Local Analysis of Heterogeneous Intracellular Transport: Slow and Fast Moving Endosomes
Abstract
1. Introduction
2. Materials and Methods
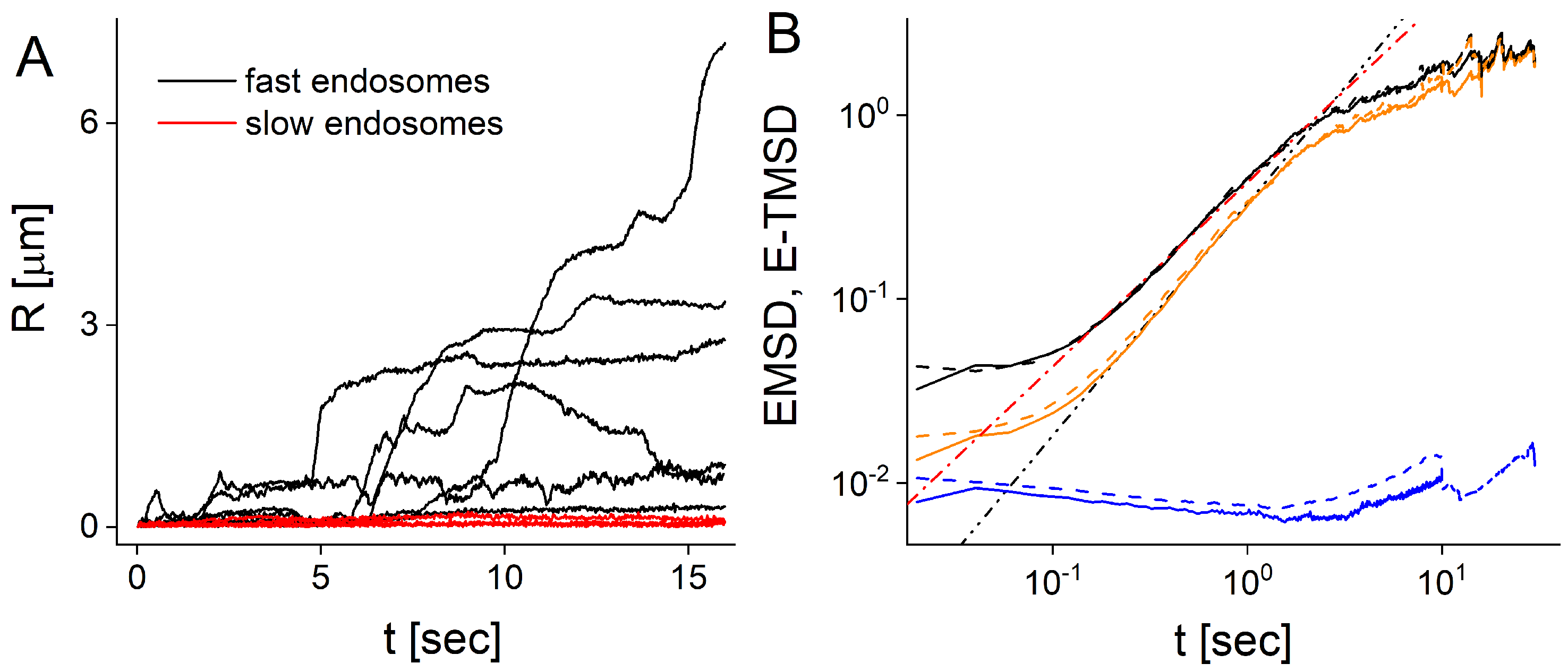
2.1. Experimental Trajectories
2.2. Splitting of Ensemble into Slow and Fast Moving Endosomes
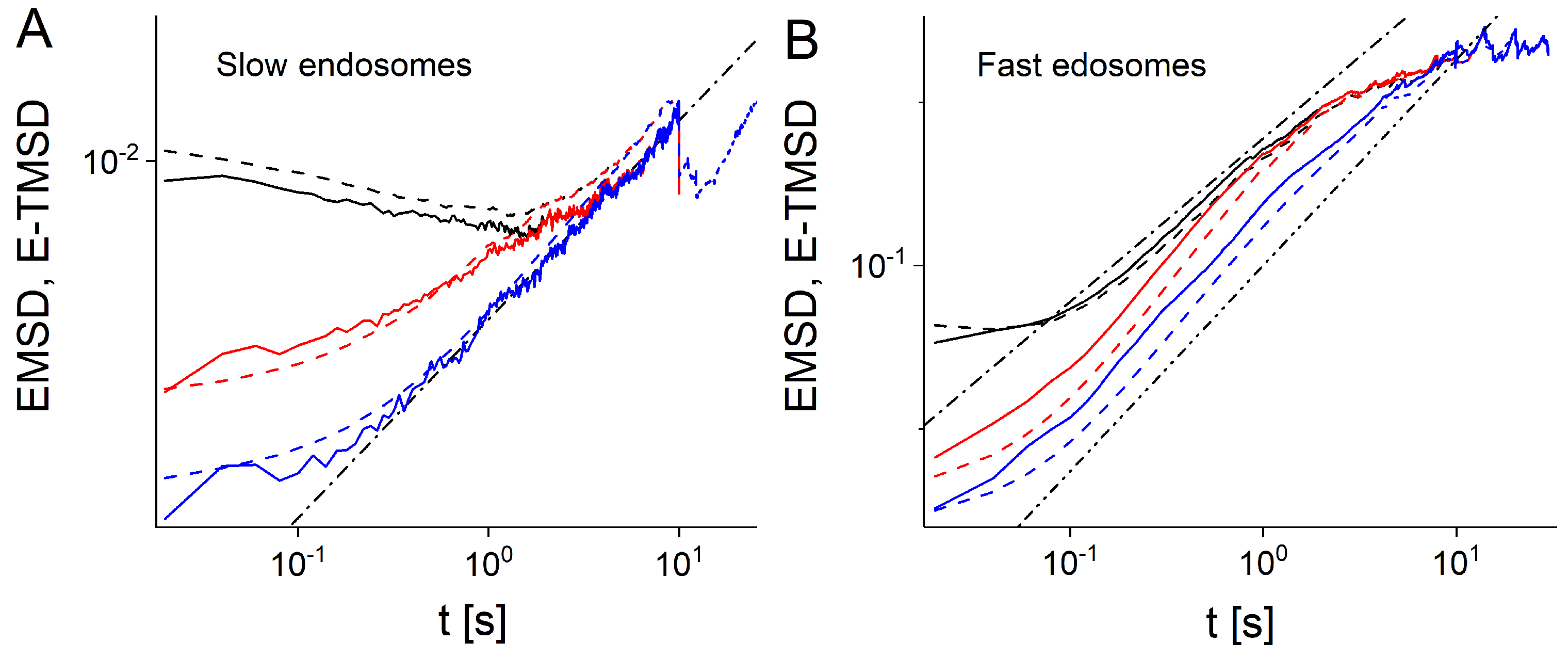
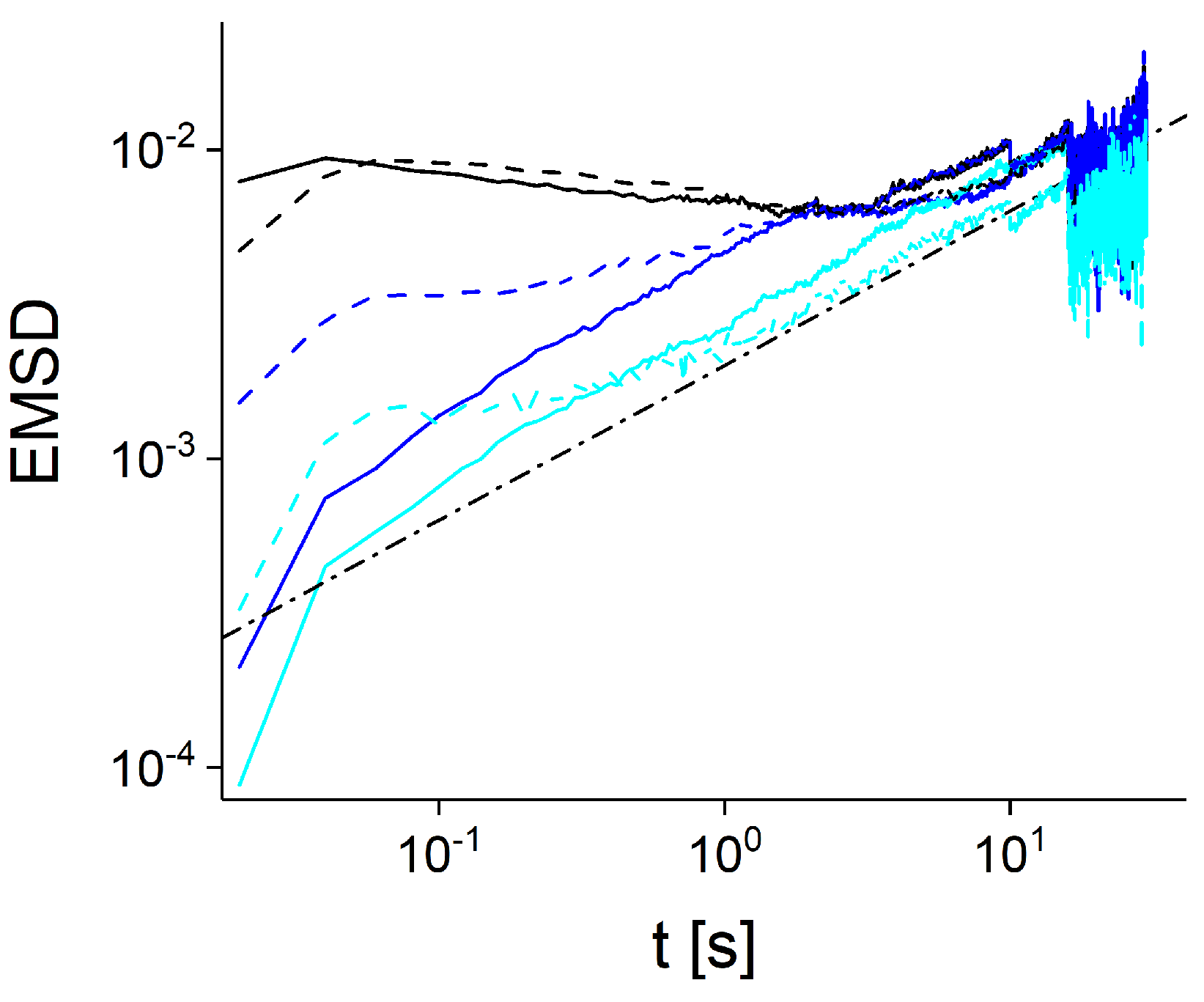
2.3. Ensemble and Time Averaged Mean Squared Displacements
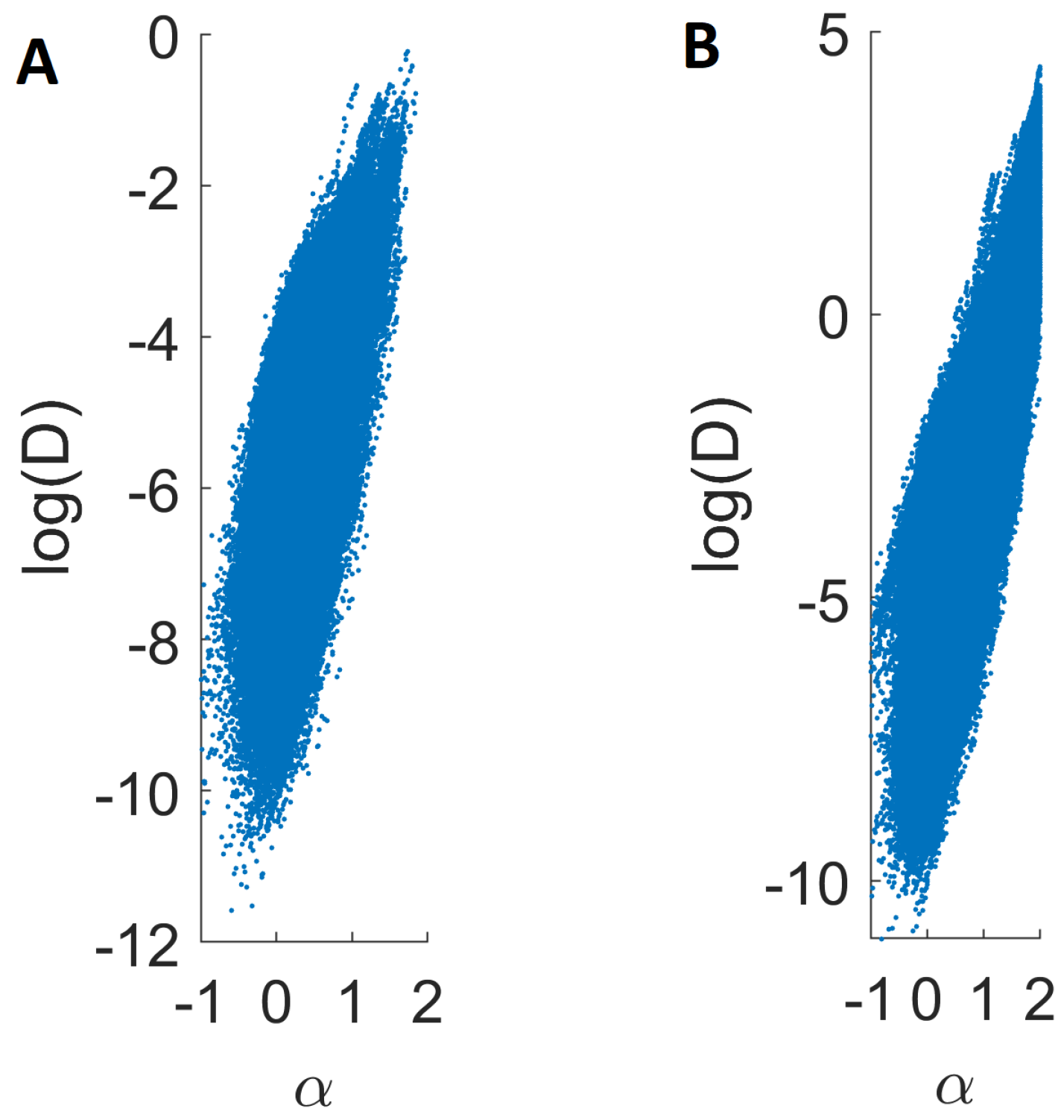
2.4. Local Analysis of Endosomal Trajectories
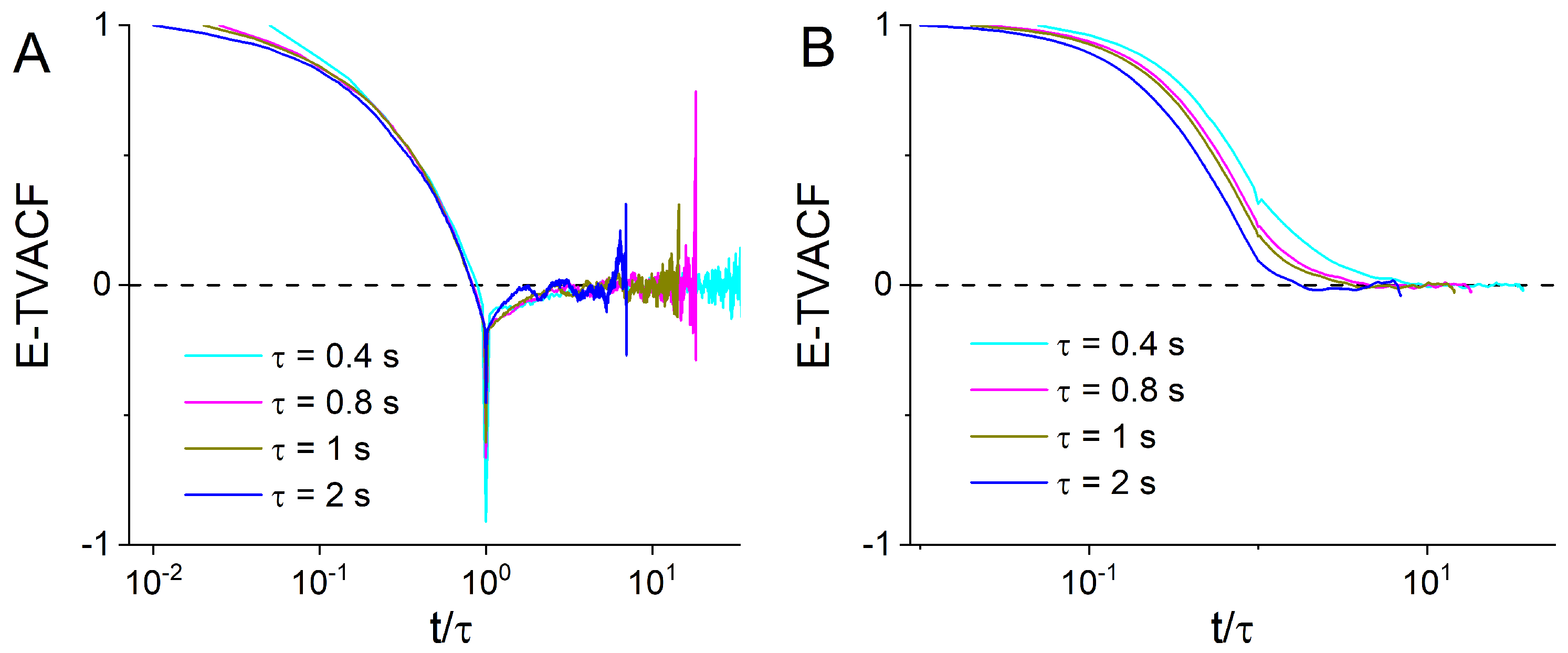
2.5. The Time and Ensemble-Time Averaged Velocity Auto-Correlation Functions
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A





References
- Klages, R.; Radons, G.; Sokolov, I.M. (Eds.) Anomalous Transport: Foundations and Applications; Wiley: Weinheim, Germany, 2004. [Google Scholar]
- Hofling, F.; Franosch, T. Anomalous transport in the crowded world of biological cells. Rep. Prog. Phys. 2013, 76, 046602. [Google Scholar] [CrossRef]
- Metzler, R.; Jeon, J.-H.; Cherstvy, A.G.; Barkai, E. Anomalous diffusion models and their properties: Non-stationarity, non-ergodicity, and ageing at the centenary of single particle tracking. Phys. Chem. Chem. Phys. 2014, 16, 24128. [Google Scholar] [CrossRef]
- Barkai, E.; Garini, Y.; Metzler, R. Strange kinetics of single molecules in living cells. Phys. Today 2012, 65, 29–35. [Google Scholar] [CrossRef]
- Arcizet, D.; Meier, B.; Sackmann, E.; Radler, J.O.; Heinrich, D. Temporal Analysis of Active and Passive Transport in Living Cells. Phys. Rev. Lett. 2008, 101, 248103. [Google Scholar] [CrossRef] [PubMed]
