Abstract
Dual-phase high entropy alloys have recently attracted widespread attention as advanced structural materials due to their unique microstructure, excellent mechanical properties, and corrosion resistance. However, their molten salt corrosion behavior has not been reported, which is critical in evaluating their application merit in the areas of concentrating solar power and nuclear energy. Here, the molten salt corrosion behavior of AlCoCrFeNi2.1 eutectic high-entropy alloy (EHEA) was evaluated in molten NaCl-KCl-MgCl2 salt at 450 °C and 650 °C in comparison to conventional duplex stainless steel 2205 (DS2205). The EHEA showed a significantly lower corrosion rate of ~1 mm/year at 450 °C compared to ~8 mm/year for DS2205. Similarly, EHEA showed a lower corrosion rate of ~9 mm/year at 650 °C compared to ~20 mm/year for DS2205. There was selective dissolution of the body-centered cubic phase in both the alloys, B2 in AlCoCrFeNi2.1 and α-Ferrite in DS2205. This was attributed to micro-galvanic coupling between the two phases in each alloy that was measured in terms of Volta potential difference using a scanning kelvin probe. Additionally, the work function increased with increasing temperature for AlCoCrFeNi2.1, indicating that the FCC-L12 phase acted as a barrier against further oxidation and protected the underlying BCC-B2 phase with enrichment of noble elements in the protective surface layer.
1. Introduction
The world economy is highly dependent on fossil fuels to meet persistent energy demands, which results in carbon emissions and negative environmental impacts. This is driving the global search for sustainable and alternative energy sources [1], solar energy being one of the most viable and clean options [2]. The global capacity for concentrating solar power (CSP) systems has increased fifteen-fold over the last two decades [3]. The solar power conversion systems in CSP operate with advanced fluids above 400 °C [4], using molten salts such as solar salt [5], Hitec salt [6], and MgNaK chlorides [7]. In particular, the ternary MgNaK chloride (MgCl2-NaCl-KCl) eutectic salt mixture has high thermal stability and is more cost effective compared to other molten salts [8,9]. This allows CSP to operate at higher temperatures for higher efficiency of thermal-to-electrical energy conversion and reduces the cost of electricity. However, degradation of structural materials in a molten salt environment remains a critical concern in CSP systems [9,10]. The high operating temperature and reactive nature of the salt significantly increase the corrosion susceptibility of the materials and reduce component lifetimes [4]. This necessitates the development of new alloys with high-temperature strength and corrosion resistance to withstand the harsh molten salt environment of CSP systems.
High-entropy alloys (HEAs) with multiple principal elements in (nearly) equimolar proportions [11,12] have attracted much attention recently because the unconventional alloying approach results in excellent mechanical properties and surface degradation resistance [13,14,15,16,17,18]. Most of the reported HEA systems have a single-phase body-centered cubic (BCC) or face-centered cubic (FCC) structure [19,20,21]. To achieve a good balance of high strength of BCC HEAs along with good ductility of FCC HEAs, eutectic high-entropy alloys (EHEAs) consisting of the dual phases in lamellar microstructure have been recently developed [22,23]. Some examples of EHEAs with excellent combination of strength and ductility include CoCrFeNiZr0.5, CoCrFeNiMnPd1.4, CoCrFeNiMnPd0.6, Nb25Sc25Ti25Zr25, AlCrFeNiMo0.2, Al1.2CrCuFeNi2 and AlCoCrFeNix [24]. In particular, the AlCoCrFeNi2.1 EHEA with lamellar arrangement of BCC (B2) and FCC (L12) solid-solution phases has shown the most promising mechanical properties [22,25,26,27]. AlCoCrFeNi2.1 EHEA showed superior pitting corrosion resistance compared to 304 stainless steel in chloride solution at room temperature [27]. This EHEA exhibited an excellent combination of high yield strength (~750 MPa) and ductility (elongation ~18%) at room temperature [22,28]. However, the molten salt corrosion behavior of dual phase HEAs for potential use in CSP systems has not been reported so far, and fundamental understanding of the underlying high-temperature corrosion mechanisms is lacking.
Here, the corrosion behavior of AlCoCrFeNI2.1 EHEA in molten NaCl-KCl-MgCl2 salt was investigated at 450 °C and 650 °C. The performance of EHEA was compared with one of the benchmark dual-phase structural alloys, namely duplex steel 2205 (DS2205). Dual-phase steels have been reported to have excellent corrosion resistance and a good combination of strength and ductility [29,30,31,32,33,34,35]. To explain the effect of eutectic microstructure on the local electrochemical activity, Scanning Kelvin Probe (SKP) analysis was utilized to measure the relative electron work function [27,36,37]. Our findings pave the way for further research on molten salt corrosion behavior of dual-phase alloys over a wide range of temperature for potential use in concentrating solar power systems.
2. Materials and Methods
The AlCoCrFeNi2.1 eutectic high-entropy alloy ingot was prepared by vacuum-arc-melting high purity constituent elements (with 99.95 wt.% elemental purity). The ingots were re-melted multiple times for homogeneity. Samples with dimensions 20 mm × 5 mm × 2 mm were cut from the alloy for counter and working electrodes. The samples were polished to 1200-grit silicon carbide paper, rinsed with distilled water, ultrasonically degreased with acetone, and dried. A schematic and experimental setup of the furnace using three electrodes for the electrochemical studies are shown in Figure 1a,b. The custom-built tube furnace consisted of three electrodes submerged up to 5 mm in the eutectic salt mixture in open air at the two temperatures of 450 °C and 650 °C. The electrochemical measurements were performed using a potentiostat (Reference 3000, Gamry, Warminster, PA, USA). A Pt sheet (15 mm × 5 mm × 0.5 mm) in a quartz tube was used as the reference electrode (RE). A porous membrane made of alumina was used at the bottom of the quartz tube to provide ionic conductivity in the molten salt. The porous membrane minimizes salt migration and provides good electrical continuity. To eliminate any mixed potentials in the three-electrode system, the counter electrode was the same alloy as the working electrode in all experiments. The surface area ratio of the counter electrode (CE) to the working electrode (WE) was kept fixed at 1 (CE:WE = 1:1) [38]. The working and counter electrodes were placed in open-ended quartz tubes to electrically isolate them during experiments.
