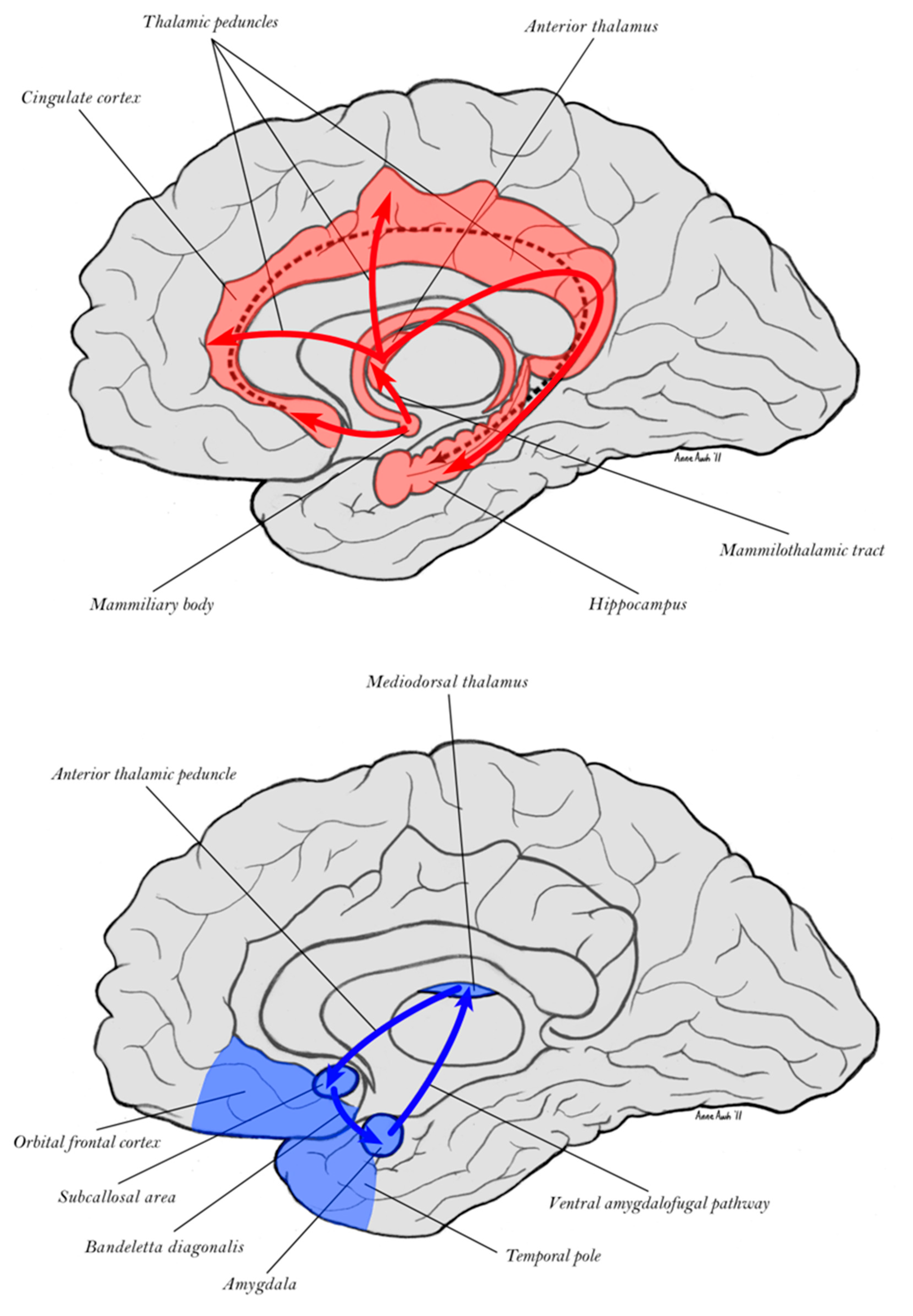
3. Adaptive Control from the Dorsal and Ventral Limbic Divisions
3.1. Dual Memory Systems
3.2. Active Inference in the Cerebral Cortex
3.3. Dorsal Excitatory and Ventral Inhibitory Controls on Attention and Working Memory
3.4. Formulating Excitatory Phasic Arousal and Inhibitory Tonic Activation in the Vertical Integration of Working Memory
3.5. Evolution of Mammalian Self-Regulation Through Weaving Excitatory and Inhibitory Architectures
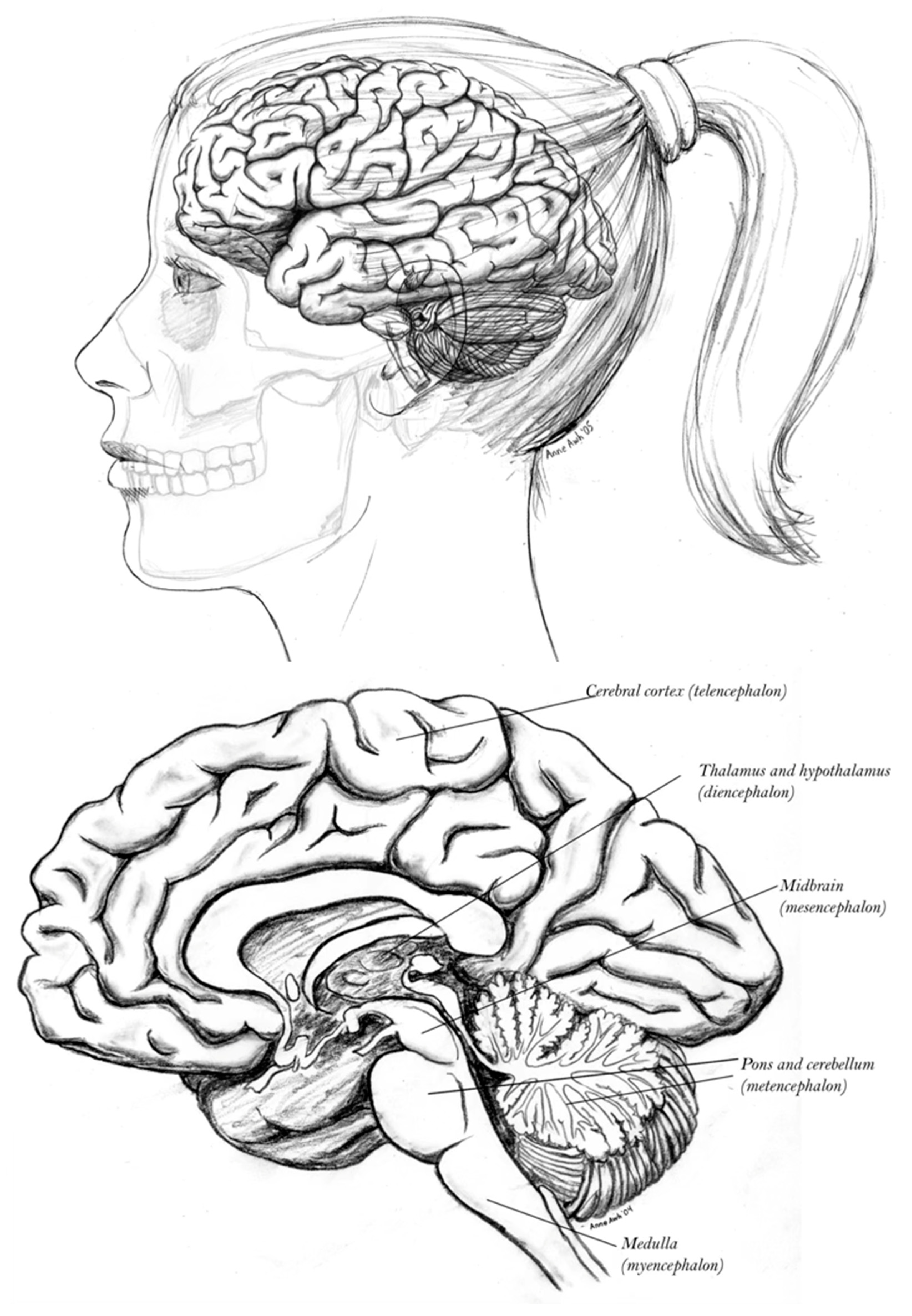
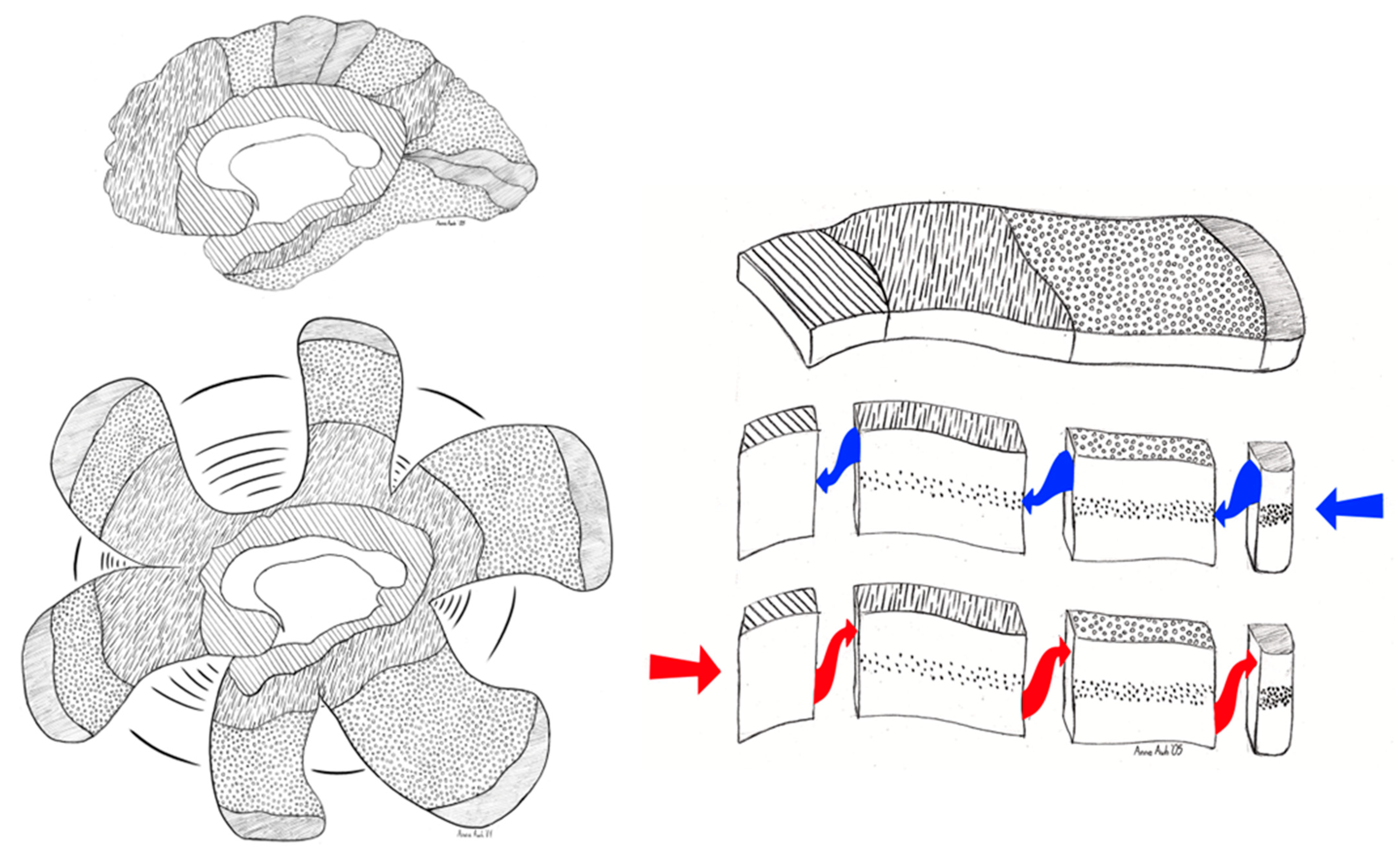
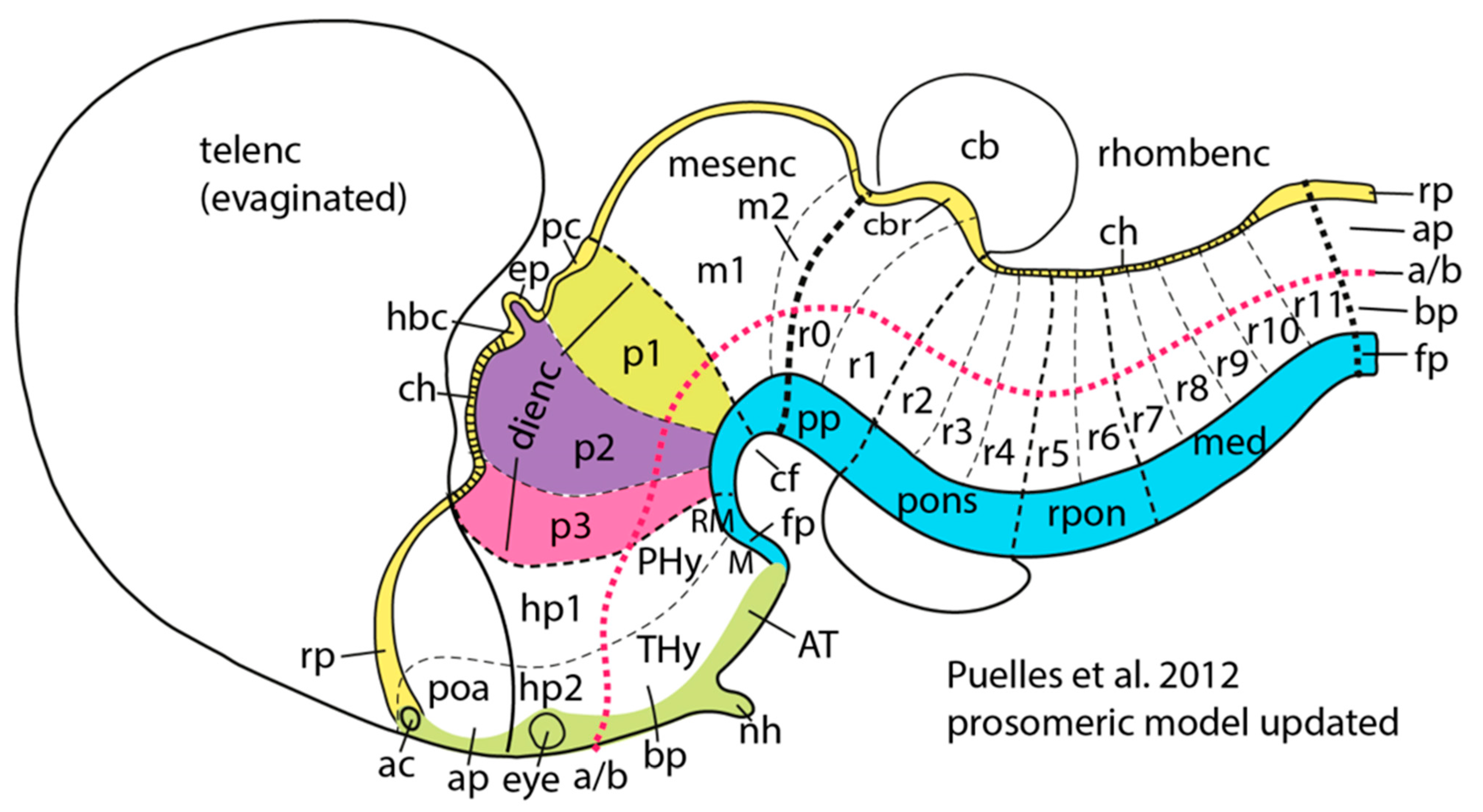
3.6. Excitatory Pallial and Inhibitory Subpallial Origins of the Human Brain
4. Inhibitory and Excitatory Neurophysiology of Memory Consolidation in Sleep