- Waigh, T.A. The Physics of Living Processes: A Mesoscopic Approach; John Wiley & Sons Ltd.: Chichester, UK, 2014. [Google Scholar]
- Rogers, S.S.; Flores-Rodriguez, N.; Allan, V.J.; Woodman, P.G.; Waigh, T.A. The first passage probability of intracellular particle trafficking. Phys. Chem. Chem. Phys. 2010, 12, 3753–3761. [Google Scholar] [CrossRef][Green Version]
- Kenwright, D.A.; Andrew, W.; Harrison, A.W.; Waigh, T.A.; Woodman, P.G.; Allan, V.J. First-passage-probability analysis of active transport in live cells. Phys. Rev. E 2012, 86, 031910. [Google Scholar] [CrossRef]
- Akimoto, T.; Yamamoto, E. Detection of transition times from single-particle-tracking trajectories. Phys. Rev. E 2017, 96, 052138. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Korabel, N.; Chen, R.; Johnston, M.; Gavrilova, A.; Allan, V.J.; Fedotov, S.; Waigh, T.A. Deciphering anomalous heterogeneous intracellular transport with neural networks. eLife 2020, 9, e52224. [Google Scholar] [CrossRef] [PubMed]
- Saxton, M.J. Single-particle tracking: The distribution of diffusion coefficients. Biophys. J. 1997, 72, 1744–1753. [Google Scholar] [CrossRef]
- Wang, B.; Anthony, S.M.; Bae, S.C.; Granick, S. Anomalous yet Brownian. Proc. Natl. Acad. Sci. USA 2009, 106, 15160. [Google Scholar] [CrossRef]
- Wang, B.; Kuo, J.; Bae, S.C.; Granick, S. When Brownian diffusion is not Gaussian. Nat. Mater. 2012, 11, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Lampo, T.J.; Stylianidou, S.; Backlund, M.P.; Wiggins, P.A.; Spakowitz, A.J. Cytoplasmic RNA-Protein Particles Exhibit Non-Gaussian Subdiffusive Behavior. Biophys. J. 2017, 112, 532–542. [Google Scholar] [CrossRef]