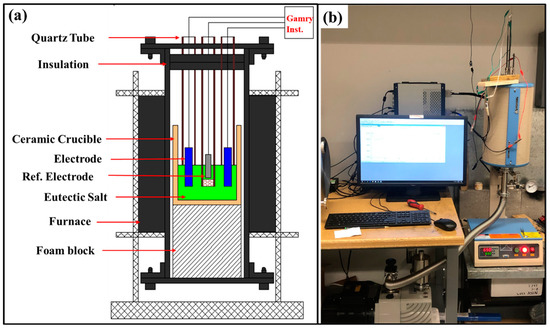
Figure 1.
(a) Schematic illustration and (b) experimental set-up of three-electrode tube furnace used in the present study for corrosion test in molten NaCl-KCl-MgCl2 salt at 450 °C and 650 °C.
Analytical grade MgCl2, NaCl, and KCl (Sigma Aldrich, St. Louis, MO, USA) were used to prepare the eutectic salt mixture in the proportion of 45.4 wt.%, 33 wt.%, and 21.6 wt.% respectively. Melting point of the salt mixture was determined experimentally to be ~380 °C, which is consistent with the value reported in the literature [39]. One application is in indirect storage system with molten salt as storage medium, often used in a parabolic trough CSP plant with maximum temperatures in the range of around 400–450 °C. Another configuration is direct storage system, often used in a tower CSP plant with molten salt heat transfer fluid (HTF), as well as TES with maximum temperatures in the range of around 600–650 °C [4,5,6,7]. The eutectic salt mixture was added in an alumina crucible to obtain a salt melt depth of ~20 mm. The NaCl-KCl-MgCl2 salt was purified by annealing at 300 °C for 24 h in high purity Ar atmosphere to remove moisture and other impurities [39]. Inductively coupled plasma-optical emission spectroscopy (ICP-OES) was used to determine impurity concentration in the eutectic salt mixture before the corrosion test as summarized in Table 1. After purification, the residual moisture in the salt is expected to be low. However, a detailed evaluation of the effect of moisture content on corrosion behavior has not been performed in the current study, but is part of a future study.

Table 1.
Concentration of main impurities in NaCl-KCl-MgCl2 eutectic salt before corrosion test (i.e., immediately following salt pre-conditioning) determined by ICP-OES.
Open circuit potential (OCP) and potentiodynamic polarization tests were conducted for each alloy. The OCP was recorded for at least 1 h after immersion to reach a stable value before the potentiodynamic polarization tests. The potentiodynamic tests were performed at a scan rate of 1 mV/s in the potential range of −0.5 to 1.5 V with respect to Pt pseudo-reference electrode. Three tests were performed for each condition.
Scanning electron microscopy (SEM, FEI Quanta-ESEM 200) equipped with energy dispersive spectroscopy (EDS) and electron backscatter diffraction (EBSD) were performed to analyze the microstructure and volume fraction of phases present in the alloys. The crystal structure of the alloys and corrosion products were determined by X-ray diffraction (XRD) using a Rigaku Ultima X-ray diffractometer with 1.54 Å Cu-Kα radiation and a scattering angle in the range of 20° to 90°. A scanning kelvin probe (SKP) microscope (Princeton Applied Research) was used to measure the relative work function distribution or Volta potential over the surface. A tungsten wire with a perpendicular amplitude of 30 µm with respect to the samples at a frequency of 80 Hz was used as a reference probe during SKP measurements. Work function was determined at a tip to sample separation of 50 µm in lab air (RH 55%).
3. Results
3.1. Microstructural Characterization
The as-cast microstructures of AlCoCrFeNi2.1 and DS2205 are shown in Figure 2a–f. The microstructure of AlCoCrFeNi2.1 consisted of lamellar arrangement of FCC (L12—light contrast) and BCC (B2—dark contrast) solid solution phases (Figure 2a) with an average grain size of ~75 ± 25 µm. Similarly, DS2205 showed a lamellar morphology of FCC (γ austenite—light contrast) and BCC (α ferrite—dark contrast) phases (Figure 2e) with an average grain size of ~110 ± 35 µm. The EBSD phase map in Figure 2b shows the lamellar structure of AlCoCrFeNi2.1 with FCC (red color ~77%) and BCC (green color ~23%) phases, while Figure 2f shows the corresponding map for DS2205. The EDS map of the as-cast AlCoCrFeNi2.1 is shown in Figure 2c. The BCC phase was found to be rich in Ni and Al, while the FCC phase was rich in Co, Cr, and Fe. Similarly, the EDS map of DS2205 in Figure 2g shows that the FCC (γ—Austenite) phase is rich in Ni and the BCC (α—Ferrite) phase is rich in Cr and Mo. The XRD pattern in Figure 2d of as-cast AlCoCrFeNi2.1 confirmed its dual-phase microstructure consisting of FCC-L12 and BCC-B2 phases. Corresponding XRD pattern for DS2205 in Figure 2h confirmed its dual-phase FCC-BCC microstructure. The chemical composition of each phase in AlCoCrFeNi2.1 and DS2205, as well as the overall composition, are summarized in Table 2. The differences in the fraction of relatively nobler elements (e.g., Co, Cr, and Ni) between the two phases in AlCoCrFeNi2.1 is significantly larger than in DS2205.
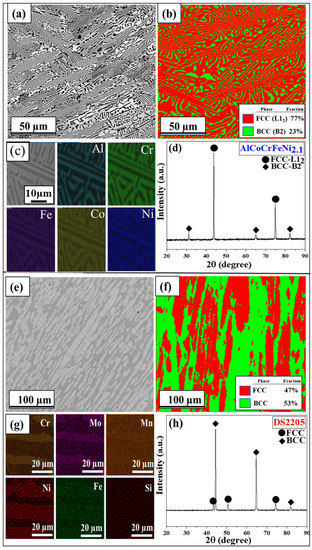
Figure 2.
SEM BSE image for (a) EHEA and (e) DS2205; EBSD phase map for (b) EHEA and (f) DS2205; EDS map for (c) EHEA and (g) DS2205; XRD pattern for (d) EHEA and (h) DS2205.

Table 2.
Chemical composition (at. %) of the phases in AlCoCrFeNi2.1 and DS2205.