4.1. Inhibitory Specification and Error-Correction in Explicit Memory
4.2. Excitatory Reconsolidation of the Bayesian Predictions of Implicit Memory
4.3. The Unconscious Sources of Waking Consciousness
5. The Criticality Hypothesis of Consciousness
5.1. Excitatory–Inhibitory Balance and Criticality
5.2. Criticality in Brain Systems
5.3. Criticality at the Edge of Active Inference
5.4. Synchronizing Vertical Integration
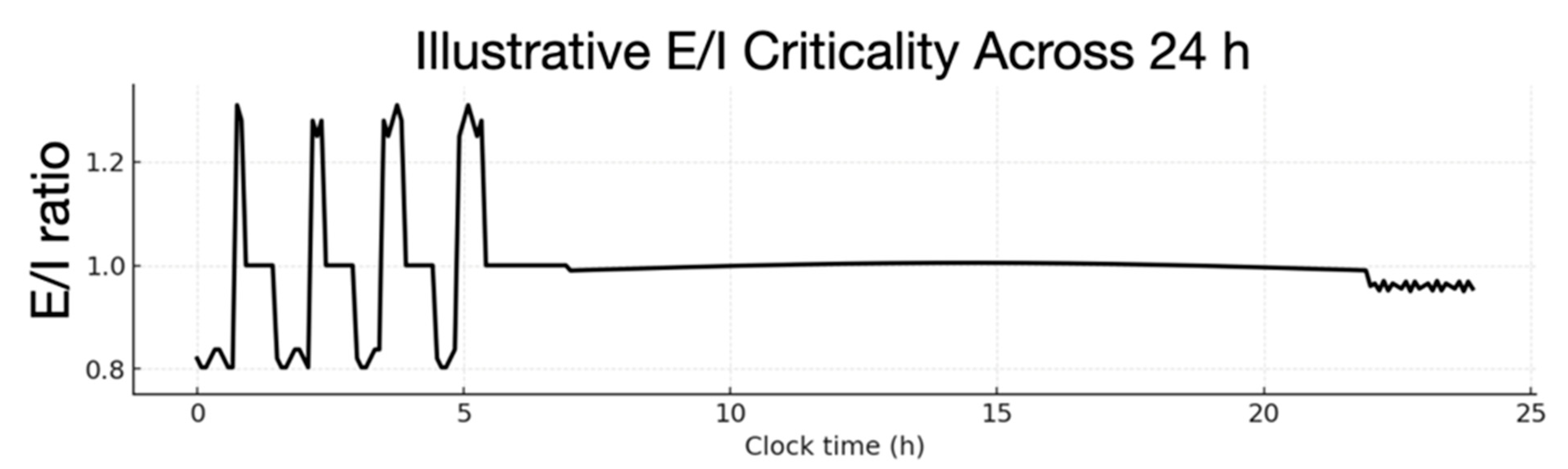
5.5. Brain Criticality and Its Variations
6. Excitatory Control of Complexity and Inhibitory Control of Accuracy in Active Inference
6.1. Terms for Formulating the E–I Gain Control of Active Inference
6.2. Overview of Active Inference and Neuronal Belief Updating
= Eq[ln q(s) − ln p(s)] − Eq[ln p(o|s)]
- The first term on the right is named complexity: it measures how far the posterior solution q(s) deviates (by the Kullback–Leibler divergence, DKL) from the original prior estimate p(s). This term minimizes free energy by keeping the updated beliefs q(s) close to the priors p(s). In other words, it scores the degree to which observations “change one’s beliefs.”
- The second term on the right is accuracy, defined as the expected likelihood that the posterior beliefs (q) successfully predict the data o. A more accurate posterior belief solution minimizes the fit error (e.g., precision-weighted prediction error) and thus minimizes F.