- Sabri, A.; Xu, X.; Krapf, D.; Weiss, M. Elucidating the Origin of Heterogeneous Anomalous Diffusion in the cytoplasm of mammalian cells. Phys. Rev. Lett. 2020, 125, 058101. [Google Scholar] [CrossRef]
- Ba, Q.; Raghavan, G.; Kiselyov, K.; Yang, G. Whole-cell scale dynamic organization of lysosomes revealed by spatial statistical analysis. Cell Rep. 2018, 23, 3591–3606. [Google Scholar] [CrossRef] [PubMed]
- Manzo, C.; Torreno-Pina, J.A.; Massignan, P.; Lapeyre, G.J., Jr.; Lewenstein, M.; Garcia-Parajo, M.F. Weak Ergodicity Breaking of Receptor Motion in Living Cells Stemming from Random Diffusivity. Phys. Rev. X 2015, 5, 011021. [Google Scholar] [CrossRef]
- Sadoon, A.A.; Wang, Y. Anomalous, non-Gaussian, viscoelastic, and age-dependent dynamics of histonelike nucleoid-structuring proteins in live Escherichia coli. Phys. Rev. E 2018, 98, 042411. [Google Scholar] [CrossRef]
- Calderon, C.P. Motion blur filtering: A statistical approach for extracting confinement forces and diffusivity from a single blurred trajectory. Phys. Rev. E 2016, 93, 053303. [Google Scholar] [CrossRef] [PubMed]
- Godoy, B.I.; Vickers, N.A.; Andersson, S.B. An Estimation Algorithm for General Linear Single Particle Tracking Models with Time-Varying Parameters. Molecules 2021, 26, 886. [Google Scholar] [CrossRef]
- Hozé, N.; Holcman, D. Statistical Methods for Large Ensembles of Super-Resolution Stochastic Single Particle Trajectories in Cell Biology. Annu. Rev. Stat. Appl. 2017, 4, 189–223. [Google Scholar] [CrossRef]
- Weron, A. Mathematical Models for Dynamics of Molecular Processes in Living Biological Cells. A Single Particle Tracking Approach. Ann. Math. Sil 2018, 32, 5–41. [Google Scholar] [CrossRef]
