Correspondence of the Symmetry of Thermodynamic Properties of Matter with the Symmetry of Equations of State
Abstract
:1. Introduction
2. The Ideal Dense Matter EOS and Symmetry of Thermodynamic Properties of Matter
2.1. The Ideal Dense Matter EOS from Thermodynamic Symmetry
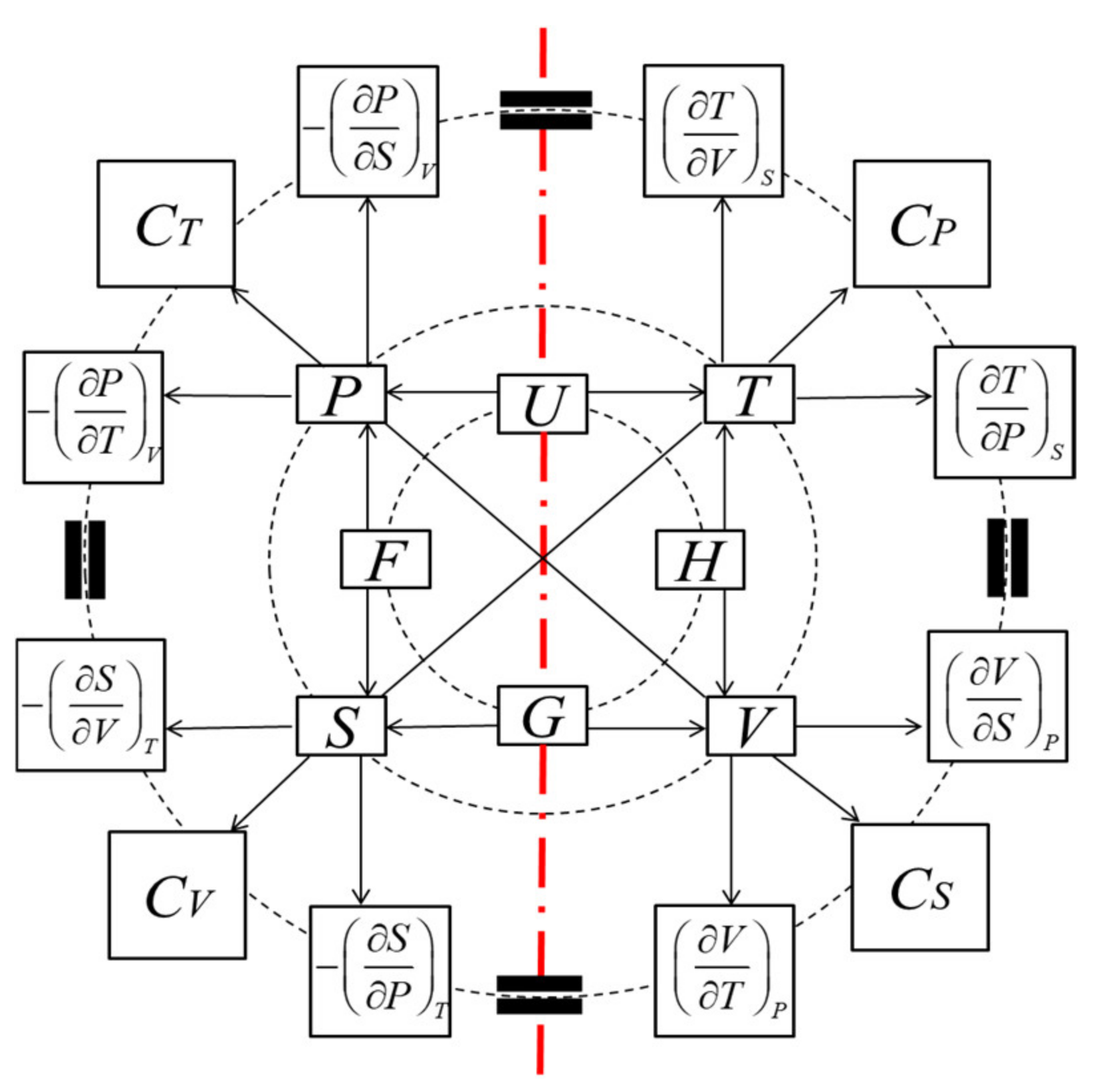
2.2. Symmetry of Thermodynamic Properties of Matter
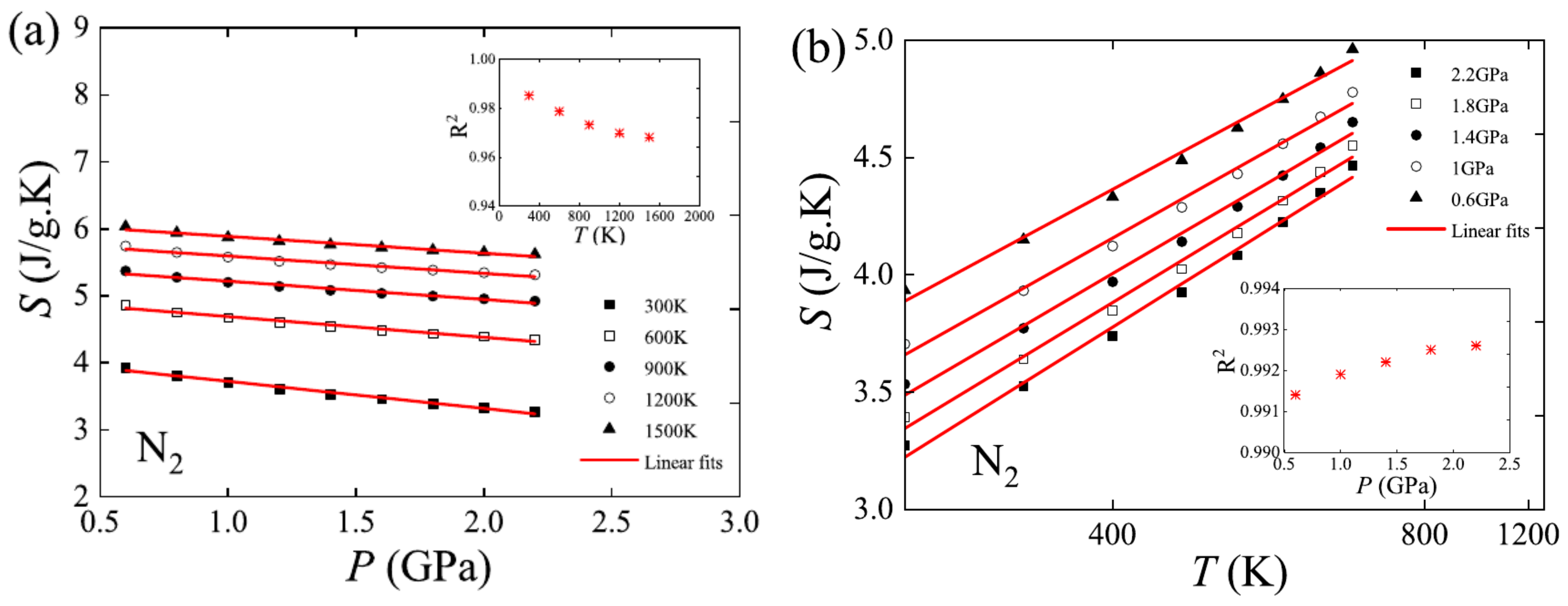
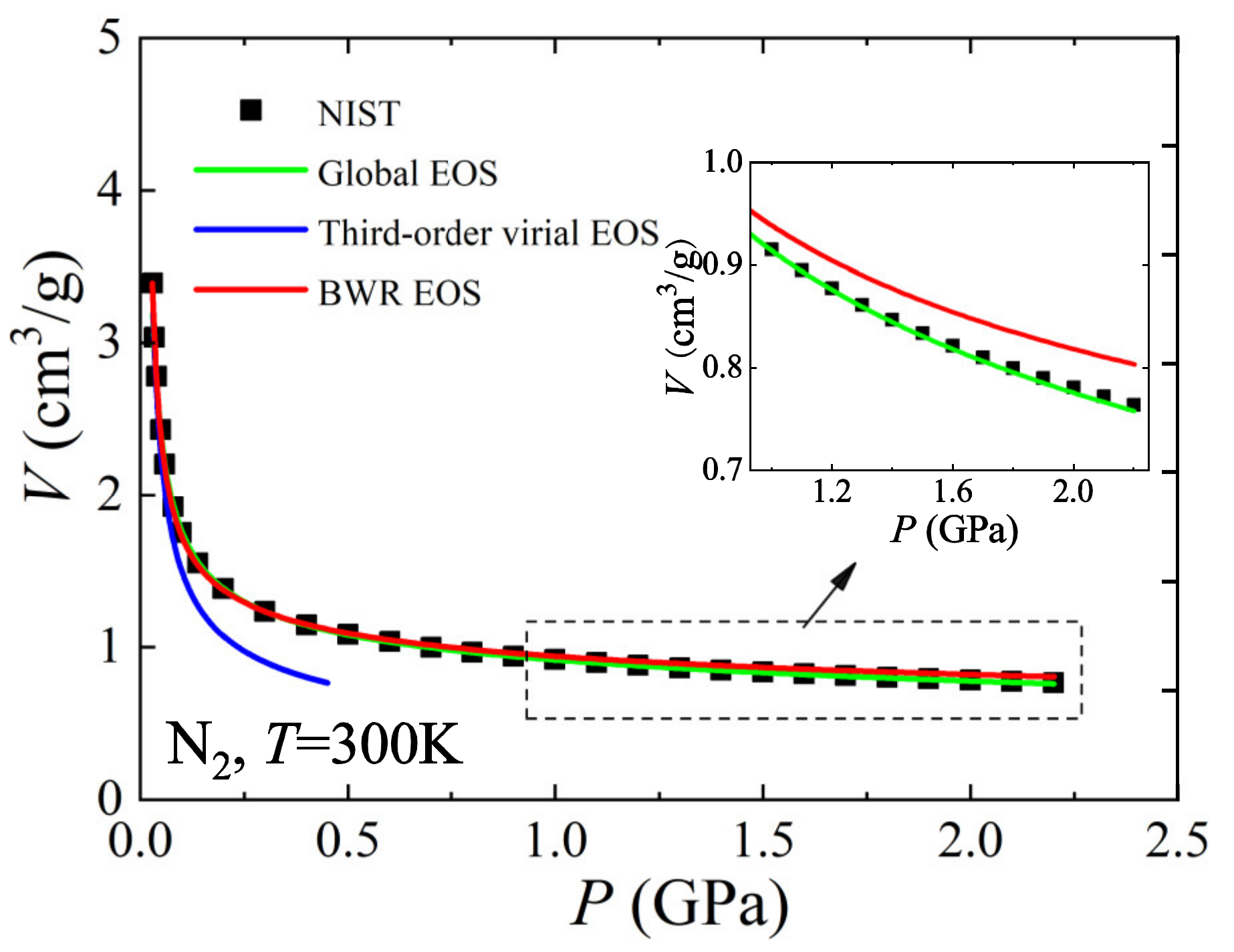
3. Developments of EOS Theory Based on Symmetry of Thermodynamic Properties of Matter
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clausius, R. The Mechanical Theory of Heat, with Its Applications to the Steam-Engine and to the Physical Properties of Bodies; John van Voorst: London, UK, 1867. [Google Scholar]
- Clausius, R. The Mechanical Theory of Heat; Macmillan and Co.: London, UK,, 1879. [Google Scholar]
- Bordoni, S. Routes towards an Abstract Thermodynamics in the late nineteenth century. Eur. Phys. J. H 2013, 38, 617–660. [Google Scholar] [CrossRef]
- Xue, T.W.; Guo, Z.Y. What is the real Clausius statement of the second law of thermodynamics? Entropy 2019, 21, 926. [Google Scholar] [CrossRef]
- Von Oettingen, A.J. Die thermodynamischen Beziehungen antithetisch entwickelt. Mem. Acad. Sci. St. Petersb. 1885, XXXII, 1–70. [Google Scholar]
- Callen, H.B. Thermodynamics and an Introduction to Thermostatistics; John Wiley & Sons: Hoboken, NJ, USA, 1985. [Google Scholar]
- Callen, H.B. Thermodynamics as a Science of Symmetry. Found. Phys. 1974, 4, 423–443. [Google Scholar] [CrossRef]