6.3. Introducing the E–I Control Gains
= Eq[ln q(s)q(E)q(I) − ln p(s)E − ln p(E,I)] − Eq[lnp(o|s)I]
= Eq[ln q(s) − E·ln p(s)] + DKL[q(E)q(I)||p(E,I)] − Eq [I·lnp(o|s)]
I = arg min F(q(s)q(E)qI(I), o)
6.4. How E and I Regulate Cerebral Networks
7. Criticality of Consciousness
7.1. Precision Dynamics of Feelings
7.2. Experience Bandwidth and Stability
7.3. Variational Free-Energy
7.4. Criticality Condition
8. Variational Dynamics of Sleep Pressure in Waking Consciousness
8.1. Memory and Cognition Consolidation
8.2. Balance of Adaptive Controls in the Stages of Sleep
9. The Criticality of Consciousness and Its Variational Control
9.1. Spanning the Specious Present
9.2. Organizing Conceptual Complexity
10. The Background Limbic Variational Dynamics That Generate Conscious Experience
10.1. Excitatory Generation of Predictions
10.2. Selective Inhibitory Specification
10.3. The Variational Backstage for the Momentary Appearances of Conscious Criticality
11. Limitations and Directions for Future Research
11.1. Affective Dynamics as the Structure of Subjectivity
11.2. Intentionality Disrupted: Absence Seizures and the Fragility of Self
11.3. Multiscale Modularity as the Substrate of Self-Referentiality
11.4. Future Directions: Toward Affective-Critical Neurocomputational Models of Selfhood
12. Conclusions: Adaptive Control of Variational Criticality
12.1. Experimental Predictions
12.2. Phenomenological Implications
12.3. The Entropic Nadir of Criticality
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baars, B.J. A Cognitive Theory of Consciousness; Cambridge University Press: New York, NY, USA, 1986. [Google Scholar]
- Dehaene, S.; Changeux, J.P. Experimental and theoretical approaches to conscious processing. Neuron 2011, 70, 200–227. [Google Scholar] [CrossRef]
- Tononi, G.; Koch, C. The neural correlates of consciousness: An update. Ann. N. Y. Acad. Sci. 2008, 1124, 239–261. [Google Scholar] [CrossRef]
- Klincewicz, M.; Cheng, T.; Schmitz, M.; Sebastián, M.Á.; Snyder, J.S. What makes a theory of consciousness unscientific? Nat. Neurosci. 2025, 28, 689–693. [Google Scholar] [CrossRef]
- Tononi, G.; Albantakis, L.; Barbosa, L.; Boly, M.; Cirelli, C.; Comolatti, R.; Ellia, F.; Findlay, G.; Casali, A.G.; Grasso, M.; et al. Consciousness or pseudo-consciousness? A clash of two paradigms. Nat. Neurosci. 2025, 28, 694–702. [Google Scholar] [CrossRef]
- Tononi, G. Consciousness as integrated information: A provisional manifesto. Biol. Bull. 2008, 215, 216–242. [Google Scholar] [CrossRef]
- James, W. Principles of Psychology; Holt: New York, NY, USA, 1890. [Google Scholar]
- Chalmers, D. The hard problem of consciousness. In The Blackwell Companion to Consciousness; Wiley: Hoboken, NJ, USA, 2007; pp. 225–235. [Google Scholar]
- Nagel, T. The View from Nowhere; Oxford University Press: New York, NY, USA, 1986. [Google Scholar]
- Nagel, T. What is it like to be a bat? In The Language and Thought Series; Harvard University Press: Cambridge, MA, USA, 1980; pp. 159–168. [Google Scholar]
- Damasio, A.R. The Feeling of What Happens: Body and Emotion in the Making of Consciousness; Mariner Books: New York, NY, USA, 2000. [Google Scholar]
- Merker, B. Consciousness without a cerebral cortex: A challenge for neuroscience and medicine. Behav. Brain Sci. 2007, 30, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Solms, M. The Hidden Spring: A Journey to the Source of Consciousness; WW Norton & Company: New York, NY, USA, 2021. [Google Scholar]
- Chanes, L.; Barrett, L.F. Redefining the role of limbic areas in cortical processing. Trends Cogn. Sci. 2016, 20, 96–106. [Google Scholar] [CrossRef]
- Chanes, L.; Barrett, L.F. The predictive brain, conscious experience, and brain-related conditions. In The Philosophy and Science of Predictive Processing; Bloomsbury Publishing: New York, NY, USA, 2020; pp. 159–169. [Google Scholar]
- Mumford, D. On the computational architecture of the neocortex. I. The role of the thalamo-cortical loop. Biol. Cybern. 1991, 65, 135–145. [Google Scholar] [CrossRef]
- Mumford, D. On the computational architecture of the neocortex. II. The role of cortico-cortical loops. Biol. Cybern. 1992, 66, 241–251. [Google Scholar] [CrossRef]
- Rao, R.P.; Ballard, D.H. Dynamic model of visual recognition predicts neural response properties in the visual cortex. Neural Comput. 1997, 9, 721–763. [Google Scholar] [CrossRef]
- Rao, R.P.; Ballard, D.H. Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nat. Neurosci. 1999, 2, 79–87. [Google Scholar] [CrossRef]
- Hinton, G.E. Learning multiple layers of representation. Trends Cogn. Sci. 2007, 11, 428–434. [Google Scholar] [CrossRef]
- Rumelhart, D.E.; McClelland, J.L. Parallel Distributed Processing: Explorations in the Microstructure of Cognition. Vol I: Foundations; MIT Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Friston, K. Functional integration and inference in the brain. Prog. Neurobiol. 2002, 68, 113–143. [Google Scholar] [CrossRef]
- Friston, K.; Breakspear, M.; Deco, G. Perception and self-organized instability. Front. Comput. Neurosci. 2012, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. A free energy principle for a particular physics. arXiv 2019, arXiv:1906.10184. [Google Scholar] [CrossRef]
- Bastos, A.M.; Usrey, W.M.; Adams, R.A.; Mangun, G.R.; Fries, P.; Friston, K.J. Canonical microcircuits for predictive coding. Neuron 2012, 76, 695–711. [Google Scholar] [CrossRef]
- Adams, R.A.; Shipp, S.; Friston, K.J. Predictions not commands: Active inference in the motor system. Brain Struct. Funct. 2013, 218, 611–643. [Google Scholar] [CrossRef] [PubMed]
- Wiese, W.; Friston, K.J. The neural correlates of consciousness under the free energy principle: From computational correlates to computational explanation. Philos. Mind Sci. 2021, 2. [Google Scholar] [CrossRef]