3.2. Electrochemical and Corrosion Behavior
Open circuit potential for the two alloys at 450 °C and 650 °C is shown in Figure 3a. DS2205 and AlCoCrFeNi2.1 showed stable potential, which gradually increased towards a more noble value and lower fluctuations after ~1 h of exposure in the molten chloride salt at 450 °C. At 650 °C, both the alloys showed relatively poor stability for the first 30 min, suggesting that an increase in temperature might have resulted in the unstable growth of surface oxide initially, before reaching steady state. Figure 3b shows the potentiodynamic polarization curves for the two alloys after stable OCP at the temperatures of 450 °C and 650 °C. The corrosion current density (Icorr) for the two alloys was determined from Tafel analysis and is summarized in Table 3. The Icorr value increased with an increase in temperature for both alloys, indicating the high dissolution rate and relatively poor protective nature of their surface passivation layer. AlCoCrFeNi2.1 showed lower Icorr at 650 °C compared to DS2205, demonstrating its excellent corrosion resistance at the higher temperature.
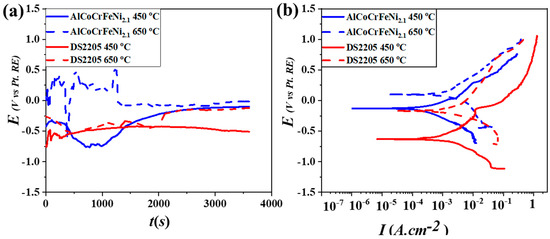
Figure 3.
(a) OCP curves of the AlCoCrFeNi2.1 and DS2205 alloys exposed to chloride salt medium at 450 °C and 650 °C; (b) potentiodynamic polarization curves of the alloys at 450 °C and 650 °C, showing better corrosion resistance of AlCoCrFeNi2.1 at 450 °C and 650 °C compared to DS2205.

Table 3.
Corrosion potential (Ecorr), corrosion current density (Icorr), and corrosion rate (CR) of AlCoCrFeNi2.1 and DS2205 alloys in NaCl-2KCl-MgCl2 salt at 450 °C and 650 °C.
Corrosion rates (CR) based on Icorr and Mass loss for the two alloys was calculated from the following equations [36,40]:
where K1 = 3.27 × 10−3 [mm/(μA·cm·year)] is a constant which is taken from ASTM G102; EW is equivalent weight in g/equivalent; ρ is density in g/cm3; and Icorr is corrosion current density in μA/cm2.
where k is constant 8.76 × 104; CR is in (mm/y), which is taken from ASTM G31; mloss is the mass loss (g) of the metal in time t (h); A is the surface area of the material exposed (cm2); and ρ is the density of the material (g/cm3). The corrosion rate based on Equations (1) and (2) for the two alloys at 450 °C and 650 °C are shown in Table 3. AlCoCrFeNi2.1 showed a significantly lower corrosion rate compared to DS2205 at both the temperatures. Poor stability of Fe-based alloys at elevated temperatures in molten chloride media due to dissolution of Cr and Fe has been reported previously [38,41]. In contrast, AlCoCrFeNi2.1 high-entropy alloy showed a higher corrosion resistance compared to dual-phase DS2205 steel possibly, due to greater stability of its constituent elements in the molten chloride salt environment.
Mass loss measurements based on immersion study was performed for determining material loss due to corrosion. Figure 4 shows the mass loss (mg/cm2) of the EHEA and DS2205 alloys after immersion in NaCl-KCl-MgCl2 at 450 °C and 650 °C for 24 h. Greater material loss was observed with an increase in temperature, which supports the potentiodynamic study (Table 3). At 450 °C, mass loss for DS2205 was ~24 mg/cm2 after 24 h, which was slightly higher than AlCoCrFeNi2.1 (~19 mg/cm2 after 24 h). At 650 °C, mass loss drastically increased for DS2205 to ~69 mg/cm2 after 24 h compared to ~38 mg/cm2 for AlCoCrFeNi2.1. Figure 4 shows the lower mass loss for AlCoCrFeNi2.1 as compared with DS2205 at both temperatures, suggesting superior corrosion resistance of the EHEA.
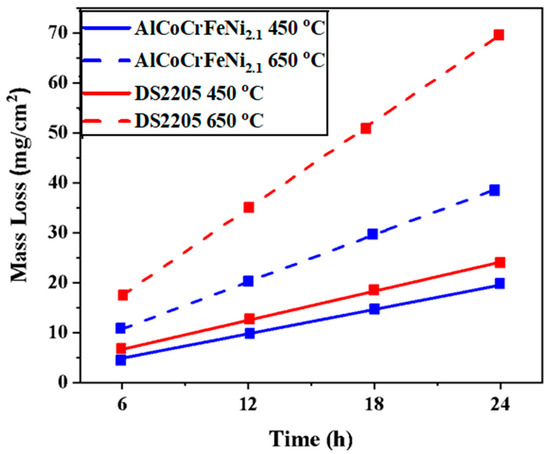
Figure 4.
Mass loss of AlCoCrFeNi2.1 and DS2205 after exposure in NaCl-KCl-MgCl2 molten salt at 450 °C and 650 °C for 24 h.
Figure 5a,b show the optical microscopy images of AlCoCrFeNi2.1 and DS2205 after corrosion tests at 450 °C and 650 °C, respectively. SEM images of the surfaces after corrosion are shown in Figure 5c–f. Selective dissolution of the B2 phase in AlCoCrFeNi2.1 was observed at 450 °C (Figure 5c). DS2205 showed preferential pitting in the BCC (α—Ferrite) regions while the FCC (γ—Austenite) phase was relatively intact (Figure 5d). At 650 °C, uniform pitting was observed all over the surface for AlCoCrFeNi2.1 (Figure 5e) in contrast to severe material loss from selective removal of the BCC (α—Ferrite) phase in DS2205 (Figure 5f). Previous studies suggest that the relative chemistry difference and phase volume fraction (FCC:BCC) determine the degree of degree of galvanic coupling in dual-phase alloys [27,42,43,44,45]. At 650 °C, duplex steel has been reported to show chloride-induced severe corrosion due to preferential attack of its ferrite phase [46]. The high-magnification SEM images in Figure 5c–f demonstrate the galvanic coupling in both AlCoCrFeNi2.1 and DS2205, with dual-phase microstructure, albeit to different extents.
