Effect of Planar Interfaces on Nucleation in Melting and Crystallization
Abstract
:1. Introduction
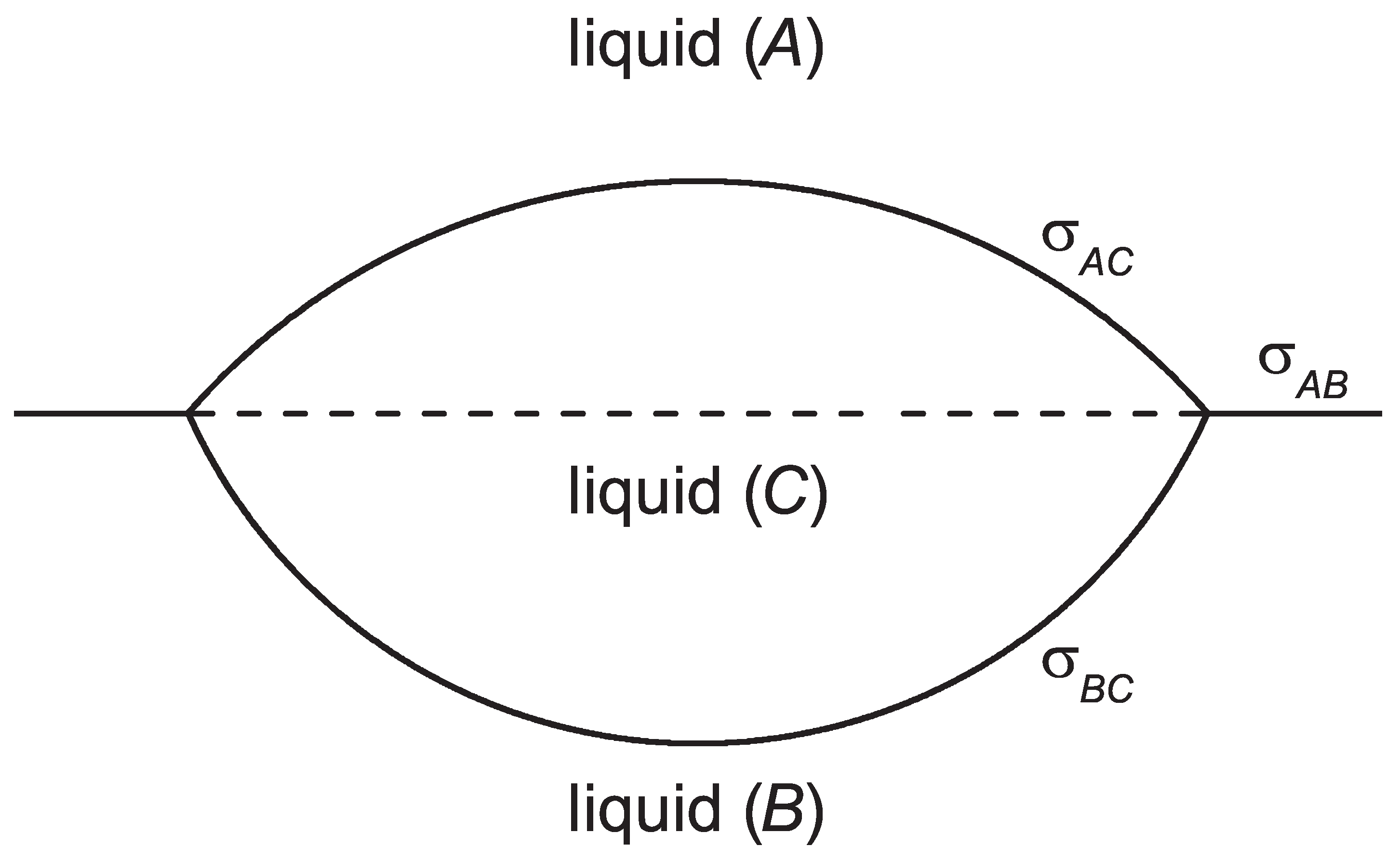
2. Theoretical Analysis of Nucleation Near Planar Interfaces
2.1. The Model: Nucleation in the Bulk
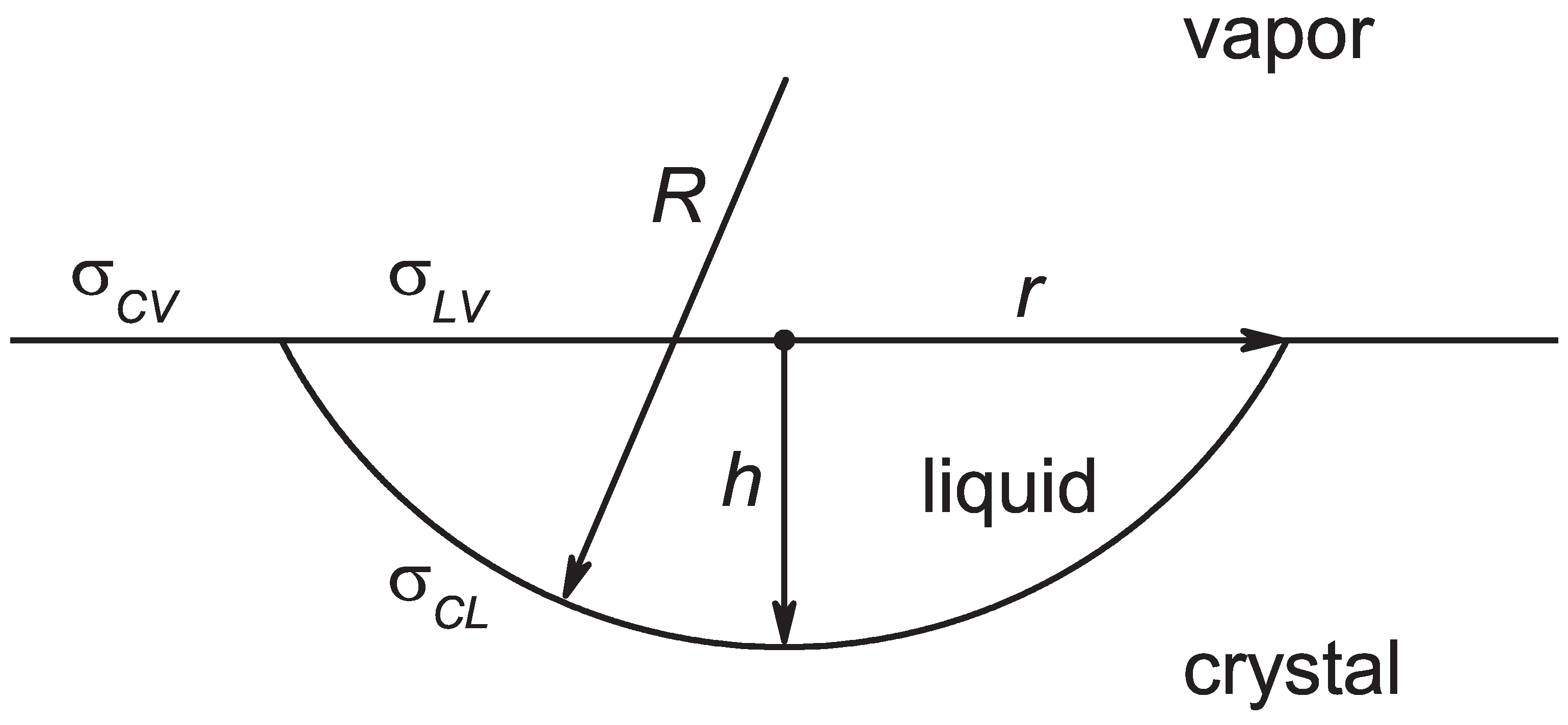
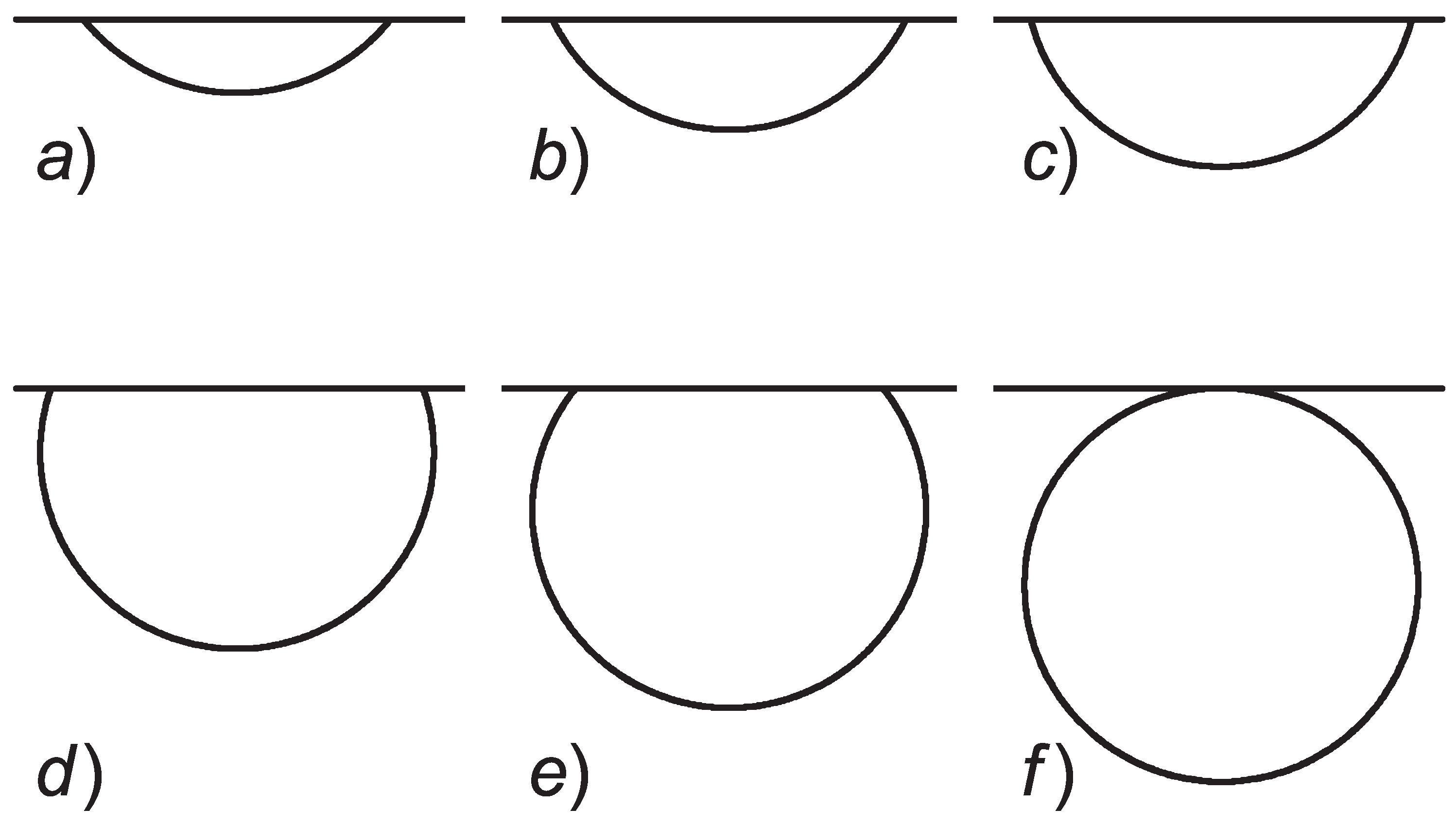
2.2. Nucleation in Melting
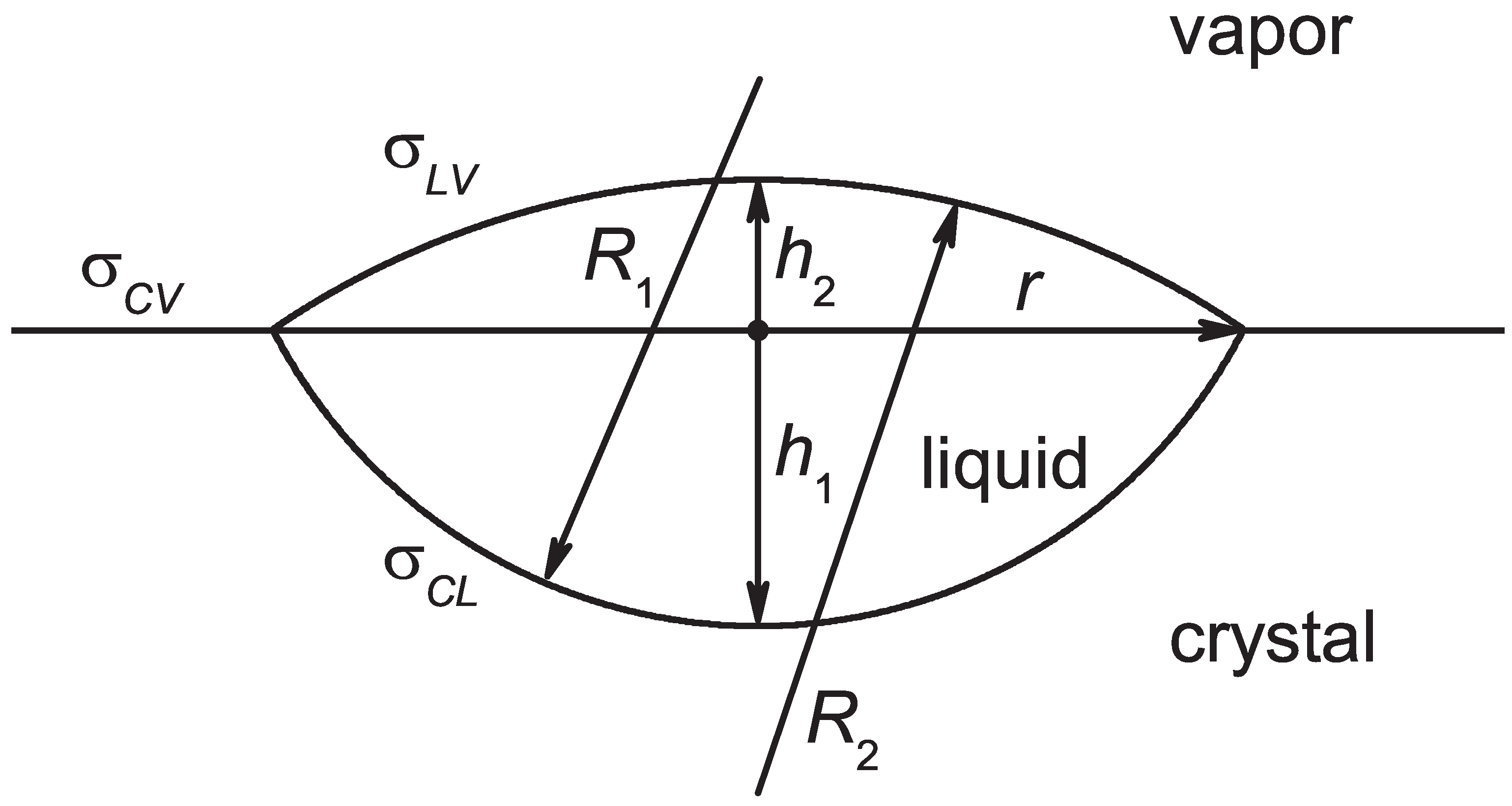
2.3. Nucleation in Crystallization
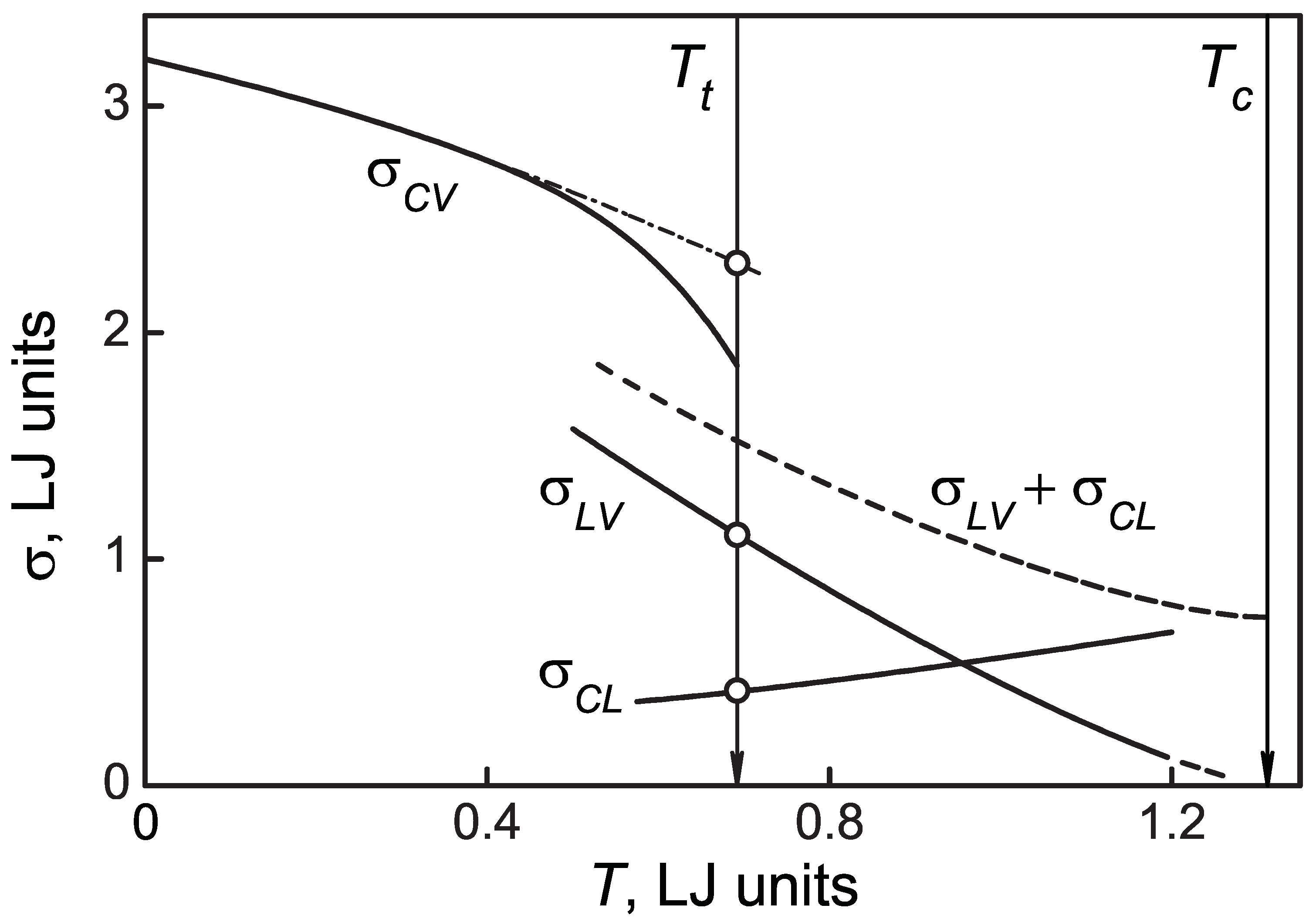
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Volmer, M. Kinetik der Phasenbildung (Engl.: Kinetics of Phase Formation); Theodor Steinkopff: Dresden, Germany, 1939. [Google Scholar]
- Skripov, V.P.; Koverda, V.P. Spontaneous Crystallization of Undercooled Liquids; Nauka Publishers: Moscow, Russian, 1984. (In Russian) [Google Scholar]
- Gutzow, I.S.; Schmelzer, J.W.P. The Vitreous State: Thermodynamics, Structure, Rheology, and Crystallization, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Gutzow, I.S.; Schmelzer, J.W.P. The Vitreous State: Thermodynamics, Structure, Rheology, and Crystallization, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Baidakov, V.G. Explosive Boiling of Superheated Cryogenic Liquids; WILEY-VCH: Berlin, Germany, 2007. [Google Scholar]