- Ernst, D.; Köhler, J.; Weiss, M. Probing the type of anomalous diffusion with single-particle tracking. Phys. Chem. Chem. Phys. 2014, 16, 7686–7691. [Google Scholar] [CrossRef] [PubMed]
- Janczura, J.; Balcerek, M.; Burnecki, K.; Sabri, A.; Weiss, M.; Krapf, D. Identifying heterogeneous diffusion states in the cytoplasm by a hidden Markov mode. New J. Phys. 2021, 23, 053018. [Google Scholar] [CrossRef]
- Metzler, R. Superstatistics and Non-Gaussian. Eur. Phys. J. Spec. Top. 2020, 229, 711–728. [Google Scholar] [CrossRef]
- Beck, C.; Cohen, E.D.B. Superstatistics. Phys. A Stat. Mech. Appl. 2003, 322, 267. [Google Scholar] [CrossRef]
- Molina-García, D.; Pham, T.M.; Paradisi, P.; Manzo, C.; Pagnini, G. Fractional kinetics emerging from ergodicity breaking in random media. Phys. Rev. E 2016, 94, 052147. [Google Scholar] [CrossRef] [PubMed]
- Maćkała, A.; Magdziarz, M. Statistical analysis of superstatistical fractional Brownian motion and applications. Phys. Rev. E 2019, 99, 012143. [Google Scholar] [CrossRef] [PubMed]
- Cherstvy, A.G.; Metzler, R. Nonergodicity, fluctuations, and criticality in heterogeneous diffusion processes. Phys. Rev. E 2014, 90, 012134. [Google Scholar] [CrossRef]
- Spakowitz, A.J. Transient Anomalous Diffusion in a Heterogeneous Environment. Front. Phys. 2019, 7, 119. [Google Scholar] [CrossRef]
- Chubynsky, M.V.; Slater, G.W. Diffusing diffusivity: A model for anomalous yet Brownian diffusion. Phys. Rev. Lett. 2014, 113, 098302. [Google Scholar] [CrossRef] [PubMed]