- Xue, T.W.; Guo, Z.Y. A general equation of state for high density matter from thermodynamic symmetry. J. Appl. Phys. 2022, 131, 044902. [Google Scholar] [CrossRef]
- Xue, T.W.; Guo, Z.Y. A global equation-of-state model from mathematical interpolation between low- and high-density limits. Sci. Rep. 2022, 12, 12533. [Google Scholar] [CrossRef] [PubMed]
- Cengel, Y.A.; Boles, M.A. Termodynamics, 5th ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Parsafar, G.A.; Spohr, H.V.; Patey, G.N. An Accurate Equation of State for Fluids and Solids. J. Phys. Chem. B 2009, 113, 11977. [Google Scholar] [CrossRef]
- Parsafar, G.; Mason, E.A. Universal equation of state for compressed solids. Phys. Rev. B 1994, 49, 304. [Google Scholar] [CrossRef]
- Holzapfel, W.B. Physics of solids under strong compression. Rep. Prog. Phys. 1996, 59, 29–90. [Google Scholar] [CrossRef]
- Rosenholm, J.B. A rational approach to basic equilibrium thermodynamics. Adv. Colloid Interface Sci. 2021, 294, 102433. [Google Scholar] [CrossRef]
- Roduner, E. What Is Heat? Can Heat Capacities Be Negative? Entropy 2023, 25, 530. [Google Scholar] [CrossRef] [PubMed]
- Van der Waals, J.D. Over de Continuiteit van den Gas-en Vloeistoftoestand. Ph.D. Dissertation, Leiden University, Leiden, The Netherlands, 1873. [Google Scholar]
- Klein, M.J. The historical origins of the van der Waals equation. Physica 1974, 73, 28–47. [Google Scholar] [CrossRef]
- Sengers, J.V.; Kayser, R.F.; Peters, C.J. Equations of State for Fluids and Fluid Mixtures; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Menikoff, R. Empirical Equations of State for Solids//ShockWave Science and Technology Reference Library; Springer: Berlin/Heidelberg, Germany, 2007; pp. 143–188. [Google Scholar]