- Slezov, V.V. Kinetics of First-Order Phase Transitions; WILEY-VCH: Berlin, Germany, 2009. [Google Scholar]
- Kelton, K.F.; Greer, A.L. Nucleation in Condensed Matter: Applications in Materials and Biology; Pergamon: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Yang, B.; Perepezko, J.H.; Schmelzer, J.W.P.; Gao, Y.; Schick, C. Dependence of crystal nucleation on prior liquid overheating by differential fast scanning calorimeter. J. Chem. Phys. 2014, 140, 104513. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, J.W.P.; Pascova, R.; Möller, J.; Gutzow, I. Surface Induced Devitrification of Glasses: The Influence of Elastic Strains. J. Non-Cryst. Solids 1993, 162, 26–39. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Möller, J.; Gutzow, I.; Pascova, R.; Müller, R.; Pannhorst, W. Surface-Energy and Structure Effects on Surface Crystallization. J. Non-Cryst. Solids 1995, 183, 215–233. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Schick, C. General concepts of crystallization: Some recent results and possible future developments. In Dielectrics and Crystallization; Ezquerra, T.A., Nogales, A., Eds.; Springer, Nature Switzerland AG: Cham, Switzerland, 2020; pp. 1–22. [Google Scholar]
- Schmelzer, J.W.P.; Tropin, T.V.; Fokin, V.M.; Abyzov, A.S.; Zanotto, E.D. Effects of Glass Transition and Structural Relaxation on Crystal Nucleation: Theoretical Description and Model Analysis. Entropy 2020, 22, 1098. [Google Scholar] [CrossRef] [PubMed]
- Ocko, B.M.; Wu, X.Z.; Sirota, E.B.; Sinha, S.K.; Gang, O.; Deutsch, M. Surface freezing in chain molecules: Normal alkanes. Phys. Rev. E 1997, 55, 3164–3182. [Google Scholar] [CrossRef] [Green Version]
- Sloutskin, E.; Wu, X.Z.; Peterson, T.B.; Gang, O.; Ocko, B.M.; Sirota, E.B.; Deutsch, M. Surface freezing in binary mixtures of chain molecules. I. Alkane mixtures. Phys. Rev. E 2003, 68, 031605. [Google Scholar] [CrossRef] [PubMed]
- Modak, V.P.; Pathak, H.; Thayer, M.; Singer, S.J.; Wyslouzil, B.E. Experimental evidence for surface freezing in supercooled n-alkane nanodroplets. Phys.Chem. Chem. Phys. 2013, 15, 6783–6795. [Google Scholar] [CrossRef] [PubMed]
- Modak, V.P.; Amaya, A.J.; Wyslouzil, B.E. Freezing of supercooled n-decane nanodroplets: From surface driven to frustrated crystallization. Phys. Chem. Chem. Phys. 2017, 19, 30181–30194. [Google Scholar] [CrossRef]
- Ubbelohde, A.R. Melting and crystal structure. Quarterly Rev. Chem. Soc. 1950, 4, 356–381. [Google Scholar] [CrossRef]
- Ubbelohde, A.R. Schmelzvorgang und Kristallstruktur (Engl.: Melting and Crystal Structure). Angew. Chem. 1965, 77, 614–618. [Google Scholar] [CrossRef]