- Sposini, V.; Chechkin, A.V.; Seno, F.; Pagnini, G.; Metzler, R. Random diffusivity from stochastic equations: Comparison of two models for Brownian yet non-Gaussian diffusion. New J. Phys. 2018, 20, 043044. [Google Scholar] [CrossRef]
- Chechkin, A.V.; Seno, F.; Metzler, R.; Sokolov, I.M. Brownian yet Non-Gaussian Diffusion: From Superstatistics to Subordination of Diffusing Diffusivities. Phys. Rev. X 2017, 7, 021002. [Google Scholar] [CrossRef]
- Wang, W.; Cherstvy, A.G.; Chechkin, A.V.; Thapa, S.; Seno, F.; Liu, X.; Metzler, R. Fractional Brownian motion with random diffusivity: Emerging residual nonergodicity below the correlation time. J. Phys. A Math. Theor. 2020, 53, 474001. [Google Scholar] [CrossRef]
- Itto, Y.; Beck, C. Superstatistical modelling of protein diffusion dynamics in bacteria. J. R. Soc. Interface 2021, 18, 20200927. [Google Scholar] [CrossRef]
- Korabel, N.; Barkai, E. Paradoxes of subdiffusive infiltration in disordered systems. Phys. Rev. Lett. 2010, 104, 170603. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, S.; Korabel, N. Self-organized anomalous aggregation of particles performing nonlinear and non-Markovian random walks. Phys. Rev. E 2015, 92, 062127. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, S.; Han, D. Asymptotic behavior of the solution of the space dependent variable order fractional diffusion equation: Ultraslow anomalous aggregation. Phys. Rev. Lett. 2019, 123, 050602. [Google Scholar] [CrossRef]
- Sandev, T.; Chechkin, A.V.; Korabel, N.; Kantz, H.; Sokolov, I.M.; Metzler, R. Distributed-order diffusion equations and multifractality: Models and solutions. Phys. Rev. E 2015, 92, 042117. [Google Scholar] [CrossRef]