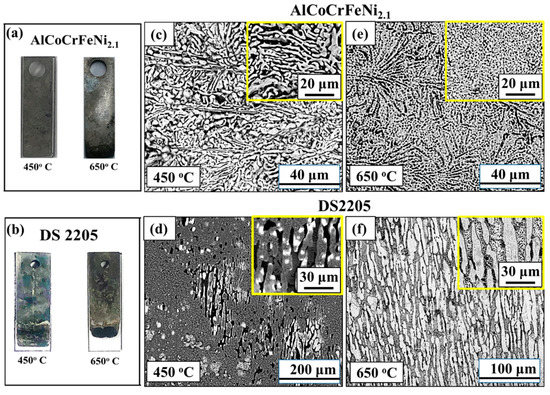
Figure 5.
Optical images for (a) AlCoCrFeNi2.1 and (b) DS2205 after electrochemical corrosion experiments in molten NaCl-KCl-MgCl2 salt; SEM image of the corroded surface at 450 °C for (c) AlCoCrFeNi2.1 EHEA and (d) DS2205; SEM image of the corroded surface at 650 °C for (e) AlCoCrFeNi2.1 EHEA and (f) DS2205. The insets in figures (c) through (f) show zoomed-in view of the corroded surfaces indicating selective removal of B2 phase in AlCoCrFeNi2.1 and BCC phase in DS2205 because of galvanic coupling.
4. Discussions
4.1. Effect of Alloy Composition on Molten Salt Corrosion Behavior
The cross-sectional morphology and element distribution of the AlCoCrFeNi2.1 and DS2205 samples are shown in Figure 6a–d by the EDS elemental maps, after immersion in molten salt at both the temperatures. For both the alloys, there was selective dissolution of some alloying elements and selective oxidation of BCC phase (B2—AlCoCrFeNi2.1 and α Ferrite—DS2205) near the surface at 450 °C and 650 °C (Figure 6). The BCC phase oxidized faster than FCC, suggesting that the BCC phase acted as micro-anodic sites with respect to the FCC phase. In AlCoCrFeNi2.1, it is noted that at 650 °C the concentration of Al, Cr, and to a lesser extent, Fe decreased (depletion), while the concentration of Co and Ni increased (enrichment). Cr depletion has been reported to be the primary form of material loss in Fe-based alloys exposed to chloride-environment [41,47]. In addition, Al loss is expected from AlCoCrFeNi2.1 because of greater stability of AlCl3 in the molten salt. At 450 °C and 650 °C, it is apparent from EDS maps shown in Figure 6 that Al and Cr are depleted from AlCoCrFeNi2.1. Although both AlCoCrFeNi2.1 and DS2205 alloys have similar Cr concertation (~20 at. %), DS2205 showed greater mass loss at both temperatures compared to AlCoCrFeNi2.1. This may be attributed to the presence of Al in AlCoCrFeNi2.1 that hinders the dissolution of the other alloy constituents in the molten salt [20]. In particular, the Cr depletion was significantly greater for DS2205 compared to AlCoCrFeNi2.1. At 450 °C, the Cr depletion depth in in AlCoCrFeNi2.1 was ~30 μm, while that for DS2205 was ~200 μm. Cr depletion depth increased significantly in DS2205 compared to AlCoCrFeNi2.1, with an increase in temperature from 450 °C to 650 °C. At 650 °C, the Cr depletion depth for AlCoCrFeNi2.1 was ~50 μm, while that for DS2205 was ~300 μm. This suggests that Al in the AlCoCrFeNi2.1 acted as a sacrificial element and reduced Cr dissolution rate in the molten salt compared to DS2205. Redox control technique has been utilized for corrosion mitigation in molten fluoride and chloride salts [48]. Dissolution of Al and Cr into the molten salt resulted in the formation of AlCl3 and CrCl2, which formed a redox couple of Al/AlCl3 and Cr/CrCl2. At the redox potential, Co, Ni, and Fe become relatively nobler and their dissolution is thermodynamically unfavorable. These findings suggest that Al/AlCl3 and Cr/CrCl2 couple may be used as redox buffer to slow or stop the dissolution of Fe, Co, and Ni from AlCoCrFeNi2.1 [49].
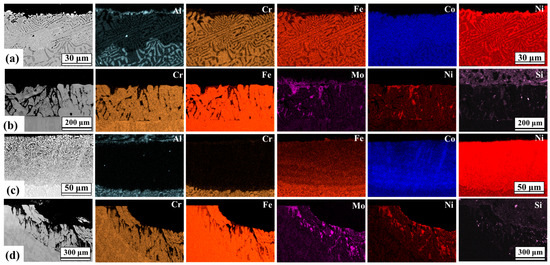
Figure 6.
Cross section EDS maps of the near-surface composition of alloys exposed to molten NaCl-KCl-MgCl2 for (a) AlCoCrFeNi2.1 at 450 °C, (b) DS2205 at 450 °C, (c) AlCoCrFeNi2.1 at 650 °C, and (d) DS2205 at 650 °C.
The chemical composition of an alloy determines the thermodynamic driving force for reaction between the molten salt and the alloy constituents [50]. Corrosion of an alloy in NaCl-KCl-MgCl2 molten salt is governed by the difference in Gibbs free energy of formation (ΔG°) for the salt constituents (NaCl, KCl, MgCl2) and corrosion products in the form of chlorides of the alloying elements (e.g., AlCl3, CrCl2, MnCl2, SiCl4, etc). Corrosion products with lower values of ΔG° are more likely to form and the associated alloying elements are likely to preferentially react with the salt and selectively leach out from the alloy. This leads to thermodynamic driving force for selective depletion of the alloying elements, i.e., dealloying at the surface [49,51]. The interaction between chlorides and moisture impurity in the salt results in the formation of highly corrosive HCl and metal oxides/hydroxides [49,51]:
The generated HCl reacts with the constituent elements of the alloy to form soluble metal chlorides as follows:
where Me is an alloying element (e.g., Al, Co, Cr, Fe, Mn, Si, etc.). Thermodynamic calculations were employed using FactSage (version 7.3, FactPS database, Montreal, Canada) to determine the potential reactions at high temperatures [48,51,52]. Based on ΔG° for formation of chloride corrosion products shown in Figure 7 (calculations at 450 °C and 650 °C), the alloying elements present in AlCoCrFeNi2.1 are expected to show high reactivity with the molten salt in the following order: Al > Cr > Fe > Co > Ni. This indicates that depletion of Al is the most favorable thermodynamically, leading to the formation of AlCl3. Similarly, for DS2205, alloying elements are expected to show high reactivity with the molten salt in the following order: Si > Mn > Cr > Fe > Ni > Mo. Thus, depletion of Si is most favorable thermodynamically in the case of DS2205, leading to the formation of SiCl4. Trace amount of salt impurities (Table 1) in NaCl-KCl-MgCl2 may also have contributed towards the relative dissolution rates of the alloying elements in the molten salt.