- Ubbelohde, A.R. Melting and Crystal Structure; Clarendon Press: Oxford, UK, 1965. [Google Scholar]
- Khaikin, S.E.; Bene, N.R. The overheating of a solid body. Acad. Sci. USSR 1939, 23, 31. [Google Scholar]
- Turnbull, D. Kinetics of solidification of supercooled liquid mercury droplets. J. Chem. Phys. 1952, 20, 411–424. [Google Scholar] [CrossRef]
- Daeges, J.; Gleiter, H.; Perepezko, J.H. Superheating of metal crystals. Phys. Lett. A 1986, 119, 79–82. [Google Scholar] [CrossRef]
- Grabaek, L.; Bohr, J.; Andersen, H.N.; Johansen, A.; Johnson, E.; Sarholt-Kristensen, L.; Robinson, I.K. Melting, growth, and faceting of lead precipitates in aluminum. Phys. Rev. B 1992, 45, 2628–2637. [Google Scholar]
- Herman, J.W.; Elsayed-Ali, H.E. Superheating of Pb (111). Phys. Rev. Lett. 1992, 69, 1228–1231. [Google Scholar] [CrossRef]
- Zhang, Z.; Jin, Z.H.; Zhang, L.H.; Sui, M.L.; Lu, K. Superheating of confined Pb thin films. Phys. Rev. Lett. 2000, 85, 1484–1487. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, D.S.; Zhigibei, L.V. Combined atomistic-continuum modeling of short-pulse laser melting and disintegration of metal films. Phys. Rev. B 2003, 68, 064114. [Google Scholar] [CrossRef] [Green Version]