- Korabel, N.; Han, D.; Taloni, A.; Pagnini, G.; Fedotov, S.; Allan, V.; Waigh, T.A. Unravelling Heterogeneous Transport of Endosomes. arXiv 2021, arXiv:2107.07760. [Google Scholar]
- Newby, J.M.; Schaefer, A.M.; Lee, P.T.; Forest, M.G.; Lai, S.K. Convolutional neural networks automate detection for tracking of submicron-scale particles in 2D and 3D. Proc. Natl. Acad. Sci. USA 2018, 115, 9026–9031. [Google Scholar] [CrossRef]
- He, W.; Song, H.; Su, Y.; Geng, L.; Ackerson, B.J.; Peng, H.B.; Tong, P. Dynamic heterogeneity and non-Gaussian statistics for acetylcholine receptors on live cell membrane. Nat. Commun. 2016, 7, 11701. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.C.; Spakowitz, A.J.; Theriot, J.A. Bacterial Chromosomal Loci Move Subdiffusively through a Viscoelastic Cytoplasm. Phys. Rev. Lett. 2010, 104, 238102. [Google Scholar] [CrossRef] [PubMed]
- Weber, S.C.; Thompson, M.A.; Moerner, W.E.; Spakowitz, A.J.; Theriot, J.A. Analytical Tools To Distinguish the Effects of Localization Error, Confinement, and Medium Elasticity on the Velocity Autocorrelation Function. Biophys. J. 2012, 102, 2443–2450. [Google Scholar] [CrossRef] [PubMed]
- Waigh, T.A. Advances in the microrheology of complex fluids. Rep. Prog. Phys. 2016, 79, 74601–74663. [Google Scholar] [CrossRef]
- Savin, T.; Doyle, P.S. Static and Dynamic Errors in Particle Tracking Microrheology. Biophys. J. 2005, 88, 623–638. [Google Scholar] [CrossRef]