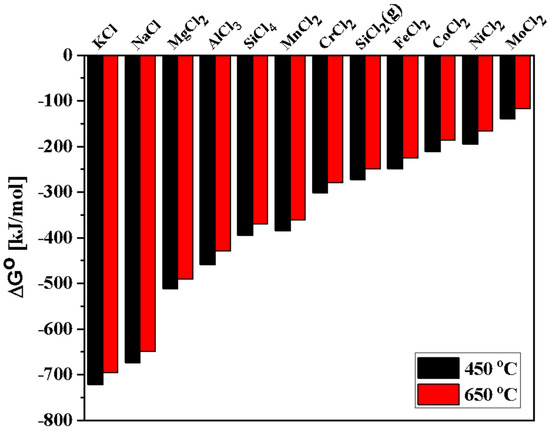
Figure 7.
Gibbs free energies of the chemical reaction between the constituent elements and 1 mol Cl−2 as a function of temperature exposed to NaCl-KCl-MgCl2 under atmospheric air.
Metallic chloride impurities (e.g., NiCl2, FeCl2, CoCl2), commonly present in NaCl-KCl-MgCl2 molten salt, may react with the alloying elements, leading to their dissolution in the melt. Ni, Co, and Fe impurity chlorides may cause depletion of Cr via the following substitution (oxidation-reduction) reactions [49,50]:
Figure 6a,c shows that there is enrichment of Co and Ni (and to some degree Fe) in the near-surface region even more than the bulk alloy, suggesting that Co, Ni, and Fe may be enriched in the Al and Cr depleted regions. Ni2+, Co2+, and Fe2+ ions were reduced to atomic Ni, Co, and Fe at the surface and their diffusion was likely assisted by vacancies created during depletion of Al and Cr. However, similar effects were not observed in case of DS2205 duplex steel, possibly because of the lack of stable redox couple of Al/AlCl3 and Cr/CrCl2. In addition, there was significant depletion of the refractory element Mo from DS2205, as shown in Figure 6b,d. Figure 7 shows relatively high ΔG° for MoCl2, so depletion of Mo due to chloride formation is not expected. Hence, depletion of Mo in DS2205 may be caused by direct reaction with moisture and oxide impurities, resulting in the formation of unstable or volatile metallic oxides as follows [53]:
4.2. Effect of Alloy Microstructure on Molten Salt Corrosion Behavior
To explain the effect of eutectic microstructure on the molten salt corrosion behavior, scanning kelvin probe (SKP) was utilized to evaluate the relative work function (WF) behavior on the surface of the alloys [27,37]. The WF may be directly related to the ability for charge transfer and represents the relative nobility of a metal surface [54]. SKP potential measured in air for several metals was found to vary linearly with their corrosion potential in aqueous solution [36,55]. Phase-specific work function for the as-cast AlCoCrFeNi2.1 and DS2205 was measured to understand the micro-galvanic corrosion and to investigate the change in galvanic activity between L12 and B2 in AlCoCrFeNi2.1 and between FCC and BCC in DS2205. Respective SKP maps are shown for DS2205 in Figure 8a and for AlCoCrFeNi2.1 in Figure 8b.
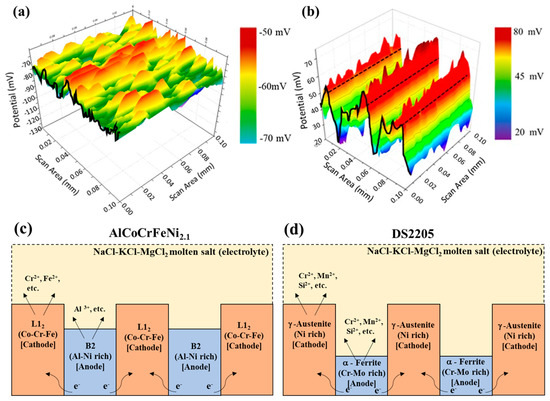
Figure 8.
Relative work-function topography obtained from scanning kelvin probe microscope of the phases to delineate the potential changes in (a) as-cast DS2205 and (b) as-cast AlCoCrFeNi2.1. Schematic illustration of the mechanism of galvanic corrosion for (c) the AlCoCrFeNi2.1 alloy Ni-rich BCC-B2 phase in the vicinity of FCC-L12; (d) the DS2205 alloy Cr-Mo-rich BCC phase in the vicinity of Ni-rich FCC phase.
The color contrast in the topographical plot indicates the relative work function potential contrast difference between the FCC and BCC phases. The FCC phase (red-colored areas) in AlCoCrFeNi2.1 (L12) and DS2205 (FCC) showed higher relative work function compared to the corresponding BCC phase (green-blue-colored areas) in AlCoCrFeNi2.1 (B2) and DS2205 (BCC). The potential contrast difference between the phases in AlCoCrFeNi2.1 was approximately ~50 mV, while in the case of DS2205 it was ~35 mV, which explains the selective dissolution seen from the EDS maps, due to the micro-galvanic cell formation in both the alloys. As schematically shown in Figure 8c,d, the high relative work function of the L12 and FCC phase indicates their higher nobility compared to the B2 and BCC phase, respectively, and agrees with the SEM-EDS data (Figure 5 and Figure 6) of selective corrosion of B2/BCC phase. Co-Cr-Fe-rich FCC-L12 may be considered the matrix in this alloy that triggers anodic dissolution of the neighboring Al-Ni-rich B2 phase. It has been reported previously that Cr-Mo-rich α—Ferrite (BCC) (Table 2) is considered anodic with respect to Ni-rich γ—Austenite (FCC) (Table 2), which is cathodic in DS2205. The overall relative work function potential of AlCoCrFeNi2.1 alloy (average ~50 mV) was higher compared to DS2205 alloy (average ~−65 mV), indicating the nobility of AlCoCrFeNi2.1 versus DS2205, and agrees with the lower corrosion rate observed in AlCoCrFeNi2.1 rather than DS2205 at both the temperatures.