- Kanel, G.I.; Fortov, V.E.; Razorenov, S.V. Shock waves in condensed-state physics. Phys. Uspekhi 2007, 50, 771–792. [Google Scholar] [CrossRef]
- Fortov, V.E.; Altshuler, L.V.; Trunin, R.F.; Funtikov, A.I. High-Pressure Shock Compression of Solids VII: Shock Waves and Extreme States of Matter; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Zhang, Y.; Xiong, H.; Elsayed-Ali, H.E. Melting and Structural Dynamics of Indium Nanoparticles Embedded in Aluminum. J. Phys. Chem. C 2020, 124, 19340–19347. [Google Scholar] [CrossRef]
- Takeya, S.; Muromachi, S.; Yoneyama, A.; Hirano, K.; Hyodo, K.; Ripmeester, J.A. Superheating of Structure I Gas Hydrates within the Structure II Cyclopentane Hydrate Shell. J. Phys. Chem. Lett. 2022, 13, 2130–2136. [Google Scholar] [CrossRef]
- Fan, X.; Chen, X.; Pan, D.; Liu, Y.; Liu, P.; Li, M. Localization and delocationzation of surface disordering in surface mediated melting. Phys. Rev. B 2021, 104, 134204. [Google Scholar] [CrossRef]
- Gibbs, J.W. On the Equilibrium of Heterogeneous Substances. In The Collected Works; Thermodynamics, Longmans & Green: New York, NY, USA; London, UK; Toronto, ON, Canada, 1928; Volume 1. [Google Scholar]
- Abyzov, A.S.; Schmelzer, J.W.P. Generalized Gibbs’s approach in heterogeneous nucleation. J. Chem. Phys. 2013, 138, 164504. [Google Scholar] [CrossRef] [PubMed]