- Towle, L.C. Empirical equations of state for solids. Appl. Phys. 1975, 8, 117–123. [Google Scholar] [CrossRef]
- Beattie, J.A.; Bridgeman, O.C. A New Equation of State for Fluids. Proc. Am. Acad. Arts Sci. 1928, 63, 229–308. [Google Scholar] [CrossRef]
- Benedict, M.; Webb, G.B.; Rubin, L.C. An empirical equation for thermodynamic properties of light hydrocarbons and their mixtures I. Methane, ethane, propane and n-butane. J. Chem. Phys. 1940, 8, 334–345. [Google Scholar] [CrossRef]
- Benedict, M.; Webb, G.B.; Rubin, L.C. An empirical equation for thermodynamic properties of light hydrocarbons and their mixtures II. Mixtures of Methane, Ethane, Propane, and n-Butane. J. Chem. Phys. 1942, 10, 747–758. [Google Scholar] [CrossRef]
- Oh, K.H.; Persson, P.A. Equation of state for extrapolation of high-pressure shock Hugoniot data. J. Appl. Phys. 1989, 65, 3852–3856. [Google Scholar] [CrossRef]
- Sun, J.X.; Wu, Q.; Cai, L.C. Equation of state for solids with high accuracy and satisfying the limitation condition at high pressure. Phys. B Condens. Matter 2006, 371, 257–271. [Google Scholar]
- Crain, R.W., Jr.; Sonntag, R.E. Nitrogen constants for the Benedict-Webb-Rubin equation of state. J. Chem. Eng. Data 1967, 12, 73–75. [Google Scholar] [CrossRef]
- Span, R.; Wagner, W. On the extrapolation behavior of empirical equations of state. Int. J. Thermophys. 1997, 18, 1415–1443. [Google Scholar] [CrossRef]