5. Conclusions
The corrosion behavior of dual-phase AlCoCrFeNi2.1 (EHEA) and duplex stainless steel 2205 (DS2205) was studied in NaCl-KCl-MgCl2 molten salt at 450 °C and 650 °C. AlCoCrFeNi2.1 EHEA showed superior molten salt corrosion resistance than DS2205, which was attributed to the following:
- (i)
- Higher Ni content in EHEA and sacrificial role of Al in reducing outward diffusion of Cr, Fe, and Ni in AlCoCrFeNi2.1 and the different reactivity of formation of chlorides, as well as enrichment of Co and Ni in the corrosion layer.
- (ii)
- Thermodynamically driven corrosion between alloying elements and molten salt constituents or impurities present within the melt. Metallic impurities such as Ni2+, Fe2+, and Co2+ enhance the corrosion resistance of AlCoCrFeNi2.1 via enrichment of impurity ions such as Ni, Co, and Fe (to lesser extent) in the corrosion layer as temperature increases.
- (iii)
- A lower volume fraction of the BCC phase, which makes it less prone to galvanic corrosion. The phase-specific work function for the as-cast AlCoCrFeNi2.1 and DS2205 suggested micro-galvanic corrosion, with nobler L12 and FCC phase in EHEA and DS 2205, respectively, compared to the B2 and BCC phases.
Author Contributions
K.P.: Conceptualization, Validation, Formal analysis, Investigation, Writing—original draft. V.H.: Formal analysis, Investigation. M.S.: Formal analysis, Investigation. C.M.: Formal analysis, Investigation, Writing—original draft. S.M. (Saideep Muskeri): Formal analysis, Investigation. S.M. (Sundeep Mukherjee): Conceptualization, Validation, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research did not receive any external funding.
Data Availability Statement
All the data obtained for this work are available upon request from the corresponding author.
Acknowledgments
We acknowledge the Advanced Materials and Manufacturing Processes Institute (AMMPI) and Materials Research Facility (MRF) at UNT.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yurtkuran, S. The Effect of Agriculture, Renewable Energy Production, and Globalization on CO2 Emissions in Turkey: A Bootstrap ARDL Approach. Renew Energy 2021, 171, 1236–1245. [Google Scholar] [CrossRef]
- Magazzino, C.; Mele, M.; Schneider, N. A Machine Learning Approach on the Relationship among Solar and Wind Energy Production, Coal Consumption, GDP, and CO2 Emissions. Renew Energy 2021, 167, 99–115. [Google Scholar] [CrossRef]
- Murdock, H.E.; Gibb, D.; Andre, T.; Sawin, J.L.; Brown, A.; Ranalder, L.; Andre, T.; Brown, A.; Collier, U.; Dent, C.; et al. Renewables 2021-Global Status Report. 2021, p. 371. Available online: https://www.ren21.net/wp-content/uploads/2019/05/GSR2021_Full_Report.pdf (accessed on 28 December 2022).
- Ding, W.; Shi, H.; Xiu, Y.; Bonk, A.; Weisenburger, A.; Jianu, A.; Bauer, T. Hot Corrosion Behavior of Commercial Alloys in Thermal Energy Storage Material of Molten MgCl2/KCl/NaCl under Inert Atmosphere. Sol. Energy Mater. Sol. Cells 2018, 184, 22–30. [Google Scholar] [CrossRef]
- Bauer, T.; Pfleger, N.; Laing, D.; Steinmann, W.D.; Eck, M.; Kaesche, S. High-Temperature Molten Salts for Solar Power Application. Molten Salts Chem. 2013, 415–438. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Arvay, A.; Hsu, K.; Kannan, A.M. Heat Transfer Fluids for Concentrating Solar Power Systems—A Review. Appl Energy 2015, 146, 383–396. [Google Scholar] [CrossRef]
- Li, Y.; Xu, X.; Wang, X.; Li, P.; Hao, Q.; Xiao, B. Survey and Evaluation of Equations for Thermophysical Properties of Binary/Ternary Eutectic Salts from NaCl, KCl, MgCl2, CaCl2, ZnCl2 for Heat Transfer and Thermal Storage Fluids in CSP. Sol. Energy 2017, 152, 57–79. [Google Scholar] [CrossRef]
- Ding, W.; Shi, Y.; Kessel, F.; Koch, D.; Bauer, T. Characterization of Corrosion Resistance of C/C–SiC Composite in Molten Chloride Mixture MgCl2/NaCl/KCl at 700 °C. Npj Mater. Degrad. 2019, 3, 42. [Google Scholar] [CrossRef]
- Kruizenga, A. Corrosion Mechanisms in Chloride and Carbonate Salts; SANDIA Report; Sandia National Laboratories (SNL): Albuquerque, NM, USA; Livermore, CA, USA, 2012. [CrossRef]
- Mehos, M.; Turchi, C.; Vidal, J.; Wagner, M.; Ma, Z.; Ho, C.; Kolb, W.; Andraka, C.; Kruizenga, A. Concentrating Solar Power Gen3 Demonstration Roadmap; Technical Report; National Renewable Energy Lab (NREL): Golden, CO, USA, 2017.