- Friston, K.J.; Wiese, W.; Hobson, J.A. Sentience and the origins of consciousness: From Cartesian duality to Markovian monism. Entropy 2020, 22, 516. [Google Scholar] [CrossRef]
- Friston, K. Am I Self-Conscious? (Or Does Self-Organization Entail Self-Consciousness?). Front. Psychol. 2018, 9, 579. [Google Scholar] [CrossRef]
- Luu, P.; Tucker, D.M.; Friston, K. From active affordance to active inference: Vertical integration of cognition in the cerebral cortex through dual subcortical control systems. Cereb. Cortex 2024, 34, 1–30. [Google Scholar] [CrossRef]
- Tucker, D.M.; Luu, P. Adaptive control of functional connectivity: Dorsal and ventral limbic divisions regulate the dorsal and ventral neocortical networks. Cereb. Cortex 2023, 33, 7870–7895. [Google Scholar] [CrossRef]
- Friston, K.J.; Lin, M.; Frith, C.D.; Pezzulo, G.; Hobson, J.A.; Ondobaka, S. Active Inference, Curiosity and Insight. Neural Comput. 2017, 29, 2633–2683. [Google Scholar] [CrossRef]
- Tucker, D.M.; Luu, P.; Friston, K.J. Adaptive consolidation of active inference: Excitatory and inhibitory mechanisms for organizing feedforward and feedback memory systems in sleep. Cereb. Cortex 2025, 35, bhaf122. [Google Scholar] [CrossRef] [PubMed]
- Limanowski, J.; Blankenburg, F. Minimal self-models and the free energy principle. Front. Hum. Neurosci. 2013, 7, 547. [Google Scholar] [CrossRef] [PubMed]
- Palmer, C.J.; Seth, A.K.; Hohwy, J. The felt presence of other minds: Predictive processing, counterfactual predictions, and mentalising in autism. Conscious. Cogn. 2015, 36, 376–389. [Google Scholar] [CrossRef] [PubMed]
- Hohwy, J. The self-evidencing brain. Noûs 2016, 50, 259–285. [Google Scholar] [CrossRef]
- Hohwy, J.; Michael, J. 16 Why should any body have a self? In The Subject’s Matter: Self-Consciousness and the Body; MIT Press: Cambridge, MA, USA, 2017; Volume 363, pp. 364–398. [Google Scholar]
- Limanowski, J. (Dis-) Attending to the body. In PPP-Philosophy and Predictive Processing; Open MIND; Frankfurt am Main: MIND Group: Frankfurt, Germany, 2017. [Google Scholar]
- Sandved-Smith, L.; Hesp, C.; Mattout, J.; Friston, K.; Lutz, A.; Ramstead, M.J. Towards a computational phenomenology of mental action: Modelling meta-awareness and attentional control with deep parametric active inference. Neurosci. Conscious. 2021, 2021, niab018. [Google Scholar] [CrossRef]
- Da Costa, L.; Sandved-Smith, L.; Friston, K.; Ramstead, M.J.; Seth, A.K. A Mathematical Perspective on Neurophenomenology. arXiv 2024, arXiv:2409.20318. [Google Scholar] [CrossRef]
- Tellegen, A. Structures of mood and personality and their relevance to assessing anxiety, with and emphasis on self-report. In Anxiety and the Anxiety Related Disorders; Tuma, A.H., Maser, J.D., Eds.; Erlbaum: Hillsdale, MI, USA, 1985; pp. 681–706. [Google Scholar]
- Tucker, D.M.; Luu, P. Cognition and Neural Development; Oxford University Press: New York, NY, USA, 2012. [Google Scholar]
- Hesp, C.; Smith, R.; Parr, T.; Allen, M.; Friston, K.J.; Ramstead, M.J. Deeply felt affect: The emergence of valence in deep active inference. Neural Comput. 2021, 33, 398–446. [Google Scholar] [CrossRef]
- Parr, T.; Friston, K.J. Uncertainty, epistemics and active inference. J. R. Soc. Interface 2017, 14, 20170376. [Google Scholar] [CrossRef]
- Luu, P.; Tucker, D.M. Continuity and change in neural plasticity through embryonic morphogenesis, fetal activity-dependent synaptogenesis, and infant memory consolidation. Dev. Psychobiol. 2023, 65, e22439. [Google Scholar] [CrossRef] [PubMed]
- Tononi, G.; Cirelli, C. Sleep function and synaptic homeostasis. Sleep. Med. Rev. 2006, 10, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.M.; Johnson, M. The Emotional Mind: How Feelings Shape Thought. 2025; Unpublished work. [Google Scholar]
- D’Esposito, M.; Postle, B.R. The cognitive neuroscience of working memory. Annu. Rev. Psychol. 2015, 66, 115–142. [Google Scholar] [CrossRef] [PubMed]
- Dewey, J. Experience and Nature. In The Later Works, 1925–1953; Boydston, J.A., Ed.; Southern Illinois University Press: Carbondale, IL, USA; Edwardsville, IL, USA, 1925; Volume 1, pp. 274–275. [Google Scholar]
- Tucker, D.M.; Luu, P.; Johnson, M. Neurophysiological mechanisms of implicit and explicit memory in the process of consciousness. J. Neurophysiol. 2022, 128, 872–891. [Google Scholar] [CrossRef]
- García-Cabezas, M.Á.; Zikopoulos, B.; Barbas, H. The Structural Model: A theory linking connections, plasticity, pathology, development and evolution of the cerebral cortex. Brain Struct. Funct. 2019, 224, 985–1008. [Google Scholar] [CrossRef]
- Tucker, D.M.; Luu, P. Motive control of unconscious inference: The limbic base of adaptive Bayes. Neurosci. Biobehav. Rev. 2021, 128, 328–345. [Google Scholar] [CrossRef]
- Gabriel, M.; Sparenborg, S.P.; Stolar, N. An executive function of the hippocampus: Pathway selection for thalamic neuronal significance code. In The Hippocampus; Isaacson, R.L., Pribram, K.H., Eds.; Plenum: New York, NY, USA, 1986; Volume 4, pp. 1–39. [Google Scholar]
- Pribram, K.H. Emotions. In Handbook of Clinical Neuropsychology; Filskov, S.K., Boll, T.J., Eds.; Wiley: New York, NY, USA, 1981; pp. 102–134. [Google Scholar]
- Aggleton, J.R.; Brown, M.W. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav. Brain Sci. 1999, 22, 425–489. [Google Scholar] [CrossRef]
- Squire, L.R. Mechanisms of memory. Science 1986, 232, 1612–1619. [Google Scholar] [CrossRef]
- Papez, J.W. A proposed mechanism of emotion. Arch. Neurol. Psychiatry 1937, 38, 725–743. [Google Scholar] [CrossRef]
- Jones, E.G. The Thalamus; Cambridge University Press: Cambridge, UK, 2007; Volume I. [Google Scholar]
- Nauta, W. A survey of the limbic system. Alcohol. Drug Res. 1985, 6, 67–68. [Google Scholar] [PubMed]
- Yakovlev, P.I. Motility, behavior and the brain. J. Nerv. Ment. Dis. 1948, 107, 313–335. [Google Scholar] [CrossRef] [PubMed]