- Etoc, F. Non-specific interactions govern cytosolic diffusion of nanosized objects in mammalian cells. Nat. Mater. 2018, 17, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Weigel, A.V.; Ragi, S.; Reid, M.L.; Chong, E.K.P.; Tamkun, M.M.; Krapf, D. Obstructed diffusion propagator analysis for single-particle tracking. Phys. Rev. E 2012, 85, 041924. [Google Scholar] [CrossRef]
- Flores-Rodriguez, N.; Rogers, S.S.; Kenwright, D.A.; Waigh, T.A.; Woodman, P.G.; Allan, V.J. Roles of dynein and dynactin in early endosome dynamics revealed using automated tracking and global analysis. PLoS ONE 2011, 6, e24479. [Google Scholar] [CrossRef]
- Zajac, A.L.; Goldman, Y.E.; Holzbaur, E.L.; Ostap, E.M. Local cytoskeletal and organelle interactions impact molecular motor-driven early endosomal trafficking. Curr. Biol. 2013, 23, 1173–1180. [Google Scholar] [CrossRef]
- Krapf, D. Mechanisms underlying anomalous diffusion in the plasma membrane. Curr. Top. Membr. 2015, 75, 167–207. [Google Scholar]
- Jeon, J.H.; Javanainen, M.; Martinez-Seara, H.; Metzler, R.; Vattulainen, I. Protein Crowding in Lipid Bilayers Gives Rise to Non-Gaussian Anomalous Lateral Diffusion of Phospholipids and Proteins. Phys. Rev. X 2016, 6, 021006. [Google Scholar] [CrossRef]
- Foret, L.; Dawson, J.E.; Villaseñor, R.; Collinet, C.; Deutsch, A.; Brusch, L.; Zerial, M.; Kalaidzidis, Y.; Jülicher, F. A general theoretical framework to infer endosomal network dynamics from quantitative image analysis. Curr. Biol. 2012, 22, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, Q.; Liang, L. Real-time visualization of clustering and intracellular transport of gold nanoparticles by correlative imaging. Nat Commun. 2017, 8, 15646. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; McMahon, S.J.; Paganetti, H.; Schuemann, J. Biological modeling of gold nanoparticle enhanced radiotherapy for proton therapy. Phys. Med. Biol. 2015, 60, 4149. [Google Scholar] [CrossRef] [PubMed]