- Abyzov, A.S.; Schmelzer, J.W.P. Heterogeneous nucleation in solutions: Generalized Gibbs’ approach. J. Chem. Phys. 2014, 140, 244706. [Google Scholar] [CrossRef]
- Rowlinson, J.S.; Widom, B. Molecular Theory of Capillarity; Clarendon Press: Oxford, UK, 1982. [Google Scholar]
- Bormashenko, E.Y. Wetting of Real Surfaces; Walter de Gruyter GmbH: Berlin, Germany; Boston, MA, USA, 2013. [Google Scholar]
- Rusanov, A.I. My Discoveries (A Review). Russ. J. General Chem. 2022, 92, 539–583. [Google Scholar] [CrossRef]
- Blander, M.; Katz, J.L. Bubble Nucleation in Liquids. Am. Inst. Chem. Eng. (AIChE) J. 1974, 21, 833–848. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S. Crystallization of glass-forming liquids: Thermodynamic driving force. J. Non-Cryst. Solids 2016, 449, 41–49. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Abyzov, A.S.; Baidakov, V.G. Entropy and the Tolman Parameter in Nucleation Theory. Entropy 2019, 21, 670. [Google Scholar] [CrossRef] [Green Version]
- Schmelzer, J.W.P. Application of the Nucleation Theorem to Crystallization of Liquids: Some General Theoretical Results. Entropy 2019, 21, 1147. [Google Scholar] [CrossRef] [Green Version]