- Blumer, D.; Benson, D.F. Personality changes with frontal and temporal lobe lesions. In Psychiatric Aspects of Neurologic Disease; Benson, D.F., Blumer, D., Eds.; Gruen and Stratton: New York, NY, USA, 1975; pp. 151–170. [Google Scholar]
- Kluver, H.; Bucy, P.C. Preliminary analysis of functions of the temporal lobes in monkeys. Arch. Neurol. Psychiatry 1939, 42, 979–1000. [Google Scholar] [CrossRef]
- Aggleton, J.P.; Passingham, R. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta). J. Comp. Physiol. Psychol. 1981, 95, 961. [Google Scholar] [CrossRef]
- Gabriel, M. Functions of anterior and posterior cingulate cortex during avoidance learning in rabbits. Prog. Brain Res. 1990, 85, 467–483. [Google Scholar]
- Goldberg, G. From intent to action: Evolution and function of the premotor systems of the forntal lobe. In The Frontal Lobes Revisited; Perceman, E., Ed.; The IRBN Press: New York, NY, USA, 1987; pp. 273–306. [Google Scholar]
- Shipp, S. The importance of being agranular: A comparative account of visual and motor cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 797–814. [Google Scholar] [CrossRef]
- Greicius, M.; Supekar, K.; Menon, V.; Dougherty, R. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cereb. Cortex 2008, 19, 72–78. [Google Scholar] [CrossRef]
- Yeo, B.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Friston, K.; FitzGerald, T.; Rigoli, F.; Schwartenbeck, P.; Pezzulo, G. Active inference and learning. Neurosci. Biobehav. Rev. 2016, 68, 862–879. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Fagerholm, E.D.; Zarghami, T.S.; Parr, T.; Hipólito, I.; Magrou, L.; Razi, A. Parcels and particles: Markov blankets in the brain. Netw. Neurosci. 2021, 5, 211–251. [Google Scholar] [CrossRef] [PubMed]
- Barbas, H.; Rempel-Clower, N. Cortical structure predicts the pattern of corticocortical connections. Cereb. Cortex 1997, 7, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Tucker, D.M.; Williamson, P.A. Asymmetric neural control systems in human self-regulation. Psychol. Rev. 1984, 91, 185–215. [Google Scholar] [CrossRef]
- Mesulam, M. Central cholinergic pathways: Neuroanatomy and some behavioral implications. In Neurotransmitters and Cortical Function: From Molecules to Mind; Avoli, M., Reader, T.A., Dykes, R.W., Gloor, P., Eds.; Plenum Press: New York, NY, USA, 1988; pp. 237–260. [Google Scholar]
- Pribram, K.H.; McGuinness, D. Arousal, activation, and effort in the control of attention. Psychol. Rev. 1975, 82, 6–149. [Google Scholar] [CrossRef]
- Vogt, B.A.; Pandya, D.N. Cingulate cortex of the rhesus monkey: II. Cortical afferents. J. Comp. Neurol. 1987, 262, 271–289. [Google Scholar] [CrossRef]
- Eidelberg, D.; Galaburda, A.M. Inferior parietal lobule: Divergent architectonic asymmetries in the human brain. Arch. Neurol. 1984, 41, 843–852. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Herkenham, M.; Thibault, J. The neostriatal mosaic: II. Patch-and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J. Neurosci. 1987, 7, 3915–3934. [Google Scholar] [CrossRef]
- Nauta, W.J. Limbic innervation of the striatum. Adv. Neurol. 1982, 35, 41–47. [Google Scholar]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670. [Google Scholar] [CrossRef] [PubMed]
- Nauta, W.J.; Smith, G.P.; Faull, R.L.; Domesick, V.B. Efferent connections and nigral afferents of the nucleus accumbens septi in the rat. Neuroscience 1978, 3, 385–401. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L. Functional implications of the prosomeric model. Biomolecules 2024, 14, 331. [Google Scholar] [CrossRef] [PubMed]
- Puelles, L. Thoughts on the development, structure and evolution of the mammalian and avian telencephalic pallium. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2001, 356, 1583–1598. [Google Scholar] [CrossRef]
- Luzzati, F. A hypothesis for the evolution of the upper layers of the neocortex through co-option of the olfactory cortex developmental program. Front. Neurosci. 2015, 9, 162. [Google Scholar] [CrossRef]
- Nauta, W.J.H.; Karten, H.J. A general profile of the vertebrate brain, with sidelights on the ancestry of cerebral cortex. In The Neurosciences; Quarton, G.C., Melnechuck, T., Adelman, G., Eds.; Rockefeller University Press: New York, NY, USA, 1970; pp. 7–26. [Google Scholar]
- Born, J.; Wilhelm, I. System consolidation of memory during sleep. Psychol. Res. 2012, 76, 192–203. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. 2010, 11, 114–126. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep and the price of plasticity: From synaptic and cellular homeostasis to memory consolidation and integration. Neuron 2014, 81, 12–34. [Google Scholar] [CrossRef]
- Walker, M. Why We Sleep: Unlocking the Power of Sleep and Dreams; Simon and Schuster: New York, NY, USA, 2017. [Google Scholar]
- Buzsáki, G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015, 25, 1073–1188. [Google Scholar] [CrossRef]
- Narikiyo, K.; Mizuguchi, R.; Ajima, A.; Shiozaki, M.; Hamanaka, H.; Johansen, J.P.; Mori, K.; Yoshihara, Y. The claustrum coordinates cortical slow-wave activity. Nat. Neurosci. 2020, 23, 741–753. [Google Scholar] [CrossRef]
- Tononi, G.; Cirelli, C. Sleep and synaptic homeostasis: A hypothesis. Brain Res. Bull. 2003, 62, 143–150. [Google Scholar] [CrossRef]
- Grossberg, S. How does a brain build a cognitive code? Psychol. Rev. 1980, 87, 1–51. [Google Scholar] [CrossRef] [PubMed]
- Luppi, P.-H.; Billwiller, F.; Fort, P. Selective activation of a few limbic structures during paradoxical (REM) sleep by the claustrum and the supramammillary nucleus: Evidence and function. Curr. Opin. Neurobiol. 2017, 44, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Basheer, R.; McKenna, J.T.; Strecker, R.E.; McCarley, R.W. Control of sleep and wakefulness. Physiol. Rev. 2012, 92, 1087–1187. [Google Scholar] [CrossRef]
- Miller, R. Cortico-hippocampal interplay: Self-organizing phase-locked loops for indexing memory. Psychobiology 1989, 17, 115–128. [Google Scholar] [CrossRef]