- Villagomez-Bernabe, B.; Currell, F.J. Physical Radiation Enhancement Effects Around Clinically Relevant Clusters of Nanoagents in Biological Systems. Sci. Rep. 2019, 9, 8156. [Google Scholar] [CrossRef]
- Sotiropoulos, M.; Henthorn, N.T.; Warmenhoven, J.W.; Mackay, R.I.; Kirkby, K.J.; Merchant, M.J. Modelling direct DNA damage for gold nanoparticle enhanced proton therapy. Nanoscale 2017, 9, 18413. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korabel, N.; Han, D.; Taloni, A.; Pagnini, G.; Fedotov, S.; Allan, V.; Waigh, T.A. Local Analysis of Heterogeneous Intracellular Transport: Slow and Fast Moving Endosomes. Entropy 2021, 23, 958. https://doi.org/10.3390/e23080958
Korabel N, Han D, Taloni A, Pagnini G, Fedotov S, Allan V, Waigh TA. Local Analysis of Heterogeneous Intracellular Transport: Slow and Fast Moving Endosomes. Entropy. 2021; 23(8):958. https://doi.org/10.3390/e23080958
Chicago/Turabian StyleKorabel, Nickolay, Daniel Han, Alessandro Taloni, Gianni Pagnini, Sergei Fedotov, Viki Allan, and Thomas Andrew Waigh. 2021. "Local Analysis of Heterogeneous Intracellular Transport: Slow and Fast Moving Endosomes" Entropy 23, no. 8: 958. https://doi.org/10.3390/e23080958
APA StyleKorabel, N., Han, D., Taloni, A., Pagnini, G., Fedotov, S., Allan, V., & Waigh, T. A. (2021). Local Analysis of Heterogeneous Intracellular Transport: Slow and Fast Moving Endosomes. Entropy, 23(8), 958. https://doi.org/10.3390/e23080958