- Brosh, E.; Shneck, R.Z.; Makov, G. Explicit Gibbs free energy equation of state for solids. J. Phys. Chem. Solids 2008, 69, 1912–1922. [Google Scholar] [CrossRef]
- Dymond, J.H.; Malhotra, R. The Tait equation: 100 years on. Int. J. Thermophys. 1988, 9, 941. [Google Scholar] [CrossRef]
- Kumar, M. High pressure equation of state for solids. Phys. B 1995, 212, 391–394. [Google Scholar] [CrossRef]
- Lee, E.; Hornig, H.; Kury, J. Adiabatic Expansion of High Explosive Detonation Products; University of California Radiation Lab.: Livermore, CA, USA, 1968. [Google Scholar]
- Baudin, G.; Serradeill, R. Review of Jones-Wilkins-Lee Equation of State. In EPJ Web of Conferences; EDP Sciences: Les Ulis, France, 2010. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, T.-W.; Guo, Z.-Y. Correspondence of the Symmetry of Thermodynamic Properties of Matter with the Symmetry of Equations of State. Entropy 2023, 25, 1532. https://doi.org/10.3390/e25111532
Xue T-W, Guo Z-Y. Correspondence of the Symmetry of Thermodynamic Properties of Matter with the Symmetry of Equations of State. Entropy. 2023; 25(11):1532. https://doi.org/10.3390/e25111532
Chicago/Turabian StyleXue, Ti-Wei, and Zeng-Yuan Guo. 2023. "Correspondence of the Symmetry of Thermodynamic Properties of Matter with the Symmetry of Equations of State" Entropy 25, no. 11: 1532. https://doi.org/10.3390/e25111532
APA StyleXue, T.-W., & Guo, Z.-Y. (2023). Correspondence of the Symmetry of Thermodynamic Properties of Matter with the Symmetry of Equations of State. Entropy, 25(11), 1532. https://doi.org/10.3390/e25111532