- Hinton, G.E.; Dayan, P.; Frey, B.J.; Neal, R.M. The “wake-sleep” algorithm for unsupervised neural networks. Science 1995, 268, 1158–1161. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M.; Siegelbaum, S.; Hudspeth, A.J.; Mack, S. Principles of Neural Science; McGraw-hill: New York, NY, USA, 2000; Volume 4. [Google Scholar]
- Munoz, M.A. Colloquium: Criticality and dynamical scaling in living systems. Rev. Mod. Phys. 2018, 90, 031001. [Google Scholar] [CrossRef]
- Chialvo, D.R. Emergent complex neural dynamics. Nat. Phys. 2010, 6, 744–750. [Google Scholar] [CrossRef]
- Hesse, J.; Gross, T. Self-organized criticality as a fundamental property of neural systems. Front. Syst. Neurosci. 2014, 8, 166. [Google Scholar] [CrossRef]
- Beggs, J.M.; Plenz, D. Neuronal avalanches in neocortical circuits. J. Neurosci. 2003, 23, 11167–11177. [Google Scholar] [CrossRef]
- Tsodyks, M.V.; Sejnowski, T. Rapid state switching in balanced cortical network models. Netw. Comput. Neural Syst. 1995, 6, 111. [Google Scholar] [CrossRef]
- Friston, K.; Kiebel, S. Predictive coding under the free-energy principle. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 1211–1221. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Hering, E. Über das Gedächtnis als Eine Allgemeine Funktion der Organisierten Materie: Vortrag Gehalten in der Feierlichen Sitzung der Kaiserlichen Akademie der Wissenschaften in Wien am XXX. Mai MDCCCLXX; Wilhelm Engelmann: Leipzig, Germany, 1912. [Google Scholar]
- Zeidman, P.; Friston, K.; Parr, T. A primer on Variational Laplace (VL). NeuroImage 2023, 279, 120310. [Google Scholar] [CrossRef]
- Fountas, Z.; Sylaidi, A.; Nikiforou, K.; Seth, A.K.; Shanahan, M.; Roseboom, W. A predictive processing model of episodic memory and time perception. Neural Comput. 2022, 34, 1501–1544. [Google Scholar] [CrossRef]
- Werner, H. The Comparative Psychology of Mental Development; Harper: New York, NY, USA, 1957. [Google Scholar]
- Dehaene, S. Consciousness and the Brain: Deciphering How the Brain Codes Our Thoughts; Viking: New York, NY, USA, 2014. [Google Scholar]
- Müller, P.M.; Miron, G.; Holtkamp, M.; Meisel, C. Critical dynamics predicts cognitive performance and provides a common framework for heterogeneous mechanisms impacting cognition. Proc. Natl. Acad. Sci. USA 2025, 122, e2417117122. [Google Scholar] [CrossRef] [PubMed]
- Isen, A.M. Positive affect, cognitive processes, and social behavior. Adv. Exp. Soc. Psychol. 1987, 20, 203–253. [Google Scholar]
- Tucker, D.M. Mind From Body: Experience From Neural Structure; Oxford University Press: New York, NY, USA, 2007. [Google Scholar]
- Hobson, J.A.; Friston, K.J. Waking and dreaming consciousness: Neurobiological and functional considerations. Prog. Neurobiol. 2012, 98, 82–98. [Google Scholar] [CrossRef]
- Brown, J. Microstructure of perception. Cogn. Brain Theory 1983, 6, 145–184. [Google Scholar]
- Tognoli, E.; Kelso, J.A.S. The Metastable Brain. Neuron 2014, 81, 35–48. [Google Scholar] [CrossRef]
- Piaget, J. The Origins of Intelligence in Children; International Universities Press: New York, NY, USA, 1936. [Google Scholar]
- Piaget, J. Genetic Epistemology; W. W. Norton & Company: New York, NY, USA, 1971. [Google Scholar]
- Axmacher, N.; Mormann, F.; Fernández, G.; Elger, C.E.; Fell, J. Memory formation by neuronal synchronization. Brain Res. Rev. 2006, 52, 170–182. [Google Scholar] [CrossRef]
- Canolty, R.T.; Edwards, E.; Dalal, S.S.; Soltani, M.; Nagarajan, S.S.; Kirsch, H.E.; Berger, M.S.; Barbaro, N.M.; Knight, R.T. High gamma power is phase-locked to theta oscillations in human neocortex. Science 2006, 313, 1626–1628. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-band oscillations—Signalling the status quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Hobson, J. The AIM Model of Dreaming, Sleeping, and Waking Consciousness; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Comrie, A.E.; Frank, L.M.; Kay, K. Imagination as a fundamental function of the hippocampus. Philos. Trans. R. Soc. B 2022, 377, 20210336. [Google Scholar] [CrossRef] [PubMed]
- Ellamil, M.; Dobson, C.; Beeman, M.; Christoff, K. Evaluative and generative modes of thought during the creative process. Neuroimage 2012, 59, 1783–1794. [Google Scholar] [CrossRef]
- Graybiel, A.M.; Matsushima, A. Striosomes and Matrisomes: Scaffolds for Dynamic Coupling of Volition and Action. Annu. Rev. Neurosci. 2023, 46, 359–380. [Google Scholar] [CrossRef]
- Meunier, D.; Lambiotte, R.; Fornito, A.; Ersche, K.; Bullmore, E.T. Hierarchical modularity in human brain functional networks. Front. Neuroinform. 2009, 3, 571. [Google Scholar] [CrossRef]
- Alemdar, E.; Poznanski, R.; Cacha, L.; Leisman, G.; Brändas, E.J. New insights into holonomic brain theory: Implications for active consciousness. J. Multiscale Neurosci. 2023, 2, 159–168. [Google Scholar] [CrossRef]
- Sbitnev, V. The edge of chaos is that where consciousness manifests itself through intermittent dynamics. Acad. Biol. 2024, 2. [Google Scholar] [CrossRef]
- Hall, C.; Van de Castle, R. The Content Analysis of Dreams; Appleton-Century-Crofts: New York, NY, USA, 1966. [Google Scholar]
- Pace-Schott, E.F.; Seo, J.; Bottary, R. The influence of sleep on fear extinction in trauma-related disorders. Neurobiol. Stress 2023, 22, 100500. [Google Scholar] [CrossRef]
- Zeithamova, D.; Bowmana, C.R. Generalization and the hippocampus: More than one story? Neurobiol. Learn. Mem. 2020, 175, 107317. [Google Scholar] [CrossRef] [PubMed]
- Gendlin, E.T. Experiencing and the Creation of Meaning: A Philosophical and Psychological Approach to the Subjective; Northwestern University Press: Evanston, IL, USA, 1997. [Google Scholar]



| Term/Symbol | Concise Definition | Typical Biological/Computational Correlate |
|---|---|---|