- Korn, G.A.; Korn, T.M. Mathematical Handbook; Mc Graw-Hill Book Company: New York, NY, USA; San Francisco, CA, USA; Toronto, ON, Canada; London, UK; Sydney, Australia, 1968. [Google Scholar]
- Schmelzer, J.W.P.; Abyzov, A.S. Crystallization of glass-forming liquids: Specific surface energy. J. Chem. Phys. 2016, 145, 064512. [Google Scholar] [CrossRef]
- Skripov, V.P.; Faizullin, M.Z. Solid-Liquid-Gas Phase Transitions and Thermodynamic Similarity; WILEY-VCH: Berlin, Germany, 2006. [Google Scholar]
- Baidakov, V.G.; Protsenko, S.P.; Kozlova, Z.R.; Chernykh, G.G. Metastable extension of the liquid-vapor phase equilibrium curve and surface tension. J. Chem. Phys. 2007, 126, 214505. [Google Scholar] [CrossRef]
- Baidakov, V.G.; Protsenko, S.P.; Tipeev, A.O. Temperature dependence of the crystal-liquid interfacial free energy and the endpoint of the melting line. J. Chem. Phys. 2013, 139, 224703. [Google Scholar] [CrossRef] [PubMed]
- Baidakov, V.G.; Tipeev, A.O.; O, A.; Protsenko, K.R. Surface free energy and some other properties of a crystal-vapor interface: Molecular dynamics simulation of a Lennard-Jones system. Chem. Phys. Lett. 2017, 680, 10–16. [Google Scholar] [CrossRef]
- Tipeev, A.O.; Rino, J.P.; Zanotto, E.D. Direct determination of Lennard-Jones crystal surface free energy by a computational cleavage method. J. Chem. Phys. 2021, 155, 094101. [Google Scholar] [CrossRef]