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and Properties of High-Entropy Alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Pole, M.; Sadeghilaridjani, M.; Shittu, J.; Ayyagari, A.; Mukherjee, S. High Temperature Wear Behavior of Refractory High Entropy Alloys Based on 4-5-6 Elemental Palette. J. Alloys Compd. 2020, 843, 156004. [Google Scholar] [CrossRef]
- Patel, K.; Sadeghilaridjani, M.; Pole, M.; Mukherjee, S. Hot Corrosion Behavior of Refractory High Entropy Alloys in Molten Chloride Salt for Concentrating Solar Power Systems. Sol. Energy Mater. Sol. Cells 2021, 230, 111222. [Google Scholar] [CrossRef]
- Ren, J.; Mahajan, C.; Liu, L.; Follette, D.; Chen, W.; Mukherjee, S. Corrosion Behavior of Selectively Laser Melted CoCrFeMnNi High Entropy Alloy. Metals 2019, 9, 1029. [Google Scholar] [CrossRef]
- Sadeghilaridjani, M.; Muskeri, S.; Pole, M.; Mukherjee, S. High-Temperature Nano-Indentation Creep of Reduced Activity High Entropy Alloys Based on 4-5-6 Elemental Palette. Entropy 2020, 22, 230. [Google Scholar] [CrossRef]
- Sadeghilaridjani, M.; Ayyagari, A.; Muskeri, S.; Hasannaeimi, V.; Salloom, R.; Chen, W.Y.; Mukherjee, S. Ion Irradiation Response and Mechanical Behavior of Reduced Activity High Entropy Alloy. J. Nucl. Mater. 2020, 529, 151955. [Google Scholar] [CrossRef]
- Sadeghilaridjani, M.; Mukherjee, S. High-Temperature Nano-Indentation Creep Behavior of Multi-Principal Element Alloys under Static and Dynamic Loads. Metals 2020, 10, 250. [Google Scholar] [CrossRef]
- Sadeghilaridjani, M.; Muskeri, S.; Hassannaeimi, V.; Pole, M.; Mukherjee, S. Strain Rate Sensitivity of a Novel Refractory High Entropy Alloy: Intrinsic versus Extrinsic Effects. Mater. Sci. Eng. A 2019, 766, 138326. [Google Scholar] [CrossRef]
- Elbakhshwan, M.; Doniger, W.; Falconer, C.; Moorehead, M.; Parkin, C.; Zhang, C.; Sridharan, K.; Couet, A. Corrosion and Thermal Stability of CrMnFeNi High Entropy Alloy in Molten FLiBe Salt. Sci. Rep. 2019, 9, 18993. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Muskeri, S.; Hasannaeimi, V.; Salloom, R.; Sadeghilaridjani, M.; Mukherjee, S. Small-Scale Mechanical Behavior of a Eutectic High Entropy Alloy. Sci. Rep. 2020, 10, 2669. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Guo, S.; Jiang, L.; Kang, H.; Wang, T.; Wen, B.; Wang, Z.; Jie, J.; Cao, Z.; et al. A Promising New Class of High-Temperature Alloys: Eutectic High-Entropy Alloys. Sci. Rep. 2014, 4, 6200. [Google Scholar] [CrossRef]
- Lu, Y.; Dong, Y.; Jiang, H.; Wang, Z.; Cao, Z.; Guo, S.; Wang, T.; Li, T.; Liaw, P.K. Promising Properties and Future Trend of Eutectic High Entropy Alloys. Scr. Mater. 2020, 187, 202–209. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Bhattacharjee, P.P.; Guo, S.; Tsuji, N. Tailoring Nanostructures and Mechanical Properties of AlCoCrFeNi 2.1 Eutectic High Entropy Alloy Using Thermo-Mechanical Processing. Mater. Sci. Eng. A 2016, 675, 99–109. [Google Scholar] [CrossRef]
- Wani, I.S.; Bhattacharjee, T.; Sheikh, S.; Lud, Y.P.; Chatterjee, S.; Bhattacharjeea, P.P.; Guo, S.; Tsujib, N. Ultrafine-Grained AlCoCrFeNi2.1 Eutectic High-Entropy Alloy. Mater. Res. Lett. 2016, 4, 174–179. [Google Scholar] [CrossRef]
- Hasannaeimi, V.; Ayyagari, A.; Muskeri, S.; Salloom, R.; Mukherjee, S. Surface Degradation Mechanisms in a Eutectic High Entropy Alloy at Microstructural Length-Scales and Correlation with Phase-Specific Work Function. Npj Mater. Degrad. 2019, 3, 16. [Google Scholar] [CrossRef]
- Choudhuri, D.; Jannotti, P.A.; Muskeri, S.; Shukla, S.; Gangireddy, S.; Mukherjee, S.; Schuster, B.E.; Lloyd, J.T.; Mishra, R.S. Ballistic Response of a FCC-B2 Eutectic AlCoCrFeNi2.1 High Entropy Alloy. J. Dyn. Behav. Mater. 2019, 5, 495–503. [Google Scholar] [CrossRef]
- Selvam, K.; Saini, J.; Perumal, G.; Ayyagari, A.; Salloom, R.; Mondal, R.; Mukherjee, S.; Grewal, H.S.; Arora, H.S. Exceptional Cavitation Erosion-Corrosion Behavior of Dual-Phase Bimodal Structure in Austenitic Stainless Steel. Tribol. Int. 2019, 134, 77–86. [Google Scholar] [CrossRef]
- Arora, H.S.; Ayyagari, A.; Saini, J.; Selvam, K.; Riyadh, S.; Pole, M.; Grewal, H.S.; Mukherjee, S. High Tensile Ductility and Strength in Dual-Phase Bimodal Steel through Stationary Friction Stir Processing. Sci. Rep. 2019, 9, 1972. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, K.; Hughes, R.; Luo, J.L. Corrosion Mechanisms and Materials Selection for the Construction of Flue Gas Component in Advanced Heat and Power Systems. Ind. Eng. Chem. Res. 2017, 56, 14141–14154. [Google Scholar] [CrossRef]
- Iacoviello, F.; Casari, F.; Gialanella, S. Effect of “475 °C Embrittlement” on Duplex Stainless Steels Localized Corrosion Resistance. Corros. Sci. 2005, 47, 909–922. [Google Scholar] [CrossRef]
- Schulz, Z.; Whitcraft, P.; Wachowiak, D. NACE-2014-4345. Availability and Economics of Using Duplex Stainless Steels. 2014. Available online: https://www.rolledalloys.com/wp-content/uploads/Availability-and-Economics-of-Using-Duplex-Stainless-Steels-rolled-alloys.pdf (accessed on 28 December 2022).