| Feed-forward (limbifugal, generative) projections | Propagation of descending predictions from higher to lower cortical levels; sets up hypotheses about upcoming input. | Cortico-cortical projections from infragranular to supragranular layers; hippocampal → neocortical replay; θ–γ “driver” coupling. |
| Feedback (limbipetal, corrective) projections | Error-correcting signals that compare predictions with sensory input and update beliefs via ascending prediction errors. | Cortico-cortical projections layer 2–3 → 4; thalamo-cortical bursting; α–β “modulator” rhythms. |
| Excitatory/Inhibitory (E–I) balance | Dynamic trade-off between excitatory drive and inhibitory restraint that keeps networks near criticality. | Pyramidal–interneuron loops; spike-time cross-correlations; LC-NE vs. VTA-DA neuromodulation. |
| Criticality | An operating regime poised at the transition between order and disorder, maximizing dynamic range and information flow. | Power-law avalanche size distributions in hdEEG/MEG; 1/f spectral slope. |
| Markov blanket | The statistical boundary or interface that shields internal states from external states while permitting exchange. | Boundaries between each hierarchical level of the Structural Model; thalamo-cortical loops. |
| Variational Free Energy | Upper bound on surprise (i.e., self information); minimized when the agent’s generative model matches sensory data. | Evidence-lower-bound (ELBO) in Bayesian machine-learning; precision-weighted prediction error in predictive coding and Bayesian filtering. |
| NREM (N1–N3)/REM | Alternating sleep macro-states: NREM supports synaptic down-selection and memory replay; REM integrates and contextualizes mnemonic traces. | Slow oscillations/spindles (NREM) vs. θ-γ coupling and PGO-waves (REM). |
| Symbol | Definition | Interpretation | Possible Neural Mechanism |
|---|---|---|---|
| o | Sensory observations | Data to be explained | Primary sensory afferents |
| s | Hidden (latent) causes/states | The causes of sensory input | States of affairs in the world |
| q(s) | Variational posterior | Posterior belief distribution in response to sensory evidence | Synaptic activity in cortical hierarchies and thalamocortical loops |
| p(s) | Prior over s | Prior beliefs or constraints | Synaptic activity and efficacy |
| E | Excitatory gain (i.e., prior precision) | Dorsal limbic, lemnothalamic regulation | Pontine θ–γ multiplexing, ACh, phasic LC-NE |
| I | Inhibitory gain (i.e., sensory precision) | Ventral limbic, collothalamic regulation | thalamic/striatal α/β bursts, tonic LC-NE, VTA D2/D4 |
| Gain | Precision | Computational Effect | Oscillatory/Neurochemical Correlate | |
|---|---|---|---|---|
| E ↑ | Prior precision | Explores narrower range of priors (reduced conceptual scope with increased central coherence) | θ–γ multiplexing, ACh, phasic DA & LC-NE | |
| I ↑ | Sensory precision | Precise, longer-lived responses to sensory perturbations | α/β bursts, tonic LC-NE, D2/D4 | |
| ρ = E/I | Precision ratio | Self-reliance vs environment reliance | Day-dreaming vs reality-checking | |
| Criticality | Regime in which E and I are balanced, so that ρ hovers near the critical regime across the diurnal cycle. | E–I phase portrait spiraling onto a limit-cycle at criticality. | ||
| Phase | Precision | Network Consequence | Behavioral Outcome |
|---|---|---|---|
| Early NREM | I dominates | Sharpens synaptic weights; down-selects noisy traces | Declarative memory stabilization |
| REM burst | E dominates | Integrates remote associations | Insight, emotional tagging |
| Late-night REM dominance | ρ oscillates near optimum E/I balance | Network at ρ ≈ 1 | Next-day critical readiness |
| Stage | Prior Precision E | Sensory Precision I | Computational Work | Phenomenological Residue |
|---|---|---|---|---|
| Stage-2 NREM (spindles) | ↘ moderate | ↗ moderate | Event segmentation: spindles carve the day’s stream into ~0.5 s packets. Hippocampal ripples that co-occur under a spindle trough bind elements that consistently follow one another—extracting hidden probabilistic contingencies. | Morning “aha” moments about order (“the light blew because the breaker was already flipped”). |
| Slow-wave NREM (SWS) | minimal | maximal | Hierarchical nesting: each slow-wave UP state embeds multiple spindles which embed ripples, building multi-timescale directed graphs of causality in medial prefrontal cortex. | Feeling of settled chronology; fewer temporal confusions in recall. |
| Phasic REM | ↑↑ broad | ↓↓ brief | Generative replay: pontine P-waves switch cortex into model-sampling. Weakly linked spindle chunks co-activate, testing counterfactual chains. Dopamine tags successful free-energy-reducing linkages. | Vivid dream scenes where remote ideas collide; creative insights on waking. |
| Tonic REM tail | moderate | moderate | Re-binding: LC/orexin micro-blips nudge sensory precision upward, copying REM-tested linkages back into hippocampus. | Dream coherence peaks just before awakening—memorable story line. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucker, D.M.; Luu, P.; Friston, K.J. The Criticality of Consciousness: Excitatory–Inhibitory Balance and Dual Memory Systems in Active Inference. Entropy 2025, 27, 829. https://doi.org/10.3390/e27080829
Tucker DM, Luu P, Friston KJ. The Criticality of Consciousness: Excitatory–Inhibitory Balance and Dual Memory Systems in Active Inference. Entropy. 2025; 27(8):829. https://doi.org/10.3390/e27080829
Chicago/Turabian StyleTucker, Don M., Phan Luu, and Karl J. Friston. 2025. "The Criticality of Consciousness: Excitatory–Inhibitory Balance and Dual Memory Systems in Active Inference" Entropy 27, no. 8: 829. https://doi.org/10.3390/e27080829
APA StyleTucker, D. M., Luu, P., & Friston, K. J. (2025). The Criticality of Consciousness: Excitatory–Inhibitory Balance and Dual Memory Systems in Active Inference. Entropy, 27(8), 829. https://doi.org/10.3390/e27080829