- Avramov, I.; Völksch, G. Near-surface crystallization of cordierite glass. J. Non-Cryst. Solids 2002, 304, 25–30. [Google Scholar] [CrossRef]
- Möller, J.; Schmelzer, J.W.P.; Gutzow, I. Elastic Stresses in Surface Crystallization of Glasses: Phase Transformations at Spike Tips. J. Non-Cryst. Solids 1997, 219, 142–148. [Google Scholar] [CrossRef]
- Schmelzer, J.W.P.; Tropin, T.V. Theory of Crystal Nucleation of Glass-forming Liquids: Some New Developments. Int. J. Appl. Glass Sci. 2022, 13, 171–198. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmelzer, J.W.P.; Tipeev, A.O. Effect of Planar Interfaces on Nucleation in Melting and Crystallization. Entropy 2022, 24, 1029. https://doi.org/10.3390/e24081029
Schmelzer JWP, Tipeev AO. Effect of Planar Interfaces on Nucleation in Melting and Crystallization. Entropy. 2022; 24(8):1029. https://doi.org/10.3390/e24081029
Chicago/Turabian StyleSchmelzer, Jürn W. P., and Azat O. Tipeev. 2022. "Effect of Planar Interfaces on Nucleation in Melting and Crystallization" Entropy 24, no. 8: 1029. https://doi.org/10.3390/e24081029
APA StyleSchmelzer, J. W. P., & Tipeev, A. O. (2022). Effect of Planar Interfaces on Nucleation in Melting and Crystallization. Entropy, 24(8), 1029. https://doi.org/10.3390/e24081029