- Donik, Č.; Kocijan, A.; Grant, J.T.; Jenko, M.; Drenik, A.; Pihlar, B. XPS Study of Duplex Stainless Steel Oxidized by Oxygen Atoms. Corros. Sci. 2009, 51, 827–832. [Google Scholar] [CrossRef]
- Ho, M.Y.; Geddes, J.; Barmatov, E.; Crawford, L.; Hughes, T. Effect of Composition and Microstructure of Duplex Stainless Steel on Adsorption Behaviour and Efficiency of Corrosion Inhibitors in 4 Molar Hydrochloric Acid. Part I: Standard DSS 2205. Corros. Sci. 2018, 137, 43–52. [Google Scholar] [CrossRef]
- Mahajan, C.; Hasannaeimi, V.; Pole, M.; Kautz, E.; Gwalani, B.; Mukherjee, S. Corrosion Mechanisms in Model Binary Metallic Glass Coatings on Mild Steel and Correlation with Electron Work Function. Corros. Sci. 2022, 207, 110578. [Google Scholar] [CrossRef]
- Leblanc, P.; Frankel, G.S. A Study of Corrosion and Pitting Initiation of AA2024-T3 Using Atomic Force Microscopy. J. Electrochem. Soc. 2002, 149, B239. [Google Scholar] [CrossRef]
- Vignarooban, K.; Pugazhendhi, P.; Tucker, C.; Gervasio, D.; Kannan, A.M. Corrosion Resistance of Hastelloys in Molten Metal-Chloride Heat-Transfer Fluids for Concentrating Solar Power Applications. Sol. Energy 2014, 103, 62–69. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Bauer, T. Corrosion Behavior of Metallic Alloys in Molten Chloride Salts for Thermal Energy Storage in Concentrated Solar Power Plants: A Review. Front. Chem. Sci. Eng. 2018, 12, 564–576. [Google Scholar] [CrossRef]
- ASTM G31-21 Standard Guide for Laboratory Immersion Corrosion Testing of Metals. Available online: https://www.astm.org/g0031-21.html (accessed on 23 January 2023).
- Gomez-Vidal, J.C.; Tirawat, R. Corrosion of Alloys in a Chloride Molten Salt (NaCl-LiCl) for Solar Thermal Technologies. Sol. Energy Mater. Sol. Cells 2016, 157, 234–244. [Google Scholar] [CrossRef]
- Örnek, C.; Engelberg, D.L. SKPFM Measured Volta Potential Correlated with Strain Localisation in Microstructure to Understand Corrosion Susceptibility of Cold-Rolled Grade 2205 Duplex Stainless Steel. Corros. Sci. 2015, 99, 164–171. [Google Scholar] [CrossRef]
- Örnek, C.; Ahmed, A.H.; Engelberg, D.L. Effect of Microstructure on Atmospheric-Induced Corrosion of Heat-Treated Grade 2205 and 2507 Duplex Stainless Steels. In Proceedings of the EuroCorr 2012, Istanbul, Turkey, 9–20 September 2012; pp. 1–10. [Google Scholar]
- Reccagni, P.; Guilherme, L.H.; Lu, Q.; Gittos, M.F.; Engelberg, D.L. Reduction of Austenite-Ferrite Galvanic Activity in the Heat-Affected Zone of a Gleeble-Simulated Grade 2205 Duplex Stainless Steel Weld. Corros. Sci. 2019, 161, 108198. [Google Scholar] [CrossRef]
- Engelberg, D.L.; Örnek, C. Probing Propensity of Grade 2205 Duplex Stainless Steel towards Atmospheric Chloride-Induced Stress Corrosion Cracking. Corros. Eng. Sci. Technol. 2014, 49, 535–539. [Google Scholar] [CrossRef]
- Schmid, A.; Mori, G.; Hönig, S.; Weil, M.; Strobl, S.; Haubner, R. Comparison of the High-Temperature Chloride-Induced Corrosion between Duplex Steel and Ni Based Alloy in Presence of H2S. Corros. Sci. 2018, 139, 76–82. [Google Scholar] [CrossRef]
- Vignarooban, K.; Xu, X.; Wang, K.; Molina, E.E.; Li, P.; Gervasio, D.; Kannan, A.M. Vapor Pressure and Corrosivity of Ternary Metal-Chloride Molten-Salt Based Heat Transfer Fluids for Use in Concentrating Solar Power Systems. Appl. Energy 2015, 159, 206–213. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, J.; Wu, W.; Zhou, W. Corrosion in the Molten Fluoride and Chloride Salts and c Applications. Prog. Mater. Sci. 2018, 97, 448–487. [Google Scholar] [CrossRef]
- Sridharan, K.; Allen, T.R. Corrosion in Molten Salts. Molten Salts Chem. 2013, 241–267. [Google Scholar] [CrossRef]
- Ding, W.; Bonk, A.; Gussone, J.; Bauer, T. Cyclic Voltammetry for Monitoring Corrosive Impurities in Molten Chlorides for Thermal Energy Storage. Energy Procedia 2017, 135, 82–91. [Google Scholar] [CrossRef]
- Pint, B.A.; McMurray, J.W.; Willoughby, A.W.; Kurley, J.M.; Pearson, S.R.; Lance, M.J.; Leonard, D.N.; Meyer, H.M.; Jun, J.; Raiman, S.S.; et al. Re-Establishing the Paradigm for Evaluating Halide Salt Compatibility to Study Commercial Chloride Salts at 600 °C–800 °C. Mater. Corros. 2019, 70, 1439–1449. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Danon, A.E.; Muránsky, O.; Karatchevtseva, I.; Zhang, Z.; Li, Z.J.; Scales, N.; Kruzic, J.J.; Edwards, L. Molten Salt Corrosion (FLiNaK) of a Ni–Mo–Cr Alloy and Its Welds for Application in Energy-Generation and Energy-Storage Systems. Corros. Sci. 2020, 164, 108306. [Google Scholar] [CrossRef]
- Ayyagari, A.; Hasannaeimi, V.; Arora, H.; Mukherjee, S. Electrochemical and Friction Characteristics of Metallic Glass Composites at the Microstructural Length-Scales. Sci. Rep. 2018, 8, 906. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, P.; Frankel, G.S. Characterization of AA2024-T3 by Scanning Kelvin Probe Force Microscopy. J. Electrochem. Soc. 2019, 145, 2285–2295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).